Human male sexual development and function require testosterone (T), the major circulating form of androgen secreted by the testes, and conversion of T in peripheral male accessory sex organs to dihydrotestosterone (DHT), a more potent androgen. The functional effects of T and DHT are mediated by high-affinity binding to the androgen receptor (AR), a ligand-activated transcription factor that interacts with DNA, coregulators, and the transcriptional machinery of chromatin.
Of the two active androgens, T predominates in muscle and DHT in prostate, appearing to provide proof-of-principle that tissue-selective ligands can be identified for AR (1). However, differences in tissue levels of 5α-reductase, the enzyme that converts T to DHT (Fig. 1), to a large extent account for tissue androgen levels. Furthermore, there is no compelling evidence that T and DHT regulate different sets of genes. Rather, more rapid binding kinetics contribute to T being a weaker androgen, requiring approximately 10-fold higher levels than DHT for the same transcriptional response (2, 3). Nevertheless, myotrophic synthetic steroid derivatives discovered over the past several decades are being used to promote anabolic effects on protein metabolism (4 – 8).
FIG. 1.
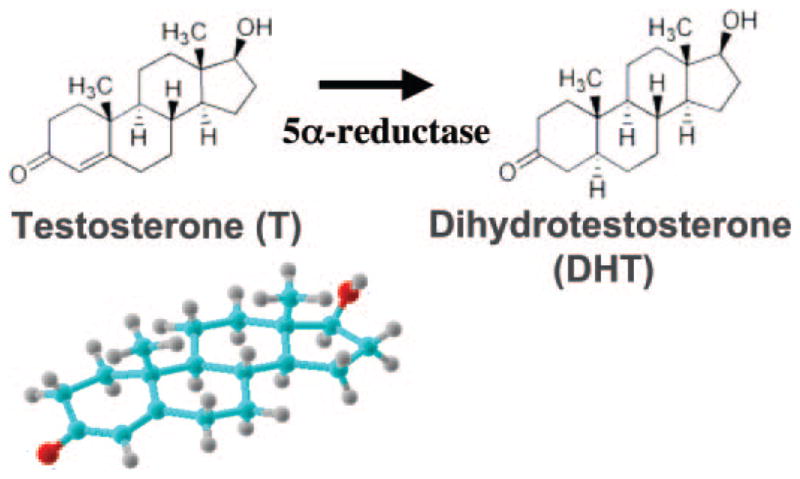
Physiologically active forms of androgen. In the male, T is produced by the testes and secreted into the blood. DHT, a more potent androgen, is produced by peripheral conversion from T by 5α-reductase. Levels of 5α-reductase are low in muscle, making T the predominant androgen, and higher in prostate where DHT predominates. The model of T was obtained from http://chemistry.umeche.maine.edu/CHY132/Ster-stat.html.
More recently, the pharmaceutical industry has been actively screening nonsteroidal ligands for anabolic activity with the intent to minimize androgenic effects in prostate and liver toxicity associated with steroid derivatives (9). Structural studies have revealed a remarkable flexibility of amino acid side chains lining the ligand binding pocket that enable steroid receptors to accommodate a wide range of chemical classes, despite an apparent rigid backbone core structure of the ligand binding domain. Among the clinically more useful receptor selective modulators is tamoxifen, an estrogen receptor antagonist used in the treatment of breast cancer (10).
It is therefore of interest that Ostrowski and co-workers at Bristol-Myers Squibb (1) have demonstrated muscle-selective effects of a high-affinity, orally active, nonsteroidal synthetic ligand, BMS-564929. Their assays in castrated rats indicate that BMS-564929 has a strikingly greater dose-dependent effect in the bulbocavernosus levator ani muscle than in prostate. The backbone structure of the BMS-564929-bound AR ligand binding domain is indistinguishable from the DHT-bound crystal structure. Like DHT, BMS-564929 makes hydrogen bond contacts to several key residues in the binding pocket required for high-affinity specific binding.
The mechanism for the apparent muscle selectivity of BMS-564929 awaits further study. One possibility is tissue-selective metabolism. In vivo hydroxylation of nonsteroidal ligands can alter the binding affinity for AR (11). Alternatively, greater activity in muscle may result from ligand-dependent, tissue-selective recruitment of coactivators to the AR transcription complex. AR activation function 1 (AF1), a predominant activation region located in the AR NH2-terminal domain (Fig. 2), is largely insensitive to ligand-specific effects beyond the requirements for agonist-induced AR nuclear transport and DNA binding (12). In contrast, activation function 2 (AF2) in the ligand binding domain lies close to the bound ligand, well positioned to respond to ligand-induced structural changes required for its interaction with α-helical motifs. AR AF2 serves as an androgen-dependent interaction site for FXXLF and WXXLF motifs present in AR that mediate the AR NH2- and carboxyl-terminal N/C interaction (13–16), FXXLF motifs in coregulatory proteins (17, 18), and as yet unidentified cofactors (18–22) and LXXLL motifs of SRC/p160 coactivators (Fig. 2) (17).
FIG. 2.
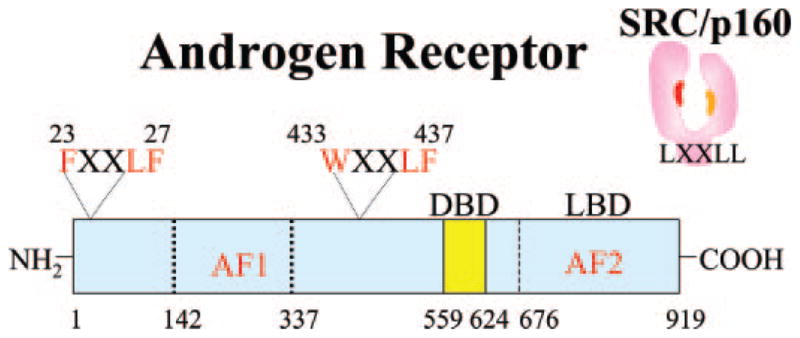
Human AR domain structure and an interacting SRC/p160 coactivator. AR contains a DNA binding domain (DBD), ligand binding domain (LBD), and NH2-terminal region. A predominant AF1 region is in the NH2-terminal domain. AF2 is a hydrophobic surface in the LBD that interacts in an androgen-dependent manner with the AR NH2-terminal FXXLF and WXXLF motifs. AR AF2 also serves as an interaction site for the LXXLL motifs in AR coregulator proteins such as the SRC/p160 coactivators (right, SRC/p160).
Crystal structures of the agonist-bound AR ligand binding domain bound with an LXXLL or FXXLF motif peptide confirm the close structural relationship between bound ligand and AF2 (23). Structural communication from the receptor interior to the exposed AF2 surface is evident from naturally occurring loss-of-function AR gene mutations that cause the androgen insensitivity syndrome (24, 25) and gain-of-function AR gene mutations in prostate cancer that increase AR FXXLF motif binding and coactivator recruitment (24). Steroid or nonsteroid ligand binding can induce subtle changes in van der Waals contacts and hydrogen bonding pathways (26) that specify motif binding at the AF2 surface and contribute to tissue-selective AR transcriptional activity. Indeed, Ostrowski et al. report amino acid contacts of BMS-564929 that differ from DHT and presumably specify tissue-selective interactions of AR with an as yet unidentified muscle cell-specific AR coregulator.
What are the caveats? Human muscle mass increases in response to T or DHT with increased myogenesis (29 –31). However, rat levator ani muscle, a frequently studied model of androgen effects in muscle because of higher AR levels compared with most skeletal muscle (27), may not necessarily be indicative of general anabolic activity (8, 28). Furthermore, if BMS-564929 is not readily metabolized and excreted, prolonged use could have undesirable effects on other organ systems including the prostate or, as shown for BMS-564929, suppression of LH in classical androgen-dependent feedback inhibition of the hypothalamic-pituitary axis. Evidence that the muscle-selective effects of BMS-564929 are inhibited by classical AR antagonists such as hydroxyflutamide and ca-sodex would provide support for a direct action through the AR.
Whether the beneficial effects of BMS-564929 aimed at improving body strength, sex drive, cognition, and overall well-being in men with hypogonadism or age-related decline in T levels outweigh adverse effects awaits the results of ongoing clinical trials. Nevertheless, in a broader sense, the development of tissue-selective nuclear receptor modulators may provide new pharmaceutical agents for treating disease and improving the quality of life.
Acknowledgments
I am grateful to Robert T. Gampe, Jr., and Frank S. French for reviewing the manuscript.
Abbreviations
- AF1
Activation function 1
- AF2
activation function 2
- AR
androgen receptor
- DHT
dihydrotestosterone
- T
testosterone
References
- 1.Ostrowski J, Kuhns JE, Lupisella JA, Manfredi MC, Beehler BC, Krystek SR, Jr, Bi Y, Sun C, Seethala R, Golla R, Sleph PG, Fura A, An Y, Kish KF, Sack JS, Mookhtiar KA, Grover GJ, Hamann LG. Pharmacological and x-ray structural characterization of a novel selective androgen receptor modulator: potent hyperanabolic stimulation of skeletal muscle with hypostimulation of prostate in rats. Endocrinology. 2007;148:4–12. doi: 10.1210/en.2006-0843. [DOI] [PubMed] [Google Scholar]
- 2.Wilson EM, French FS. Binding properties of androgen receptors: evidence for identical receptors in rat testis, epididymis, and prostate. J Biol Chem. 1976;251:5620–5629. [PubMed] [Google Scholar]
- 3.Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990;126:1165–1172. doi: 10.1210/endo-126-2-1165. [DOI] [PubMed] [Google Scholar]
- 4.Edgren RA. A comparative study of the anabolic and androgenic effects of various steroids. Acta Endocrinol. 1963;44(Suppl 87):1–21. doi: 10.1530/acta.0.044s0003. [DOI] [PubMed] [Google Scholar]
- 5.Krüskemper HL. Anabolic steroids. New York: Academic Press; 1968. [Google Scholar]
- 6.Kochakian CD. Definition of androgens and protein anabolic steroids. Pharmacol Ther. 1975;1:149–177. doi: 10.1016/0306-039x(75)90002-1. [DOI] [PubMed] [Google Scholar]
- 7.Toth M, Zakar T. Relative binding affinities of testosterone, 19-nortestosterone and their 5 α-reduced derivatives to the androgen receptor and to other androgen-binding proteins: a suggested role of 5 α-reductive steroid metabolism in the dissociation of “myotropic” and “androgenic” activities of 19-nortestosterone. J Steroid Biochem. 1982;17:653– 660. doi: 10.1016/0022-4731(82)90567-2. [DOI] [PubMed] [Google Scholar]
- 8.Kopera H. The history of anabolic steroids and a review of clinical experience with anabolic steroids. Acta Endocrinologica Suppl. 1985;271:11–18. doi: 10.1530/acta.0.109s00011. [DOI] [PubMed] [Google Scholar]
- 9.Negro-Vilar A. Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium. J Clin Endocrinol Metab. 1999;54:3459–3462. doi: 10.1210/jcem.84.10.6122. [DOI] [PubMed] [Google Scholar]
- 10.McKenna NJ, O’Malley BW. An issue of tissues: divining the split personalities of selective estrogen receptor modulators. Nat Med. 2000;6:960–962. doi: 10.1038/79637. [DOI] [PubMed] [Google Scholar]
- 11.Peets EA, Henson MF, Neri R. On the mechanism of the anti-androgenic action of flutamide (α-α-α-trifluoro-2-methyl-4′-nitro-m-propionotoluidide) in the rat. Endocrinology. 1974;94:532–540. doi: 10.1210/endo-94-2-532. [DOI] [PubMed] [Google Scholar]
- 12.Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266:510–518. [PubMed] [Google Scholar]
- 13.He B, Kemppainen JA, Wilson EM. FXXLF and WXXLF sequences mediate the NH2-terminal interaction with the ligand binding domain of the androgen receptor. J Biol Chem. 2000;275:22986–22994. doi: 10.1074/jbc.M002807200. [DOI] [PubMed] [Google Scholar]
- 14.Langley E, Zhou ZX, Wilson EM. Evidence for an anti-parallel orientation of the ligand-activated human androgen receptor dimer. J Biol Chem. 1995;270:29983–29990. doi: 10.1074/jbc.270.50.29983. [DOI] [PubMed] [Google Scholar]
- 15.Langley E, Kemppainen JA, Wilson EM. Intermolecular NH2-/carboxyl-terminal interactions in androgen receptor dimerization revealed by mutations that cause androgen insensitivity. J Biol Chem. 1998;273:92–101. doi: 10.1074/jbc.273.1.92. [DOI] [PubMed] [Google Scholar]
- 16.Schaufele F, Carbonell X, Guerbadot M, Borngraeber S, Chapman MS, Ma AA, Miner JN, Diamond MI. The structural basis of androgen receptor activation: intramolecular and intermolecular amino-carboxy interactions. Proc Natl Acad Sci USA. 2005;102:9802–9807. doi: 10.1073/pnas.0408819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He B, Minges JT, Lee LW, Wilson EM. The FXXLF motif mediates androgen receptor-specific interactions with coregulators. J Biol Chem. 2002;277:10226–10235. doi: 10.1074/jbc.M111975200. [DOI] [PubMed] [Google Scholar]
- 18.Hsu CL, Chen YL, Yeh S, Ting HJ, Hu YC, Lin H, Wang X, Chang C. The use of phage display technique for the isolation of androgen receptor interacting peptides with (F/W)XXL(F/W) and FXXLY new signature motifs. J Biol Chem. 2003;278:23691–23698. doi: 10.1074/jbc.M211908200. [DOI] [PubMed] [Google Scholar]
- 19.Hur E, Pfaff SJ, Payne ES, Gron H, Buehrer BM, Fletterick RJ. Recognition and accommodation at the androgen receptor coactivator binding interface. PLoS Biol. 2004;2:E274. doi: 10.1371/journal.pbio.0020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estebanez-Perpina E, Moore JM, Mar E, Delgado-Rodrigues E, Nguyen P, Baxter JD, Buehrer BM, Webb P, Fletterick RJ, Guy RK. The molecular mechanisms of coactivator utilization in ligand-dependent transactivation by the androgen receptor. J Biol Chem. 2005;280:8060– 8068. doi: 10.1074/jbc.M407046200. [DOI] [PubMed] [Google Scholar]
- 21.van de Wijngaart DJ, van Royen ME, Hersmus R, Pike AC, Houtsmuller AB, Jenster G, Trapman J, Dubbink HJ. Novel FXXFF and FXXMF motifs in androgen receptor cofactors mediate high affinity and specific interactions with the ligand-binding domain. J Biol Chem. 2006;281:19407–19416. doi: 10.1074/jbc.M602567200. [DOI] [PubMed] [Google Scholar]
- 22.Chang CY, Abdo J, Hartney T, McDonnell DP. Development of peptide antagonists for the androgen receptor using combinatorial peptide phage display. Mol Endocrinol. 2005;19:2478–2490. doi: 10.1210/me.2005-0072. [DOI] [PubMed] [Google Scholar]
- 23.He B, Gampe RT, Kole AJ, Hnat AT, Stanley TB, An G, Stewart EL, Kalman RI, Minges JT, Wilson EM. Structural basis for androgen receptor interdomain and coactivator interactions suggests a transition in nuclear receptor activation function dominance. Mol Cell. 2004;16:425– 438. doi: 10.1016/j.molcel.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 24.He B, Gampe RT, Hnat AT, Faggart JL, Minges JT, French FS, Wilson EM. Probing the functional link between androgen receptor coactivator and ligand binding sites in prostate cancer and androgen insensitivity. J Biol Chem. 2006;281:6648– 6663. doi: 10.1074/jbc.M511738200. [DOI] [PubMed] [Google Scholar]
- 25.Ghali SA, Gottlieb B, Lumbroso R, Beitel LK, Elhaji Y, Wu J, Pinsky L, Trifiro MA. The use of androgen receptor amino/carboxyl-terminal interaction assays to investigate androgen receptor gene mutations in subjects with varying degrees of androgen insensitivity. J Clin Endocrinol Metab. 2003;88:2185–2193. doi: 10.1210/jc.2002-021324. [DOI] [PubMed] [Google Scholar]
- 26.Bohl CE, Miller DD, Chen J, Bell CE, Dalton JT. Structural basis for accommodation of nonsteroidal ligands in the androgen receptor. J Biol Chem. 2005;280:37747–37754. doi: 10.1074/jbc.M507464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenberg E, Gordan GS. The levator ani muscle of the rat as an index of myotrophic activity of steroidal hormones. J Pharmacol Exp Ther. 1950;99:38– 44. [PubMed] [Google Scholar]
- 28.Wilson JD. Androgen abuse by athletes. Endocr Rev. 1988;9:181–199. doi: 10.1210/edrv-9-2-181. [DOI] [PubMed] [Google Scholar]
- 29.Bhasin S, Woodhouse L, Storer TW. Proof of the effect of testosterone on skeletal muscle. J Endocrinol. 2001;170:27–38. doi: 10.1677/joe.0.1700027. [DOI] [PubMed] [Google Scholar]
- 30.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 31.Asthana S, Bhasin S, Butler RN, Fillit H, Finkelstein J, Harman SM, Holstein L, Korenman SG, Matsumoto AM, Morley JE, Tsitouras P, Urban R. Masculine vitality: pros and cons of testosterone in treating the andropause. J Gerontol A Biol Sci Med Sci. 2004;59:461–465. doi: 10.1093/gerona/59.5.m461. [DOI] [PubMed] [Google Scholar]


