Abstract
Malaria is a mosquito-borne disease caused by parasites of the obligate intracellular Apicomplexa family, the most deadly of which, Plasmodium falciparum, prevails in Africa. Malaria imposes a huge health burden on the world’s most vulnerable populations, claiming the lives of nearly a million children and pregnant women each year in Africa alone. Although there is keen interest in eradicating malaria, we do not yet have the necessary tools to meet this challenge, including an effective malaria vaccine and adequate vector control strategies. Here we review what is known about the mechanisms at play in immune resistance to malaria in both the human and mosquito hosts at each step in the parasite’s complex life cycle with a view towards developing the tools that will contribute to the prevention of disease and death and ultimately the goal of malaria eradication. In so doing we hope to inspire immunologists to participate in defeating this devastating disease.
Keywords: malaria, mosquito immunity, human immunity, human adaptive immunity, human innate immunity
Introduction
Malaria is a deadly disease. Each year P. falciparum infections cause approximately 225 million cases of malaria and nearly one million deaths, the majority of which are among African children and pregnant women (1). P. falciparum is transmitted from person to person by the bite of an infected female Anopheles mosquito, primarily Anopheles gambiae in Africa. A. gambiae is an extraordinarily efficient, highly adapted vector that feeds nearly exclusively on humans and has a long life span.
P. falciparum has a complex life cycle that involves critical developmental stages in both the human and mosquito hosts. We now appreciate that both the human and mosquito immune systems mount responses to P. falciparum infections and that these responses dictate the course of the disease in humans and the ability of the mosquito to transmit the parasite. The striking feature of immune resistance to malaria is that it develops only after years in malaria endemic areas, even in areas of high transmission where children may be exposed to hundreds of infectious mosquito bites each year (2). In areas of intense transmission, children become resistant to the most severe forms of malaria by the age of five or so, however, they remain susceptible to uncomplicated episodes of febrile malaria until late childhood or early adolescence when they transition to a malaria resistant state and rarely suffer from clinical malaria (3). The length of time required to develop resistance to malaria is remarkable when compared to the rapid acquisition of immunity to many viral diseases including measles, rubella and smallpox after a single infection. Even though resistance to disease is eventually acquired with cumulative malaria exposure, resistance to liver infection is rarely if ever achieved such that adults living in endemic areas frequently have asymptomatic infections (3). Thus, the acquisition of immunity to malaria in humans is complex involving early resistance to severe disease, followed by resistance to uncomplicated disease but rarely, if ever involving resistance to infection. In contrast to the human host that combats malaria through both adaptive and innate immune mechanisms, the mosquito has only innate immune mechanisms to control parasite infection, but these are remarkably complex and may provide insights into mechanisms at play in the human host.
In this review we describe our current understanding of the acquisition of immunity to both uncomplicated and severe malaria in humans and the nature of the mosquito’s innate immune response to parasite infection. Due to length restrictions we focus our discussion on P. falciparum malaria although another Plasmodium species, P. vivax, is an important pathogen outside of Africa and we refer the reader to an excellent review of the acquisition of immunity to this parasite (4). At present we do not have a malaria vaccine (5) or adjunctive therapies to treat severe disease (6) nor adequate vector control strategies (7) that would decrease disease and death and aid eradication efforts. A clearer understanding of human and mosquito immunity to this deadly infection will undoubtedly contribute to efforts to develop these tools. We preface this description with a discussion of the evolution of P. falciparum and its human host to highlight the importance of the influence of host and parasite genetics on the outcome of Plasmodium infections. Recently, there have been renewed calls for eradication of malaria and to begin we consider how a better understanding of the cellular and molecular basis of human and mosquito immune mechanisms in malaria may contribute to eradication efforts.
A renewed call for malaria eradication
In the fall of 2007 at the Gates Malaria Forum, Bill and Melinda Gates proposed a sweeping new plan to eradicate malaria (8). The proposal shocked the malaria research field as there had been little discussion of eradication since earlier programs launched in the 1950s failed. The proposal to eradicate malaria immediately begged the questions: do we have the necessary tools to even make an attempt? For smallpox, an eradication success story, and for polio, a virus that may be well on its way to eradication, the only tool necessary was a vaccine. At present, we do not have a licensed vaccine that would block malaria transmission and the front running malaria vaccine candidate, RTS,S, appears to confer only short-lived, partial efficacy (30–50%) against clinical malaria in African infants and children (9). We currently have effective anti-malarial drugs that treat the blood stage of the disease and thus decrease transmission (6). However, explosive malaria epidemics would be possible prior to eradication when drug resistance emerges, as seems inevitable (6). The Gates proposal for malaria eradication was based on the idea that each incremental improvement in malaria control would be additive, ultimately resulting in eradication. Experience teaches us otherwise. Attempts at eradication in the 1950s were driven by the Macdonald equation for vector control that predicted that if mosquito populations could be reduced to critical levels malaria transmission would be prevented (10). However, in reality even with the wonder of the insecticide DDT, it was not possible to stop malaria transmission in Africa (11). Even in areas of relatively low transmission such as in India, elimination was not achieved through mosquito control. In India malaria nearly disappeared in the 1960s but then returned reaching over six million cases in 1976 (12). The lesson drawn is wherever mosquitoes persist, explosive malaria epidemics are always possible. Indeed, it has been calculated that when the reproductive rate (a complicated factor that takes into account a variety of parameters including the number of infected mosquitoes, how often they feed and how long they live) reaches 100, a rate not uncommon in many areas in Africa, infections can explode, going from 0.1% to 50% infected individuals in a mere 100 days (13). If conventional vector control methods have not succeeded in blocking malaria transmission, what other vector control tools might be developed? Transmission blocking vaccines that induce in the human host antibodies to parasite proteins that are essential in parasite development in the mosquito host are under development but thus far have not shown the ability to induce longlasting transmission blocking antibodies in humans (5). An alternative approach is to modify the mosquito population so that it has no vector capacity. Although such modifications clearly face enormous challenges and will likely require years of research to identify genes that if modified would lead to refractoriness and then to develop genetic techniques to manipulate mosquito populations, such a long term effort may be well worthwhile. Without the ability to modify mosquito populations, eradication may simply never be possible. Despite the focus of the Gates Foundation on developing tools for eradication, it is important to bear in mind that there remains a desperate need for a vaccine that would reduce malaria disease and death even one that did not block transmission. Knowledge of the mechanisms underlying the acquisition of immunity to natural malaria infection will undoubtedly aid in the development of such a vaccine.
The evolutionary dance of P. falciparum and its hosts
In discussing the immune mechanisms at play in controlling malaria it is helpful to consider that P. falciparum is estimated to be greater than 100,000 years old, as old as humans (14), suggesting that the human immune system and the parasite co-evolved. Because of the enormous selective pressure imposed by the high mortality of P. falciparum infections in children and pregnant women, malaria has had a tremendous impact in shaping the human genome, perhaps more than any other pathogen (15). One of the best-studied examples of the evolutionary pressure of P. falciparum on the human genome is the Hemoglobin S variant (HbS). In the homozygous state, HbS results in sickle cell anemia that is lethal in West African children. Nonetheless, HbS is maintained at a frequency of 10–20% because in the heterozygous state HbAS confers protection against severe malaria (16). One theme that will run through this review is that malaria is likely to have had a significant influence in shaping the immune mechanisms in both the human and mosquito hosts allowing the parasite’s persistence in both hosts and its transmission, presumably in exchange for some advantage to its hosts. As part of the co-evolution of P. falciparum and its hosts the parasite has likely evolved a myriad of mechanisms to evade and disable its hosts’ immune responses. The P. falciparum genome contains approximately 5,400 genes (17) however, remarkably, we are aware of only a handful of mechanisms in humans and in mosquitoes that impact host immunity, leaving a great deal for discovery.
P. falciparum life cycle
P. falciparum has a complex life cycle involving both human and mosquito hosts (Figure 1). The cycle begins when a parasite-infected female Anopheles mosquito probes human skin as it prepares to take a blood meal. Its saliva contains a highly motile differentiated form of the P. falciparum parasite called a sporozoite and a small number of these (10–100) are injected in the skin where they may remain for hours if not days (18). The infection sporozoites cross the endothelium of the capillaries in the skin, enter the blood and travel to the liver. In the liver the sporozoites traverse Kupffer cells and hepatocytes then invade a small number of hepatocytes (19). Within hepatocytes the sporozoites replicate over a week’s time, increasing in number up to 40 thousand fold, and differentiate giving rise to a large number of asexual blood stage parasites called merozoites. Up to this point in the parasite’s life-cycle individuals are unaware that they are infected as there are no clinical symptoms. Merozoites are released into the blood stream via the budding of parasite-filled, hepatocyte-derived vesicles called merosomes, clearing the liver of parasites (20). In the blood stream, free merozoites begin 48 h cycles of invasion of RBCs, replication, RBC rupture and release of merozoites and invasion of RBCs. Parasitemia often exceeds densities of 50,000 infected RBCs (iRBCs) per microliter of blood. A variety of parasite products are released upon iRBC lysis that correlate with the onset of the malaria symptoms including headaches, fever and lethargy. During RBC infection merozoites dramatically remodel the RBC membrane expressing several parasite-encoded proteins on the iRBC surface (21). Key among these are the P. falciparum erythrocyte membrane protein 1s (PfEMP1s) encoded by P. falciparum var genes (21). Each parasite genome contains approximately 60 var genes that encode antigencially distinct PfEMP1 proteins that are clonally expressed by the parasite. PfEMP1s bind to a variety of ligands on endothelial cells and function to sequester the iRBC in the venules of various tissues, thus saving the iRBC from entering the spleen where they would be destroyed. The PfEMP1-mediated sequestration of iRBCs in tissues, for example in the brain and in the placenta, has been implicated in the pathogenesis of severe malaria in children and in pregnant women. The life cycle of P. falciparum in the human host is completed when, in a poorly understood process, the asexual blood-stage parasites differentiate into male and female gametocytes that are taken up in a mosquito’s blood meal. In the mosquito midgut, male and female gametes fuse to form ookinetes that cross the midgut epithelium and ultimately differentiate into sporozoites that invade the mosquito’s salivary glands, completing the parasite’s life cycle in the invertebrate host.
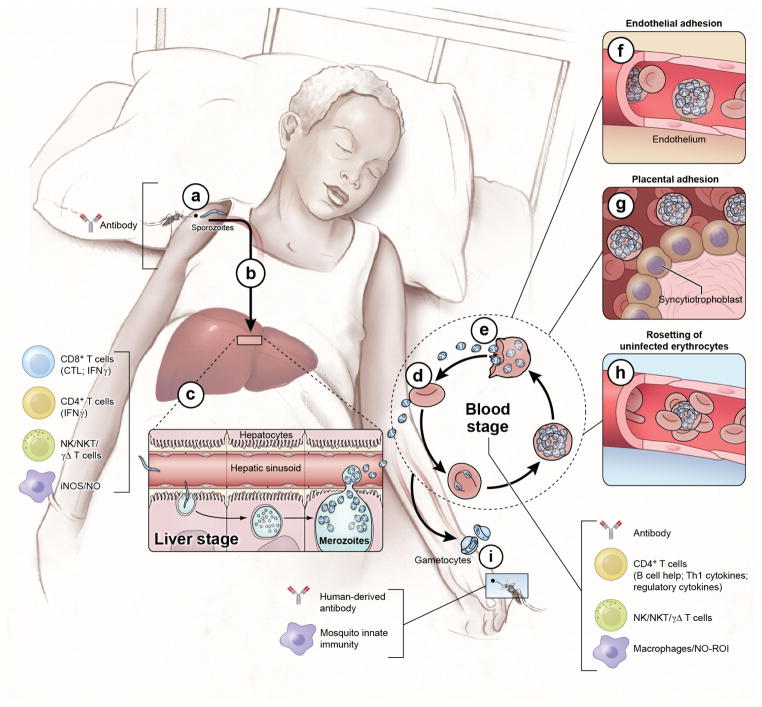
Figure 1. The Plasmodium life cycle.
The Plasmodium life cycle in humans includes the asymptomatic liver stage, the blood-stage which causes disease, and the sexual gametocyte blood-stage which infects mosquitoes that transmit the parasite. Infection begins when a female Anopheles mosquito injects saliva that contains sporozoites into the skin and blood as it takes a blood meal (a). At this point the infection is clinically silent and there is no evidence for naturally acquired immunity. However, immunization with attenuated whole sporozoites induces sterilizing immunity and in this case, the only known immune effector that can reduce or block sporozoites in the skin are antibodies. In mouse models sporozoites have been shown to enter draining lymph nodes from the skin where they are presented by DCs and prime CD8+ T cells. The highly motile sporozoites migrate to the liver, transverse Kupffer cells and invade a small number of hepatocytes (b). In humans the infection continues to be clinically and immunologically silent at the liver stage and sterilizing immunity is not naturally acquired. However, in humans and in mice immunization with attenuated sporozoites induces sterilizing immunity that appears to rely on adaptive CD8 + and CD4 + T cells, the innate production of iNOS and NOS and on NK, NKT and γδ T cells. Each sporozoites infected hepatocyte gives rise to tens of thousands of asexual parasites called merozoites (c). Approximately one week after hepatocyte invasion merozoites exit the liver into the bloodstream and begin a 48 hr cycle (d) of RBC invasion, replication, RBC rupture, and merozoite release (e). Clinical symptoms of malaria only occur during the blood-stage and can begin as early as three days after the release of merozoites from the liver. Inside RBCs the parasite dramatically remodels the RBC including the export of variant surface antigens (VSAs) such as PfEMP1 to the RBC surface. VSAs act as receptors for a variety of endothelial cell ligands and mediate binding of iRBCs to the microvascular endothelium of various organs (f) allowing parasites to avoid splenic clearance. However, the sequestration of iRBCs in the microvaculature promotes the inflammation and circulatory obstruction associated with clinical syndromes of severe malaria including cerebral malaria with iRBC sequestration in the brain and pregnancy-associated malaria with iRBCs in the placenta (g). VSA-mediated rosetting of iRBCs to uninfected RBCs may also contribute to disease (h). Coincident with the rupture of merozoites iRBC and the release of merozoites and variety of merozoites products are inflammation and the clinical symptoms of malaria. Both adaptive and innate immune responses are readily detected. The key immune effector at this stage is antibodies. CD4+ T cell cytokine producing T cells also play a role as do NK, NKT and γδ T cells and macrophages through the production of NO and NOI. A small number of blood-stage parasites differentiate into sexual gametocytes which are taken up by mosquitos in blood meals (i). In the mosquito the gametes fuse, ultimately forming sporozoites that enter the mosquito salivary gland to complete the life cycle (see Fig. 3). In the mosquito innate immune mechanisms serve to control parasite development. Immunization of the vertebrate host with proteins expressed by the parasite in the mosquito host results in the production of antibodies that are taken up by the mosquito with the blood meal and have been shown to block parasite development and consequently block transmission.
Human immunity and malaria
Studying human immunity to malaria
Animal models of malaria have provided and will undoubtedly continue to provide important insights into the immunobiology of Plasmodium infection (22), but ultimately, despite obvious experimental limitations, it is critical to investigate the immune response to Plasmodium infection in humans. In contrast to what is typically available to researchers of other infectious diseases, the malaria research community has access to longitudinal ‘models’ of both experimental and natural P. falciparum infection in humans (23–25). Under strictly controlled conditions, volunteers are exposed to the bites of laboratory-reared P. falciparum-infected mosquitoes. In previously unexposed individuals, parasites become detectable in the blood by microscopy approximately 11 days after infection at which point curative anti-malarial therapy is administered. Since the 1980s over 1500 volunteers have been experimentally infected with P. falciparum without serious adverse events, although P. falciparum-naïve volunteers often experience transient malaria symptoms such as fever, chills, headache and myalgias. A model of direct intravenous administration of iRBCs in healthy volunteers has also been developed allowing investigations of the early immunological responses to blood-stage infections while strictly controlling the size of the blood-stage inoculum (24). The highly controlled and predictable nature of human experimental P. falciparum infections allows for high-resolution immunological analyses during the skin, liver and early blood-stages of the parasite life cycle.
The study of immune responses to natural infection in individuals of all ages is also feasible in malaria endemic areas due in part to the patterns and intensities of P. falciparum transmission. Transmission patterns vary across endemic areas from sporadic to seasonal to year round depending on the temperature, rains and mosquito breeding, and transmission intensities vary from low to high. It is possible to take advantage of these variations to design field-based immunological studies tailored to these transmission patterns and intensities. For example, in areas of intense seasonal malaria, such as in much of West Africa, the intensity of transmission is so great that every individual will predictably be exposed to hundreds of infectious mosquito bites over the course of a transmission season. Cohorts can be enrolled during the six month dry season during which there is no malaria transmission and then followed through the malaria season. The predictable timing and intensity of P. falciparum transmission from year to year allow for multi-year cohort studies in which P. falciparum infections and clinical malaria episodes can be reliably detected through active parasitological and clinical surveillance and relevant biospecimens can be collected at time points before, during, and after asymptomatic and symptomatic P. falciparum infections (25). Importantly, such designs allow study subjects to serve as their own healthy pre-infection controls and permit two general types of questions to be addressed: 1) What immune parameters or profiles correlate prospectively with protection from malaria?, and 2) How do acute and chronic P. falciparum infections modulate the human immune response?
These unique and powerful clinical research models, informed by experimental findings in animal studies, and in conjunction with technological advances that enhance our ability to interrogate perturbations to the human immune system, have the potential to rapidly improve our understanding of malaria immunity.
The immune response to the skin and liver stages of Plasmodium infection
In humans, the skin and liver stages of P. falciparum infection are clinically silent as they fail to induce significant dermal, hepatic or systemic inflammation. Not unexpectedly, the lack of a robust innate immune response to the skin and liver stages is associated with a correspondingly muted induction of antibody, CD4+ and CD8+ T cell responses to the infecting sporozoites in individuals exposed to natural P. falciparum infections in endemic areas (reviewed in (26)). Accordingly, there is no convincing evidence for naturally-acquired immunity capable of completely neutralizing the parasite at the skin or liver stages. Indeed, even after decades of repeated P. falciparum exposures, adults in malaria-endemic areas are at the same risk of becoming infected with parasites as young children (27). The cellular and molecular basis of this clinical silence and paucity of sterilizing immunity is only poorly understood. The relatively weak innate and adaptive immune response to the skin and liver stages may reflect the low inoculum of parasites in the mosquitoes’ saliva (10 – 100 sporozoites). The inherent immune-regulatory environment of skin (28) and liver (29), may also be exploited by the parasite to evade immune mechanisms. Normal skin has a particularly high proportion of regulatory T cells (Tregs) in the steady state (28) and a meta-analysis of malaria vaccine studies suggested that Plasmodium-specific Tregs are induced during the skin stage of infection, potentially mediating immune tolerance to sporozoites as well as to the subsequent blood-stage of infection (30). Consistent with these results are experiments in mice that show that skin Tregs and DCs are mobilized within 30 minutes of sporozoite inoculation and that DCs downregulate MHC II and CD86 suggesting a tolerogenic response (31). The immunoregulatory environment of the skin may be reinforced by chronic immune activation or immune dysregulation due to repeated exposures to sporozoites and liver stage antigens (32). Plasmodium-specific mechanisms that disable innate immune defenses, such as sporozoite-mediated disruption of Kupffer cells’ respiratory burst in the liver (33) may also affect pre-erythrocytic immunity.
In striking contrast to the lack of pre-erythrocytic immunity in natural infection, sterilizing immunity to Plasmodium infection can be readily induced in mice (34), non-human primates (35) and humans (36) through exposure to radiation-attenuated (RA) sporozoites that are able to infect hepatocytes but cannot replicate in the hepatocyte and do not give rise to blood-stage infections. Sterilizing immunity can also be induced by exposure to genetically-attenuated parasites (37) which also invade hepatocytes but do not replicate to produce blood-stage merozoites, and by exposure to sporozoites under chloroquine anti-malarial chemoprophylaxis (38). Chloroquine kills only blood-stage parasites and thus exposure to sporozoites under chloroquine treatment allows full exposure to sporozoites and liver-stage parasites and only transient exposure to blood-stage parasites. The number of RA sporozoites and route of exposure to RA sporozoites appears critical to induce immunity. Early studies showed sterilizing immunity to RA sporozoites required the bites of over 1,000 infective mosquitoes. A recent trial in humans showed that subcutaneous RA sporozoite immunization did not confer protection (39), however, intravenous RA sporozoite immunization in animal models was protective (39). Thus far, most experimental exposures to sporozoites have only been shown to be effective when individuals are challenged with homologous sporozoites and in endemic areas individuals would rarely be infected with the same parasite clone given the extreme genetic diversity (40). Even though whole sporozoite-induced sterile immunity does not have a natural counterpart, there is considerable interest in understanding the mechanisms underlying the discordant acquisition of immunity to experimental and natural P. falciparum infection to aid in efforts to develop whole sporozoite vaccines for use in humans.
In humans, RA sporozoite exposure induces antibodies, CD4+ and CD8+ T cells that react to several sporozoite and liver stage antigens (41–43), but the relative contribution and antigen specificity of these putative effector mechanisms remains unclear. Volunteers exposed to sporozoites under chloroquine treatment develop complete protection against homologous sporozoite challenge (38) that lasts at least 28 months (44). Exposure elicits both antibody and T cell responses against sporozoite and blood-stage antigens (38) and protection has been linked to pluripotent effector memory T cells producing interferon-γ (IFN-γ), tumor necrosis factor (TNF), and interleukin-2 (IL-2) (38) that are most likely directed against liver rather than blood-stage antigens (45).
Animal models have allowed detailed characterization of the immune mechanisms at play in the skin (reviewed in (46)) and in the liver (47) during Plasmodium infection that may provide insights for the ongoing development of whole sporozoites based vaccines. In mice, within minutes to hours of RA sporozoite inoculation into the dermis, 10–25% of sporozoites reach the liver via the bloodstream while 15–25% enter skin-draining lymph nodes (reviewed in (46)). As early as 48 hours after sporozoite inoculation, Plasmodium-specific CD8+ T cells are detectable in skin draining lymph nodes (48). Surgical removal of skin-draining lymph nodes or inhibition of T cell egress from these lymph nodes significantly decreases the number of T cells reaching the liver and disrupts CD8+ T cell-mediated killing of intracellular liver stage parasites (48) that involves both cytolytic (i.e. perforin/granzyme) and non-cytolytic (i.e. IFN-γ - induced nitrous oxide) pathways (reviewed in (49, 50)). A key question is: How are CD8+ T cells primed to sporozoites antigens? Recent studies using parasites carrying a mutant circumsporozoite protein (CSP) containing the ovalbumin-derived SIINFEKL epitope and a variety of mouse mutants showed that DCs phagocytose sporozoite antigen and cross-present parasite-derived peptides on MHC class I to CD8+ T cells (51). Accordingly, in vivo depletion of CD11c+ DCs abrogates priming of Plasmodium-specific CD8+ T cells (52). Antigen presentation may not be limited to DCs as sporozoites traverse Kupffer cells in the liver prior to hepatocyte invasion and shed parasite proteins into the cell cytoplasm that may also be presented to CD8+ T cells (51). Surprisingly to date only a few T cell antigenic peptides derived from sporozoites have been identified. Recent studies, using liver-stage specific epitope profiling, revealed three new CD8+ T cell epitopes for the mouse Plasmodium species (53, 54). Clearly, additional approaches are needed to identify T cell epitopes to allow better characterization of protective and nonprotective T cell responses.
Concerning the length of time required to prime T cells it is interesting that sporozoite molecules may be retained in skin draining lymph nodes as well as in the spleen and liver for up to two months after exposure to RA sporozoites or infected mosquito bites (55). Although previous studies suggested that CD8+ T cell priming only requires short (<24h) exposure to antigen (56) prolonged antigen exposure might be required to optimize the liver-stage specific CD8+ T cell response by maximizing the expansion of effector cell populations (55). Indeed, high levels of CD8+ T cells are required to kill intrahepatic parasites (57), and similarly in humans, the magnitude of vaccine-induced CD8+ T cell responses specific for pre-erythrocytic antigens correlated with protection (58). Only one infected hepatocyte escaping the cytotoxic T cell response might be enough to initiate a blood-stage infection. This is a likely reason why extremely high frequencies of memory CD8+ T cells are required to induce sterile immunity after immunization with irradiated sporozoites (57). RA sporozoite-induced antibodies have also been implicated as effectors in liver stage immunity in mice (reviewed in (59)) through enhancing sporozoite clearance, decreasing sporozoite motility (60) or blocking hepatocyte invasion (61). Undoubtedly, animal models will continue to inform further investigations that also take advantage of recent advances in human skin immunobiology (62) and humanized mouse liver models (63).
The early observation of RA sporozoite-induced protection from Plasmodium infection spurred the pursuit of not only whole sporozoite-based vaccines but of sporozoite protein subunit vaccines as well. The most advanced candidate in this regard is RTS,S, a subunit vaccine which targets CSP, a surface protein on sporozoites that is critical for hepatocyte invasion (reviewed in (64)). RTS,S induces both antibodies and CD4+ T cells (not CD8+ T cells), but unambiguous correlates of protection have yet to be identified (65). Remarkably, RTS,S vaccination reliably confers sterile protection in ~50% of malaria-naïve adults (64). However, in African infants and children the protective efficacy of RTS,S is 30–50% against clinical malaria (9, 66) while durable protection from infection per se is not observed. Similarly, a vaccine targeting the liver stage antigen, thrombospondin related adhesion protein (TRAP), that relies on the generation of CD8+ T cell immunity showed partial efficacy in malaria naïve adults (58) but not in African children (67) or adults (68).
The question that emerges from these observations is why do malaria vaccine candidates appear to be more effective in naïve adults than in the target populations, namely African children? There are several possible explanations, but no real answers. The malaria-naïve subjects are drawn primarily from Europe and the U.S. and may have important genetic differences, most notably in the MHC HLA alleles (69) and other immune related genes including the innate systems Toll like receptors (TLRs). Co-infections with other pathogens are common in malaria endemic areas and these may modulate the development of Plasmodium-specific immunity (70). In addition, the nutritional status (71) and composition of gut microbiota in African children (72) may have profound immunodulatory effects on host immunity to malaria, particularly the immune microenvironment in the liver (73) which drains portal blood.
Given the increased focus on the development of vaccines that aim to induce sterile protection by targeting the sporozoite and liver stage of infection for the purpose of eradication, studies aimed at identifying the mechanisms underlying refractoriness to the natural acquisition of sterile immunity and vaccine failures are badly needed. Such studies will benefit from the development of several new tools. Antigen-specific Class I and Class II MHC tetramers would aid in defining the extent to which P. falciparum-specific T cells are engaged and function in individuals in endemic areas versus sporozoite exposed naive individuals. Biomarkers reflective of the liver stage parasite burden would allow the relative contributions of effective liver-stage immunity from early blood-stage immunity to be disentangled and the immune mechanism at play in each to be better understood.
The response to blood-stage infection
The clinical manifestations of malaria are caused by asexual blood-stage parasites as they replicate in the blood (Figure 1). Although complex and incompletely understood (6), malaria pathogenesis is generally thought to be driven by two distinct processes: sequestration and inflammation. Sequestration involves the binding of iRBCs to receptors on vascular endothelium via specific parasite-host interactions, causing microvascular obstruction, local ischemia and inflammation in the brain and other vital organs (74, 75). P. falciparum also induces a systemic inflammatory response akin to bacterial sepsis (76) which may exacerbate iRBC sequestration by upregulating vascular adhesion molecules such as ICAM-1 (77). The question of whether inflammation or sequestration is the central initiating event in malaria pathogenesis is a matter of ongoing debate (78, 79), but it seems likely that these processes occur in parallel and are mutually reinforcing.
In areas of intense P. falciparum transmission, resistance to severe life-threatening malaria (discussed below) is generally acquired by the age of five years, whereas children remain susceptible to repeated bouts of febrile malaria through adolescence, eventually achieving near complete clinical immunity to blood-stage parasites by adulthood ((3); Figure 2). Here we focus on two immunological processes that play crucial roles in controlling P. falciparum blood-stage parasites and the disease they cause: inflammation and its regulation, and the antibody response. In previously unexposed individuals, blood-stage P. falciparum parasites invariably induce fever and other signs and symptoms of malaria (80), a process driven by the production of proinflammatory cytokines and chemokines such as IL-1β, IL-6, IL-8, IL-12(p70), IFN-γ and TNF (81–83). Recent work has begun to identify the P. falciparum pathogen-associated molecular pattern molecules (PAMPs) that trigger inflammation through pattern recognition receptors (PRRs) expressed by immune cells. These PAMPs include GPI anchors (TLR2) (84), hemozoin (NLRP3 inflammasome) (85), CpG-containing DNA motifs bound to hemozoin (TLR9) (86) and AT-rich DNA motifs (unidentified cytosolic receptor) (87). A handful of Plasmodium proteins have also been identified as drivers of inflammation through specific interactions with host receptors. For example, particular PfEMP1s have been recently implicated in parasite sequestration in brain endothelium in severe cerebral malaria in children (88–90). The receptor for these PfEMP1s was recently identified as the endothelial protein C receptor (EPCR) that mediates cytoprotective effects through activated protein C suggesting that PfEMP1 binding to EPCR may block its protective function and contribute to the pathology of cerebral malaria (75). Merozoite surface protein 1 (MSP1) that is shed from the merozoite surface as the parasite invades RBCs was shown to bind to S100P a member of the pro-inflammatory S100 protein family and to block S100P activity (91). These studies were recently extended to show MSP1 binds to all S100 family members (Waisberg unpublished observation). The extent to which heterogeneity in the host inflammatory response and disease severity is the result of polymorphisms in these and other proteins, the expression of specific subsets of PfEMP1s, or variability in the structure, configuration or quantity of Plasmodium PAMPs is an area that requires further study.
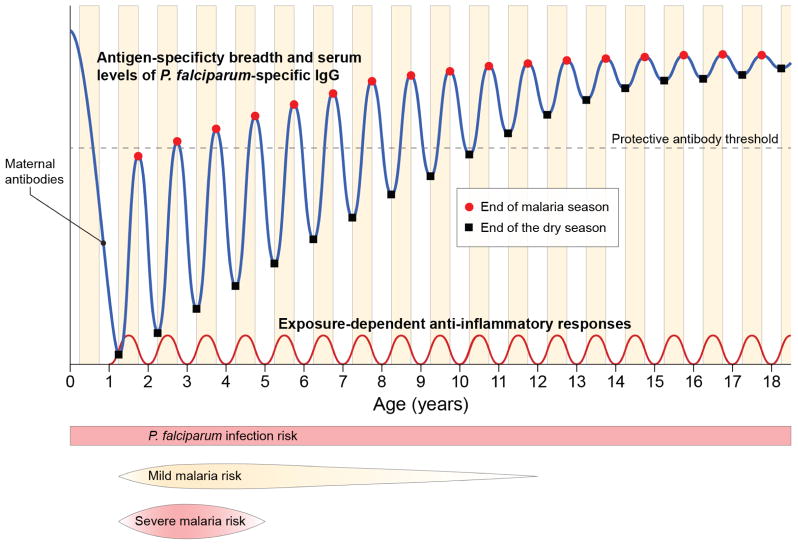
Figure 2. The acquisition of immunity to malaria in the context of intense seasonal P. falciparum transmission.
In areas of intense P. falciparum transmission immunity to severe life-threatening malaria is generally acquired by the age of five years, whereas children remain susceptible to repeated episodes of febrile malaria into adolescence, eventually acquiring near complete immunity to the symptoms of malaria by adulthood while remaining susceptible to becoming infected with blood-stage parasites. The mechanisms of immunity to severe malaria are unclear but may involve the acquisition of ‘strain-specific’ antibodies that neutralize key P. falciparum variant antigens that drive the pathogenesis of severe disease (e.g. subsets of PfEMP1s that mediate sequestration) and the induction of ‘strain-transcendent’ regulatory mechanisms that control excessive P. falciparum-induced inflammation, both of which may depend on ongoing P. falciparum exposure to be maintained. In young children P. falciparum-specific antibody responses to acute infection are generally short-lived, but with each year of exposure there is a gradual increase in the breadth of antigen specificity and serum levels of P. falciparum-specific IgG that persists in the absence of transmission (i.e. during the dry season in the case of seasonal malaria), only reliably conferring protection against malaria symptoms when an ill-defined threshold is surpassed.
Work in animal models demonstrates a role for various innate immune cells in sensing early blood-stage infection, promoting inflammation, inhibiting parasite growth and shaping adaptive immune responses (reviewed in (92)) including mast cells (93), neutrophils (94), NK cells, NKT cells and γδ T cells ((95)). Human NK cells are a particularly important early source of IFNγ in response to iRBCs in vitro, a central cytokine in the immune response to malaria that promotes the destruction of iRBCs by activated macrophages. In humans, γδ T cells contribute to early IFNγ production to a lesser degree, while later IFNγ production is dominated by αβ T cells (96).
On the one hand, Plasmodium-induced inflammation likely plays an important role in the early control of parasite replication, for example, IFN-γ and TNF kill blood-stage P. falciparum parasites through the induction of NO and other toxic radicals (97), but on the other hand excessive inflammation has been linked to severe and fatal malaria (98) which occurs in a minority of individuals who have little or no prior malaria exposure (16). However, in endemic areas where individuals are repeatedly infected, blood-stage infections commonly cause a mild febrile illness or no symptoms at all (3), consistent with the long-standing hypothesis that tolerance to Plasmodium-induced inflammation can develop (99). This notion is supported by early studies in humans which showed that the risk of fever decreases with serial experimental Plasmodium infections (80) and that Plasmodium infection induces cross-tolerance to endotoxin (100). Consistent with this hypothesis, mouse models of non-lethal malaria show that Plasmodium-induced inflammation (TNF and IFN-γ) peaks 5–10 days after infection (101, 102) and then diminishes due to the production the regulatory cytokines TGF-β (101) and IL-10 (102) even before parasitemia decreases. In humans, cross-sectional studies show that higher ratios of pro- to anti-inflammatory cytokines in serum during acute malaria are associated with more severe disease (81, 103), although this is not a consistent finding(104). What remains unknown is whether malaria-induced inflammation is attenuated within individuals as they are repeatedly re-exposed to P. falciparum infections in endemic areas, and the extent to which this contributes to clinical immunity (105). Also unclear are the immunoregulatory mechanisms and cells that mediate control of P. falciparum-induced inflammation in humans (106, 107) and the longevity of regulatory responses in the absence of ongoing P. falciparum exposure.
A longitudinal study of children in Mali (Crompton, unpublished) provides some insight into these questions. Transcriptome analysis of leukocytes from healthy children before the malaria season and the same children seven days after treatment of their first febrile malaria episode of the ensuing season (when malaria symptoms had resolved) showed that key mediators of P. falciparum-induced inflammation and fever (IL-1β, TNF, IL-8) are downregulated following treatment relative to the healthy pre-malaria baseline. Stimulation of these children’s leukocytes in vitro with P. falciparum iRBCs at the same time points showed downregulation of proinflammatory cytokines/chemokines and upregulation of the anti-inflammatory cytokines TGF-β and IL-10 seven days after treatment relative to the healthy baseline. P. falciparum-specific CD4+Foxp3− T cells which co-produced IFNγ and TNF, were found to be the major source of IL-10. The ability of T cells to upregulate IL-10 was lost in children who were no longer exposed to ongoing P. falciparum transmission, but partially maintained in children with persistent asymptomatic infection. This finely-tuned regulatory response reduces the production of potentially harmful pyrogenic and inflammatory mediators without abolishing critical antiparasite effectors. Thus, children acquire the ability to mount P. falciparum-specific anti-inflammatory responses that control inflammation during subsequent P. falciparum exposures, which likely contributes to protection from fever and immunopathology.
Until recently the cellular source of IL-10 in P. falciparum-infected individuals was unclear (106). The finding in Mali that CD4+Foxp3− Th1 cells are the major source of IL-10 (Crompton unpublished) corroborates a cross-sectional study in the Gambia in which FOXP3− CD45RO+CD4+ T cells, rather than Tregs, were identified as the only substantial source of IL-10 in children with malaria (104). As in Mali, this same study showed that a significant proportion of IL-10-producing CD4+ T cells co-produced IFN-γ, and this cell population was significantly increased in children with mild versus severe malaria (104). Similar ‘self-regulating’ CD4+ Th1 cells (108) have been observed in other infections in humans (109, 110) and animal models (111, 112) pointing towards a protective role for this cell population. The ability to control excessive P. falciparum-induced inflammation in early life may be the key adaptation that confers protection from potentially life-threatening disease in young children who have yet to acquire protective antibodies, which are only reliably acquired after many years of P. falciparum exposure (113).
In 1961, Cohen et al. established that antibodies play a central role in blood-stage malaria immunity by showing that the transfer of purified IgG from malaria-immune adults to children with acute malaria led to rapid and profound reductions in parasite numbers in the blood and resolution of fever (114). Over fifty years later, we have yet to fully understand which of the over 5000 P. falciparum proteins (17) are targets of protective antibodies, the precise mechanisms by which these antibodies protect and why they are only acquired after years of repeated infections—all critically important questions for blood-stage malaria vaccine development, an effort which has been largely empiric and unsuccessful to date (115). Progress toward addressing these questions has been catalyzed in recent years by advances in basic B cell biology and the development of new tools to probe B cell responses to Plasmodium infection.
The inefficient acquisition of antibodies that protect against malaria has been attributed in part to the genetic diversity of many P. falciparum proteins (116) and the parasite’s ability to clonally vary the proteins it expresses on the surface of iRBCs (117), such that it may take years living in an endemic area for an individual to be exposed to a sufficient number of parasite clones to generate a protective repertoire of antibodies. This highly successful immune evasion strategy may underpin the limited efficacy of blood-stage vaccines that have targeted polymorphic merozoite proteins (118). To be successful going forward, it is likely that blood-stage vaccines will need to induce antibodies specific for either conserved proteins that are critical to parasite survival, or to a large repertoire of clonally variant antigens expressed on the surface of iRBCs, such as the PfEMP1 family.
Hope for the former possibility was recently bolstered by the discovery of the P. falciparum reticulocyte-binding protein homologue 5 (PfRH5), a conserved protein that is essential for merozoite invasion of erythrocytes (119–121). Moreover, antibodies raised against either PfRH5 (122, 123) or its erythrocyte receptor, basigin, (121) inhibit parasite invasion of erythrocytes in vitro. Although PfRH5 appears to be a relatively poor immunogen in the context of natural P. falciparum infection (122) (possibly explaining why it is more conserved than other merozoite proteins), naturally-acquired antibodies specific for PfRH5 correlate with protection from malaria in longitudinal studies in Papua New Guinea (124) and in Mali (Crompton, unpublished). In principle, if immunization with PfRH5 reliably blocks merozoite invasion of RBCs, it would not only prevent disease, but may also disrupt the parasite’s life cycle and prevent transmission to mosquitos.
The development of a vaccine that would confer protection against malaria by inducing immunity to clonally variant P. falciparum antigens is a formidable challenge. However, evidence from studies in malaria endemic areas suggests that the clonally variant PfEMP1 family is a major target of humoral immunity to malaria (125) and that repeated P. falciparum infections may elicit a protective repertoire of PfEMP1-specific antibodies (126). How might this immunity be replicated through vaccination? One approach may be to immunize with whole killed blood-stage parasites that have been genetically modified to express the entire PfEMP1 repertoire of a given parasite simultaneously (127). This approach could complement ongoing efforts to develop killed or attenuated whole parasite blood-stage vaccines (128), which intriguingly, appear to mediate protection through CD4+ T cells, IFNγ and nitric oxide (NO) rather than antibodies (129).
In addition to genetic polymorphism and antigenic variation, there is mounting evidence that Plasmodium evades humoral immunity through dysregulation of CD4+ T cell and B cell function. It is well established that long-lived protective antibody responses depend on the generation of memory B cells (MBCs) and long-lived plasma cells (LLPCs), a process which relies on CD4+ T cell help (130). Although there is heterogeneity in the magnitude, quality, and longevity of antibody responses following infection or vaccination, in general, antibody responses are long-lived, even after one or a few exposures (131). For instance, the estimated half-life of the IgG response to the measles vaccine is >300 years (131). In contrast, several studies have reported that P. falciparum–specific antibodies decline to undetectable or nearly undetectable levels within 3–9 months of documented malaria episodes in children (reviewed in (113). A study using protein arrays corroborated and extended this finding by showing that children generate IgGs specific for hundreds of P. falciparum antigens in response to a single malaria season but rapidly lose most of what they gain during the subsequent six-month dry season, a period of little to no malaria transmission (132). The same study showed that with each year of P. falciparum exposure, the level of P. falciparum-specific IgG persisting through the dry season increases incrementally until young adulthood when IgG levels are maintained at high levels (corresponding temporally with the acquisition of malaria immunity) and fluctuate less in response P. falciparum infection. These data suggest that the gradual acquisition of clinical immunity to malaria may reflect the need for repeated infections to “fill” the P. falciparum–specific LLPC compartment to the point where steady-state antibody levels exceed a protective threshold.
It has long been suspected that malaria might not generate MBCs based on anecdotal reports of waning immunity in the absence of P. falciparum exposure as well as inconsistent antibody boosting upon reinfection (133), even to conserved antigens (134). However, several studies have now shown that P. falciparum–specific MBCs are generated in response to infection, albeit inefficiently, as their prevalence appears to be relatively low among adults (~30–50%) (135–138), even those with a history of documented P. falciparum infections. Once acquired, however, MBCs appear to be long-lived (137) and may persist longer than antibodies in the absence of ongoing P. falciparum exposure (139). The findings for P. falciparum–induced MBCs stand in contrast to studies that show, for example, that smallpox vaccine–specific MBCs are generated and persist in nearly all vaccinees for ≥50 years in the absence of antigen re-exposure (140). Therefore it appears that P. falciparum infection can generate long-lived MBCs but less efficiently, at least compared with vaccines for other pathogens.
The relatively inefficient acquisition of P. falciparum–specific LLPCs and MBCs may be related to dysregulated B cell antigen-driven differentiation. Altered B cell function is well described in other infections, for example, HIV and hepatitis C virus infection are associated with an increase in B cells identified by the cell surface markers CD19+CD20+CD21−CD27−CD10− and Fc receptor–like-4+ (141, 142). In HIV-infected individuals, these cells are hyporesponsive or “exhausted” and may contribute to the humoral deficiencies associated with HIV (141). An increase in a phenotypically similar subset of B cells has been observed in malaria–exposed children and adults across genetically and geographically diverse populations (reviewed in (113)). In the context of malaria, this B cell subset is referred to as “atypical” (143) rather than exhausted because the function of these cells and whether they are beneficial or detrimental in malaria remains unclear. That P. falciparum per se may drive this response has been suggested by the observation of differential expansion of atypical MBCs in age-matched children living under similar conditions in rural Kenya with the exception of P. falciparum exposure (144). Intriguingly, at the single-cell level, P. falciparum exposure has recently been associated with the acquisition of atypical MBCs that differed in their Ig gene repertoire from classical MBCs, suggesting that these two populations develop from different precursors (145).
Malaria may also dysregulate helper CD4+ T cell responses. PD-1, a marker of T cell exhaustion, is upregulated on CD4+ T cells in children following P.falciparum infection (146). Although the functional significance of this observation in human is unclear, Butler et al. reported that P. yoelii infection in mice induces phenotypic and functional CD4+ T cell exhaustion, and that in vivo blockade of the PD-1 ligand PD-L1 and the inhibitory receptor LAG-3 restored CD4+ T cell function, augmented the number of T follicular helper and germinal center B cells, increased antibody levels, and ultimately enhanced the clearance of blood-stage parasites (146).
Malaria-associated B cell dysregulation may be driven by direct interactions between P. falciparum products and B cells. For example, the cysteine-rich interdomain regions 1α of PfEMP1 has been implicated as a T cell–independent polyclonal B cell activator and Ig binding protein (147). There is also evidence that systemic mediators of B cell differentiation and survival such as BAFF are modulated during P. falciparum infection (148, 149). Moreover, chronic exposure to P. falciparum PAMPs could possibly result in tolerance of PRRs expressed on B cells and DCs, which play a critical role in enhancing B cell responses. Indeed, recent clinical trials show that the TLR9 agonist CpG enhances the IgG and MBC response to blood-stage vaccine candidates in malaria-naive adults (150) but not in adults chronically exposed to P. falciparum (151). Further studies are needed to define how P. falciparum exposure modulates PRR function and how this might influence homologous and heterologous LLPC and MBC responses.
The immune response in severe malaria
Each year there are approximately 225 million cases of malaria in Africa, the vast majority of which are uncomplicated and are resolved with time even without treatment with anti-malarial drugs. However, in a small portion of cases, approximately 1% almost exclusively in young children or in older individuals with little or no prior exposure, the infection becomes severe and life threatening resulting in nearly a million deaths each year (1). Malaria during pregnancy can also be severe resulting in substantial maternal, fetal and infant morbidity. Because of space limitation we focus our discussion to severe malaria in young children and refer the reader to an excellent review on immunity to malaria in pregnancy (152). Most severe malaria deaths in children are due to three overlapping clinical syndromes, malaria with impaired consciousness (cerebral malaria), malaria with respiratory distress due to severe metabolic acidosis and severe anemia (153). Although the pathophysiology is incompletely understood, severe cerebral malaria, one of the most deadly forms of severe malaria, is associated with ischemia caused by sequestration of iRBC in the brain and microvascular damage, edema, blood brain barrier breakdown, and immune cell activation and recruitment resulting in inflammation and oxidative stress (reviewed in (154, 155)). Indeed, in children the pathology of severe disease has been consistently linked to excessive inflammatory responses including the production of the pro-inflammatory cytokines TNF-α, INF-γ, IL-1β and IL-6 and a linked reduced production of the anti-inflammatory cytokine IL-10. At present we do not have a clear picture of the sequence of events responsible for the progression from uncomplicated malaria to severe disease or of the events that trigger severe disease. Currently, it is not possible to distinguish children who are at high risk to progress from uncomplicated to severe malaria and we have no specific therapies that are effective for children who present with severe disease. Clearly a better understanding of the immune mechanisms at play in severe disease would contribute to efforts to development of such diagnostics and therapies.
It is not clear how resistance to severe disease is acquired or if the protective immune mechanisms at play in uncomplicated and severe disease are similar. That immunity to severe malaria is not the same as immunity to uncomplicated malaria is suggested by the observations that young children that survive an episode of non-cerebral severe disease have a life-long immunity to severe disease but remain susceptible to uncomplicated malaria until adolescence (2). In addition, HbS protects against severe malaria (16) but not against uncomplicated malaria (90). It is possible that children who develop severe disease are relatively naïve to malaria, having little exposure early in life. This begs the question: how many malaria infections are necessary to achieve protection from severe disease? The answer is not clear as determining the risk of severe malaria in relationship to infections requires large cohorts to capture the relatively rare cases of severe disease and such studies have simply not been done. However, a recent nested case control study in Kenya provided evidence that children who suffer severe malaria do not have fewer infections early in life as compared to community controls (156). Cerebral malaria is rare in very young children in areas of high year round transmission suggesting that immunity to cerebral malaria is acquired early under the cover of passively acquired maternal antibodies (157). It is also possible that the mechanisms underlying protection may be more complicated and relate to the brain physiology of young children and their ability to mount an inflammatory response. Based on models of the relationships of exposure to infection and resistance to severe disease, resistance to non-cerebral severe malaria has been proposed to occur after only a few infections, even after a single infection in children protected by maternal antibodies (158). If so, this would suggest that the acquisition of immunity to uncomplicated versus severe disease are quite different or at very least occur over a very different time scale.
Are there known genetic factors that are protective in severe disease? Is susceptibility to severe disease in children due to inborn errors in immunity leading to failure to control inflammation? It has been proposed that in populations widely exposed to pathogens from birth, including malaria, life threatening diseases in children, such as severe malaria, result from collections of single-gene variants effecting immunity to primary infection (159). Indeed, Casanova and colleagues modeled malaria mortality with age for simple mendelian inheritance of genetic susceptibility and concluded that a few dozen gene variants could account for the human genetic contribution to severe malaria (159). The question is: What are these genes? Although malaria has been recognized to have a strong impact on the human genome, the full impact of human genetics on resistance to severe disease remains largely unexplored (15). Linkage studies have provided evidence for a variety of genes including HbS and other hemoglobin variants that influence the severity of malaria, many of which are likely to affect RBC functions necessary for parasite invasion or sequestration of iRBC not immunity per se. In addition, there are a number of immune genes that show association with resistance or susceptibility to malaria, including those encoding MHC molecules, FcγRIIB, components of both the type I and type II interferon responses, IL-12 and NO synthase (NOS) (15). However, few of these associations have been tested in more than one endemic settings and when tested results have been variable.
The sequencing of the human genome in 2003 allowed genome wide association studies (GWAS) for severe malaria. The first GWAS study of severe malaria carried out in the The Gambia identified only HbS to be associated with resistance (160) even though HbS accounts for only approximately 8–10% of resistance. The second GWAS study of severe malaria carried out in Ghana identified two novel resistance loci, a RBC calcium pump (ATP2B4) and an endothelial tight junction protein (MARVELD3) (161) and confirmed HbS and blood group O as protective. Thus, for malaria it would appear that many resistance genes remain to be discovered.
It is reasonable to assume that features of the infecting parasite would also contribute to severe disease analogous to virulence factors in other pathogens. However, thus far, only a few such features have been identified. The overall total parasite burden in children with uncomplicated malaria as measured by the concentration of the malaria produced protein, histidine rich protein 2, in the plasma predicts progress to severe disease but the factors that determine parasite loads are not known (162). In terms of particular parasite gene products that contribute to severe disease only the PfEMP1s have been identified. Placental malaria in pregnant women is caused by parasites that express one particular PfEMP1, var2CSA, that enables them to sequester in the placenta where the var2CSA ligand for chondroitin sulfate A (CSA) is selectively expressed, causing damage to the placenta, fetus and mother (163). In addition, one particular PfEMP1 that binds to endothelia protein C receptor is associated with cerebral malaria (88, 164, 165) and brain autopsies of Malawian children who died of cerebral malaria showed loss of EPCR at sites of sequestered iRBCs (75).
Because severe disease is difficult to study in children, most investigations of the cellular and molecular basis of severe malaria have been restricted to mouse models, in particular, a mouse model of cerebral malaria. At present, the value of the mouse cerebral malaria model to understanding cerebral disease in children is controversial (166). Nonetheless, the hope is that studies in mice will provide clues as to the immune mechanisms underlying the disease that can contribute to the development of adjunctive therapies in cerebral disease in children. In the best studied model, infection with the mouse adapted parasite, P. berghei ANKA, results in rapid death of susceptible C57BL/6 mice within six to eight days. As for the human disease, the pathogenesis of cerebral malaria in mice appears to be due to a combination of parasite sequestration in the brain and parasite-induced hyper- inflammation (reviewed in (154)). However, the order and importance of events leading to mouse cerebral malaria are still incompletely understood and sequestration of iRBCs and hyper-inflammation are not sufficient to explain completely the pathogenesis of cerebral malaria in mice (167). Inflammatory responses are required for the development of cerebral malaria and both adaptive and innate immune cells appear to play critical roles in the inflammatory process. For example, the depletion of CD4+ and CD8+ T cells is protective against cerebral malaria (168) as is depletion of neutrophils (169), however, the mechanisms by which these contribute to severe disease is not completely understood. Recently, malaria antigen-specific CD8+ T cells recognizing peptides derived from Plasmodium glideosome-associated protein 50 (GAP50) were shown to expand during infection in mice, migrate to the brain and damage the blood brain barrier upon recognizing GAP50 peptides cross-presented by brain endothelial cells (170) suggesting that the first event in cerebral malaria may be the activation and recruitment of CD8+ T cells by parasite antigens. Other immune cells in the brain are activated during cerebral malaria and play distinct roles in the pathogenesis. For example, brain macrophage activation precedes CD8+ T cells accumulation and proliferation in cerebral malaria (171). Clearly, there is a need to better understand the cellular and molecular basis of severe malaria to allow the development of adjunctive therapies.
The mouse model of cerebral disease has allowed for an analysis of genes that modulate severe disease. Genetic deficiencies have been described that confer resistance to cerebral malaria including, among many others, ones that affect PAMP detection (172–174), inflammatory cytokine production (175, 176) and leukocyte adhesion (177). One particularly interesting class of genes that function as resistance factors in mouse cerebral malaria are those that are susceptibility genes in mouse models of the autoimmune disease, systemic lupus erythematosus (SLE). SLE is interesting in the context of malaria immunity as in humans SLE is six to eight times more common in African American women as compared to women of European descent (178), suggesting that the African genome contains SLE susceptibility genes. Such genes may have been selected for their protective effect in severe malaria, although thus far SLE susceptibility genes have not been identified in the African genome. In mice two SLE susceptibility genes, a deficiency in Fcγ receptor II B (FcγRIIB) (179), an inhibitory receptor that can serve to control inflammation, and a multiplication of the gene encoding toll like receptor 7 (TLR7tg) (180) the intracellular receptor for RNA on the C57/BL background result in lethal autoimmune disease by six months of age. Remarkably, these two mouse strains when infected at four to six weeks of age, before the development of any symptoms of autoimmune disease, were completely protected against cerebral malaria (172) despite the fact that parasitemia develops equivalently to that in wild type mice. The effect of FcγRIIB-deficiency was of particular interest as a human loss of function polymorphism of FcγRIIB is more frequent in Africans (181, 182) and associated with SLE risk in Asia (183). The immune parameter that best explained the ability of the SLE-susceptible mice to resist cerebral disease was their production of large quantities of the anti-inflammatory cytokine, IL-10 (172). This observation is reminiscent of the finding that malaria in Malian children induced a switch from pro- to anti-inflammatory responses with IL-10 predominating. We hypothesize that early in life as the effect of the autoimmune susceptibility genes begin to manifest in inflammation, the immune system counters with an anti-inflammatory response that confers the protection to cerebral malaria that we observed. This inflammatory-anti-inflammatory response may teeter-totter back and forth with age, with inflammation inevitably winning out.
If the African genome contains SLE-susceptibility genes, then a high prevalence of autoimmune disease would be expected. Paradoxically, autoimmune disease is strikingly absent from malaria endemic areas of Africa (184), leading Greenwood and colleagues to speculate that malaria suppresses autoimmunity (185). Evidence for their hypothesis was first provided by experiments showing infection of autoimmune susceptible NZB and (NZB×NZW)F1 mice with non-lethal parasites protected from autoimmune disease (186). The effect of malaria infections on autoimmune disease does not appear to be unique in that infection with γ-herpes virus also seems to protect against SLE (187). One possible explanation for these observations is that infections, like malaria, induce inflammatory responses that provoke anti-inflammatory responses that are protective in autoimmune disease. Outside of the African pathogen environment, in the U.S. for example, unchecked SLE-susceptibility genes lead to SLE. Thus, we speculate that susceptibility to severe disease in African children may be the result of a combination of inborn errors in key components of the immune control of inflammation and the child’s exposure to pathogens that have the ability to induce anti-inflammatory immune responses.
Mosquito immunity and malaria
The lack of adaptive immunity in insects, the relatively simple organization of their immune system and the ability to disrupt gene function in adult stages makes them powerful models to understand the basic molecular mechanisms that mediate innate immune responses (188). This is particularly true of anopheline mosquitoes, the natural vector of malaria, as models of anti-plasmodial responses. Understanding the interactions of the mosquito immune system that affect Plasmodium parasites is critical to elucidate the factors that sustain transmission in the field, but can also provide new insights into molecular mechanisms that may also be operating in the human host.
Epithelial responses to the gut microbiota and Plasmodium infection
Mosquitoes become infected when they ingest blood from a host that contains Plasmodium gametocytes (Figure 3). Fertilization takes place in the lumen of the midgut and the parasite matures into a motile form, the ookinete, in the complex environment of a blood meal undergoing digestion, at a time when the gut microbiota is proliferating. Epithelial cells need to protect the host from pathogenic organisms, but must do so without mounting immune responses against the normal flora. Recent studies found that an immune-modulatory peroxidase (IMPer) is secreted by midgut cells of A. gambiae and, together with dual oxidase (Duox), catalyzes the formation of a dityrosine network on the luminal surface of midgut epithelial cells (189). This network prevents activation of gut immune responses by immune elicitors from commensal microbes. The dityrosine network also enhances Plasmodium parasite transmission, because it allows parasites to develop in the midgut lumen without activating NOS expression. Disruption of this barrier by silencing either IMPer or Duox results in strong and effective pathogen-specific immune responses (190).
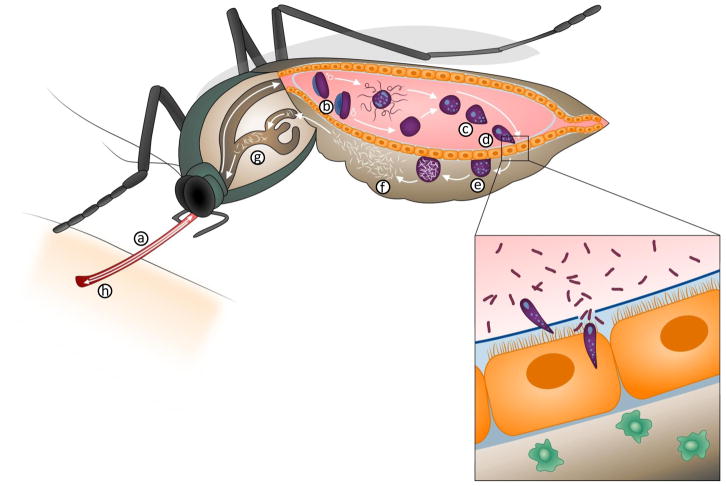
Figure 3. Plasmodium life cycle in the mosquito.
Mosquitoes become infected when they ingest gametocytes (a) that transform into mature gametes in the midgut lumen (b). Fertilization takes place, giving rise to a zygote (c) that matures into an ookinete, a motile stage that invades the mosquito midgut (d). Ookinetes transform into oocysts (e) when they reach the midgut basal lamina and begin to divide continuously, generating thousands of sporozoites that are released into the mosquito circulatory system (f), invade the salivary glands (g) and are injected into a new host when the mosquito takes a second blood meal (h). (Insert) When ookinetes invade the midgut, they disrupt the barriers, such as the peritrophic matrix (blue line in inset), that prevent direct contact between the gut micribiota and epithelial cells and inflict damage on the invaded midgut cell. Midgut invasion triggers the release of a hemocyte differentiation factor (HDF) that increases the number of circulating phagocytic cells called granulocytes (green cells). This priming enhances the immune response to subsequent Plasmodium infections.
Innate immune memory response to Plasmodium midgut invasion
The innate immune system has an ancestral origin and has been thought to be hard-wired and unable to adapt to the environment. Insects lack adaptive immunity and rely on the innate immune system to mount defense responses against pathogenic organisms. However, memory-like responses have been described in several insects, a phenomenon that has been termed ‘immune priming’ (191, 192). Although these studies challenge the dogma that invertebrates lack the ability to mount adaptive responses, a functional mechanism that would allow the innate immune system to “remember” a pathogen remained elusive. Recent studies also revealed that natural killer cells, central players of the vertebrate innate immune system, are capable of intrinsic immunological memory (193–195); but the mechanism(s) mediating these responses remain to be defined.
A recent study in A. gambiae mosquitoes revealed that a strong priming response is established when Plasmodium ookinetes breach the natural gut barriers and along with bacteria come in close contact with invaded epithelial midgut cells (196). The mosquito’s immune system “learns” from this exposure and establishes a systemic state of enhanced immune surveillance. Upon re-exposure to a similar insult, the immune system mounts a more effective antibacterial response that indirectly harms Plasmodium parasites through a by-stander effect. What the immune system “remembers” is the interaction between bacteria and parasite-invaded midgut cells (196).
Immune priming was found to be mediated by a hemocyte differentiation response that persists for the lifespan of the mosquito (196). Exposure to Plasmodium/bacteria infection increased the proportion of circulating granulocytes, a macrophage-like phagocytic cell, and triggered a differentiation process that altered the morphology and binding properties of these cells. Following priming, a Hemocyte Differentiation Factor(s) (HDF) was released, and transfer of hemolymph from challenged mosquitoes induced hemocyte differentiation in naïve recipient mosquitoes. Furthermore, HDF transfer was sufficient to confer enhanced antiplasmodial immunity (196). Understanding the molecular mechanism(s) that mediate hemocyte differentiation and trigger a long-lasting state of enhanced immunity in insects will undoubtedly provide new insights into the evolution of innate immune memory responses.
Epithelial responses to Plasmodium and complement activation
To complete their development in the mosquito, Plasmodium ookinetes must traverse the midgut epithelium and avoid being detected and lysed by the mosquito complement-like system. Mosquito midgut cells invaded by ookinetes are known to mount defense responses, such as expression of high levels of NOS (197, 198). It has been proposed that these epithelial responses are harmful to the parasite and that the rate of nitration is a major determinant of Plasmodium survival (198–200). Biochemical studies in this system revealed that nitration in Plasmodium-invaded cells is a two-step process in which the induction of NOS expression is followed by an increase in peroxidase activity (200, 201), similar to what has been described for myeloperoxidase-mediated nitration in vertebrate macrophages (202, 203).
As ookinetes complete their traversal of the mosquito midgut, they come in contact with components of the mosquito complement-like system present in the mosquito hemolymph (the serum-like fluid in the circulatory system of insects). The thioester-containing protein 1 (TEP1) is similar to vertebrate complement factor C3, and circulates as a stable complex associated with two proteins of the leucine-rich repeat (LRR) family, LRIM1 and APL1 (204, 205). It is clear that TEP1 binds to the surface of some parasites and is a critical component of a lytic complex (206). However, because all ookinetes come in contact with TEP1 circualting in the hemolyph, it has been difficult to understand why TEP1 binds to the surface of some ookinetes and triggers lysis, while other parasites are spared.
An A. gambiae heme peroxidase (HPX2) and NADPH oxidase 5 (NOX5) were recently identified as key enzymes induced in ookinete-invaded midgut cells that, together with NOS, mediate protein nitration (207). The HPX2/NOX5 system potentiates NO toxicity and is critical for mosquitoes to mount an effective anti-plasmodial response. Furthermore, these studies revealed that epithelia nitration and TEP1-mediated lysis work sequentially, and that epithelial nitration serves as an opsonization-like system that promotes activation of the mosquito complement cascade.
Signaling pathways regulating mosquito anti-plasmodial immunity
Immune signaling pathways are among the most widely studied mechanisms of mosquito immune activation. Comparative whole genome sequencing analysis revealed that the genes mediating intracellular signaling are highly conserved, particularly between mosquitoes and Drosophila, providing a useful starting point to identify genes comprising individual pathways (208–212). There are four signaling cascades that have been linked to immune activation and the killing of Plasmodium parasites: the Toll, Imd, JNK and STAT pathways (199, 213–217). Gene silencing by systemic injection of dsRNA into the mosquito circulatory system has been a powerful tool to disrupt or over-activate specific signaling cascades. The effect of silencing positive regulators, such as transcription factors or genes involved in signal transduction, or negative regulators that enhance signaling on Plasmodium infection has been very informative. The Toll and Imd pathways target the ookinete stage of the parasite and promote activation of the mosquito TEP1 complement-like system (213–215). Interestingly, the Toll pathway is more effective against P. berghei (rodent malaria parasite), while the Imd pathway limits infection with the human malaria parasite P. falciparum in several different anopheline mosquitoes (214). Recent experiments revealed that the transfer of hemocytes in which Toll signaling has been enhanced by silencing cactus (a suppressor gene), confers the anti-plasmodial activity against P. berghei parasites; indicating that hemocytes play an active role in regulating complement activation through the Toll pathway (L. Garver and C. Barillas-Mury, unpublished). The JNK pathway also promotes TEP1-mediated lysis by the complement-like system by regulating the expression of HPX2 and NOX5, the two enzymes that mediate midgut nitration, and by regulating the basal level of TEP1 expression in hemocytes (A. Garver and C. Barillas-Mury, unpublished). It is noteworthy that these three signaling pathways, Toll, Imd and JNK converge with TEP1 as a key effector of the complex that lyses the parasite. This is reminiscent of the convergence of the classic, alternative and lectin pathways of complement activation in vertebrates. The STAT pathway targets Plasmodium parasites after they cross the midgut and transform into the oocysts (217). This late-phase immune response limits oocyst survival trough the induction of NOS expression.
Undoubtedly, the different signaling pathways interact as a complex network that operates in different compartments and higher levels of organization and complexity are becoming apparent. For example, a recent study revealed that activation of the Imd or Toll pathways induces expression of different splicing factors that, in turn, generate specific isoforms of AgDscam, a hyper variable pattern recognition receptor protein, through differential gene splicing (218). Furthermore, expression of these different AgDscam isoforms results in species-specific anti-plasmodial responses (218, 219).
Some of the future challenges are to identify the effector genes mediating the responses of the different signaling cascades and to determine the tissues in which they are produced. In some cases it is possible to transfer cells (such as the hemocyte transfer experiments described before) in which different pathways have been activated and establish their effect on Plasmodium survival. Broad expression analysis of specific tissues in which a given pathway is disrupted or overactive can also be informative and genetic systems to drive tissue-specific overexpression or silencing are also being developed. This detailed analysis will also inform vector control strategies based on manipulating immunological responses. For example, it would make it possible to over-express or silence certain pathway components using tissue-specific promoters that are transiently activated at the precise time, in order to maximize their effect on the parasite, while avoiding detrimental effects to the mosquito.
Immune evasion by Plasmodium parasites
It is clear from animal model systems that mosquitoes have a complex immune system, capable of mounting robust and effective anti-plasmodial responses. However, there are several reports that disrupting the complement-like system has a modest (214, 220) or no effect (211, 212) when A. gambiae is infected with some strains of the human parasite P. falciparum.
An A. gambiae strain selected to be refractory (R) to the simian malaria, P. cynomolgi, eliminates most Plasmodium species including P. falciparum strains from the New World by forming a melanotic capsule (deposition of melanin, a black insoluble pigment) around the dead parasites. However, the P. strain is highly susceptible to infection with some P. falciparum strains of African origin where A. gambiae is the natural vector (213). Disruption of the complement-like system prevents elimination of the P. falciparum 7G8 strain from Brazil, but has no effect on the intensity or prevalence of infection with NF54 parasites from Africa, indicating that the African parasite lines are able to evade the A. gambiae immune system (212). Co-infection experiments revealed that the immune response (or lack thereof) to a parasite strain did not affect the fate of other parasites present in the same mosquito midgut (212); suggesting that parasite survival is determined by genetic differences between P. falciparum strains (212).
A combination QTL mapping, using a genetic cross between the African GB4 strain that survives in R mosquitoes and the Brazilian 7G8 strain that is eliminated, linkage group selection and functional genomics was recently used to identify Pfs47 as the parasite gene that allows P. falciparum parasites to evade the mosquito immune system (214). Disruption of Pfs47 (in a NF54 genetic background that normally survives) resulted in parasite killing and melanization, that could be prevented by silencing TEP1; indicating the Pfs47 is necessary for NF54 parasites to evade destruction by the mosquito complement system (214). Pfs47 is required for P. falciparum to evade two well-defined anti-plasmodial responses mediated by the complement-like system, parasite killing/melanization and lysis without melanization. Genetic complementation of the Pfs47 KO line with the NF54 allele of Pfs47 completely reversed the melanization phenotype in the R strain, confirming that this gene allele is sufficient for parasites to evade the mosquito immune system (214).
Interestingly, Pfs47 is a highly polymorphic gene that exhibits strong population structure and extreme fixation in non-African regions (215, 216). Our findings suggest that the population structure of Pfs47 may be due to adaptation of P. falciparum to the different anopheles vector species present in different geographical regions of the world. It appears that the evolution of Pfs47 promoted P. falciparum survival in A. gambiae mosquitoes and could be responsible for the very high rates of malaria transmission in Sub-Saharan Africa. Disruption of the immunomodulatory activity of Pfs47 may be an effective target to reduce human malaria transmission.
There are ten members of the 6-cystein protein family in P. falciparum (217). The function of four of them that are expressed in the asexual erythrocytic stages of the parasite in the human host is unknown (217, 218). It is well-established that P. falciparum parasites are able to evade the vertebrate immune system and elicit ineffective immune memory responses, resulting in persistent and recurrent infections (219–221). This raises the intriguing possibility that other members of this protein family may also have an immune-modulatory function in humans.
Summary and road forward
It is an exciting time in malaria immunity research. We now have the tools to make significant progress in understanding the cellular and molecular mechanisms underlying several long standing phenomenon in malaria immunity. In humans these include the paucity of sterilizing immunity to the liver stage, the relatively rapid acquisition of immunity to severe disease, the acquired control of inflammation, the slow acquisition of ultimately protective antibody immunity and the susceptibility to severe disease of only a small fraction of exposed children. In mosquitoes, these include the genetic resistance to infection in mosquito populations and acquired innate immune control of parasites in individual mosquitoes. It is a time ripe for new investigators to enter the field and contribute to the defeat of this devastating disease.
References
- 1.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–31. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–32. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 3.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 4.Mueller I, Galinski MR, Tsuboi T, Arevalo-Herrera M, Collins WE, King CL. Natural acquisition of immunity to Plasmodium vivax: epidemiological observations and potential targets. Adv Parasitol. 2013;81:77–131. doi: 10.1016/B978-0-12-407826-0.00003-5. [DOI] [PubMed] [Google Scholar]
- 5.Riley EM, Stewart VA. Immune mechanisms in malaria: new insights in vaccine development. Nat Med. 2013;19:168–78. doi: 10.1038/nm.3083. [DOI] [PubMed] [Google Scholar]
- 6.Miller LH, Ackerman HC, Su XZ, Wellems TE. Malaria biology and disease pathogenesis: insights for new treatments. Nat Med. 2013;19:156–67. doi: 10.1038/nm.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller LH, Pierce SK. Perspective on malaria eradication: is eradication possible without modifying the mosquito? J Infect Dis. 2009;200:1644–5. doi: 10.1086/646612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts L, Enserink M. Malaria. Did they really say … eradication? Science. 2007;318:1544–5. doi: 10.1126/science.318.5856.1544. [DOI] [PubMed] [Google Scholar]
- 9.The RTSS Clinical Trials Partnership. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284–95. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, McKenzie FE. Ross, macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog. 2012;8:e1002588. doi: 10.1371/journal.ppat.1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molineaux L, Gramiccia G. The Garki Project: Research on the Epidemiology and Control of Malaria in the Sudan Savanna of West Africa. Geneva: World Health Organization; 1980. p. 311. [Google Scholar]
- 12.Sharma VP. Re-emergence of malaria in India. Indian J Med Res. 1996;103:26–45. [PubMed] [Google Scholar]
- 13.Macdonald G. The epidemiology and control of malaria. Oxford University Press; 1957. Epidemics during or after eradication of malaria; p. 59. [Google Scholar]
- 14.Mu J, Duan J, Makova KD, Joy DA, Huynh CQ, Branch OH, Li WH, Su XZ. Chromosome-wide SNPs reveal an ancient origin for Plasmodium falciparum. Nature. 2002;418:323–6. doi: 10.1038/nature00836. [DOI] [PubMed] [Google Scholar]
- 15.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171–92. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molineaux L. The impact of parasitic diseases and their control on mortality, with emphasis on malaria and Africa. In: Vallin J, Lopez A, editors. Health policy, social policy, and mortality prospects. Liege: Ordina Editions; 1985. pp. 13–44. [Google Scholar]
- 17.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gueirard P, Tavares J, Thiberge S, Bernex F, Ishino T, Milon G, Franke-Fayard B, Janse CJ, Menard R, Amino R. Development of the malaria parasite in the skin of the mammalian host. Proc Natl Acad Sci U S A. 2010;107:18640–5. doi: 10.1073/pnas.1009346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, Nussenzweig RS, Nussenzweig V, Rodriguez A. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291:141–4. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- 20.Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R, Heussler VT. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science. 2006;313:1287–90. doi: 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- 21.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–9. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 22.Wykes MN, Good MF. What have we learnt from mouse models for the study of malaria? Eur J Immunol. 2009;39:2004–7. doi: 10.1002/eji.200939552. [DOI] [PubMed] [Google Scholar]
- 23.Sauerwein RW, Roestenberg M, Moorthy VS. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol. 2011;11:57–64. doi: 10.1038/nri2902. [DOI] [PubMed] [Google Scholar]
- 24.Engwerda CR, Minigo G, Amante FH, McCarthy JS. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol. 2012;28:515–21. doi: 10.1016/j.pt.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Tran TM, Samal B, Kirkness E, Crompton PD. Systems immunology of human malaria. Trends Parasitol. 2012;28:248–57. doi: 10.1016/j.pt.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Offeddu V, Thathy V, Marsh K, Matuschewski K. Naturally acquired immune responses against Plasmodium falciparum sporozoites and liver infection. Int J Parasitol. 2012;42:535–48. doi: 10.1016/j.ijpara.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Tran TM, Li S, Doumbo S, Doumtabe D, Huang CY, Dia S, Bathily A, Sangala J, Kone Y, Traore A, Niangaly M, Dara C, Kayentao K, Ongoiba A, Doumbo OK, Traore B, Crompton PD. An Intensive Longitudinal Cohort Study of Malian Children and Adults Reveals No Evidence of Acquired Immunity to Plasmodium falciparum Infection. Clin Infect Dis. 2013;57:40–7. doi: 10.1093/cid/cit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda T, Miyachi Y, Kabashima K. Regulatory T cells in cutaneous immune responses. J Dermatol Sci. 2011;63:75–82. doi: 10.1016/j.jdermsci.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–63. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 30.Guilbride DL, Gawlinski P, Guilbride PD. Why functional pre-erythrocytic and bloodstage malaria vaccines fail: a meta-analysis of fully protective immunizations and novel immunological model. PLoS One. 2010;5:e10685. doi: 10.1371/journal.pone.0010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Silva HB, Caetano SS, Monteiro I, Gomez-Conde I, Hanson K, Penha-Goncalves C, Olivieri DN, Mota MM, Marinho CR, D’Imperio Lima MR, Tadokoro CE. Early skin immunological disturbance after Plasmodium-infected mosquito bites. Cell Immunol. 2012;277:22–32. doi: 10.1016/j.cellimm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Good MF, Doolan DL. Malaria vaccine design: immunological considerations. Immunity. 2010;33:555–66. doi: 10.1016/j.immuni.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Usynin I, Klotz C, Frevert U. Malaria circumsporozoite protein inhibits the respiratory burst in Kupffer cells. Cell Microbiol. 2007;9:2610–28. doi: 10.1111/j.1462-5822.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 34.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–2. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 35.Gwadz RW, Cochrane AH, Nussenzweig V, Nussenzweig RS. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull World Health Organ. 1979;57(Suppl 1):165–73. [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, Gordon DM, Stoute JA, Church LW, Sedegah M, Heppner DG, Ballou WR, Richie TL. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–64. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan AM, Wang R, Kappe SH. Genetically engineered, attenuated whole-cell vaccine approaches for malaria. Hum Vaccin. 2010;6:107–13. doi: 10.4161/hv.6.1.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, Spaarman L, de Mast Q, Roeffen W, Snounou G, Renia L, van der Ven A, Hermsen CC, Sauerwein R. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468–77. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 39.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, Richman A, Velmurugan S, Reyes S, Li M, Tucker K, Ahumada A, Ruben AJ, Li T, Stafford R, Eappen AG, Tamminga C, Bennett JW, Ockenhouse CF, Murphy JR, Komisar J, Thomas N, Loyevsky M, Birkett A, Plowe CV, Loucq C, Edelman R, Richie TL, Seder RA, Hoffman SL. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science. 2011;334:475–80. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 40.Barry AE, Schultz L, Buckee CO, Reeder JC. Contrasting population structures of the genes encoding ten leading vaccine-candidate antigens of the human malaria parasite, Plasmodium falciparum. PLoS One. 2009;4:e8497. doi: 10.1371/journal.pone.0008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trieu A, Kayala MA, Burk C, Molina DM, Freilich DA, Richie TL, Baldi P, Felgner PL, Doolan DL. Sterile protective immunity to malaria is associated with a panel of novel P. falciparum antigens. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M111.007948. M111 007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krzych U, Lyon JA, Jareed T, Schneider I, Hollingdale MR, Gordon DM, Ballou WR. T lymphocytes from volunteers immunized with irradiated Plasmodium falciparum sporozoites recognize liver and blood stage malaria antigens. J Immunol. 1995;155:4072–7. [PubMed] [Google Scholar]
- 43.Doolan DL, Southwood S, Freilich DA, Sidney J, Graber NL, Shatney L, Bebris L, Florens L, Dobano C, Witney AA, Appella E, Hoffman SL, Yates JR, 3rd, Carucci DJ, Sette A. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc Natl Acad Sci U S A. 2003;100:9952–7. doi: 10.1073/pnas.1633254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roestenberg M, Teirlinck AC, McCall MB, Teelen K, Makamdop KN, Wiersma J, Arens T, Beckers P, van Gemert G, van de Vegte-Bolmer M, van der Ven AJ, Luty AJ, Hermsen CC, Sauerwein RW. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet. 2011;377:1770–6. doi: 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]
- 45.Bijker EM, Bastiaens GJ, Teirlinck AC, van Gemert GJ, Graumans W, van de Vegte- Bolmer M, Siebelink-Stoter R, Arens T, Teelen K, Nahrendorf W, Remarque EJ, Roeffen W, Jansens A, Zimmerman D, Vos M, van Schaijk BC, Wiersma J, van der Ven AJ, de Mast Q, van Lieshout L, Verweij JJ, Hermsen CC, Scholzen A, Sauerwein RW. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A. 2013;110:7862–7. doi: 10.1073/pnas.1220360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinnis P, Zavala F. The skin: where malaria infection and the host immune response begin. Semin Immunopathol. 2012;34:787–92. doi: 10.1007/s00281-012-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindner SE, Miller JL, Kappe SH. Malaria parasite pre-erythrocytic infection: preparation meets opportunity. Cell Microbiol. 2012;14:316–24. doi: 10.1111/j.1462-5822.2011.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med. 2007;13:1035–41. doi: 10.1038/nm1628. [DOI] [PubMed] [Google Scholar]
- 49.Hafalla JC, Silvie O, Matuschewski K. Cell biology and immunology of malaria. Immunol Rev. 2011;240:297–316. doi: 10.1111/j.1600-065X.2010.00988.x. [DOI] [PubMed] [Google Scholar]
- 50.Overstreet MG, Cockburn IA, Chen YC, Zavala F. Protective CD8 T cells against Plasmodium liver stages: immunobiology of an ‘unnatural’ immune response. Immunol Rev. 2008;225:272–83. doi: 10.1111/j.1600-065X.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cockburn IA, Tse SW, Radtke AJ, Srinivasan P, Chen YC, Sinnis P, Zavala F. Dendritic cells and hepatocytes use distinct pathways to process protective antigen from plasmodium in vivo. PLoS Pathog. 2011;7:e1001318. doi: 10.1371/journal.ppat.1001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hafalla JC, Bauza K, Friesen J, Gonzalez-Aseguinolaza G, Hill AV, Matuschewski K. Identification of targets of CD8(+) T cell responses to malaria liver stages by genome-wide epitope profiling. PLoS Pathog. 2013;9:e1003303. doi: 10.1371/journal.ppat.1003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy SC, Kas A, Stone BC, Bevan MJ. A T-cell response to a liver-stage Plasmodium antigen is not boosted by repeated sporozoite immunizations. Proc Natl Acad Sci U S A. 2013;110:6055–60. doi: 10.1073/pnas.1303834110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cockburn IA, Chen YC, Overstreet MG, Lees JR, van Rooijen N, Farber DL, Zavala F. Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites. PLoS Pathog. 2010;6:e1000877. doi: 10.1371/journal.ppat.1000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–22. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog. 2010;6:e1000998. doi: 10.1371/journal.ppat.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster DP, Dunachie S, Vuola JM, Berthoud T, Keating S, Laidlaw SM, McConkey SJ, Poulton I, Andrews L, Andersen RF, Bejon P, Butcher G, Sinden R, Skinner MA, Gilbert SC, Hill AV. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc Natl Acad Sci U S A. 2005;102:4836–41. doi: 10.1073/pnas.0406381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duffy PE, Sahu T, Akue A, Milman N, Anderson C. Pre-erythrocytic malaria vaccines: identifying the targets. Expert Rev Vaccines. 2012;11:1261–80. doi: 10.1586/erv.12.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34:991–6. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Sinnis P, Coppi A. A long and winding road: the Plasmodium sporozoite’s journey in the mammalian host. Parasitol Int. 2007;56:171–8. doi: 10.1016/j.parint.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Meglio P, Perera GK, Nestle FO. The multitasking organ: recent insights into skin immune function. Immunity. 2011;35:857–69. doi: 10.1016/j.immuni.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013 doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 64.Casares S, Brumeanu TD, Richie TL. The RTS,S malaria vaccine. Vaccine. 2010;28:4880–94. doi: 10.1016/j.vaccine.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 65.Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malar J. 2009;8:312. doi: 10.1186/1475-2875-8-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–75. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 67.Bejon P, Mwacharo J, Kai O, Mwangi T, Milligan P, Todryk S, Keating S, Lang T, Lowe B, Gikonyo C, Molyneux C, Fegan G, Gilbert SC, Peshu N, Marsh K, Hill AV. A phase 2b randomised trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS Clin Trials. 2006;1:e29. doi: 10.1371/journal.pctr.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moorthy VS, Imoukhuede EB, Milligan P, Bojang K, Keating S, Kaye P, Pinder M, Gilbert SC, Walraven G, Greenwood BM, Hill AS. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS medicine. 2004;1:e33. doi: 10.1371/journal.pmed.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zevering Y, Houghten RA, Frazer IH, Good MF. Major population differences in T cell response to a malaria sporozoite vaccine candidate. Int Immunol. 1990;2:945–55. doi: 10.1093/intimm/2.10.945. [DOI] [PubMed] [Google Scholar]
- 70.Hartgers FC, Yazdanbakhsh M. Co-infection of helminths and malaria: modulation of the immune responses to malaria. Parasite Immunol. 2006;28:497–506. doi: 10.1111/j.1365-3024.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- 71.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS medicine. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Molloy MJ, Bouladoux N, Belkaid Y. Intestinal microbiota: shaping local and systemic immune responses. Semin Immunol. 2012;24:58–66. doi: 10.1016/j.smim.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 74.Kraemer SM, Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr Opin Microbiol. 2006;9:374–80. doi: 10.1016/j.mib.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JE, Avril M, Brazier AJ, Freeth J, Jespersen JS, Nielsen MA, Magistrado P, Lusingu J, Smith JD, Higgins MK, Theander TG. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature. 2013;498:502–5. doi: 10.1038/nature12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clark IA, Alleva LM, Mills AC, Cowden WB. Pathogenesis of malaria and clinically similar conditions. Clin Microbiol Rev. 2004;17:509–39. doi: 10.1128/CMR.17.3.509-539.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–9. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 78.White NJ, Turner GD, Day NP, Dondorp AM. Lethal malaria: marchiafava and bignami were right. J Infect Dis. 2013;208:192–8. doi: 10.1093/infdis/jit116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clark IA, Alleva LM. Is human malarial coma caused, or merely deepened, by sequestration? Trends Parasitol. 2009;25:314–8. doi: 10.1016/j.pt.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 80.Collins WE, Jeffery GM. A retrospective examination of secondary sporozoite- and trophozoite-induced infections with Plasmodium falciparum: development of parasitologic and clinical immunity following secondary infection. Am J Trop Med Hyg. 1999;61:20–35. doi: 10.4269/tropmed.1999.61-020. [DOI] [PubMed] [Google Scholar]
- 81.Day NP, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TT, Mai NT, Phu NH, Sinh DX, White NJ, Ho M. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis. 1999;180:1288–97. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 82.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–7. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walther M, Woodruff J, Edele F, Jeffries D, Tongren JE, King E, Andrews L, Bejon P, Gilbert SC, De Souza JB, Sinden R, Hill AV, Riley EM. Innate immune responses to human malaria: heterogeneous cytokine responses to blood-stage Plasmodium falciparum correlate with parasitological and clinical outcomes. J Immunol. 2006;177:5736–45. doi: 10.4049/jimmunol.177.8.5736. [DOI] [PubMed] [Google Scholar]
- 84.Krishnegowda G, Hajjar AM, Zhu J, Douglass EJ, Uematsu S, Akira S, Woods AS, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–16. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, Olivier M. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5:e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen KA, Lamphier M, Olivier M, Bartholomeu DC, Gazzinelli RT, Golenbock DT. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104:1919–24. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharma S, DeOliveira RB, Kalantari P, Parroche P, Goutagny N, Jiang Z, Chan J, Bartholomeu DC, Lauw F, Hall JP, Barber GN, Gazzinelli RT, Fitzgerald KA, Golenbock DT. Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity. 2011;35:194–207. doi: 10.1016/j.immuni.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Claessens A, Adams Y, Ghumra A, Lindergard G, Buchan CC, Andisi C, Bull PC, Mok S, Gupta AP, Wang CW, Turner L, Arman M, Raza A, Bozdech Z, Rowe JA. A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc Natl Acad Sci U S A. 2012;109:E1772–81. doi: 10.1073/pnas.1120461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurtzhals JA, Adabayeri V, Goka BQ, Akanmori BD, Oliver-Commey JO, Nkrumah FK, Behr C, Hviid L. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet. 1998;351:1768–72. doi: 10.1016/S0140-6736(97)09439-7. [DOI] [PubMed] [Google Scholar]
- 90.Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. The Lancet infectious diseases. 2012;12:457–68. doi: 10.1016/S1473-3099(12)70055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waisberg M, Cerqueira GC, Yager SB, Francischetti IM, Lu J, Gera N, Srinivasan P, Miura K, Rada B, Lukszo J, Barbian KD, Leto TL, Porcella SF, Narum DL, El-Sayed N, Miller LH, Pierce SK. Plasmodium falciparum merozoite surface protein 1 blocks the proinflammatory protein S100P. Proc Natl Acad Sci U S A. 2012;109:5429–34. doi: 10.1073/pnas.1202689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stevenson MM, Riley EM. Innate immunity to malaria. Nat Rev Immunol. 2004;4:169–80. doi: 10.1038/nri1311. [DOI] [PubMed] [Google Scholar]
- 93.Guermonprez P, Helft J, Claser C, Deroubaix S, Karanje H, Gazumyan A, Darasse-Jeze G, Telerman SB, Breton G, Schreiber HA, Frias-Staheli N, Billerbeck E, Dorner M, Rice CM, Ploss A, Klein F, Swiecki M, Colonna M, Kamphorst AO, Meredith M, Niec R, Takacs C, Mikhail F, Hari A, Bosque D, Eisenreich T, Merad M, Shi Y, Ginhoux F, Renia L, Urban BC, Nussenzweig MC. Inflammatory Flt3l is essential to mobilize dendritic cells and for T cell responses during Plasmodium infection. Nat Med. 2013;19:730–8. doi: 10.1038/nm.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Porcherie A, Mathieu C, Peronet R, Schneider E, Claver J, Commere PH, Kiefer-Biasizzo H, Karasuyama H, Milon G, Dy M, Kinet JP, Louis J, Blank U, Mecheri S. Critical role of the neutrophil-associated high-affinity receptor for IgE in the pathogenesis of experimental cerebral malaria. J Exp Med. 2011;208:2225–36. doi: 10.1084/jem.20110845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hansen DS, D’Ombrain MC, Schofield L. The role of leukocytes bearing Natural Killer Complex receptors and Killer Immunoglobulin-like Receptors in the immunology of malaria. Curr Opin Immunol. 2007;19:416–23. doi: 10.1016/j.coi.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 96.Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol. 2010;184:6043–52. doi: 10.4049/jimmunol.1000106. [DOI] [PubMed] [Google Scholar]
- 97.Schofield L, Mueller I. Clinical immunity to malaria. Current molecular medicine. 2006;6:205–21. doi: 10.2174/156652406776055221. [DOI] [PubMed] [Google Scholar]
- 98.Grau GE, Taylor TE, Molyneux ME, Wirima JJ, Vassalli P, Hommel M, Lambert PH. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–91. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 99.Sinton JA. Immunity or Tolerance in Malarial Infections: (Section of Comparative Medicine) Proc R Soc Med. 1938;31:1298–302. doi: 10.1177/003591573803101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rubenstein M, Mulholland JH, Jeffery GM, Wolff SM. Malaria Induced Endotoxin Tolerance. Proc Soc Exp Biol Med. 1965;118:283–7. doi: 10.3181/00379727-118-29820. [DOI] [PubMed] [Google Scholar]
- 101.Omer FM, Riley EM. Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J Exp Med. 1998;188:39–48. doi: 10.1084/jem.188.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun. 1999;67:4435–42. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis. 2002;185:971–9. doi: 10.1086/339408. [DOI] [PubMed] [Google Scholar]
- 104.Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, Deininger S, Lawrence E, Ngwa-Amambua A, Jayasooriya S, Cheeseman IH, Gomez-Escobar N, Okebe J, Conway DJ, Riley EM. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 2009;5:e1000364. doi: 10.1371/journal.ppat.1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riley EM, Wahl S, Perkins DJ, Schofield L. Regulating immunity to malaria. Parasite Immunol. 2006;28:35–49. doi: 10.1111/j.1365-3024.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 106.Finney OC, Riley EM, Walther M. Regulatory T cells in malaria--friend or foe? Trends Immunol. 2010;31:63–70. doi: 10.1016/j.it.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 107.Spence PJ, Langhorne J. T cell control of malaria pathogenesis. Curr Opin Immunol. 2012;24:444–8. doi: 10.1016/j.coi.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 108.O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–8. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 109.Gerosa F, Nisii C, Righetti S, Micciolo R, Marchesini M, Cazzadori A, Trinchieri G. CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin Immunol. 1999;92:224–34. doi: 10.1006/clim.1999.4752. [DOI] [PubMed] [Google Scholar]
- 110.Nylen S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, Sacks D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med. 2007;204:805–17. doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–97. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–83. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Portugal S, Pierce SK, Crompton PD. Young lives lost as B cells falter: what we are learning about antibody responses in malaria. J Immunol. 2013;190:3039–46. doi: 10.4049/jimmunol.1203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 115.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010;120:4168–78. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takala SL, Plowe CV. Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming ‘vaccine resistant malaria’. Parasite Immunol. 2009;31:560–73. doi: 10.1111/j.1365-3024.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scherf A, Lopez-Rubio JJ, Riviere L. Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol. 2008;62:445–70. doi: 10.1146/annurev.micro.61.080706.093134. [DOI] [PubMed] [Google Scholar]
- 118.Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, Guindo AB, Traore K, Traore I, Kouriba B, Diallo DA, Diarra I, Daou M, Dolo A, Tolo Y, Sissoko MS, Niangaly A, Sissoko M, Takala-Harrison S, Lyke KE, Wu Y, Blackwelder WC, Godeaux O, Vekemans J, Dubois MC, Ballou WR, Cohen J, Thompson D, Dube T, Soisson L, Diggs CL, House B, Lanar DE, Dutta S, Heppner DG, Jr, Plowe CV. A field trial to assess a blood-stage malaria vaccine. N Engl J Med. 2011;365:1004–13. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hayton K, Gaur D, Liu A, Takahashi J, Henschen B, Singh S, Lambert L, Furuya T, Bouttenot R, Doll M, Nawaz F, Mu J, Jiang L, Miller LH, Wellems TE. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe. 2008;4:40–51. doi: 10.1016/j.chom.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baum J, Chen L, Healer J, Lopaticki S, Boyle M, Triglia T, Ehlgen F, Ralph SA, Beeson JG, Cowman AF. Reticulocyte-binding protein homologue 5 - an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int J Parasitol. 2009;39:371–80. doi: 10.1016/j.ijpara.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 121.Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, Mboup S, Ndir O, Kwiatkowski DP, Duraisingh MT, Rayner JC, Wright GJ. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–7. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Douglas AD, Williams AR, Illingworth JJ, Kamuyu G, Biswas S, Goodman AL, Wyllie DH, Crosnier C, Miura K, Wright GJ, Long CA, Osier FH, Marsh K, Turner AV, Hill AV, Draper SJ. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun. 2011;2:601. doi: 10.1038/ncomms1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Williams AR, Douglas AD, Miura K, Illingworth JJ, Choudhary P, Murungi LM, Furze JM, Diouf A, Miotto O, Crosnier C, Wright GJ, Kwiatkowski DP, Fairhurst RM, Long CA, Draper SJ. Enhancing blockade of Plasmodium falciparum erythrocyte invasion: assessing combinations of antibodies against PfRH5 and other merozoite antigens. PLoS Pathog. 2012;8:e1002991. doi: 10.1371/journal.ppat.1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Richards JS, Arumugam TU, Reiling L, Healer J, Hodder AN, Fowkes FJ, Cross N, Langer C, Takeo S, Uboldi AD, Thompson JK, Gilson PR, Coppel RL, Siba PM, King CL, Torii M, Chitnis CE, Narum DL, Mueller I, Crabb BS, Cowman AF, Tsuboi T, Beeson JG. Identification and Prioritization of Merozoite Antigens as Targets of Protective Human Immunity to Plasmodium falciparum Malaria for Vaccine and Biomarker Development. J Immunol. 2013;191:795–809. doi: 10.4049/jimmunol.1300778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chan JA, Howell KB, Reiling L, Ataide R, Mackintosh CL, Fowkes FJ, Petter M, Chesson JM, Langer C, Warimwe GM, Duffy MF, Rogerson SJ, Bull PC, Cowman AF, Marsh K, Beeson JG. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J Clin Invest. 2012;122:3227–38. doi: 10.1172/JCI62182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–60. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jiang L, Mu J, Zhang Q, Ni T, Srinivasan P, Rayavara K, Yang W, Turner L, Lavstsen T, Theander TG, Peng W, Wei G, Jing Q, Wakabayashi Y, Bansal A, Luo Y, Ribeiro JM, Scherf A, Aravind L, Zhu J, Zhao K, Miller LH. PfSETvs methylation of histone H3K36 represses virulence genes in Plasmodium falciparum. Nature. 2013;499:223–7. doi: 10.1038/nature12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McCarthy JS, Good MF. Whole parasite blood stage malaria vaccines: a convergence of evidence. Hum Vaccin. 2010;6:114–23. doi: 10.4161/hv.6.1.10394. [DOI] [PubMed] [Google Scholar]
- 129.Pinzon-Charry A, McPhun V, Kienzle V, Hirunpetcharat C, Engwerda C, McCarthy J, Good MF. Low doses of killed parasite in CpG elicit vigorous CD4+ T cell responses against blood-stage malaria in mice. J Clin Invest. 2010;120:2967–78. doi: 10.1172/JCI39222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol. 2010;185:3117–25. doi: 10.4049/jimmunol.1001155. [DOI] [PubMed] [Google Scholar]
- 131.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–15. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 132.Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, Tan X, Doumbo S, Doumtabe D, Kone Y, Narum DL, Liang X, Doumbo OK, Miller LH, Doolan DL, Baldi P, Felgner PL, Pierce SK. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A. 2010;107:6958–63. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar J. 2007;6:82. doi: 10.1186/1475-2875-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fonjungo PN, Elhassan IM, Cavanagh DR, Theander TG, Hviid L, Roper C, Arnot DE, McBride JS. A longitudinal study of human antibody responses to Plasmodium falciparum rhoptry-associated protein 1 in a region of seasonal and unstable malaria transmission. Infect Immun. 1999;67:2975–85. doi: 10.1128/iai.67.6.2975-2985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dorfman JR, Bejon P, Ndungu FM, Langhorne J, Kortok MM, Lowe BS, Mwangi TW, Williams TN, Marsh K. B cell memory to 3 Plasmodium falciparum blood-stage antigens in a malaria-endemic area. J Infect Dis. 2005;191:1623–30. doi: 10.1086/429671. [DOI] [PubMed] [Google Scholar]
- 136.Nogaro SI, Hafalla JC, Walther B, Remarque EJ, Tetteh KK, Conway DJ, Riley EM, Walther M. The breadth, but not the magnitude, of circulating memory B cell responses to P. falciparum increases with age/exposure in an area of low transmission. PLoS One. 2011;6:e25582. doi: 10.1371/journal.pone.0025582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wipasa J, Suphavilai C, Okell LC, Cook J, Corran PH, Thaikla K, Liewsaree W, Riley EM, Hafalla JC. Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 2010;6:e1000770. doi: 10.1371/journal.ppat.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weiss GE, Traore B, Kayentao K, Ongoiba A, Doumbo S, Doumtabe D, Kone Y, Dia S, Guindo A, Traore A, Huang CY, Miura K, Mircetic M, Li S, Baughman A, Narum DL, Miller LH, Doumbo OK, Pierce SK, Crompton PD. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog. 2010;6:e1000912. doi: 10.1371/journal.ppat.1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ndungu FM, Olotu A, Mwacharo J, Nyonda M, Apfeld J, Mramba LK, Fegan GW, Bejon P, Marsh K. Memory B cells are a more reliable archive for historical antimalarial responses than plasma antibodies in no-longer exposed children. Proc Natl Acad Sci U S A. 2012;109:8247–52. doi: 10.1073/pnas.1200472109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 141.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Charles ED, Green RM, Marukian S, Talal AH, Lake-Bakaar GV, Jacobson IM, Rice CM, Dustin LB. Clonal expansion of immunoglobulin M+CD27+ B cells in HCV-associated mixed cryoglobulinemia. Blood. 2008;111:1344–56. doi: 10.1182/blood-2007-07-101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, Kayentao K, Ongoiba A, Doumbo OK, Pierce SK. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183:2176–82. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, Crompton PD, Marsh K, Ndungu FM. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol. 2013;190:1038–47. doi: 10.4049/jimmunol.1202438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Muellenbeck MF, Ueberheide B, Amulic B, Epp A, Fenyo D, Busse CE, Esen M, Theisen M, Mordmuller B, Wardemann H. Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J Exp Med. 2013;210:389–99. doi: 10.1084/jem.20121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–95. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Donati D, Mok B, Chene A, Xu H, Thangarajh M, Glas R, Chen Q, Wahlgren M, Bejarano MT. Increased B cell survival and preferential activation of the memory compartment by a malaria polyclonal B cell activator. J Immunol. 2006;177:3035–44. doi: 10.4049/jimmunol.177.5.3035. [DOI] [PubMed] [Google Scholar]
- 148.Nduati E, Gwela A, Karanja H, Mugyenyi C, Langhorne J, Marsh K, Urban BC. The plasma concentration of the B cell activating factor is increased in children with acute malaria. J Infect Dis. 2011;204:962–70. doi: 10.1093/infdis/jir438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scholzen A, Sauerwein RW. How malaria modulates memory: activation and dysregulation of B cells in Plasmodium infection. Trends Parasitol. 2013;29:252–62. doi: 10.1016/j.pt.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 150.Crompton PD, Mircetic M, Weiss G, Baughman A, Huang CY, Topham DJ, Treanor JJ, Sanz I, Lee FE, Durbin AP, Miura K, Narum DL, Ellis RD, Malkin E, Mullen GE, Miller LH, Martin LB, Pierce SK. The TLR9 ligand CpG promotes the acquisition of Plasmodium falciparum-specific memory B cells in malaria-naive individuals. J Immunol. 2009;182:3318–26. doi: 10.4049/jimmunol.0803596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Traore B, Kone Y, Doumbo S, Doumtabe D, Traore A, Crompton PD, Mircetic M, Huang CY, Kayentao K, Dicko A, Sagara I, Ellis RD, Miura K, Guindo A, Miller LH, Doumbo OK, Pierce SK. The TLR9 agonist CpG fails to enhance the acquisition of Plasmodium falciparum-specific memory B cells in semi-immune adults in Mali. Vaccine. 2009;27:7299–303. doi: 10.1016/j.vaccine.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. The Lancet infectious diseases. 2007;7:105–17. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 153.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 154.Grau GE, Craig AG. Cerebral malaria pathogenesis: revisiting parasite and host contributions. Future Microbiol. 2012;7:291–302. doi: 10.2217/fmb.11.155. [DOI] [PubMed] [Google Scholar]
- 155.Shikani HJ, Freeman BD, Lisanti MP, Weiss LM, Tanowitz HB, Desruisseaux MS. Cerebral malaria: we have come a long way. Am J Pathol. 2012;181:1484–92. doi: 10.1016/j.ajpath.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lundblom K, Murungi L, Nyaga V, Olsson D, Rono J, Osier F, Ogada E, Montgomery S, Scott JA, Marsh K, Farnert A. Plasmodium falciparum infection patterns since birth and risk of severe malaria: a nested case-control study in children on the coast of Kenya. PLoS One. 2013;8:e56032. doi: 10.1371/journal.pone.0056032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lackritz EM, Campbell CC, Ruebush TK, 2nd, Hightower AW, Wakube W, Steketee RW, Were JB. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet. 1992;340:524–8. doi: 10.1016/0140-6736(92)91719-o. [DOI] [PubMed] [Google Scholar]
- 158.Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med. 1999;5:340–3. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 159.Alcais A, Quintana-Murci L, Thaler DS, Schurr E, Abel L, Casanova JL. Life-threatening infectious diseases of childhood: single-gene inborn errors of immunity? Ann N Y Acad Sci. 2010;1214:18–33. doi: 10.1111/j.1749-6632.2010.05834.x. [DOI] [PubMed] [Google Scholar]
- 160.Jallow M, Teo YY, Small KS, Rockett KA, Deloukas P, Clark TG, Kivinen K, Bojang KA, Conway DJ, Pinder M, Sirugo G, Sisay-Joof F, Usen S, Auburn S, Bumpstead SJ, Campino S, Coffey A, Dunham A, Fry AE, Green A, Gwilliam R, Hunt SE, Inouye M, Jeffreys AE, Mendy A, Palotie A, Potter S, Ragoussis J, Rogers J, Rowlands K, Somaskantharajah E, Whittaker P, Widden C, Donnelly P, Howie B, Marchini J, Morris A, Sanjoaquin M, Achidi EA, Agbenyega T, Allen A, Amodu O, Corran P, Djimde A, Dolo A, Doumbo OK, Drakeley C, Dunstan S, Evans J, Farrar J, Fernando D, Hien TT, Horstmann RD, Ibrahim M, Karunaweera N, Kokwaro G, Koram KA, Lemnge M, Makani J, Marsh K, Michon P, Modiano D, Molyneux ME, Mueller I, Parker M, Peshu N, Plowe CV, Puijalon O, Reeder J, Reyburn H, Riley EM, Sakuntabhai A, Singhasivanon P, Sirima S, Tall A, Taylor TE, Thera M, Troye-Blomberg M, Williams TN, Wilson M, Kwiatkowski DP. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nat Genet. 2009 doi: 10.1038/ng.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Timmann C, Thye T, Vens M, Evans J, May J, Ehmen C, Sievertsen J, Muntau B, Ruge G, Loag W, Ansong D, Antwi S, Asafo-Adjei E, Nguah SB, Kwakye KO, Akoto AO, Sylverken J, Brendel M, Schuldt K, Loley C, Franke A, Meyer CG, Agbenyega T, Ziegler A, Horstmann RD. Genome-wide association study indicates two novel resistance loci for severe malaria. Nature. 2012;489:443–6. doi: 10.1038/nature11334. [DOI] [PubMed] [Google Scholar]
- 162.Fox LL, Taylor TE, Pensulo P, Liomba A, Mpakiza A, Varela A, Glover SJ, Reeves MJ, Seydel KB. Histidine-Rich Protein 2 Plasma Levels Predict Progression to Cerebral Malaria in Malawian Children With Plasmodium falciparum Infection. J Infect Dis. 2013 doi: 10.1093/infdis/jit176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–4. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 164.Avril M, Tripathi AK, Brazier AJ, Andisi C, Janes JH, Soma VL, Sullivan DJ, Jr, Bull PC, Stins MF, Smith JD. A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells. Proc Natl Acad Sci U S A. 2012;109:E1782–90. doi: 10.1073/pnas.1120534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lavstsen T, Turner L, Saguti F, Magistrado P, Rask TS, Jespersen JS, Wang CW, Berger SS, Baraka V, Marquard AM, Seguin-Orlando A, Willerslev E, Gilbert MT, Lusingu J, Theander TG. Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proc Natl Acad Sci U S A. 2012;109:E1791–800. doi: 10.1073/pnas.1120455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Craig AG, Grau GE, Janse C, Kazura JW, Milner D, Barnwell JW, Turner G, Langhorne J. The role of animal models for research on severe malaria. PLoS Pathog. 2012;8:e1002401. doi: 10.1371/journal.ppat.1002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Milner DA., Jr Rethinking cerebral malaria pathology. Curr Opin Infect Dis. 2010;23:456–63. doi: 10.1097/QCO.0b013e32833c3dbe. [DOI] [PubMed] [Google Scholar]
- 168.Belnoue E, Kayibanda M, Vigario AM, Deschemin JC, van Rooijen N, Viguier M, Snounou G, Renia L. On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol. 2002;169:6369–75. doi: 10.4049/jimmunol.169.11.6369. [DOI] [PubMed] [Google Scholar]
- 169.Chen L, Zhang Z, Sendo F. Neutrophils play a critical role in the pathogenesis of experimental cerebral malaria. Clin Exp Immunol. 2000;120:125–33. doi: 10.1046/j.1365-2249.2000.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Howland SW, Poh CM, Gun SY, Claser C, Malleret B, Shastri N, Ginhoux F, Grotenbreg GM, Renia L. Brain microvessel cross-presentation is a hallmark of experimental cerebral malaria. EMBO Mol Med. 2013;5:984–99. doi: 10.1002/emmm.201202273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pais TF, Chatterjee S. Brain macrophage activation in murine cerebral malaria precedes accumulation of leukocytes and CD8+ T cell proliferation. J Neuroimmunol. 2005;163:73–83. doi: 10.1016/j.jneuroim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 172.Waisberg M, Tarasenko T, Vickers BK, Scott BL, Willcocks LC, Molina-Cruz A, Pierce MA, Huang CY, Torres-Velez FJ, Smith KG, Barillas-Mury C, Miller LH, Pierce SK, Bolland S. Genetic susceptibility to systemic lupus erythematosus protects against cerebral malaria in mice. Proc Natl Acad Sci U S A. 2011;108:1122–7. doi: 10.1073/pnas.1017996108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Togbe D, Schofield L, Grau GE, Schnyder B, Boissay V, Charron S, Rose S, Beutler B, Quesniaux VF, Ryffel B. Murine cerebral malaria development is independent of toll-like receptor signaling. Am J Pathol. 2007;170:1640–8. doi: 10.2353/ajpath.2007.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Coban C, Ishii KJ, Uematsu S, Arisue N, Sato S, Yamamoto M, Kawai T, Takeuchi O, Hisaeda H, Horii T, Akira S. Pathological role of Toll-like receptor signaling in cerebral malaria. Int Immunol. 2007;19:67–79. doi: 10.1093/intimm/dxl123. [DOI] [PubMed] [Google Scholar]
- 175.Rudin W, Favre N, Bordmann G, Ryffel B. Interferon-gamma is essential for the development of cerebral malaria. Eur J Immunol. 1997;27:810–5. doi: 10.1002/eji.1830270403. [DOI] [PubMed] [Google Scholar]
- 176.Engwerda CR, Mynott TL, Sawhney S, De Souza JB, Bickle QD, Kaye PM. Locally up-regulated lymphotoxin alpha, not systemic tumor necrosis factor alpha, is the principle mediator of murine cerebral malaria. J Exp Med. 2002;195:1371–7. doi: 10.1084/jem.20020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Favre N, Da Laperousaz C, Ryffel B, Weiss NA, Imhof BA, Rudin W, Lucas R, Piguet PF. Role of ICAM-1 (CD54) in the development of murine cerebral malaria. Microbes Infect. 1999;1:961–8. doi: 10.1016/s1286-4579(99)80513-9. [DOI] [PubMed] [Google Scholar]
- 178.Molokhia M, McKeigue P. Systemic lupus erythematosus: genes versus environment in high risk populations. Lupus. 2006;15:827–32. doi: 10.1177/0961203306070007. [DOI] [PubMed] [Google Scholar]
- 179.Bolland S, Ravetch JV. Spontaneous Autoimmune Disease in Fc[gamma]RIIB-Deficient Mice Results from Strain-Specific Epistasis. Immunity. 2000;13:277–85. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 180.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–10. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Clatworthy MR, Willcocks L, Urban B, Langhorne J, Williams TN, Peshu N, Watkins NA, Floto RA, Smith KG. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc Natl Acad Sci U S A. 2007;104:7169–74. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Willcocks LC, Carr EJ, Niederer HA, Rayner TF, Williams TN, Yang W, Scott JA, Urban BC, Peshu N, Vyse TJ, Lau YL, Lyons PA, Smith KG. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2010;107:7881–5. doi: 10.1073/pnas.0915133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Niederer HA, Willcocks LC, Rayner TF, Yang W, Lau YL, Williams TN, Scott JA, Urban BC, Peshu N, Dunstan SJ, Hien TT, Phu NH, Padyukov L, Gunnarsson I, Svenungsson E, Savage CO, Watts RA, Lyons PA, Clayton DG, Smith KG. Copy number, linkage disequilibrium and disease association in the FCGR locus. Hum Mol Genet. 2010;19:3282–94. doi: 10.1093/hmg/ddq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Greenwood BM. Autoimmune disease and parasitic infections in Nigerians. Lancet. 1968;2:380–2. doi: 10.1016/s0140-6736(68)90595-3. [DOI] [PubMed] [Google Scholar]
- 185.Greenwood BM, Herrick EM, Voller A. Can parasitic infection suppress autoimmune disease? Proc R Soc Med. 1970;63:19–20. doi: 10.1177/003591577006300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Greenwood BM, Herrick EM, Voller A. Suppression of autoimmune disease in NZB and (NZB × NZW) F1 hybrid mice by infection with malaria. Nature. 1970;226:266–7. doi: 10.1038/226266a0. [DOI] [PubMed] [Google Scholar]
- 187.Larson JD, Thurman JM, Rubtsov AV, Claypool D, Marrack P, van Dyk LF, Torres RM, Pelanda R. Murine gammaherpesvirus 68 infection protects lupus-prone mice from the development of autoimmunity. Proc Natl Acad Sci U S A. 2012;109:E1092–100. doi: 10.1073/pnas.1203019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 189.Jaramillo-Gutierrez G, Molina-Cruz A, Kumar S, Barillas-Mury C. The Anopheles gambiae oxidation resistance 1 (OXR1) gene regulates expression of enzymes that detoxify reactive oxygen species. PLoS One. 2010;5:e11168. doi: 10.1371/journal.pone.0011168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–8. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 2007;3:e26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. Proc Biol Sci. 2009;276:145–51. doi: 10.1098/rspb.2008.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Raulet DH. Natural killer cells: remembrances of things past. Curr Biol. 2009;19:R294–6. doi: 10.1016/j.cub.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sun JC, Lanier LL. Natural killer cells remember: an evolutionary bridge between innate and adaptive immunity? Eur J Immunol. 2009;39:2059–64. doi: 10.1002/eji.200939435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-Mury C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329:1353–5. doi: 10.1126/science.1190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc Natl Acad Sci U S A. 1998;95:5700–5. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Han YS, Thompson J, Kafatos FC, Barillas-Mury C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J. 2000;19:6030–40. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Han YS, Barillas-Mury C. Implications of Time Bomb model of ookinete invasion of midgut cells. Insect Biochem Mol Biol. 2002;32:1311–6. doi: 10.1016/s0965-1748(02)00093-0. [DOI] [PubMed] [Google Scholar]
- 200.Kumar S, Gupta L, Han YS, Barillas-Mury C. Inducible peroxidases mediate nitration of anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion. J Biol Chem. 2004;279:53475–82. doi: 10.1074/jbc.M409905200. [DOI] [PubMed] [Google Scholar]
- 201.Kumar S, Barillas-Mury C. Ookinete-induced midgut peroxidases detonate the time bomb in anopheline mosquitoes. Insect Biochem Mol Biol. 2005;35:721–7. doi: 10.1016/j.ibmb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 202.Pfeiffer S, Lass A, Schmidt K, Mayer B. Protein tyrosine nitration in cytokine-activated murine macrophages. Involvement of a peroxidase/nitrite pathway rather than peroxynitrite. J Biol Chem. 2001;276:34051–8. doi: 10.1074/jbc.M100585200. [DOI] [PubMed] [Google Scholar]
- 203.But PG, Murav’ev RA, Fomina VA, Rogovin VV. Oxides of nitrogen (NO* and NO2-) as cofactors of the myeloperoxidase system. Izv Akad Nauk Ser Biol. 2004:269–73. [PubMed] [Google Scholar]
- 204.Povelones M, Waterhouse RM, Kafatos FC, Christophides GK. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324:258–61. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fraiture M, Baxter RH, Steinert S, Chelliah Y, Frolet C, Quispe-Tintaya W, Hoffmann JA, Blandin SA, Levashina EA. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe. 2009;5:273–84. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 206.Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–70. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- 207.de Oliveira GA, Lieberman J, Barillas-Mury C. Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science. 2012;335:856–9. doi: 10.1126/science.1209678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, Dong Y, Jiang H, Kanost MR, Koutsos AC, Levashina EA, Li J, Ligoxygakis P, Maccallum RM, Mayhew GF, Mendes A, Michel K, Osta MA, Paskewitz S, Shin SW, Vlachou D, Wang L, Wei W, Zheng L, Zou Z, Severson DW, Raikhel AS, Kafatos FC, Dimopoulos G, Zdobnov EM, Christophides GK. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316:1738–43. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Muller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, von Mering C, Xu J, Zheng L, Bork P, Kafatos FC. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–65. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- 210.Zdobnov EM, von Mering C, Letunic I, Torrents D, Suyama M, Copley RR, Christophides GK, Thomasova D, Holt RA, Subramanian GM, Mueller HM, Dimopoulos G, Law JH, Wells MA, Birney E, Charlab R, Halpern AL, Kokoza E, Kraft CL, Lai Z, Lewis S, Louis C, Barillas-Mury C, Nusskern D, Rubin GM, Salzberg SL, Sutton GG, Topalis P, Wides R, Wincker P, Yandell M, Collins FH, Ribeiro J, Gelbart WM, Kafatos FC, Bork P. Comparative genome and proteome analysis of Anopheles gambiae and Drosophila melanogaster. Science. 2002;298:149–59. doi: 10.1126/science.1077061. [DOI] [PubMed] [Google Scholar]
- 211.Barillas-Mury C, Han YS, Seeley D, Kafatos FC. Anopheles gambiae Ag-STAT, a new insect member of the STAT family, is activated in response to bacterial infection. EMBO J. 1999;18:959–67. doi: 10.1093/emboj/18.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Barillas-Mury C, Charlesworth A, Gross I, Richman A, Hoffmann JA, Kafatos FC. Immune factor Gambif1, a new rel family member from the human malaria vector, Anopheles gambiae. EMBO J. 1996;15:4691–701. [PMC free article] [PubMed] [Google Scholar]
- 213.Frolet C, Thoma M, Blandin S, Hoffmann JA, Levashina EA. Boosting NFkappaB- dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity. 2006;25:677–85. doi: 10.1016/j.immuni.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 214.Garver LS, Dong Y, Dimopoulos G. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 2009;5:e1000335. doi: 10.1371/journal.ppat.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Garver LS, Bahia AC, Das S, Souza-Neto JA, Shiao J, Dong Y, Dimopoulos G. Anopheles Imd pathway factors and effectors in infection intensity-dependent anti- Plasmodium action. PLoS Pathog. 2012;8:e1002737. doi: 10.1371/journal.ppat.1002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Manske M, Miotto O, Campino S, Auburn S, Almagro-Garcia J, Maslen G, O’Brien J, Djimde A, Doumbo O, Zongo I, Ouedraogo JB, Michon P, Mueller I, Siba P, Nzila A, Borrmann S, Kiara SM, Marsh K, Jiang H, Su XZ, Amaratunga C, Fairhurst R, Socheat D, Nosten F, Imwong M, White NJ, Sanders M, Anastasi E, Alcock D, Drury E, Oyola S, Quail MA, Turner DJ, Ruano-Rubio V, Jyothi D, Amenga-Etego L, Hubbart C, Jeffreys A, Rowlands K, Sutherland C, Roper C, Mangano V, Modiano D, Tan JC, Ferdig MT, Amambua-Ngwa A, Conway DJ, Takala-Harrison S, Plowe CV, Rayner JC, Rockett KA, Clark TG, Newbold CI, Berriman M, MacInnis B, Kwiatkowski DP. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487:375–9. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nsango SE, Pompon J, Xie T, Rademacher A, Fraiture M, Thoma M, Awono-Ambene PH, Moyou RS, Morlais I, Levashina EA. AP-1/Fos-TGase2 Axis Mediates Wounding-induced Plasmodium falciparum Killing in Anopheles gambiae. J Biol Chem. 2013;288:16145–54. doi: 10.1074/jbc.M112.443267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gupta L, Molina-Cruz A, Kumar S, Rodrigues J, Dixit R, Zamora RE, Barillas-Mury C. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe. 2009;5:498–507. doi: 10.1016/j.chom.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dong Y, Cirimotich CM, Pike A, Chandra R, Dimopoulos G. Anopheles NF-kappaB- regulated splicing factors direct pathogen-specific repertoires of the hypervariable pattern recognition receptor AgDscam. Cell Host Microbe. 2012;12:521–30. doi: 10.1016/j.chom.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dong Y, Taylor HE, Dimopoulos G. AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol. 2006;4:e229. doi: 10.1371/journal.pbio.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Portugal S, Pierce SK, Crompton PD. Young lives lost as B cells falter: What we’re learning about antibody responses to malaria. J Immunol. 2013 doi: 10.4049/jimmunol.1203067. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]