Abstract
Suicide represents a major health problem world-wide. Nevertheless, the understanding of the neurobiological underpinnings of suicidal behavior remains far from complete. We compared suicide attempters to non-attempters, and high vs. low lethality attempters, to identify brain regions associated with suicidal behavior in patients with psychotic disorders. 489 individuals with schizophrenia, schizoaffective disorder, or psychotic bipolar disorder I and 262 healthy controls enrolled in the B-SNIP study were studied. Groups were compared by attempt history and the highest medical lethality of previous suicide attempts. 97 patients had a history of a high lethality attempt, 51 of a low lethality attempt and 341 had no attempt history. Gray matter volumes were obtained from 3T structural MRI scans using FreeSurfer. ANCOVAs were used to examine differences between groups, followed by Hochberg multiple comparison correction. Compared to non-attempters, attempters had significantly less gray matter volume in bilateral inferior temporal and superior temporal cortices, left superior parietal, thalamus and supramarginal regions, right insula, superior frontal and rostral middle frontal regions. Among attempters, a history of high lethality attempts was associated with significantly smaller volumes in the left lingual gyrus and right cuneus. Compared to non-attempters, low lethality attempters had significant decreases in the left supramarginal gyrus, thalamus and the right insula. Structural brain abnormalities may distinguish suicide attempters from non-attempters and high from low lethality attempters among individuals with psychotic disorders. Regions in which differences were observed are part of neural circuitries that mediate inhibition, impulsivity and emotion, visceral, visual and auditory perception.
Keywords: imaging, suicide, schizophrenia
BACKGROUND
Suicidal behavior includes self-directed injurious acts with a variable degree of intent to end one’s own life (Mann, 2003). Suicide is one of the leading causes of death worldwide and the 10th leading cause of death in the United States. In 2007, it was the 7th leading cause of death for males, and 15th leading cause of death for females. The total number of suicide deaths that year in the US was 34,598. Suicide was the third leading cause of death for young people ages 15 to 24 (http://www.nimh.nih.gov/health/publications/suicide-in-the-us-statistics-and-prevention/index.shtml).The risk of suicide is particularly high among patients with schizophrenia: about 5% commit suicide (Palmer et al, 2005), and between 18% and 55% attempt it (Siris, 2001). Furthermore, suicide risk is especially elevated in the first 6-12 months following the first episode of psychosis (Fedyszyn et al, 2010). Because psychotic illnesses typically occur in the otherwise physically healthy teenage or early adolescent years of an individual’s life, suicide is the leading cause of premature death in the psychotic-spectrum population (Robinson et al, 2010).
Recently, there has been a growing interest in understanding the diverse factors mediating suicidal behavior (Jimenez-Treviño & Blasco-Fontecilla, 2011, Amitai & Apter, 2012), which are likely to involve interaction between genetic, environmental, and developmental factors (Brezo et al, 2008, Zalsman, 2010, Pandey GN, 2011). Attempters appear particularly sensitive to social disapproval and have reduced ability to forecast positive outcomes of their actions, resulting in choosing options with high immediate reward (van Heeringen et al, 2011). Impulsive-aggressive traits, as well as depression, appear to be associated with suicidality (Giegling et al, 2009, Turecki, 2005). Nevertheless, clinical correlates of suicide are admittedly diverse, and also present in a large number of non-attempters (Aguilar et al, 2003).
Brain imaging studies have started to yield information on neuroanatomical correlates of suicidal behavior. The lack of consensus concerning reported brain regions is not surprising given the early state of knowledge regarding these phenomena and their link with neuroanatomy, and the heterogeneity in risk factors for suicide. Specifically, an initial MRI study, although limited by small sample size (n=37) and absence of healthy comparison subjects, has shown gray matter reduction in the left superior temporal and orbitofrontal cortices in attempters with schizophrenia (Aguilar et al, 2008). Thus far, regions of interest identified in the above mentioned study are fewer compared with the numerous brain abnormalities that have been found to be associated with suicidality in other disorders (Monkul et al, 2006, Ahearn et al, 2001, Hwang et al, 2010). Further studies are needed to establish whether regions differ or are similar across the entire spectrum of psychosis diagnoses and to establish findings in a larger sample size.
This paper investigated regional brain volumes in groups of patients with psychotic disorders, specifically individuals with either schizophrenia (SZ), schizoaffective (SZA) or psychotic bipolar disorder I (BP-P), recruited as part of the Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP) study. We separated these subjects into groups of attempters and non-attempters and tested if our results were diagnosis-specific or similar across the psychosis spectrum. We then divided the attempters into high and low lethality attempters, in order to see if there are any volumetric differences between these groups. Additionally, we compared low lethality attempters to non-attempters in our attempt to minimize the possibility that the observed differences were the result of the attempt itself. Our main hypothesis was that structural brain abnormalities might be associated with suicidal behavior in patients with psychotic disorders. We also sought to examine whether demographic (age, sex, race, socioeconomic status), clinical (diagnosis) and neurobehavioral measures (cognition, impulsivity, social functioning) differed across these groups.
MATERIALS AND METHODS
Subjects
Our subject pool consists of 489 individuals with either SZ, SZA, or BP-P and 262 Healthy Controls (HC) recruited as part of the B-SNIP study, a multisite collaborative research consortium (Baltimore, Chicago, Dallas, Detroit, Boston and Hartford). Sites used identical diagnostic and clinical assessment techniques, and shared approaches to recruitment (Tamminga et al, in press). The study protocol was approved by each site’s local Institutional Review Board. All volunteers provided written informed consent after receiving a full explanation of the study procedures.
Consensus diagnoses using the Diagnostic and Statistical Manual of Mental Disorders-IV-TR (American Psychiatric Association, 2000) were established by trained clinical raters and senior diagnosticians with all clinical data and Structured Clinical Interviews for DSM Diagnoses (SCID) (Spitzer et al, 1992) interviews: inter-rater reliability was > 0.90 among raters. Probands were recruited and evaluated when stable and optimally treated as judged clinically (e.g., no recent significant increase in core symptoms from their stable baseline that requires a change in treatment such as increased dose of medication, drug change, or hospitalization). Recruitment was stratified on key variables so that probands and HC were frequency matched for age, sex ratio, and head of the household’s socio-economic status.
Subjects were excluded if they had a serious medical or neurological illness affecting brain function, mental retardation, current substance abuse within the last month, or substance dependence within the last 3 months. HC with psychotic or mood disorders confirmed by DSM-IV SCID and those with family histories of psychotic or bipolar disorders in their first degree relatives were also excluded from the study.
Assessment and Classification of Suicidal Behavior
Medical lethality of suicide attempts was assessed with Beck’s Lethality Scale (LS) (Beck et al, 1975), which has previously been used in other studies to assess suicidal behavior in patients with psychotic disorders (Radomsky et al, 1999). The LS was administered only to probands, since none of the HC included in our study had an attempt history and its measurements were based both on self-report and on the patient’s medical records. The LS is completed by an interviewer based on clinical examination, medical records, and information from the treatment team. It measures the medical lethality of any history of suicide attempt for one of eight possible methods (sedative drugs, non-sedative drugs and other substances, shooting, immolation, drowning, cutting, jumping, hanging) on an ordinal scale from 0 (minimal or no damage) to 8 (death). The LS has demonstrated good inter-rater reliability (intra-class correlation coefficient = 0.80) (Lester & Beck, 1975).
Groups were compared by attempt status and highest medical lethality of previous suicide attempts. 97 patients had a history of a high lethality suicide attempt (score of 2 or more on Lethality Scale for a prior attempt indicating significant medical sequelae of an attempt), 51 had a history of a low lethality suicide attempt and 341 had no history of a suicide attempt. We defined low lethality as minimal or no medically significant (a score of 1 on LS) self-harm as a result of the attempt. More specifically, in this group, we included subjects who were conscious but sleepy after having used sedative drugs, having cuts with minimal bleeding, or having minor bruises after having jumped. The high lethality group consists of subjects whose attempt resulted in more serious self-harm, such as being comatose after having used sedative drugs, bleeding of major veins with danger of considerable blood loss without surgical intervention and fractures of the extremities after having jumped. Out of the 148 attempters and the 341 non attempters, 5 attempters and 32 non attempters were not taking medications (antipsychotics or mood stabilizers) at the time of the assessment. Furthermore, the aforementioned groups were compared across the psychosis spectrum as a whole (SZ and SZA and BP-P), as well as according to their specific diagnosis (SZ or SZA or BP-P).
We also assessed cognitive function using the Brief Assessment of Cognition in Schizophrenia (BACS) (Keefe et al, 2004), social function using the Birchwood Social Functioning Scale (SFS) (Birchwood et al, 1990), socioeconomic status (SES) using the Hollingshead two-factor social economic rating scale (Hollingshead, 1957) and impulsivity using the Barratt Impulsiveness Scale (BIS-11) (Patton et al, 1995).
Imaging
High-resolution isotropic T1-weighted sequences (TR=6.7 msec, TE= 3.1 msec, 8° flip angle, 256×240 matrix size, total scan duration=10:52.6 minutes, 170 sagittal slices, 1mm slice thickness, 1×1×1.2 mm3 voxel resolution) were obtained following the Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocol (http://www.loni.ucla.edu/ADNI). All images went through a rigorous data quality control scheme. First, images were opened and converted to nifti format and checked for scanner artifacts by trained raters (NT, ITM, CIG, JS). If the images passed the pre-check, they were run through an automatic reconstruction with skull stripping in FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). Then, the skull stripped brains were again checked, this time for any remaining dura or sinus that can interfere with accurate segmentation. If any non-brain tissue is found, trained raters carefully edit the images manually. All raters were reliable above 95%. When the images are sufficiently clean for segmentation, as determined by an independent rater, the brains are run through a second and third automatic reconstruction with segmentation and the gray matter surface area, thickness, and volume are extracted for analysis.
Statistical Analysis
First, data were checked for normality before conducting parametric comparisons. ANCOVAs were used to examine differences between groups, co-varying for age, sex, site, and intracranial volume, followed by Hochberg corrections for multiple comparisons (Benjamini & Hochberg, 1995). Effect sizes for these comparisons were calculated using Cohen’s d.
RESULTS
Demographics and sample characteristics
Table 1 provides the diagnostic and demographic characteristics of the study groups. Attempters were significantly more often females. Age and diagnostic distributions did not differ, but the low lethality attempters showed a trend for being less likely from the “other” race group.
Table 1.
CLINICAL AND DEMOGRAPHIC FEATURES OF NON-ATTEMPTERS, HI LETHALITY ATTEMPTERS, LOW LETHALITY ATTEMPTERS AND HEALTHY CONTROLS
| Non-Attempters | Hi Lethality Attempters | Low Lethality Attempters | HC | Test Statistic | p-value | |
|---|---|---|---|---|---|---|
| n | 341 | 97 | 51 | 262 | ||
| Age Mean ± SD | 35.9 ± 13.3 | 35.6 ± 11.7 | 36.9 ± 12.2 | 38.1 ± 12.5 | F=1.65 | 0.18 |
| Sex (M:F) | 185:156 | 36:61 | 14:37 | 129:133 | χ2=18.65 | 0.00 |
| Diagnosis (SZ:SZA:BPP) | 141:79:121 | 35:31:31 | 20:11:20 | 0:0:0 | χ2=16.44 | 0.29 |
| Race (AA:CA:OT) | 123:200:18 | 43:45:9 | 18:32:1 | 71:168:23 | χ2=24.22 | 0.06 |
| SES Mean ± SD | 41.1 ± 16.2 | 42.0 ± 15.9 | 39.4 ± 17.3 | 39.2 ± 15.3 | F=0.90 | 0.44 |
SD= Standard Deviation M= Male, F=Female AA= African-American, CA= Caucasian, OT= Other SES= Socioeconomic Status
We found that sex, race, age, site and diagnosis had a significant effect on regional brain volumes, but there were no significant sex by group, race by group, age by group, site by group or diagnosis by group interactions.
Table 2 shows that attempters and non-attempters did not differ significantly in cognitive functioning (BACS), impulsivity (BIS Total), socio-economic status (SES) or social functioning (SFS).
Table 2.
GROUP COMPARISONS BETWEEN ATTEMPTERS (n=78), NON-ATTEMPTERS (n=208) AND HC (n=177) ON COGNITION, IMPULSIVITY, SOCIO-ECONOMIC STATUS AND SOCIAL FUNCTIONING
| HC Mean Value | Attempters Mean Value | HC – Attempters Cohen’s d p-value | Non Attempters Mean Value | HC – Non Attempters Cohen’s d p-value | Non Attempters - Attempters Cohen’s d p-value | |
|---|---|---|---|---|---|---|
| MEASURE | ||||||
| BACS Composite (Z-score) | 0.11 | -1.03 | 0.89*** | -1.35 | 1.08*** | -0.21 |
| BIS Total | 54.06 | 67.46 | -1.32*** | 67.52 | -1.22*** | -0.06 |
| SES | 38.5 | 40.56 | -0.15 | 41.76 | -0.29* | 0.14 |
| SFS | 156.72 | 126.56 | 1.4*** | 127.29 | 1.29*** | 0.07 |
Adjusted p-values reported – Hochberg method used for multiple comparisons:
“.” < 0.1,
< 0.05,
< 0.01,
< 0.001
Table 3 compares attempt rates and lethality according to diagnosis. We found that psychotic-spectrum patients with a history of a suicide attempt - independent of the lethality of the attempt - are more likely to have been diagnosed with SZA. In addition, we found that the degree of medical lethality of a suicide attempt varied by diagnosis. Specifically, psychotic patients with a history of a low lethality suicide attempt were more likely to have been diagnosed with BP-P and those with a history of a high lethality suicide attempt were more likely to have been diagnosed with SZA.
Table 3.
COMPARISONS OF ATTEMPT RATES AND LETHALITY ACCORDING TO DIAGNOSIS
| Total Attempters/ Total Patients | Hi Lethality Attempters/ Total Patients | Low Lethality Attempters/ Total Patients | |
|---|---|---|---|
| SZ | 55/196 (28.1%) | 35/196 (17.9%) | 20/196 (10.2%) |
| SZA | 42/121 (34.7%) | 31/121 (25.6%) | 11/121 (9.1%) |
| BPP | 51/172 (29.7%) | 31/172 (18.0%) | 20/172 (11.6%) |
Comparisons across the psychotic spectrum
When comparing attempters, non-attempters and HC across the psychosis spectrum, attempters were found to have significantly less gray matter volume in various temporal, parietal and frontal regions, as well as in the left thalamus. More specifically, attempters had significantly smaller volumes of bilateral inferior temporal, and superior temporal, right insula, superior frontal and rostral middle frontal regions and left superior parietal, and supramarginal regions, when compared to non-attempters. Both attempters and non-attempters when separately compared to HC had significantly decreased volumes in the same regions, with the exception of the left thalamus whose volume was not significant different between non-attempters and HC. None of the brain regions were larger in the attempters than HC.
Comparisons within diagnoses
When compared according to diagnosis, BP-P attempters had significantly smaller volumes in the left supramarginal and right fusiform gyri compared to BP-P non-attempters and HC. BP-P non-attempters showed no significant differences when compared to HC. SZ attempters had significant decrease in the left thalamus compared to SZ non-attempters and HC. SZ non-attempters showed no significant differences when compared to HC. SZA attempters had significantly smaller volumes of the right accumbens, bilateral inferior temporal, right lingual and left supramarginal areas and significant increased volume of the left medial orbitofrontal gyrus when compared to SZA non-attempters. When compared to HC, SZA attempters had significant reductions in the right lingual, right accumbens, bilateral inferior temporal, and left supramarginal areas and SZA non-attempters in the left medial orbitofrontal and bilateral inferior temporal gyri.
Brain structure and medical lethality of suicide attempt
Among suicide attempters across the psychosis spectrum, high lethality attempters showed significantly diminished gray matter concentrations compared to low lethality attempters in the left lingual area and right cuneus.
Diagnosis-specific significant differences were also observed between high and low lethality attempters. In particular, we observed that BP-P high lethality attempters had significant reductions in the left caudal anterior cingulate, right inferior parietal, left inferior temporal and right middle temporal areas. High lethality attempters with SZ showed significant reductions in the left lingual, bilateral pericalcarine, right cuneus and right lateral occipital areas, while SZA high lethality attempters had significant increase in the left rostral middle frontal area.
Low lethality attempters vs. non-attempters
Compared to non-attempters, low lethality attempters across the psychosis spectrum had significantly smaller volumes in the left supramarginal area, left thalamus and right insula.
When comparing the above-mentioned groups according to diagnosis, BP-P low lethality attempters had significantly increased volume of the right middle temporal gyrus. SZ low lethality attempters showed significantly increased volume of the left pericalcarine area and SZA low lethality attempters had significantly decreased volume of the left supramarginal area.
All the above differences survived Hochberg corrections for multiple comparisons.
DISCUSSION
There were no significant demographic and diagnostic differences between attempters and non-attempters. By contrast, we found significant differences in gray matter volume between attempters and non-attempters, between high and low lethality attempters, and between low lethality attempters and non-attempters both when examining effects across the psychosis spectrum (SZ and SZA and BP-P) and when examining effects in each specific diagnosis (SZ or SZA or BP-P). The above observations may suggest that structural changes are a sensitive marker of suicidal behavior. These observations were unlikely to be accounted for by sex or age effects. There were no diagnosis by group interactions, but some differences were seen in the structure- suicidal behavior relations across the SZ, SZA and BP-P groups.
The possibility that the observed differences could be the result of suicide attempts rather than pre-existing brain abnormalities cannot be ruled out completely since these structural MRIs were by definition acquired after attempts. However, since low-lethality attempts were less likely to cause brain abnormalities, it is noteworthy that a comparison of low-lethality attempters to non-attempters found significant structural differences even when high-lethality attempters were excluded. Moreover, we co-varied for several factors, such as sex, age, site, and total intracranial volume and thereby addressed the potential confounding effects of these measures.
The anatomical differences observed between attempters and non-attempters point to disruptions in neural networks that may underlie the different components of suicidal behavior in psychotic disorders. Specifically, the rostral middle frontal gyrus is part of the prefrontal cortex which is implicated in cognitive analysis, foresight and weighing possible consequences of behavior, considering the future and making predictions, impulse control and delaying gratification, inhibiting inappropriate behavior and initiating appropriate behavior. Impulsivity in particular has been associated with increased suicide risk in patients with schizophrenia (Hawton et al, 2005). Abnormalities in fronto-thalamic pathways may also result in impaired cognition and emotion processing. Such disturbances might contribute to the risk for suicidal behavior by disinhibiting emotional responses and disrupting the ability to plan adaptive behavior to stressful events (Jia et al, 2012).
Alterations in superior temporal gyri have been associated with the presence of auditory hallucinations (García-Martí et al, 2008), a distressing experience which can be a precipitant for suicidal ideation or attempts (Fialko et al, 2006). In addition, the superior temporal cortex (gyrus and sulcus) is part of a complex face processing system and is involved in the perception of emotion (Campbell et al, 1990). Functional MRI studies suggest that these structures mediate a “reflexive” response to visual social inputs, especially negative visual stimuli (Buchheim et al, 2008). The insular cortex is involved in subjective evaluation of one’s affective state and the internalization of perceptions of oneself by others (New et al, 2008). Negative affect resulting from perceived rejection and social disappointment has been found to be the most common precipitant of suicidal behavior in borderline personality disorder (Soloff et al, 2012). The same factors could increase suicidality in patients with psychosis.
While they need to be replicated in independent samples, the results of this study could be of significant clinical interest. If the observed alterations in specific brain regions are replicated, they could be of corroborative value for clinicians while assessing suicide risk of the patient. Furthermore, these regions could be investigated as biomarkers for the pathophysiology of suicidal behavior in individuals with psychosis. Eventually these alterations could represent a neurobiological signature of risk of suicidality across psychotic disorders.
The major strength of our study is its large sample size and the examination of the relation between brain structure and suicidal behavior across the psychosis spectrum. To the best of our knowledge, ours is the first imaging study to investigate volumetric brain differences related to suicidality in either SZA or BP-P subjects. However, our study is limited by the lack of examination of those with only suicidal ideation, as this was not recorder during the SCID-IV-TR interview. Another limitation is the absence of the important class of attempters who completed suicide. Even though the current severity of psychotic symptoms was assessed with the PANSS (Positive and Negative Symptoms Scale), the severity of the psychotic symptoms at the time of suicide attempt is unknown. It should also be noted that the high- and low-lethality attempter groups were selected based only on the highest medical lethality of the suicide attempt without taking into account the different methods used or other previous attempts: as a result, highly lethal methods may have presented to clinical attention as low-lethality attempts because of limited actual medical consequences, and vice versa. Despite this possibility, the dose-response relationship between attempt lethality and structural change is striking and validates the distinction being made in the LS scale between low- and high-lethality attempts. Furthermore, because the LS relies in part on self-report, a possible interpretation of our results is that the observed structural changes observed might reflect differences associated with proneness to self-reporting suicidality, or differences in degree of intentionality of self-harming behavior. However, two studies suggest that the degree of intent is not necessarily associated with the lethality of the attempt (Brown et al, 2004, Plutchik et al, 1989).
We caution that the results of our study do not establish causality between brain structural alterations and suicidal behavior. Brain structural alterations probably account for only a small part of the variation underlying suicidal behavior. There are many risk factors underlying suicidal behavior in patients with psychosis. Future studies examining the relationship between brain changes on the one hand, and family history of suicide, genetic factors and substance abuse on the other hand are necessary in order to gain a more comprehensive understanding of the complex phenomenon of suicidal behavior in patients with psychotic disorders. The functional and neurochemical correlates of the observed structural changes also need to be determined.
Figure 1. GROUP COMPARISONS ON BILATERAL SUPERIORAL TEMPORAL VOLUME.
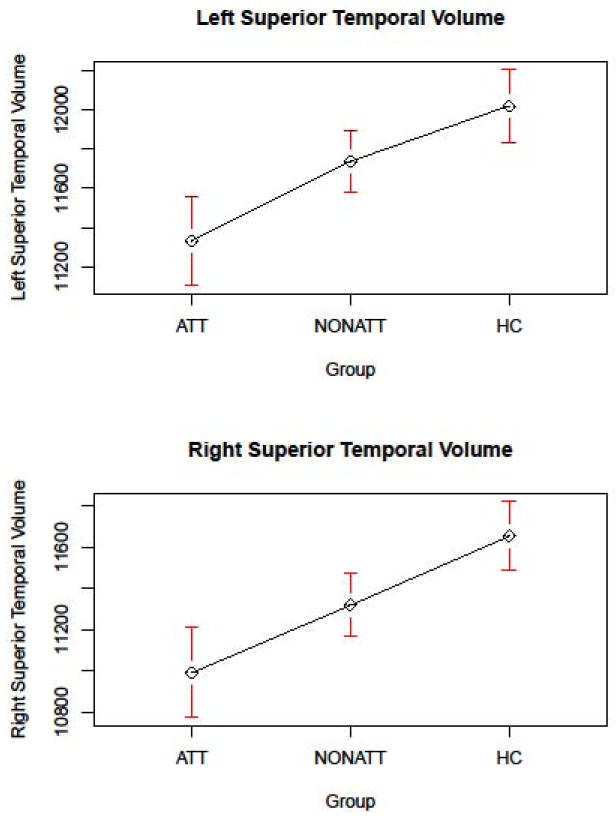
ATT=Attempters, NONATT=Non Attempters, HC=Healthy Controls
Figure 2. EFFECT SIZES OF SIGNIFICANT VOLUMETRIC DIFFERENCES.
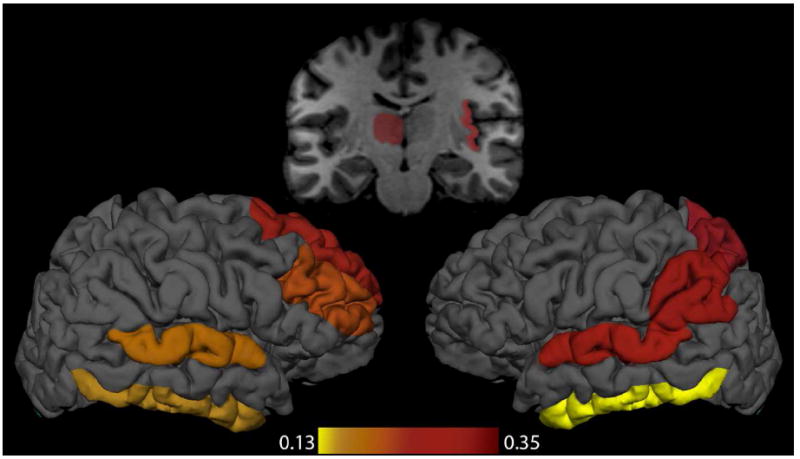
In the above figure, regions where we observed volumetric differences between attempters and nonattempters are colored according to a scale based on Cohen’s d effect size.
Table 4.
GROUP COMPARISONS BETWEEN ATTEMPTERS, NON-ATTEMPTERS AND HC ON REGIONAL BRAIN VOLUME
| HC Mean Regional Volume | Attempters Mean Regional Volume | HC – Attempters Cohen’s d p-value | Non Attempters Mean Regional Volume | HC – Non Attempters Cohen’s d p-value | Non Attempters - Attempters Cohen’s d p-value | |
|---|---|---|---|---|---|---|
| ACROSS PSYCHOSIS SPECTRUM | ||||||
| R Rostral Middle Frontal | 15899.74 | 14983.74 | 0.33*** | 15537.15 | 0.13* | 0.20* |
| R Superior Frontal | 21287.35 | 19916.76 | 0.44*** | 20691.79 | 0.19*** | 0.24* |
| L Superior Parietal | 13060.31 | 12301.23 | 0.38*** | 12841.94 | 0.10* | 0.25* |
| L Supramarginal | 10934.96 | 10132.07 | 0.45*** | 10625.54 | 0.17** | 0.28** |
| L Thalamus | 7075.84 | 6640.83 | 0.43*** | 6979.64 | 0.09 | 0.33* |
| L Inferior Temporal | 11186.59 | 10125.28 | 0.51*** | 10417.37 | 0.35*** | 0.13* |
| R Inferior Temporal | 10853.87 | 9962.81 | 0.48*** | 10305.72 | 0.27*** | 0.17* |
| L Superior Temporal | 12021.72 | 11328.30 | 0.39*** | 11736.26 | 0.16** | 0.24* |
| R Superior Temporal | 11655.76 | 10988.87 | 0.40*** | 11318.89 | 0.20*** | 0.19* |
| R Insula | 6832.06 | 6380.14 | 0.48*** | 6657.27 | 0.18** | 0.29* |
| BP-P | ||||||
| L Supramarginal | 10934.96 | 10182.10 | 0.43* | 10741.50 | 0.11 | 0.34* |
| R Fusiform | 10041.56 | 9269.04 | 0.51** | 9929.24 | 0.06 | 0.42* |
| SZ | ||||||
| L Thalamus | 7075.84 | 6564.66 | 0.49** | 7076.19 | -0.00 | 0.50** |
| SZA | ||||||
| L Medial Orbitofrontal | 5165.29 | 4884.62 | 0.32 | 4746.22 | 0.50*** | -0.18* |
| R Lingual | 6947.66 | 6177.37 | 0.77*** | 6609.07 | 0.32 | 0.40* |
| L Supramarginal | 10934.96 | 9872.40 | 0.60*** | 10269.06 | 0.37. | 0.25* |
| R Accumbens | 717.52 | 659.42 | 0.43* | 734.46 | -0.11 | 0.50* |
| L Inferior Temporal | 11186.59 | 9631.37 | 0.76*** | 10089.95 | 0.52** | 0.22* |
| R Inferior Temporal | 10853.87 | 9407.32 | 0.78*** | 10003.44 | 0.43* | 0.30* |
Adjusted p-values reported – Hochberg method used for multiple comparisons:
“.” < 0.1,
< 0.05,
< 0.01,
< 0.001
Acknowledgments
This research was supported by funding from NIMH through MH077851 (CT), MH078113 (MK), MH077945 (GP), MH077852 (GT), and MH077862 (JS).
We express gratitude to the patients and their families who contributed their time and effort to participate in this study.
Footnotes
Christoforos I Giakoumatos
Contribution: conception and design, analysis and interpretation of data, preparing the first draft of the article, final approval of the version to be published
Neeraj Tandon, BS
Contribution: analysis and interpretation of data, drafting the article, article review, final approval of the version to be published
Jai Shah, MD
Contribution: interpretation of data, drafting the article, article review, final approval of the version to be published
Ian T Mathew
Contribution: analysis and interpretation of data, article review, final approval of the version to be published
Roscoe O Brady, MD, PhD
Contribution: interpretation of data, article review, final approval of the version to be published
Brett A Clementz, PhD
Contribution: conception and design of the B-SNIP study, article review, final approval of the version to be published
Godfrey D Pearlson, MD
Contribution: conception and design of the B-SNIP study, article review, final approval of the version to be published
Gunvant K Thaker, MD
Contribution: conception and design of the B-SNIP study, article review, final approval of the version to be published
Carol A Tamminga, MD
Contribution: conception and design of the B-SNIP study, article review, final approval of the version to be published
John A Sweeney, PhD
Contribution: conception and design of the B-SNIP study, article review, final approval of the version to be published
Matcheri S Keshavan, MD
Contribution: conception and design of the B-SNIP study, article review, final approval of the version to be published
CONFLICTS OF INTEREST
Carol A Tamminga, MD
Disclosures: Intracellular Therapies (ITI, Inc.), PureTech Ventures, Eli Lilly Pharmaceuticles, Sunovion, Astellas, Merck ad hoc consulting,International Congress on Schizophrenia Research - unpaid volunteer, NAMI unpaid volunteer, American Psychiatric Association - Deputy Editor, and Finnegan Henderson Farabow Garrett & Dunner, LLP – Expert Witness
John A Sweeney, PhD
Disclosures: Takeda, Pfizer, and Lilly consulting, and a Janssen research grant
Matcheri S Keshavan, MD
Disclosures: Grants from GSK and Synovion
All other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christoforos I Giakoumatos, Department of Psychiatry, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, cgiakoum@bidmc.harvard.edu.
Neeraj Tandon, Department of Psychiatry, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, ntandon@bidmc.harvard.edu.
Jai Shah, Department of Psychiatry, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts; Department of Psychiatry, Yale University, New Haven, Connecticut, jai.shah@yale.edu.
Ian T Mathew, Department of Psychiatry, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, imathew@caregroup.org.
Roscoe O Brady, Department of Psychiatry, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, robrady@bidmc.harvard.edu.
Brett A Clementz, Departments of Psychology and Neuroscience, Bio-Imaging Research Center, University of Georgia, Athens, Georgia, brett.clementz@gmail.com.
Godfrey D Pearlson, Olin Neuropsychiatry Research Center, Hartford Hospital/Institute of Living, Hartford, Connecticut; Department of Psychiatry, Yale University, New Haven, Connecticut; Department of Neurobiology, Yale University, New Haven, Connecticut, gpearls@harthosp.org.
Gunvant K Thaker, Department of Psychiatry, University of Maryland School of Medicine, Baltimore, Maryland, gthaker@mprc.umaryland.edu.
Carol A Tamminga, Department of Psychiatry, University of Texas Southwestern Medical School, Dallas, Texas, carol.tamminga@utsouthwestern.edu.
John A Sweeney, Department of Psychiatry, University of Illinois at Chicago, Chicago, Illinois; Department of Psychiatry, University of Texas Southwestern Medical School, Dallas, Texas, John.Sweeney@utsouthwestern.edu.
Matcheri S Keshavan, Department of Psychiatry, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, mkeshava@bidmc.harvard.edu.
References
- Aguilar EJ, Leal C, Acosta FJ, Cejas MR, Fernández L, Gracia R. A psychopathological study of a group of schizophrenic patients after attempting suicide. Are there two different clinical subtypes? European Psychiatry. 2003;18(4):190–192. doi: 10.1016/s0924-9338(03)00047-6. [DOI] [PubMed] [Google Scholar]
- Aguilar EJ, García-Martí G, Martí-Bonmatí L, Lull JJ, Moratal D, Escartí MJ, Robles M, González JC, Guillamón MI, Sanjuán J. Left orbitofrontal and superior temporal gyrus structural changes associated to suicidal behavior in patients with schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32(7):1673–1676. doi: 10.1016/j.pnpbp.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Ahearn EP, Jamison KR, Steffens DC, Cassidy F, Provenzale JM, Lehman A, Weisler RH, Carroll BJ, Krishnan KR. MRI correlates of suicide attempt history in unipolar depression. Biological Psychiatry. 2001;50(4):266–270. doi: 10.1016/s0006-3223(01)01098-8. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington: 2000. text rev. [Google Scholar]
- Amitai M, Apter A. Social aspects of suicidal behavior and prevention in early life: a review. International Journal of Environmental Research and Public Health. 2012;9(3):985–994. doi: 10.3390/ijerph9030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Beck R, Kovacs M. Classification of suicidal behaviors: I. Quantifying intent and medical lethality. American Journal of Psychiatry. 1975 doi: 10.1176/ajp.132.3.285. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995 [Google Scholar]
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. British Journal of Psychiatry. 1990;157:853–9. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Brezo J, Klempan T, Turecki G. The genetics of suicide: a critical review of molecular studies. Psychiatric Clinics of North America. 2008 doi: 10.1016/j.psc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Brown GK, Henriques GR, Sosdjan D, Beck AT. Suicide intent and accurate expectations of lethality: predictors of medical lethality of suicide attempts. Journal of Consulting and Clinical Psychology. 2004;72(6):1170–1174. doi: 10.1037/0022-006X.72.6.1170. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Erk S, George C, Kächele H, Kircher T, Martius P, Pokorny D, Ruchsow M, Spitzer M, Walter H. Neural correlates of attachment trauma in borderline personality disorder: a functional magnetic resonance imaging study. Psychiatry Research. 2008;163(3):223–235. doi: 10.1016/j.pscychresns.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Campbell R, Heywood CA, Cowey A, Regard M, Landis T. Sensitivity to eye gaze in prosopagnosic patients and monkeys with superior temporal sulcus ablation. Neuropsychologia. 1990;28(11):1123–1142. doi: 10.1016/0028-3932(90)90050-x. [DOI] [PubMed] [Google Scholar]
- Fedyszyn IE, Robinson J, Matyas T, Harris MG, Paxton SJ. Temporal pattern of suicide risk in young individuals with early psychosis. Psychiatry Research. 2010;175(1-2):98–103. doi: 10.1016/j.psychres.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Fialko L, Freeman D, Bebbington PE, Kuipers E, Garety PA, Dunn G, Fowler D. Understanding suicidal ideation in psychosis: findings from the Psychological Prevention of Relapse in Psychosis (PRP) trial. Acta Psychiatrica Scandinavica. 2006;114(3):177–186. doi: 10.1111/j.1600-0447.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- García-Martí G, Aguilar EJ, Lull JJ, Martí-Bonmatí L, Escartí MJ, Manjón JV, Moratal D, Robles M, Sanjuán J. Schizophrenia with auditory hallucinations: a voxel-based morphometry study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32(1):72–80. doi: 10.1016/j.pnpbp.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Giegling I, Olgiati P, Hartmann AM, Calati R, Möller HJ, Rujescu D, Serretti A. Personality and attempted suicide. Analysis of anger, aggression and impulsivity. Journal of Psychiatric Research. 2009;43(16):1262–1271. doi: 10.1016/j.jpsychires.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Hawton K, Sutton L, Haw C, Sinclair J, Deeks JJ. Schizophrenia and suicide: systematic review of risk factors. British Journal of Psychiatry. 2005;187:9–20. doi: 10.1192/bjp.187.1.9. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two Factor Index of Social Position. 1957 [Google Scholar]
- Hwang J-P, Lee T-W, Tsai S-J, Chen TJ, Yang CH, Lirng JF, Tsai CF. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. Journal of geriatric psychiatry and neurology. 2010;23(3):171–184. doi: 10.1177/0891988710363713. [DOI] [PubMed] [Google Scholar]
- Jia Z, Wang Y, Kuang W, Wu Q, Lui S, Huang X, Chan R, Sweeney JA, Gong Q. Impaired fronto-thalamic circuitry in major depression with a history of suicidal behavior. Presented at the Organization of Human Brain Mapping conference; Beijing, China. 2012. [Google Scholar]
- Jimenez-Treviño L, Blasco-Fontecilla H. Endophenotypes and suicide behaviour. Actas Espanolas de Psiquiatria. 2011 [PubMed] [Google Scholar]
- Keefe RSE, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophrenia Research. 2004;68(2-3):283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Lester D, Beck AT. Suicidal intent, medical lethality of the suicide attempt, and components of depression. Journal of Clinical Psychology. 1975;31(1):11–12. doi: 10.1002/1097-4679(197501)31:1<11::aid-jclp2270310104>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Neurobiology of suicidal behaviour. Nature Reviews Neuroscience. 2003;4(10):819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, Sassi RB, Mallinger AG, Keshavan MS, Soares JC. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Molecular Psychiatry. 2006;12(4):360–366. doi: 10.1038/sj.mp.4001919. [DOI] [PubMed] [Google Scholar]
- New AS, Goodman M, Triebwasser J, Siever LJ. Recent Advances in the Biological Study of Personality Disorders. Psychiatric Clinics of North America. 2008;31(3):441–461. doi: 10.1016/j.psc.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Palmer BA, Pankratz VS, Bostwick JM. The lifetime risk of suicide in schizophrenia: a reexamination. Archives of General Psychiatry. 2005;62(3):247–253. doi: 10.1001/archpsyc.62.3.247. [DOI] [PubMed] [Google Scholar]
- Pandey GN. Neurobiology of adult and teenage suicide. Asian Journal of Psychiatry. 2011 Mar 1;4(1):2–13. doi: 10.1016/j.ajp.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Plutchik R, van Praag HM, Picard S, Conte HR, Korn M. Is there a relation between the seriousness of suicidal intent and the lethality of the suicide attempt? Psychiatry Research. 1989;27(1):71–79. doi: 10.1016/0165-1781(89)90011-5. [DOI] [PubMed] [Google Scholar]
- Radomsky ED, Haas GL, Mann JJ, Sweeney JA. Suicidal behavior in patients with schizophrenia and other psychotic disorders. American Journal of Psychiatry. 1999;156(10):1590–1595. doi: 10.1176/ajp.156.10.1590. [DOI] [PubMed] [Google Scholar]
- Robinson J, Harris MG, Harrigan SM, Henry LP, Farrelly S, Prosser A, Schwartz O, Jackson H, McGorry PD. Suicide attempt in first-episode psychosis: a 7.4 year follow-up study. Schizophrenia Research. 2010;116(1):1–8. doi: 10.1016/j.schres.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Siris SG. Suicide and schizophrenia. Journal of Psychopharmacology. 2001 doi: 10.1177/026988110101500209. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA. Structural brain abnormalities and suicidal behavior in borderline personality disorder. Journal of Psychiatric Research. 2012;46(4):516–525. doi: 10.1016/j.jpsychires.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of General Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. Clinical Phenotypes of Psychosis in the Bipolar and Schizophrenia Network on Intermediate Phenotyopes (B-SNIP) American Journal of Psychiatry. doi: 10.1176/appi.ajp.2013.12101339. in press. [DOI] [PubMed] [Google Scholar]
- Turecki G. Dissecting the suicide phenotype: the role of impulsive-aggressive behaviours. Journal of Psychiatry & Neuroscience. 2005;30(6):398–408. [PMC free article] [PubMed] [Google Scholar]
- van Heeringen C, Bijttebier S, Godfrin K. Suicidal brains: a review of functional and structural brain studies in association with suicidal behaviour. Neuroscience and Biobehavioral Reviews. 2011;35(3):688–698. doi: 10.1016/j.neubiorev.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Zalsman G. Timing is critical: gene, environment and timing interactions in genetics of suicide in children and adolescents. European Psychiatry. 2010;25(5):284–286. doi: 10.1016/j.eurpsy.2010.01.007. [DOI] [PubMed] [Google Scholar]


