Abstract
Crohn’s disease (CD) is a multifactorial pathology associated with the presence of adherent-invasive Escherichia coli (AIEC) and NLRP3 polymorphic variants. The presence of intracellular E. coli in other intestinal pathologies (OIP) and the role of NLRP3-inflammasome in the immune response activated by these bacteria have not been investigated. In this study, we sought to characterize intracellular strains isolated from patients with CD, ulcerative colitis (UC) and OIP, and analyze NLRP3-inflammasome role in the immune response and bactericidal activity induced in macrophages exposed to invasive bacteria. For this, intracellular E. coli isolation from ileal biopsies, using gentamicin-protection assay, revealed a prevalence and CFU/biopsy of E. coli higher in biopsies from CD, UC and OIP patients than in controls. To characterization of bacterial isolates, pulsed-field gel electrophoresis (PFGE) patterns, virulence genes, serogroup and phylogenetic group were analyzed. We found out that bacteria isolated from a given patient were closely related and shared virulence factors; however, strains from different patients were genetically heterogeneous. AIEC characteristics in isolated strains, such as invasive and replicative properties, were assessed in epithelial cells and macrophages, respectively. Some strains from CD and UC demonstrated AIEC properties, but not strains from OIP. Furthermore, the role of NLRP3 in pro-inflammatory cytokines production and bacterial elimination was determined in macrophages. E. coli strains induced IL-1β through NLRP3-dependent mechanism; however, their elimination by macrophages was independent of NLRP3. Invasiveness of intracellular E. coli strains into the intestinal mucosa and IL-1β production may contribute to CD and UC pathogenesis.
Keywords: E. coli, Crohn’s disease, IL-1β, NLRP3, inflammasome
INTRODUCTION
Inflammatory bowel diseases (IBD) comprise a variety of relapsing and remitting clinical conditions, all a result of chronic inflammation of the gastrointestinal tract. Crohn’s disease (CD) and ulcerative colitis (UC) are the major forms of IBD (Abraham and Cho, 2009). High IBD incidence has been reported in Europe and North America, and although there is sparse data from South America, clinical experience suggests increasing rates of IBD in recent years (Cosnes et al., 2011; Figueroa et al., 2005).
CD can affect any area of the gastrointestinal tract; however, the terminal ileum and colon are more frequently involved. Characteristics of CD are patchy inflammation, full-thickness lesions of the intestinal wall, and granulomas (Louis et al., 2001; Odze, 2003). UC, unlike CD, is characterized by continuous and symmetrical damage of the colonic intestinal mucosa (Abraham and Cho, 2009; Louis et al., 2001; Odze, 2003). Both pathologies present elevated proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6. In CD, there is a predominance of Th1 cytokines, whereas UC seems to be a Th2-like cytokine-mediated disease (Strober and Fuss, 2011). Although the aetiology of IBD remains poorly understood, several environmental, immunological, and genetic factors have been linked to the disease risk (Abraham and Cho, 2009). Recent studies have suggested that intestinal microbiota composition contribute to CD pathogenesis. Compared to normal individuals, CD patien’s microbiota display reduced diversity and altered composition (Walker et al., 2011). E. coli are commensal bacteria that colonize the human gastrointestinal tract. However, some E. coli pathovars have acquired virulence factors, presumably increasing their propensity to cause enteric disease. Six categories of classic diarrheagenic E. coli (DEC) have been described (Kaper et al., 2004). Likewise, analysis of CD patient-derived tissue has identified bacteria named adherent-invasive E. coli (AIEC) (Hansen et al., 2010) as potential contributors to CD pathogenesis (Carvalho et al., 2009; Darfeuille-Michaud et al., 2004; Nash et al., 2010). Previous studies showed that 22-65% of CD patients harbour AIEC, compared to 6-9% in controls (Darfeuille-Michaud et al., 2004; Glasser et al., 2001; Sasaki et al., 2007). These E. coli strains are characterized by the absence of specific virulence factors characteristic of classic DEC, similarity to extra-intestinal E. coli pathovars and capability to adhere and to invade intestinal epithelial cells and macrophages in vitro (Glasser et al., 2001; Martinez-Medina et al., 2009a; Nash et al., 2010). The mechanism by which AIEC accesses to the mucosa is not completely defined yet. It has been proposed that AIEC adhere via FimH, the terminal subunit of the type 1 pilus, to carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) abnormally expressed in ileal mucosa of CD patients (Barnich et al., 2007). Additionally, AIEC has been also shown to adhere and translocate through Peye’s Patches via long polar fimbriae (Chassaing et al., 2011). The above findings have led to the hypothesis that AIEC represents a bacterial pathotype associated with CD (Eaves-Pyles et al., 2008; Glasser et al., 2001), however a pathogenic role in CD of these bacteria is controversial. AIEC strains NRG857c, HM605, and LF82, isolated from CD patients, have been sequenced and used as reference pathotype (Clarke et al., 2011; Miquel et al., 2010; Nash et al., 2010). However none of them show defined virulence determinant genes. On the other hand, changes in intestinal microbiota have also been observed in other inflammatory pathologies (OIP) of the intestine, such as irritable bowel syndrome (IBS) or diverticulitis, whose aetiology has not been completely elucidated (Strate et al., 2012). However, presence of intracellular E. coli with adherent-invasive properties has not been studied in OIP.
Multiple variants of pattern-recognition receptor (PRR) genes that sense pathogen-associated molecular patterns (PAMPs) have been associated with IBD (Kaser et al., 2010; Shih and Targan, 2008), including NOD2 (Nucleotide-binding oligomerization domain containing 2), TLR4 (Toll-like receptor 4) and NLRP3 (NOD-like receptor family, pyrin domain containing 3) (Peeters et al., 2007; Shen et al., 2010; Villani et al., 2009). Several genetic variants in the non-coding region of NLRP3 and decreased expression of the receptor have been associated with increased susceptibility to CD (Villani et al., 2009). NLRP3 is one of the sensors capable to induce the formation of the multi-protein complex inflammasome, which activates caspase-1 through adaptor protein ASC (apoptosis-associated speck-like protein containing a carboxy-terminal CARD) (Franchi et al., 2012), promoting IL-1β and IL-18 processing and secretion. Different microbial stimuli, such as E. coli RNA and enterotoxin, have been shown to activate NLRP3 (Brereton et al., 2011; Sander et al., 2011). Although the inflammasome has been involved in some bacterial elimination by macrophages (Pereira et al., 2011), a link between NLRP3-inflammasome and mucosa-associated E. coli driven-inflammatory responses has not been investigated.
This study aimed to assess intracellular E. coli prevalence in mucosal from patients with CD, UC, OIP and controls as well as to characterize virulence genes, clonality among strains and AIEC properties. Moreover, the relationship between NLRP3-inflammasome and intracellular E.coli driven-inflammatory responses by macrophages, cytokine induction and microbicidal ability was determined.
MATERIALS AND METHODS
Patients
E. coli strains included in this study were isolated from ileal specimens of Chilean patients: 34 with CD; 57 with UC; 17 with other intestinal pathologies (OIP), and 22 control individuals, subjected to colonoscopy for colon cancer evaluation. Patients were diagnosed based on standardized clinical, endoscopic and histological criteria. All patients undergoing colonoscopy were older than 18 years and were not under antimicrobial treatment for at least 2 weeks before the procedure. Clinical characteristics of patients included in the study are shown in Supplementary Table 1. In the case of UC, endoscopic activity was determined in the damage area using the endoscopic Mayo Score and for CD the Simple Endoscopic Score for Crohn’s Disease (SES-CD).
Patients were recruited at Clínica Las Condes and Hospital Clínico Pontificia Universidad Católica de Chile in Santiago, Chile from July 2010 to January 2011. The study was approved by the Institutional Review Board of Clínica Las Condes, Faculty of Medicine Universidad de Chile and Hospital Clínico Pontificia Universidad Católica de Chile, as well as the Ethics Committee of the North Metropolitan Health Service, Santiago, Chile, and informed consent was obtained for all patients and control subjects.
Bacterial strains and culture conditions
E. coli strain isolation from intestinal biopsies of patients with CD, UC, OIP and controls for this study is detailed in “E. coli isolation” section. E. coli reference strains HS, HB101, NRG857c and HM605 were used to compare genotypic and phenotypic characteristic with clinical isolates. HS strain (Levine et al., 1978) (GenBank CP000802) is a commensal bacteria and was used as negative control to invasiveness of epithelial cells. HB101 is a non-pathogenic E. coli and was used as negative control of adhesiveness as it lacks fimbriae to adhere to epithelial cells (Boyer and Roulland-Dussoix, 1969; Del Canto et al., 2012; Saldaña et al., 2009). AIEC reference strains NRG857c and HM605 were kindly provided by A. Torres and I. Henderson, respectively (Nash et al., 2010; Clarke et al., 2011). All strains were cultured in LB agar at 37°C with 5% CO2 or Luria Bertani broth (LB) Miller medium (Sigma-Aldrich, St Louis, MO, USA).
E. coli isolation
Intracellular bacteria were isolated from ileal mucosa biopsies by gentamicin protection assay. Tissues were suspended in Hank’s balanced salt solution (HBSS) 1X (Gibco BRL, Grand Island, NY, USA) supplemented with 100 μg/ml gentamicin (Sigma-Aldrich, St Louis, MO, USA), and incubated for 1 h at 37°C, to eliminate extracellular microbiota and favour invasive bacteria isolation. Samples were then washed with phosphate buffered saline (PBS) 1X (Calbiochem, Merck Biosciences Ltd, Nottingham, Darmstadt, Germany) and lysed in 100 μl of 1% Triton-X-100/PBS. Dilutions of tissue homogenate were made, and the total volume of homogenate and dilutions were plated on MacConkey agar (Oxoid ltd, Wade Road, Basingstoke, Hampshire, UK) and incubated for 18 h at 37°C. Individual bacterial colonies were grown in Luria Bertani broth (LB) Miller medium (Sigma-Aldrich, St Louis, MO, USA) under aerobic conditions for 18 h at 37°C. Grown bacteria were biochemically analysed for E. coli identification and the stored in 30% glycerol at −80°C.
Cells
To determine bacterial invasion of epithelial cells and survival in macrophages, Caco-2 and RAW264.7 cell lines were used, respectively. Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics (Gibco BRL, Grand Island, NY) at 37°C and 5% CO2.
To determine cytokine production and bacterial clearance by macrophages exposed to E. coli strains, bone marrow-derived macrophages (BMDMs) from Asc−/−, Nlrp3−/−, and wild-type (WT) mice were used, as has been previously described (Kanneganti et al., 2006; Ozören et al., 2006). Animal manipulation was conducted according approved University of Michigan Committee on the use and care of animal’s protocols. Mice housed in a pathogen-free facility were used for bone-marrow-derived macrophages isolation, as previously described (Davies and Gordon, 2005). Briefly, femurs and tibias were removed from euthanized mice, marrow cavity plugs were washed out, and bone marrow cells were suspended in DMEM supplemented with 25% L cell-conditioned medium, 15% calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin, plated and cultured at 37°C and 5% CO2. After 5-6 days, resulting bone-marrow-derived macrophages were replated one day before the experimental procedure. Immortalized BMDMs from Nlrp3 deficient and WT mice, previously described (Halle et al., 2008), were maintained in RPMI-1640 (HyClone, Laboratories Inc, Utah, USA) supplemented with 10% FBS, and antibiotics at 37°C and 5%CO2.
Virulence genotyping by PCR
For virulence genotyping, a multiplex PCR protocol was used to determine genes specifically associated with defined classic DEC (EPEC, ETEC, EIEC, EHEC, DAEC, EAEC) pathotypes (Vidal et al., 2005). In addition, extra-intestinal pathogenic E. coli (ExPEC) associated genes, such as fimH, papC, neuC, ibeA, hlyA, cnfI, sfaDE, iucD, fimA, and cdtB, previously described for AIEC strains (Martinez-Medina et al., 2009b) were assessed by monoplex PCR (Blanco et al., 1997; Ewers et al., 2007; Johnson and Stell, 2000; Moulin-Schouleur et al., 2006; Tiba et al., 2008).
Pulse-Field Gel Electrophoresis
The genetic relationship among isolated E. coli strains, commensal HS, and reference AIEC strains NRG857c and HM605 was analysed by pulse-field gel electrophoresis (PFGE) as described previously (Céspedes et al., 2011). Salmonella braenderup was used as a gel loading control. Briefly, genomic DNA (gDNA) was subjected to enzymatic digestion using XbaI. DNA fragments were separated by PFGE on a CHEF-DRIII system (Bio-Rad Laboratories, Richmond, CA, USA) using 1% agarose gel (pulsed-field certified agarose; BioRad Laboratories, Richmond, CA, USA) and 0.5X TBE buffer (45 mM Tris, 45 mM boric acid, 1 mM EDTA [pH 8.0]) at 6 V/cm and 14°C. The Gel Compare software (Applied Maths, Kortrijk, Belgium) was used for gel analysis. Similarity indices were estimated using the Dice method, with a band position tolerance of 1 or 1.5%, based on the Unweighted Pair Group Method with Arithmetic Mean (UPGMA). The similarity percentage cut-off to distinguish clonally distinct groups was 95%.
Phylogenetic group and serogroups determination
The major E. coli phylogenetic group (A, B1, B2, and D) was determined by PCR (Clermont et al., 2000). O antigen was determined using antisera raised against O1 to O185 serogroups, as previously described (Guinée et al., 1972). All antisera, kindly provided by Dr. Blanco Laboratory, LREC, Universidad de Santiago de Compostela, Lugo, Spain, were adsorbed with cross-reacting antigens to remove non-specific agglutinins. E. coli isolates that did not react with any O antisera were classified as nontypeable (NT).
Infection assays
Cell invasion analyses were carried out in Caco-2 cells cultured in DMEM without antibiotics, and maintained in 5% CO2 and 37°C. Cell monolayers were infected with E.coli strains at multiplicity of infection (MOI) of 10, for 3h at 37 °C. After the infection period, cells were washed with 1× PBS and placed in fresh medium supplemented with gentamicin (3 mg/ml), incubated for 1 h at 37°C, and lysed with 1% Triton-X-100/PBS. Lysate serial dilutions were plated on LB agar (Merck KGaA, Darmstadt, Germany) and incubated at 37°C overnight. Colonies were counted the next day and invasion percentage was determined as intracellular bacterial content at 3 h post-infection (h.p.i) in relation to the initial inoculum. We considered invasive strains those with an invasion level that exceeded by at least a factor of five that of commensal strain HS (≥0.65%), which is within the invasion range previously described. (Darfeuille-Michaud et al., 2004) Cell adhesion analysis was also carried out in Caco-2 cells using similar infection conditions as described for invasion assays, but omitting the gentamicin treatment. The E. coli strain, HB101 was used as a negative control. Adhesion percentage was determined as recovered bacterial content at 30 min post-infection in relation to the initial inoculum.
RAW 264.7 cells, bone marrow-derived or immortalized macrophages from Asc, Nlrp3 deficient and WT mice were infected with E. coli strains (MOI = 20) for 2h at 37°C. Cells were then washed in PBS and placed in fresh medium supplemented with gentamicin (3mg/ml). Intracellular bacterial content was determined at 3, 24, and 48 h.p.i. at 37°C and the ratio between bacterial content at each period and content at 3 h.p.i. was determined.
Cytokine induction by intracellular E. coli strains
Bone marrow-derived or immortalized macrophages from Asc, Nlrp3 deficient and WT mice were infected with mucosa-isolated E. coli, HS, or NRG857c strains and TNF-α and IL-1β induction was determined in cell supernatants by enzyme-linked immunosorbent assay (ELISA) kit (R&D System, Minneapolis, MN, USA). Asc, Nlrp3 deficient or WT macrophages were infected with E. coli strains (MOI = 10) for 2h at 37 °C, then washed in PBS, placed in fresh medium supplemented with gentamicin (3mg/ml), and incubated for 24h at 37°C.
Immunoblotting
For caspase-1 detection, cells were lysed with LDB supplemented with Complete Mini Protease Inhibitor Cocktail tablets (Roche® Applied Science, Indianapolis, IN, USA). Protein concentration was determined by Bradford assay (BioRad, Hercules, CA, USA). Proteins were resolved by 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and electrotransferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). Non-specific binding was blocked with 5% non-fat milk in TBS-Tween (2 mM Tris-HCl, pH 7.6, 13.7 mM NaCl). Detection of caspase-1 was determined using rabbit anti-mouse caspase-1 that was a gift from Dr. Vandanabeele (Ghent University, Ghent, Belgium), followed by horseradish peroxidase-conjugated anti-rabbit antibody (Sigma-Aldrich, Saint Louis, MO, USA). Immuno-reactive bands were visualized with SuperSignal® West Femto Maximum Sensitivity Substrate (Thermo Scientific, N Meridian, Rockford, USA).
Statistical analysis
To compare E. coli content in biopsies from controls and patients with CD, UC, or OPI, the Kruskal-Wallis two-tailed test and Dunn’s Multiple Comparison test were used, with a significance of <0.05.To compare the prevalence of E. coli in different diseases in regard to control Fisher test with two-sided p-values (p<0.05) was used. To compare cytokine levels in conditioned media of Asc, Nlrp3 deficient or WT macrophages (n=3), an unpaired t-test was used, with a significance of <0.05 (*<0.05, **<0.01, ***<0.001).
RESULTS
Intracellular E. coli in intestinal mucosa: prevalence and content in patients with Crohn’s disease, ulcerative colitis, and other inflammatory pathologies
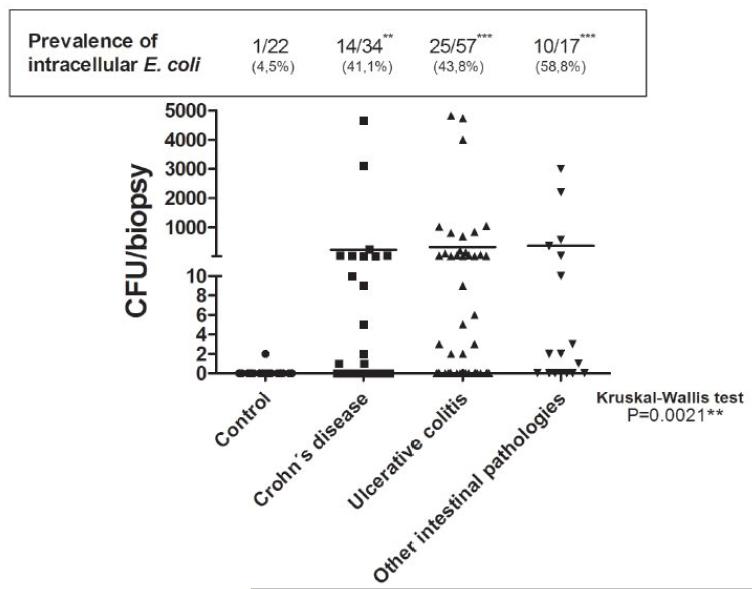
The prevalence of intracellular E. coli isolated from ileal biopsies, defined as the percentage of patients presenting bacterial colonies by gentamicin protection assay, was 14/34 (41.1%) CD, 25/57 (43.8%) UC, 10/17 (58.8%) OIP, and 1/22 (4.5%) controls (p<0.05) (Figure 1). Notably, the number of E. coli in CD, UC, and OIP patients was higher than in controls (p=0.0021) (Figure 1). Colony forming unit (CFU) counts were highly variable in each group of patients and between them, ranging from 1-4,700 in CD, 1-4,800in UC, and 1-3,000 in OIP patients, whereas only 2 CFU/biopsy of intracellular E. coli were identified in one control subject (Figure 1).
Figure 1. Intracellular E. coli in intestinal biopsies from CD, UC and OIP patientes.
Ileal biopsies from patients with CD (n=34), UC (n=57), OIP (n=17), and controls (n=22) were incubated with gentamicin for 1h, and homogenates were then plated in MacConkey agar. Graph shows the bacterial content represented as colony-forming units (CFU) of identified intracellular E. coli in each biopsy and mean bacteria in each group (Kruskal-Wallis two-tailed test, Dunn’s Multiple Comparison test was used, significance level set at <0.05). Prevalence of putative invasive E. coli is expressed as the percentage of patients with the bacteria in each group; Fisher’s exact test with a two-sided p-value was used to evaluate statistical significance as compared to the control group (p<0.05, CD p**=0.0023, UC p***=0.0005, OIP p***=0.0003).
In terms of clinical characteristics, 36% and 60% of CD patients containing bacteria or not were women, respectively. In CD patients, there was no correlation between the presence of bacteria and disease activity or intestinal area affected. Furthermore, no association between the presence of E. coli and gender was identified in UC and OIP patients. However, there was an association between the presence of E. coli and disease activity in UC patients (p 0.0277), in which 88% of those containing bacteria were in an active state of the disease compared to 66% of those patients without bacteria in ileal mucosa.
Genetic and phenotypic characterization of clinical isolates of E. coli strains
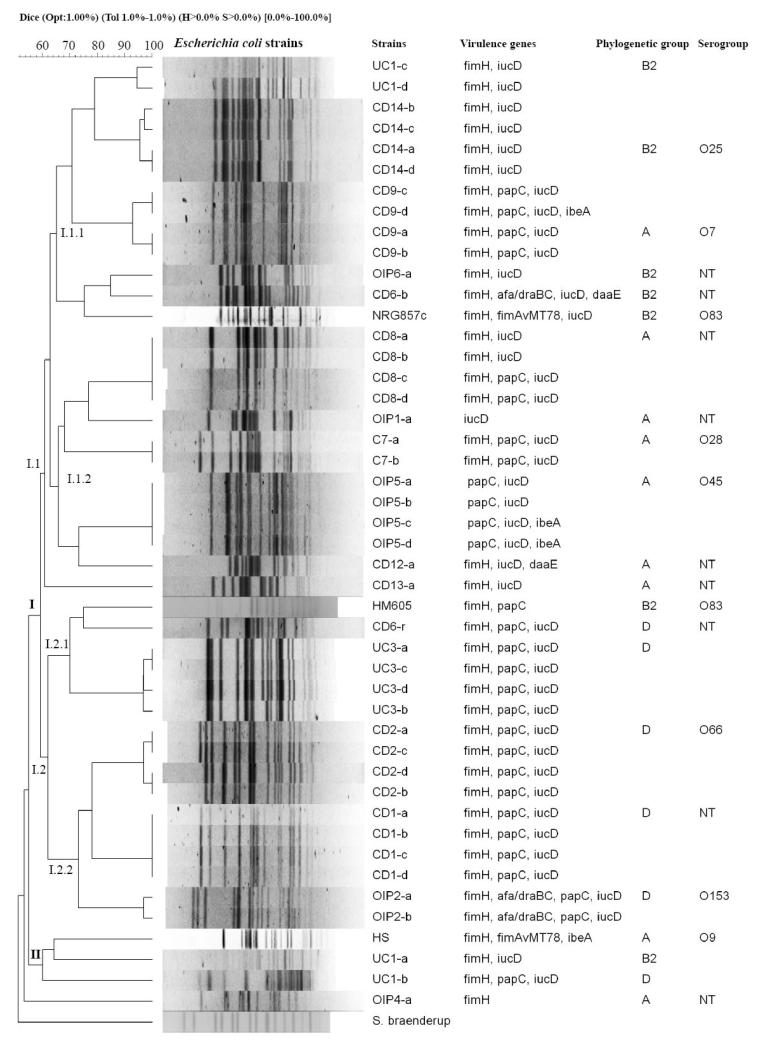
PFGE analysis and virulence genes typically associated with classic DEC or ExPEC (as described in methods section) were conducted in a total of 46 intracellular E. coli strains, 24 isolated from 8 CD patients, 9 from 5 patients with OIP, 8 from two UC patients, 2 from one control, as well as strains HS, NRG857c and HM605 (Figure 2). PFGE analysis and virulence gene identification showed that most isolates were clonal or closely related within a single patient, sharing virulence genes; however, strains were genetically heterogeneous among patients. Of the total E. coli strain tested, 46 were grouped into two major clusters, according to PFGE analysis (Figure 2); cluster I contains 42 strains derived from 8 CD, 2 UC and 4 OIP patients, control, NRG875c and HM605. Cluster II contains 3 strains, two derived from a UC patient and HS. The OIP4-a strain and Salmonella braenderup were excluded from both clusters according to PFGE analysis. Several genes including fimH, papC, and iucD that are characteristic of ExPEC, and previously described in AIEC reference strains, were common in most isolates (Figure 2). Results of phylogenetic group determination show that strains from clusters I.1.1, I.1.2 and I.2 mostly belong to groups B2, A and D, respectively (Figure 2). E. coli strains (CD6-b and CD12-a) isolated from a CD patient (cluster I) harboured the daaE gene, which is characteristic of DAEC pathotype (Figure 2).
Figure 2. Genetic variability among E. coli isolates from patients with CD, UC and OIP.
Dendrogram based on pulsed-field gel electrophoresis (PFGE) of E. coli strain DNA digested with XbaI. PFGE analysis identified two groups of genetically-related strain clusters (I and II). Cluster A harboured the greatest number of strains and was in turn divided into I.1 (I.1.1 and I.1.2) and I.2 (I.2.1 and I.2.2). The right side of the figure shows the strain name (patient code-clone), virulence genes identified in each strain by PCR, phylogenetic group and serogroup. E. coli isolates were compared to HS commensal bacteria, and NRG857c and HM605 reference AIEC strains.
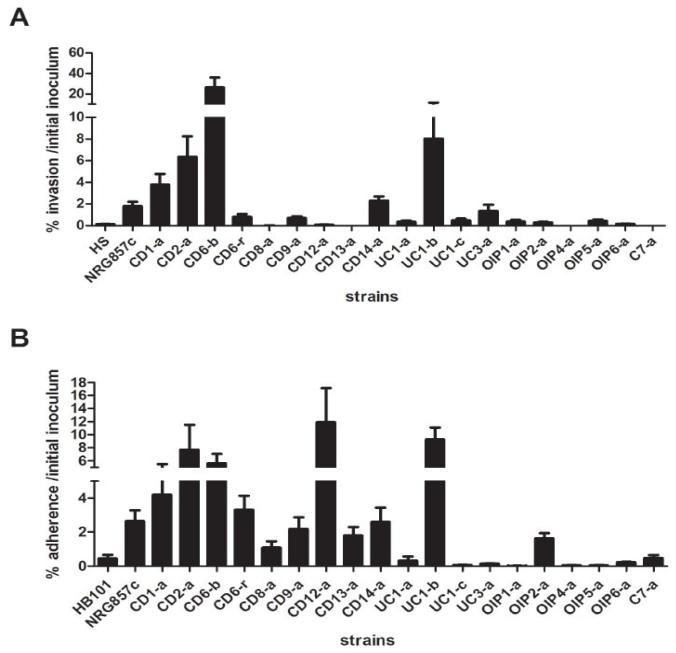
To evaluate phenotypic characteristics of isolated intracellular E. coli, Caco-2 cells were infected with 19 strains selected to represent those isolated from all group of patients and control. Figure 3A shows invasiveness properties of intracellular strains in Caco-2 cells, whereas the HS strain has a low invasive capacity and was considered a negative control (0.130%) and the NRG857c strain showed an invasiveness of 1.783%. Isolated E. coli strains from patients showed variable invasiveness; higher values were observed for CD1-a, CD2-a, CD6-b, CD6-r, CD9-a, CD14-a, UC1-b and UC3-a compared to HS strain (Figure 3A). Of these, CD1-a, CD2-a CD6-b and UC1-b strains were 2-15 times more invasive than NRG857c. Those obtained from different groups (C7-a, CD8-a, CD12-a, CD13-a, UC-1a, UC1-c, OIP1-a, OIP2-a, OIP4-a, OIP5-a and OIP6-a) showed a low invasive ability comparable to HS (Figure 3A).
Figure 3. Invasion and adhesion of isolated E. coli strains in epithelial cells.
(A) To evaluate invasion, Caco-2 cells were infected with representative E. coli strains isolated patient-derived tissue with a MOI=10 for 3h and then incubated with gentamicin for 1h. Graph shows the percentage of inoculum surviving gentamicin treatment. Graph represents mean and standard errors of three independent experiments in duplicate. (B) The capacity of isolated E. coli to adhere to Caco-2 cells was evaluated using a MOI=10 for 30 min. Graph shows the percentage of adhered bacteria to epithelial cells related to the initial inoculum. Data represents mean and standard errors of three independent experiments in duplicate.
We then studied another phenotypic feature of intracellular E. coli strains related to the adhesion capacity to epithelial cells (Figure 3B). HB101 strain showed a 0.442% of adhesion and was used to determine a threshold of negative response. We found that NRG857c has an adherence to Caco-2 cells of 2.657% while CD1-a, CD2-a, CD6-b, CD6-r, CD12-a and UC1b strains showed higher values (Figure 3B).
To further phenotypically characterize intracellular E. coli strains, we selected some of those from CD, UC patients and controls, and assessed their survival inside RAW 264.7 macrophages. Table 1 shows mean recovered CFUs at 3h.p.i and the percentage of bacterial content at 8 and 24 h.p.i compared to the initial bacterial uptake (at 3h.p.i). The control HS strain did not survive inside macrophages, as was found to be eliminated at 24 h.p.i. Macrophages infected with strains CD1-a, CD2-a, CD6-b, CD6-r, CD8-a, CD9-a and UC1-b contained more than 20% of the phagocytosed bacteria at 24 h.p.i. Especially noteworthy was the CD2-a strain, that almost 100% of the phagocytosed bacteria was recovered, suggesting its strong ability to survive inside macrophages.
Table 1. Ability of E. coli strain isolated from patients to survive inside RAW 264.7 macrophages.
The table shows the initial uptake defined as the mean CFU resisting gentamicin treatment after 3h.p.i, and the percentage of bacterial content at 8 and 24 h.p.i related to the initial uptake (*). HS strain was used as negative control. Mean and standard deviation indicated correspond to three independent experiments, performed in duplicate.
| Strain | initial uptake (3 h.p.i) | % 8 h.p.i* | % 24 h.p.i* |
|---|---|---|---|
| HS | 90,833 ± 10,368 | 54.39 ± 26.48 | 0.24 ± 0.22 |
| NRG857c | 1,073,333 ± 295,945 | 60.12 ± 19.13 | 0.56 ± 0.70 |
| C7-a | 26,070 ± 18,176 | 86.54 ± 21.23 | 1.39 ± 1.93 |
| CD1-a | 266,667 ± 60,422 | 102.08 ± 8.68 | 39.74 ± 14.70 |
| CD2-a | 555,556 ± 197,390 | 112.10 ± 35.22 | 98.88 ± 21.89 |
| CD6-b | 143,889 ± 87,754 | 100.39 ± 20.89 | 43.47 ± 14.45 |
| CD6-r | 272,333 ± 257,595 | 110.18 ± 25.74 | 59.76 ± 16.01 |
| CD8-a | 33,019 ± 59,267 | 101.59 ± 19.15 | 56.99 ± 23.68 |
| CD9-a | 443,333 ± 153,930 | 112.11 ± 30.22 | 20.97 ± 11.04 |
| CD12-a | 42,222 ± 33,973 | 43.59 ± 18.81 | 0.27 ± 0.24 |
| CD13-a | 62,855 ± 114,635 | 55.47 ± 18.05 | 0.41 ± 0.56 |
| CD14-a | 168,611 ± 54,410 | 126.74 ± 43.97 | 8.58 ± 8.37 |
| UC1-b | 509,815 ± 388,578 | 133.75 ± 60.45 | 20.58 ± 15.76 |
| UC3-a | 20,501 ± 21,178 | 103.69 ± 36.08 | 8.99 ± 2.13 |
Intracellular E. coli isolated from ileal mucosa induces release of pro-inflammatory cytokines by macrophages through NLRP3-dependent signalling pathway
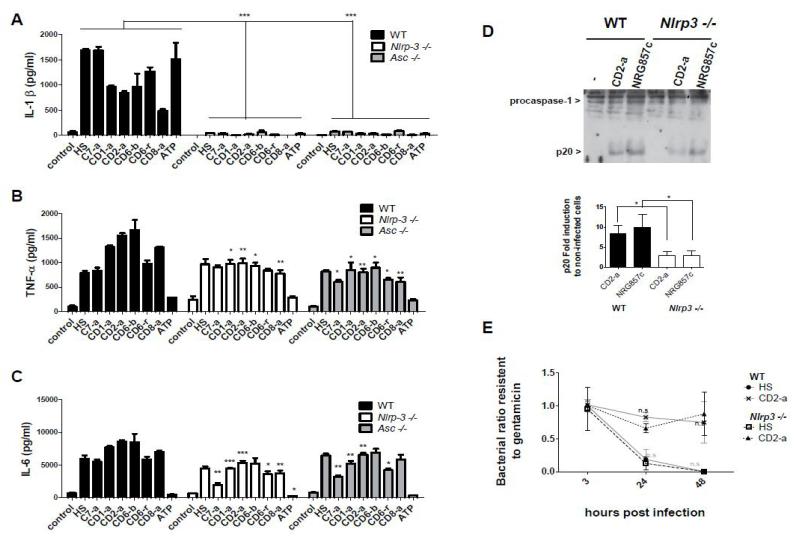
We next determined if infection of BMDMs from Asc, Nlrp3 knockout or WT mice with selected E. coli, with or without adherent-invasive properties, induced the activation of the NLRP3-inflammasome. We found that these bacteria induced substantial production of IL-1β and this response was abolished in NLRP3- or ASC-deficient macrophages (Figure 4A). In contrast, the production of TNF-α and IL-6 was partially reduced in macrophages deficient in NLRP3 or ASC (Figure 4B and 4C). Further analysis of the role of inflammasome in IL-1β induction by these bacteria, infection of macrophages with CD2-a or NRG857c strains showed that caspase-1 activation was significantly reduced in NLRP-3 macrophages (Figure 4D). In addition, and to investigate the role of NLRP3 in the ability of macrophages to eliminate E. coli strains, we infected WT and NLRP3-deficient macrophages with CD2-a, that highly survived in macrophages, and HS strain. However, survival of intracellular or commensal E. coli inside macrophages was not dependent on the NLRP3-inflammasome (Figure 4E).
Figure 4. IL-1β production by macrophages infected with isolated E. coli depends on the NLRP3 inflammasome.
(A) Bone marrow-derived macrophages from Nlrp3−/−,Asc−/− or WT mice cells were primed with LPS for 4 h. After that, cells were infected with E. coli isolated from CD (CD1-a, CD2-a, CD6-b, CD6-r, CD8-a) patients, control (C7-a) or HS strain (MOI=10) for 1 h and then treated with gentamicin. Twenty-four h.p.i, supernatants were analysed for IL-1β TNF-α and (C) IL-6 secretion by ELISA. (D) As an indicator of caspase-1 activation, content of caspase-1 p20 subunit was determined in WT or Nlrp3−/− macrophages primed with LPS for 4 h, infected with CD2-a or NRG857c strains (MOI=10) for two h and then treated with gentamicin overnight. Extracts were prepared from cell and supernatant, and immunobloted using a caspase-1 antibody. Arrows denote procaspase-1 and its processed p20 subunit. Graph bar shows content of caspase-1 p20 subunit related to procaspase-1 in each condition expressed as the fold induction in infected to non-infected cells. Graph represents mean and standard errors of three independent experiments (E) Bacterial clearance ability of Nlrp3−/− or WT macrophages infected with CD2-a or HS strains was evaluated at 3, 24 and 48 h.p.i. Graph shows the ratio of gentamicin-resistant bacteria to initial uptake (3 h.p.i). Graph represents mean and standard errors of three independent experiments, performed in duplicate. No significant differences were found in bacterial clearance between Nlrp3−/− and WT macrophages, infected with the same strain, using Fisher’s exact test with a two-sided p-value.
DISCUSSION
In this work, we show that E. coli strains colonize the mucosa of patients with CD, UC and OIP, but AIEC characteristics were identified only in isolates from IBD patients. Moreover, E. coli isolated from CD are able to induce the secretion of IL-1β via NLRP3-inflammasome. It has been reported that E. coli with AIEC properties is found in the intestinal mucosa of a significant proportion of CD patients (Darfeuille-Michaud et al., 2004; Martinez-Medina et al., 2009b), compared to UC patients and control subjects, including those with diverticulitis and IBS (Martinez-Medina et al., 2009b). Similarly, a higher content of total mucosa-associated and intracellular E. coli was observed in IBD compared to control subjects (Elliott et al., 2013). In our study, E. coli strains were isolated, using gentamicin protection assay, from patients with CD, UC and OIP comprising diverticulitis, chronic diarrhoea, and IBS. Comparable prevalence of E. coli was found in all patient groups compared to controls. However, intracellular E. coli with invasiveness and adhesiveness capacity to epithelial cells and survival in macrophages, were those exclusively isolated from IBD patients’ mucosa. It is likely that these strains may contribute to the intestinal inflammation in CD and UC, given the induction of pro-inflammatory cytokines and their ability to invade and survive within host cells. The presence of mucosa-associated E. coli in patients with intestinal disorders of different aetiology (IBD and OIP) may indicate that persistent inflammatory environment or changes in the microbiota could favour their accumulation in the intestine. Alternatively, epithelial barrier disruption in the different intestinal disorders studied may be associated with increased expression of CEACAM6, favouring the adhesion of E. coli strains having or not AIEC characteristics, via FimH.
Isolated colonies from the same patient, in most cases, were closely genetically related, whereas those from different individuals exhibited wide genetic diversity. Comparative analysis of strains by PFGE showed clusters that included bacteria isolated from patients with different intestinal pathologies carrying the same virulence genes described for AIEC strains, such as fimH, papC, and iucD (Martinez-Medina et al., 2009b) and most of them lack virulence genes of classic DEC (Boudeau et al., 1999), except CD6-b and CD12-a. In particular, these two strains carry the daaE gene, a fimbria characteristic of DAEC, and coincidently both show high adhesion capacity to epithelial cells. Furthermore, invasion capacity of CD6-b strain suggests that it could be the result of horizontal transfer of daaE gene. Morevoer, CD6-b is genetically related to the reference AIEC strain NRG857c as shown by PFGE analysis, reinforcing the hypothesis that it is an adherent-invasive strain with DAEC features. Moreover, as CD12-a strain contained the daaE virulence gene, highly adhered and poorly invaded to epithelial cells, it can be more associated with a classic DAEC pathotype.
The analysis showed that 8/19 of the strains isolated from patient-derived tissues (CD1-a, CD2-a, CD6-b, CD6-r, CD9-a, CD14-a, UC1-a, UC3-a) have AIEC properties such as invade epithelial cells and survive within macrophages as previously reported (Boudeau et al., 1999; Nash et al., 2010). These bacteria were identified in the cluster I.1.1 and I.2 in the PFGE analysis, and were categorized mainly as phylogenetic group B2 and D, similar to previously described in other AIEC strains (Martinez-Medina et al., 2009b). A remarkable capacity to invade epithelial cells and survive inside macrophages was seen for CD2-a strain, compared to NGR857c strain. However, further studies will be necessary to understand the molecular basis for the enhanced virulence displayed by CD2-a strain.
Some intracellular E. coli strains isolated from patient-derived ileal mucosa (CD1-a, CD2-a, CD6-r, and CD6-b) induce high levels of TNF-α and IL-6 in macrophages, and also activate caspase-1 and IL-1β secretion through NLRP3-inflammasome. However, these responses were comparable to those induced by other E. coli strains lacking AIEC characteristics, such as CD8-a, C7-a or commensal HS, suggesting that E. coli has a common feature to induce pro-inflammatory cytokines via shared PAMPs. On the other hand, NLRP3-deficient macrophages induce partially lower levels of TNF-α and IL-6 than WT cells. This response could be explained for the discrete IL-1β nduction by NLRP3-deficient macrophages, which can promote further NF-kB activation, and therefore increase TNF-α and IL-6 production, through an alternative pathway independent of PRRs. It is striking to note that strains with AIEC-like properties seem to produce less IL-1β, but higher TNF-α and IL-6 levels as compared to those with non-AIEC-like strains (HS, C7-a). However, a necessity to evaluate this trend with more strains is imminent for us to identify significant differences to associate the role of a distinctly cytokine balance evoked between E. coli strains holding or not AIEC features.
On the other hand, NLRP3 activation by Gram-positive bacteria can enhance microbicidal capacity of macrophages, promoting phagolysosome acidification (Sokolovska et al., 2013). However, our results show no association between NLRP3 and E. coli elimination in macrophages.
In conclusion, we have shown that a cohort of patients with CD, as well as UC and OIP have E. coli strains associated with the intestinal mucosa in a higher proportion than controls. However, only E. coli strains associated to IBD bear virulence genes, invasiveness and survival inside macrophages, constituting a difficult-to-control pro-inflammatory stimulus. E. coli isolates induced production of IL-1β mediated via the NLRP3-inflammasome.
Since polymorphisms in NLRP3, associated with lower expression of the protein, are common in CD patients (Villani et al., 2009), it is possible that dysfunctional NLRP3 activity early in disease evolution may result in a weak innate inflammatory response to intracellular E. coli, inducing low levels of IL-1β (Marks et al., 2006; Moráin et al., 1981). Deficient production of this cytokine may impair the efficiency of bacterial clearance in the intestinal mucosa by a poor recruitment of neutrophil and monocytes; this, in turn, may promote chronic and sustained inflammation associated with CD and other IBD disorders.
Supplementary Material
Acknowledgments
Miguel ÓRyan for her helpful critical reading of the manuscript and Alfredo Torres for providing us the NRG857c sequenced AIEC strain. Funding support from FONDECYT 1120577 (MH), FONDECYT 1110260 (RV), DA-CLC 2010 (RQ), CONICYT, National Institutes of Health grants AI063331 and AI064748 (GN) and MECESUP UCH 0714 fellowships (MDLF).
REFERENCES
- Abraham C, Cho JH. Inflammatory bowel disease. The New England journal of medicine. 2009;361:2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnich N, Carvalho FA, Glasser A, Darcha C, Jantscheff P, Allez M, Peeters Harald, Bommelaer G, Desreumaux P, Colombel J, Darfeuille-michaud A. CEACAM6 acts as a receptor for mucosa colonization in Crohn disease. Journal of Clinical Investigation. 2007;117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco M, Blanco JE, Alonso MP, Mora A, Balsalobre C, Muñoa F, Juárez A, Blanco J. Detection of pap, sfa and afa adhesin-encoding operons in uropathogenic Escherichia coli strains: relationship with expression of adhesins and production of toxins. Research in microbiology. 1997;148:745–55. doi: 10.1016/s0923-2508(97)82450-3. [DOI] [PubMed] [Google Scholar]
- Boudeau Jerome, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Croh’s disease. Infection and immunity. 1999;67:4499–509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer HW, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. Journal of molecular biology. 1969;41:459–72. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brereton CF, Sutton CE, Ross PJ, Iwakura Y, Pizza M, Rappuoli R, Lavelle EC, Mills KHG. Escherichia coli heat-labile enterotoxin promotes protective Th17 responses against infection by driving innate IL-1 and IL-23 production. Journal of immunology. 2011;186:5896–906. doi: 10.4049/jimmunol.1003789. [DOI] [PubMed] [Google Scholar]
- Carvalho F. a, Barnich N, Sivignon A, Darcha C, Chan CHF, Stanners CP, Darfeuille-Michaud Arlette. Croh’s disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. The Journal of experimental medicine. 2009;206:2179–89. doi: 10.1084/jem.20090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Rolhion N, De Vallée A, Salim SY, Prorok-Hamon M, Neut C, Campbell BJ, Söderholm JD, Hugot J, Colombel J-F, Darfeuille-Michaud Arlette. Crohn disease--associated adherent-invasive E. coli bacteria target mouse and human Peyer’s patches via long polar fimbriae. The Journal of clinical investigation. 2011;121:966–75. doi: 10.1172/JCI44632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DJ, Chaudhuri RR, Martin HM, Campbell BJ, Rhodes JM, Constantinidou C, Pallen MJ, Loman NJ, Cunningham AF, Browning DF, Henderson IR. Complete genome sequence of the Crohn’s disease-associated adherent-invasive Escherichia coli strain HM605. Journal of bacteriology. 2011;193:4540. doi: 10.1128/JB.05374-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont O, Bonacorsi S,, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Applied and environmental microbiology. 2000;66:4555–8. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794.e4. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Céspedes S, Salgado P, Valenzuela P, Vidal R, Oñate A. a. Characterization of genomic island 3 and genetic variability of Chilean field strains of Brucella abortus. Journal of clinical microbiology. 2011;49:2461–9. doi: 10.1128/JCM.01176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darfeuille-Michaud Arlette, Boudeau Jérôme, Bulois P, Neut C, Glasser Anne-Lise, Barnich N, Bringer M-A, Swidsinski A, Beaugerie L, Colombel J-F. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- Davies JQ, Gordon S. Isolation and culture of murine macrophages. Methods in molecular biology (Clifton, N.J.) 2005;290:91–103. doi: 10.1385/1-59259-838-2:091. [DOI] [PubMed] [Google Scholar]
- Del Canto F, Botkin DJ, Valenzuela P, Popov V, Ruiz-Perez F, Nataro JP, Levine Myron M, Stine OC, Pop M, Torres AG, Vidal R. Identification of Coli Surface Antigen 23, a novel adhesin of enterotoxigenic Escherichia coli. Infection and immunity. 2012;80:2791–801. doi: 10.1128/IAI.00263-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves-Pyles T, Allen C. a, Taormina J, Swidsinski A, Tutt CB, Jezek GE, Islas-Islas M, Torres AG. Escherichia coli isolated from a Crohn’s disease patient adheres, invades, and induces inflammatory responses in polarized intestinal epithelial cells. International journal of medical microbiology : IJMM. 2008;298:397–409. doi: 10.1016/j.ijmm.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Elliott TR, Hudspith BN, Wu G, Cooley M, Parkes G, Quiñones B, Randall L, Mandrell RE, Fagerquist CK, Brostoff J, Rayment NB, Boussioutas A, Petrovska L, Sanderson JD. Quantification and Characterization of Mucosa-Associated and Intracellular Escherichia coli in Inflammatory Bowel Disease. Inflammatory bowel diseases. 2013;19:2326–2338. doi: 10.1097/MIB.0b013e3182a38a92. [DOI] [PubMed] [Google Scholar]
- Ewers C, Li G, Wilking H, Kiessling S, Alt K, Antáo E-M, Laturnus C, Diehl I, Glodde S, Homeier T, Böhnke U, Steinrück H, Philipp H-C, Wieler LH. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: how closely related are they? International journal of medical microbiology : IJMM. 2007;297:163–76. doi: 10.1016/j.ijmm.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Figueroa C, Quera P,R, Valenzuela E,J, Jensen B,C. [Inflammatory bowel disease: experience of two Chilean centers] Revista médica de Chile. 2005;133:1295–304. [PubMed] [Google Scholar]
- Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nature immunology. 2012;13:325–32. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser AL, Boudeau Jerome, Barnich N, Perruchot MH, Colombel JF, Darfeuille-Michaud A. Adherent invasive Escherichia coli strains from patients with Crohn’s disease survive and replicate within macrophages without inducing host cell death. Infection and immunity. 2001;69:5529–37. doi: 10.1128/IAI.69.9.5529-5537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinée P. a,, Agterberg CM, Jansen WH. Escherichia coli O antigen typing by means of a mechanized microtechnique. Applied microbiology. 1972;24:127–31. doi: 10.1128/am.24.1.127-131.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald K. a, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature immunology. 2008;9:857–65. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R, Thomson JM, El-Omar EM, Hold GL. The role of infection in the aetiology of inflammatory bowel disease. Journal of gastroenterology. 2010;45:266–76. doi: 10.1007/s00535-009-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Stell a L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. The Journal of infectious diseases. 2000;181:261–72. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- Kanneganti T-D, Ozören N, Body-Malapel M, Amer A, Park J-H, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Núñez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–6. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nature reviews. Microbiology. 2004;2:123–40. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annual review of immunology. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM, Bergquist EJ, Nalin DR, Waterman DH, Hornick RB, Young CR, Sotman S. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;1:1119–22. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Croh’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–82. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DJB, Harbord MWN, MacAllister R, Rahman FZ, Young J, Al-Lazikani B, Lees W, Novelli M, Bloom S, Segal AW. Defective acute inflammation in Crohn’s disease: a clinical investigation. Lancet. 2006;367:668–78. doi: 10.1016/S0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- Martinez-Medina M, Mora Azucena, Blanco Miguel, López C, Alonso María Pilar, Bonacorsi Stéphane, Nicolas-Chanoine M-H, Darfeuille-Michaud Arlette, Garcia-Gil J, Blanco Jorge. Similarity and divergence among adherent-invasive Escherichia coli and extraintestinal pathogenic E. coli strains. Journal of clinical microbiology. 2009a;47:3968–79. doi: 10.1128/JCM.01484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Medina M, Aldeguer X, Lopez-Siles M, González-Huix F, López-Oliu C, Dahbi G, Blanco Jesus E, Blanco Jorge, Garcia-Gil LJ, Darfeuille-Michaud Arlette. Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn’s disease. Inflammatory bowel diseases. 2009b;15:872–82. doi: 10.1002/ibd.20860. [DOI] [PubMed] [Google Scholar]
- Miquel S, Peyretaillade E, Claret L, De Vallée A, Dossat C, Vacherie B, Zineb EH, Segurens B, Barbe V, Sauvanet P, Neut C, Colombel J-F, Medigue C, Mojica FJM, Peyret P, Bonnet R, Darfeuille-Michaud Arlette. Complete genome sequence of Crohn’s disease-associated adherent-invasive E. coli strain LF82. PloS one. 2010;5:e12714. doi: 10.1371/journal.pone.0012714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moráin CO, Segal AA, Walker D, Levi AJ. Abnormalities of neutrophil function do not cause the migration defect in Crohn’s disease. Gut. 1981;22:817–22. doi: 10.1136/gut.22.10.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin-Schouleur M, Schouler C, Tailliez P, Kao M-R, Brée A, Germon P, Oswald E, Mainil J, Blanco Miguel, Blanco Jorge. Common virulence factors and genetic relationships between O18:K1:H7 Escherichia coli isolates of human and avian origin. Journal of clinical microbiology. 2006;44:3484–92. doi: 10.1128/JCM.00548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JHE, Villegas A, Kropinski AM, Aguilar-Valenzuela R, Konczy P, Mascarenhas M, Ziebell K, Torres AG, Karmali M. a,, Coombes BK. Genome sequence of adherent-invasive Escherichia coli and comparative genomic analysis with other E. coli pathotypes. BMC genomics. 2010;11:667. doi: 10.1186/1471-2164-11-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odze R. Diagnostic problems and advances in inflammatory bowel disease. Modern pathology. 2003;16:347–58. doi: 10.1097/01.MP.0000064746.82024.D1. [DOI] [PubMed] [Google Scholar]
- Ozören N, Masumoto J, Franchi L, Kanneganti T-D, Body-Malapel M, Ertuk I, Jagirdar R, Zhu L, Inohara N, Bertin J, Coyle A, Grant EP, Nuñez G. Distinct Roles of TLR2 and the Adaptor ASC in IL-1 /IL-18 Secretion in Response to Listeria monocytogenes. The Journal of Immunology. 2006;176:4337–4342. doi: 10.4049/jimmunol.176.7.4337. [DOI] [PubMed] [Google Scholar]
- Peeters H, Bogaert S, Laukens D, Rottiers P, De Keyser F, Darfeuille-Michaud A, Glasser A-L,, Elewaut D, De Vos M. CARD15 variants determine a disturbed early response of monocytes to adherent-invasive Escherichia coli strain LF82 in Croh’s disease. International journal of immunogenetics. 2007;34:181–91. doi: 10.1111/j.1744-313X.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- Pereira MSF, Morgantetti GF, Massis LM, Horta CV, Hori JI, Zamboni DS. Activation of NLRC4 by flagellated bacteria triggers caspase-1-dependent and -independent responses to restrict Legionella pneumophila replication in macrophages and in vivo. Journal of immunology (Baltimore, Md. : 1950) 2011;187:6447–55. doi: 10.4049/jimmunol.1003784. [DOI] [PubMed] [Google Scholar]
- Saldaña Z, Erdem AL, Schüller S, Okeke IN, Lucas M, Sivananthan A, Phillips AD, Kaper JB, Puente JL, Girón J. a. The Escherichia coli common pilus and the bundle-forming pilus act in concert during the formation of localized adherence by enteropathogenic E. coli. Journal of bacteriology. 2009;191:3451–61. doi: 10.1128/JB.01539-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander LE, Davis MJ, Boekschoten MV,, Amsen D, Dascher CC, Ryffel B, Swanson J. a, Müller M, Blander JM. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature. 2011;474:385–9. doi: 10.1038/nature10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Sitaraman SV, Babbin B. a, Gerner-Smidt P, Ribot EM, Garrett N, Alpern J. a, Akyildiz A, Theiss AL, Nusrat A, Klapproth J.-M. a. Invasive Escherichia coli are a feature of Croh’s disease. Laboratory investigation. 2007;87:1042–54. doi: 10.1038/labinvest.3700661. [DOI] [PubMed] [Google Scholar]
- Shen X, Shi R, Zhang H, Li K, Zhao Y, Zhang R. The Toll-like receptor 4 D299G and T399I polymorphisms are associated with Crohn’s disease and ulcerative colitis: a meta-analysis. Digestion. 2010;81:69–77. doi: 10.1159/000260417. [DOI] [PubMed] [Google Scholar]
- Shih DQ, Targan SR. Immunopathogenesis of inflammatory bowel disease. World journal of gastroenterology : WJG. 2008;14:390–400. doi: 10.3748/wjg.14.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovska A, Becker CE, Ip WKE, Rathinam V. a K., Brudner M, Paquette N, Tanne A, Vanaja SK, Moore KJ, Fitzgerald K. a, Lacy-Hulbert A, Stuart LM. Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nature immunology. 2013;14:543–53. doi: 10.1038/ni.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strate LL, Modi R, Cohen E, Spiegel BMR. Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. The American journal of gastroenterology. 2012;107:1486–93. doi: 10.1038/ajg.2012.194. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–67. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiba MR, Yano T, Leite DDS. Genotypic characterization of virulence factors in Escherichia coli strains from patients with cystitis. Revista do Instituto de Medicina Tropical de São Paulo. 2008;50:255–60. doi: 10.1590/s0036-46652008000500001. [DOI] [PubMed] [Google Scholar]
- Vidal M, Kruger E, Durán C, Lagos R, Levine M, Prado V, Toro C, Vidal R. Single multiplex PCR assay to identify simultaneously the six categories of diarrheagenic Escherichia coli associated with enteric infections. Journal of clinical microbiology. 2005;43:5362–5. doi: 10.1128/JCM.43.10.5362-5365.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani A-C, Lemire M, Fortin G, Louis Edouard, Silverberg MS, Collette C, Baba N, Libioulle C, Belaiche Jacques, Bitton A, Gaudet D, Cohen A, Langelier D, Fortin PR, Wither JE, Sarfati M, Rutgeerts P, Rioux JD, Vermeire S, Hudson TJ, Franchimont D. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nature genetics. 2009;41:71–6. doi: 10.1038/ng285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AW, Sanderson JD, Churcher C, Parkes GC, Hudspith BN, Rayment N, Brostoff J, Parkhill J, Dougan G, Petrovska L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC microbiology. 2011;11:7. doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.