Abstract
Mutations in the gene encoding cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia (PSACH), a severe dwarfing condition. Pain, a significant complication, has generally been attributed to joint abnormalities and erosion and early onset osteoarthritis. Previously, we found that the Inflammatory-related transcripts were elevated In growth plate and articular cartilages, Indicating that Inflammation plays an important role in the chondrocyte disease pathology and may contribute to the overall pain sequelae. Here, we describe the effects of D469-delCOMP expression on the skeleton and growth plate chondrocytes with the aim to define a treatment window and thereby reduce pain. Consistent with the human PSACH phenotype, skeletal development of D469del-COMP mice was normal and similar to controls at birth. By postnatal day 7 (P7), the D469del-COMP skeleton, limbs, skull and snout were reduced and this reduction was progressive during postnatal growth, resulting in a short-limbed dwarfed mouse. Modulation of prenatal and postnatal expression of D469del-COMP showed minimal retention/cell death at P7 with some retention/cell death by P14, suggesting that earlier treatment intervention at the time of PSACH diagnosis may produce optimal results. Important and novel findings were an increase In Inflammatory proteins generally starting at P21 and that exercise exacerbates Inflammation. These observations suggest that pain in PSACH may be related to an intrinsic inflammatory process that can be treated symptomatically and is not related to early joint erosion. We also show that genetic ablation of CHOP dampens the inflammatory response. observed in mice expressing D469del-COMP. Toward identifying potential treatments, drugs known to decrease cellular stress (lithlum, phenyl butyric add, and valproate) were assessed. Interestingly, all diminished the chondrocyte pathology but had untoward outcomes on mouse growth, development, and longevity. Collectively, these results define an early treatment window in which chondrocytes can be salvaged, thereby potentially Increasing skeletal growth and decreasing pain.
Keywords: COMP, CELL DEATH, GROWTH PLATE, MUTATION, GENETICS, CHONDRODYSTROPHY
Introduction
Pseudoachondroplasia (PSACH) is a well-characterized skeletal dysplasia that was first described in 1959.(1,2) Interestingly, newborns with PSACH are indistinguishable from unaffected babies; the diagnosis is generally made around 2 to 3 years of age when linear growth slows and a painful waddling gait develops, indicating that clinical manifestations of PSACH occur during postnatal development.(3,4) Joint pain, the most debilitating clinical complication, is generally attributed to joint erosion and osteoarthritis with joint replacements frequently needed in early adulthood.(3,4)
PSACH is caused by mutations in cartilage oligomeric matrix protein (COMP), which is a large extracellular matrix protein and the fifth member of the thrombospondin gene family.(5,6) We and others have shown that COMP is retained in the endoplasmic reticulum (ER) of growth plate chondrocytes, causing massive enlargement of the rough ER (rER) cisternae.(7,8) Moreover, we have reported that in the presence of mutant COMP, other extracellular matrix proteins, including types II and IX collagens and matrilin-3, prematurely assemble an intracellular matrix.(7–9) Despite decades of study, progress toward understanding the pathologic mechanisms underlying this process has been limited because previous observations have relied on cross-sectional information gleamed from cultured PSACH chondroids or human biopsies.(10) As a result, these types of studies provide little information about the development of the chondrocyte pathology, thereby limiting the development of potential treatment options.
To circumvent this roadblock, we generated a transgenic mouse with doxycycline (DOX)-inducible chondrocyte-specific expression of the common D469del-COMP mutation, which is responsible for approximately 30% of PSACH cases.(11–13) We have previously shown that this D469del-COMP mouse recapitulates critical cellular and clinical features of PSACH by postnatal day 28 (P28; 4 weeks of age) including (1) retention of CaMP and other extracellular matrix proteins, (2) the presence of intracellular matrix in the rER cisternae, (3) increased chondrocyte apoptosis, and (4) limb shortening.(9, 14) In this study, we describe evidence for inflammation associated with the chondrocyte pathology and the changes in murine skeletal development from birth to 4 weeks. We show that although mutant COMP does not disturb neonatal bone formation, it has a significant negative impact on chondrocyte viability and longevity by invoking inflammation. This important and novel observation may explain some of the clinical symptoms such as early childhood joint pain in PSACH. Additionally, we show the unfolded protein response (UPR) through the CCAAT/enhancer binding protein (C/EBP) homologous protein (CHOP) pathway plays a significant role in the pathology, suggesting that treatment modalities modifying this ER response might ameliorate some of the clinical complications. To address this question, we tested three therapies known to decrease ER stress and thereby increase chondrocyte viability.
Subjects and Methods
Generation of bigenic mice
Plasmids containing expression cassettes for mutant D469del-COMP under control of a tetracycline-inducible (TRE) promoter and recombinant tetracycline controlled trans-activation factor (rtTA) under control of type II collagen promoter were generated as described.(9) Standard breeding was used to generate bigenic animals in C57BL/6 genetic background. Mice were administered DOX (500 ng/ml) through drinking water (with 5% wt/vol sucrose) prenatally and postnatally either continually or for defined periods of time. The Animal Welfare Committee at the University of Texas Medical School at Houston approved these studies.
Skeletal preparations
Mice were collected and the soft tissue removed from the skeleton. Skeletons were fixed overnight in 95% vol/vol ethanol. Skeletons are stained for48 hours with Alcian blue (0.015% wt/vol solution in 20% vol/vol acetic acid and 80% vol/vol methanol), transferred to 95% vol/vol ethanol for 2 hours, and then 2% vol/vol KOH for 3 hours. The mineralized bone was then stained with Alizarin red (0.005% wt/vol in 1% KOH vol/vol) for 3 hours. Skeletons were cleared in 1% KOH in 20% vol/vol glycerol for 48 hours and stored in a glycerol ethanol mixture (1:1). At least six C57BL/6 and D469del-COMP mice were examined at each age.
Skull measurements
Skulls were obtained from 8 control and 8 D469del-COMP mice, the soft tissue was removed and stored in 70% vol/vol ethanol and subjected to Xradia scanning (Xradia, Pleasanton, CA, USA). Measurements were made from landmark locations in the skull using Xradia software as described.(15) A t test was used to compare the measurements from control and D469del-COMP mice skulls.
Immunostaining
Hind limbs from D469del-COMP and C57BL/6 control mice were collected and tibial growth plates were analyzed as described.(9) Briefly, the limbs were fixed in 95% vol/vol ethanol for COMP (Abeam, Cambridge, MA, USA; ab11056-rat, 1:100), eosinophil peroxidase (EPX; Santa Cruz Biotechnology, Santa Cruz, CA, USA; sc-19147, 1:50). YM1 (Stem Cell Technologies, BC, Canada; 01404, 1:100), C-C chemokine receptor type 5 (CCR5; Novus Biologicals; NBP1-41434, 1:100), interleukin 1 (IL1) (Abeam; ab7632, 1:200), IL16 (Santa Cruz Biotechnology; SC7902, 1:100), IL18 (Abcam; ab197664, 1:200), or tumor necrosis factor α (TNFα) (Abcam; ab6671, 1:200) immunostaining or in 10% wt/vol formalin for terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) staining. The COMP antibody generated in rat is specific for human COMP and does not cross-react with endogenous mouse COMPo At least 3 CS7BL/6 and D469del-COMP mice were examined at each age.
Transcriptome analysis
Total RNA (300 ng) from knee joints was purified using TRIzol (Life Technologies Corp.) and Qiagen RNAeasy columns (Qiagen, Valencia, CA, USA) as described.(14) Briefly, RNA was amplified using the Illumina Total Prep RNA Amplification Kit (Illumina, San Diego, CA, USA), and assessed by RNA integrity number (RIN) number. For each group, 3 independent RNA sample animals were examined to control for variation in expression. An aliquot of 1.5 μg of amplified products were loaded onto Illumina Sentrix Beadchip Array Mouse WG-6.v2 arrays, hybridized at 58°C in an Illumina Hybridization Oven for 17 hours, washed, and incubated with Streptavidin-Cy3 to detect biotin-labeled cRNA on the arrays. Arrays were dried and scanned with BeadArray Reader (Illumina). Data were analyzed using GenomeStudio software (Illumina). Clustering and pathway analysis were performed with GenomeStudio software and Ingenuity Pathway Analysis software (Ingenuity Systems, Inc., Redwood City, CA, USA), respectively. A p value of < 0.05 was considered significant. The mRNAs were classified into functional categories.
mRNA quantification
Total RNA was isolated from the rat chondrosarcoma (RCS) cells using TRIzol and treated with RNAse-free DNAse1 (Ambion, Inc.). cDNA was made using iScript kit (Bio-Rad Laboratories, Hercules, CA, USA) as per the manufacturer’s instructions. Levels of mRNAs were measured by quantitative PCR using SYBR Green kit (Applied Biosystems, Inc.). The primers are as follows: CCR5 forward 3′-ATGGATTTTCAAGGGTCAGTTCC-5′, CCR5 reverse 3′-CTGAGCCGCAATTTGTTTCAC-5′, EPX forward 3′-TTCAGCCCTTCATGTTCCG-5′, EPX reverse 3′-TCGATGCCACCTTCATGTATG-5′, hypoxanthine phosphoribosyltransferase 1 (HPRT1) forward 3′-CCTCATGGACTGATTATGGACAG-5′, HPRT1 reverse 3′-TCAGCAAAGAACTTATAGCCCC-5′, alpha-synuclein (SNCA) forward 3′-GGGAGTCCTCTATGTAGGTTCC-5′, and SNCA reverse 3′-TCCAACATTTGTCACTTGCTCT-5′. ABI TaqMan assays were used to quantify eosinophil-associated ribonuclease 6 (EAR6; Mn04213770_gl), HPRT1 (Mn01545399_ml), and WD repeat and FYVE domain containing 1 (WDFY1; Mn00840455_ml) mRNA. Relative changes in mRNA levels were assessed using the comparative threshold cycle (CT) method. All the measurements were normalized to the endogenous Hprt1 mRNA.
Exercise protocol
Three to five male mice D469del-COMP and C57BL/6 controls were subjected to an exercise protocol and were allowed to run from P21 to P42. The rodent wheel was held in place by a heavy metal plate and the distance run by each mouse was recorded using a CatEye magnetic bicycle monitor (Osaka, Japan). Daily distances were recorded both manually and digitally from Monday through Friday, and distance traveled on Saturday and Sunday was recorded with the digital monitor only. All housing conditions and experiments were conducted in compliance with University of Texas Medical School at Houston animal care standards.
Drug administration
Each drug was mixed into DOX water according to dosages and administered through drinking water beginning at birth to 1 month of age. Valproate (valproic acid sodium salt; Sigma, St. Louis, MO, USA), Li (lithium carbonate; Chemetall, New Providence, NJ, USA), and PBA (4-phenylbutyric acid; Sigma) were administered at 5 mg/L, 125 mg/L, and 10g/L, respectively.
Results
D469del-COMP mouse pathology mimics human PSACH pathology
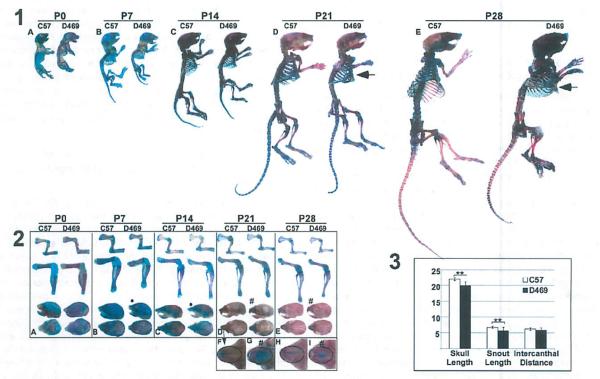
In previous work, we have shown that D469del-COMP reduces the mouse hind limb length by 12% at 1 month of age.(14) Here, we define the development of skeletal abnormalities relative to the timing of D469del-COMP retention and the loss in growth plate chondrocytes. D469del-COMP and control mice were collected at birth (P0), P7, P14, P21, and P28, and the skeletons were stained with Alizarin red and Alcian blue to visualize bone and cartilage, respectively. As shown in Fig. 1, the skeleton and limbs at birth were similar for both D469del-COMP and control mice (Fig. 1A, panels 1 and 2). By P7, the skeleton of the D469del-COMP mouse was slightly reduced compared to the C57BL/6 control (Fig. 1 B, panels 1 and 2). The reduction in the size of the D469del-COMP skeleton compared to the C57BL/6 control progressed from P7 to P28. At P28, the difference between the D469del-COMP mouse and control was most marked (Fig. 1 E, panels 1 and 2) at a time correlated with high levels of growth plate chondrocyte death.(14) Moreover, there was a marked progression in the development of pectus carinatum at P28 (arrow in Fig. 1D, E, panel 1). Pectus carinatum is a frequent finding in PSACH.(3) Additionally, an exaggerated curvature in the thoracic and lumbar spine was observed in most animals by P28 and may translate to scoliosis that is observed in PSACH.
Fig. 1.
D469del-COMP mice are shorter than control mice. Panel 1: Skeletal preparations from birth (P0) to 1 month or age (P28) are shown for control CS7BL/6 mice (on left A–E) and D469del-COMP mice (on right A–E). Mice were collected, soft tissue was removed, and the skeleton was fixed and stained with Alcian blue (cartilage) and Alizarin red (bone). D469del-COMP skeleton was shorter than controls starting at P7 and were 12% shorter by 28 days. Arrow marks the pectus carinatum. Panel 2: Limb and skulls were dissected away from skeletal preparations. For comparison, control and D469del-COMP images were equally magnified at each age. Control C57BL/6 limbs are on the left and D469del-COMP are on the right in sections A–E. D469del-COMP and control limbs are similar until P7 and then are reduced at all ages examined. The skull and snout of D469del-COMP mice was also shorter (shown by “•” compared to the C57BL/6 and there appears to be more cartilage (shown by “#,” Aldan blue staining) in the D469del-COMP snout (G:I) compare to controls (F:H) at P21 to P28 (shown by “#”). Panel 3: 8y P28, the skull and snout of D469del-COMP was Significantly shorter than the control (**p ≤ 0.01). D469 = D469del-COMP.
A reduction in snout length was observed by P7 in the D469del-COMP skulls and more cartilage was detected in the snout of P2l and P28 D469del-COMP skulls (Fig. 1A–I, panel 2). To quantify these changes, the skull length, snout length, and intercanthal distance were measured as described.(15) The total skull and snout lengths were reduced by 10% and 15%, respectively, in the D469del-COMP mice while intercanthal distance was equivalent (Fig. 1C. These results indicate that D469del-COMP expression progressively reduces skeletal growth.
Limiting the duration of D469del-COMP expression reduces growth plate chondrocyte death
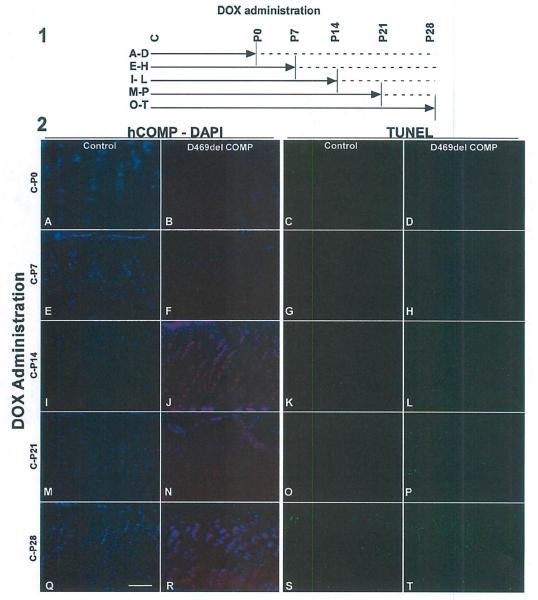
Next, we assessed the effect of limiting the duration of D469del-COMP expression on intracellular retention of mutant COMP and chondrocyte cell death by modulating expression with DOX. In these experiments, expression of D469del-COMP was induced from conception to birth by administration of DOX to the pregnant dams. After birth, exposure to DOX was continued until P0, P7, P14, P21, or P28, and the growth plates of all mice were assessed at P28 (Fig. 2A–T, panel 1). DOX exposure in the prenatal period to P7 evoked very little intracellular D469del-COMP retention and cell death (Fig. 2E–H, panel 2 similar to those that did not receive DOX Fig. 2A–D). However, by P14, D469del-COMP was retained in all chondrocytes and chondrocyte death was greater than the control and was present throughout the growth plate (Fig. 2I–L, panel 2). Expression of D469del-COMP until P21 or P28 resulted In the same pathology (Fig. 2M–T, panel 2). These results suggest that 14 days of postnatal expression of D469del-COMP in mice is necessary to elicit the PSACH chondrocyte pathology.
Fig. 2.
Chondrocyte cell death increases with duration of D469del-COMP expression. Panel 1: (A–T) DOX was administered for weekly intervals according to the schematic. Solid line indicates DOX administration to mice (500 ng/ml); dotted line indicates no DOX. All treatments were started at conception (shown as “C”)and continued until P0, P7, P14, P21, or P28. Growth plates from all animals were analyzed at P28. Panel 2: D469del-COMP (red) and DAPI (blue-nuclei) staining of D469del-COMP (B, F, J, N, and R) and control C57BL/6 (A, E, I, M, and Q) growth plates are shown. Very little intracellular D469del-COMP was observed with DOX administration from birth to P7. TUNEL staining of D469del-COMP (D, H, L, P, and T) and control C57BL/6 (C, G, K, O, and S) growth plates are shown. Cell death increased dramatically in D469del-COMP growth plates after 2 weeks of DOX (L, P, and T) compared to controls (K, O, and S). Bar = 500 μm.
Inflammation develops in a time dependent manner in D469del-COMP growth plate chondrocytes
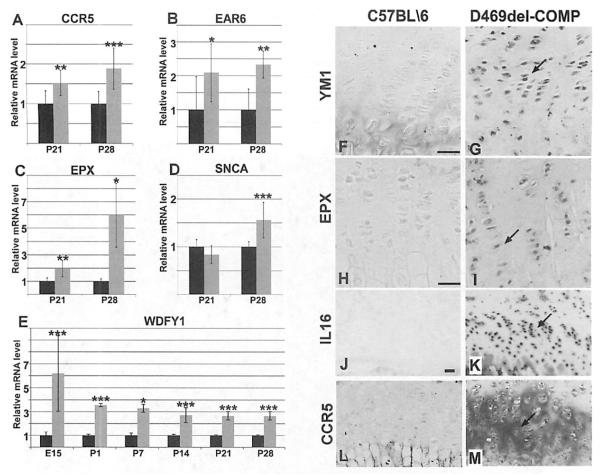
Previously, we identified a spike in the expression of inflammatory-related mRNAs beginning at P21 in the D469del-COMP mouse growth plate, suggesting that an inflammatory process was occurring.(14) In these studies, temporal expression of inflammatory markers was examined using immunostaining and qRT-PCR.(14) Control and D469del-COMP mice were administered DOX at conception and limbs were collected at embryonic day 15.5 (E15.5), P1, P7, P14, P21, and P28 for RNA extraction and immunostaining. Based on our previous microarray analysis, we selected several inflammation-related mRNAs for quantitation: EAR6, EPX, CCR5, SNCA, and WDFY1. As shown in Fig. 3, EAR6 and EPX mRNAs are both increased in the D469del-COMP mice. EAR6 mRNA was elevated at P21 and P28 in the D469del-COMP mice (Fig. 3B). EPX was sixfold higher in D469del-COMP mice compared to controls at P28 (Fig. 3C. In addition to increases in eosinophil related transcripts, CCR5, a chemokine receptor, WDFY1, a macrophage chemoattractant, and SNCA, a chaper-oneβ-like protein that is associated with chronic inflammation, were also expressed at significantly higher levels in the D469del-COMP mice (Fig. 3A, D, E).(16–19) These results indicate that the inflammation process begins by P21, when intracellular retention of D469del-COMP was pronounced, and continues through P28. Inflammation lags behind intracellular accumulation of mutant COMP in chondrocytes perhaps because secretory cells tolerate ER stress for a longer period prior to causing cellular damage.(20)
Fig.3.
Proinflammatory transcripts and proteins are elevated in D469del-COMP. mRNA levels of (CRS (A), EAR6 (B), EPX (C), SNCA (D), and WDFY1 (E) were assessed using qRT-PCR. C57BL/6 levels are set to 1 and shown in black bars and D469del-COMP levels are shown in gray. ***p > 0.0005, **P > 0.05, and *p > 0.05. CCR, EAR6, EPX, and SNCA mRNA levels were not elevated at earlier ages. (F–K) Tibias were collected from P28 mice for immunostaining. The D469del-COMP tibias showed more intense YM1, EPX, IL16, and CCR5 immunostaining (G, I, K, M) than C57BL/6 control (F, H, J, L). Bar = 100 μm. Arrows indicate positive immunostaining.
Immunostaining was used to confirm the presence of inflammatory-related proteins, EPX, CCR5, IL16, and YM1 in the growth plate and articular cartilage. Increased EPX immunostaining was present in D469del-COMP mice at P28 compared to controls (Fig. 3H, I). Consistent with upregulation of CCR5 and IL 16 mRNA, positive immunostaining for both CCR5 and IL16 protein was observed at P28 (Fig. 3J–M). YM1, a macrophage protein, was increased in both the growth plate and articular cartilage of the D469del-COMP mice at P28 (Fig. 3F compared to G; and Fig. 5, panel 1). Additionally, IL18 was assessed because IL18 mRNA was Increased 1.7-fold at P21 and has an established role in chondrocyte inflammation and cartilage destruction.(21–24) As shown in Fig. 4, IL18 immunostaining is more intense in the D469del-COMP growth plate compared to control (Fig. 4E, F).
Fig. 5.
A–D panel 2 shows wild-type controls with little to no YM1 immunostaining YM1 indicates that inflammation is present in D469del-COMP cartilages and is exacerbated by exercise. Panel 1: Tibias from P28 mice were collected and immunostained with YM1 antibodies. The D469del-COMP growth plate and articular cartilage showed elevated YM1 immunostaining (C, D) compared to the C57BL/6 controls (A, B). Panel 2: D469del-COMP and control C67BL/6 mice were administered DOX and housed with (runner) and without (non-runner) access to a running wheel from P21 to P42. Tibias were collected at P42 and immunostained for YM1. Running increased expression of YM1 in the D469del-COMP articular cartilage (E compared to F, G, and H). Bar = 100 μm.
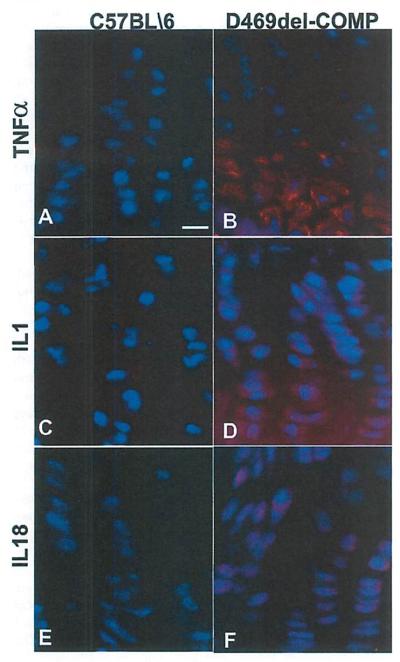
Fig. 4.
TNFα, IL1, and IL18 mediate inflammation in the D469del-COMP cartilages. Tibial growth plates were collected from P28 mice for immunostaining with TNFα, IL1, and IL18 antibodies. The D469del-COMP growth plate is immunopositive for TNFα, IL1, and IL18 (B, D, F compared to A, C, E), Bar = 100 μm.
To identify the mediator of this inflammatory process, we evaluated two common pro-inflammatory proteins, TNFα and IL1, which are downstream of IL18. Substantial TNFα expression was observed in the hypertrophic zone in D469del-COMP growth plate (Fig. 4A compared to Fig. 4B). In contrast, IL1 was present throughout the tibial growth plate of the D469del-COMP mice (Fig. 4D compared to Fig. 4C. These results show that the inflammation process begins by P21, when intracellular retention of D469del-COMP was pronounced, and continues through P28 indicating that inflammation plays a role in the murine chondrocyte disease pathology.
Exercise exacerbates inflammation in the articular cartilage
Joint inflammation can be caused by extensive joint wear and injury. To examine if physical activity further increases inflammation in the D469del-COMP mice, males were provided with a running wheel from weaning at P21 through P42 and the limbs were collected for immunostaining and analyzed as described in the previous section. YM1 was increased in the articular cartilage of running D469del-COMP mice compared to the D469del-COMP non-runner (Fig. 5E compared to Fig. 5F–H, panel 2). Fig. 5A–D panel 2 shows wild-type controls with little to no YM1 immunostaining. Based on these results, joint inflammation in PSACH patients may be aggravated by physical activity.
Absence of CHOP results in decreased levels of inflammatory markers in D469del-COMP growth plate chondrocytes
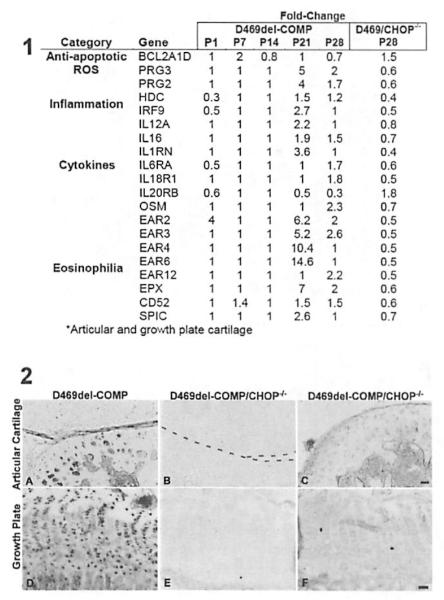
CHOP or DNA-damage-inducible transcript 3 (DDIT3) is a proapoptotic transcription factor that is Induced by ER stress.(25) CHOP null mice challenged with ER stress reagents have dramatically reduced levels of ER stress-induced apoptosis.(25) Previously, we have shown that the absence of CHOP at P28 is associated with export of D469del-COMP to the extracellular matrix, reduced chondrocyte death, and normalization of proliferating cell nuclear antigen (PCNA).(14) Because the absence of CHOP reduces many of the negative consequences of D469del-COMP, the inflammation status of D469del-COMP/CHOP−/− mice was evaluated using microarray analysis and immunostaining. Two proinflammatory mRNAs, histidine decarboxylase (HOC) and interferon regulatory factor 9 (IRF9), were increased in the D469del-COMP mice at P21 and P28, but in the absence of CHOP, these transcripts were decreased (Fig. 6, panel 1). Cytokines IL12A, IL16, ILRN, IL6RA, IL18r1, and oncostatin M (OSM) were reduced in the absence of CHOP, whereas these cytokines were elevated in the D469del-COMP mice at P21 and P28 (Fig. 6, panel 1). In contrast, the cytokine receptor 1L20RB was increased in the absence of CHOP compared to D469del-COMP mice (Fig. 6, panel 1). Eosinophil-related transcripts, EAR2, EAR3, EAR4, EAR6, EAR12, EPX, CD52, and SPIC, were increased l.4-fold to 14.6-fold In the D469del-COMP mice and decreased in the D469del-COMP/CHOP−/− mice at P28 (Fig. 6, panel 1). Interestingly, two reactive oxygen species (ROS)-related transcripts. proteoglycan 2 (PRG2) and proteoglycan 3 (PRG3), were decreased in the absence of CHOP (Fig. 6, panel 1). BCL2A1D, an antiapoptotic transcript, was increased 1.5-fold in the absence of CHOP. Finally, YM1 expression was reduced in articular cartilage and growth plate chondrocyte D469del-COMP/CHOP−/− mice compared to D469del-COMP controls at P28 (Fig. 6A compared to B and C, D compared to E and F; panel 2). Taken together, loss of CHOP reduces inflammation, ROS, and apoptosis signaling, and confirms that CHOP plays a central role in the PSACH chondrocyte pathology.
Fig. 6.
Absence of CHOP reduced inflammation in D469del-COMP hind limbs. Panel 1 :A variety of mRNAs involved in survival, ROS, inflammation, cytokines, and eosinophilia are altered in the absence of CHOP. mRNA levels from control mice were set to 1. Fold change values below 1 indicate decrease in expression and values above 1 indicate an increase in expression. No change in expression = 1. D469/CHOP−/− = D469del-COMP/CHOP−/−; * = growth plate and articular cartilage. Panel 2: Tibias were collected from D469del-COMP and D469del-COMP/CHOP−/− P28 mice for immunostaining. The D469del-COMP tibias showed more intense YM1 immunostaining in the articular cartilage and growth plate (A, D) than D469del-COMP/CHOP−/− mice (B, C, E, and F). The tibias shown in B, C, E, and F display the range of YM1 staining in the D469del-COMP/CHOP−/− mice. Bar = 100 μm.
ER stress reduction drugs decrease cellular stress but negatively affect growth plate morphology and skeletal growth
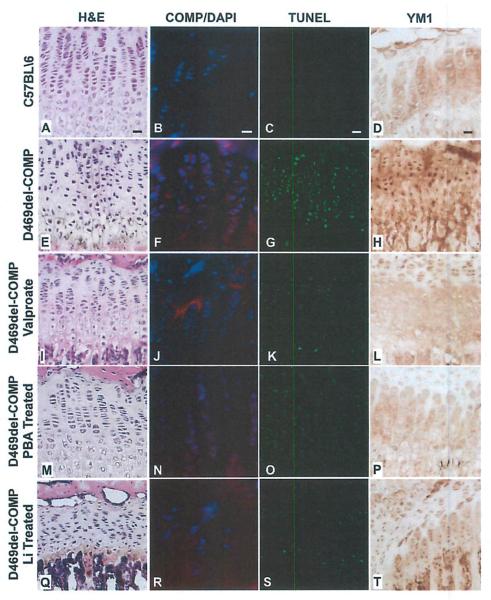
Because we have shown that CHOP plays a key role in intracellular retention of D469del-COMP and the accompanying inflammation, we hypothesized that ER stress reduction drugs might reduce the D469del-COMP chondrocyte pathology. We tested three psychotropic U.s. Food and Drug Administration (FDA)-approved drugs, valproate, lithium (Li), and phenyl butyric acid (PBA), which are used to treat mood disorders and decrease ER stress through diverse mechanisms.(26,27) Valproate, Li, and PBA administration was started at birth and continued until P28. As shown in Fig. 7, all three drugs decreased intracellular D469del-COMP retention, TUNEL staining, and YM1 immunostaining. Although valproate reduced TUNEL staining by approximately 50% and improved growth plate architecture, the skull and limb lengths were reduced by additional 9% (data not shown). Lithium reduced pup viability (data not shown) and disrupted growth plate organization and this may be attributed to lithium exposure to newborn pups (Fig. 7), Nursing mothers are discouraged from taking lithium due to negative side effects in infants and newborns.(28,29) PBA produced only minimal reductions in intracellular D469del-COMP retention and TUNEL staining (Fig. 7). Together these results show that although UPR cellular response can be dampened, the adverse side effects associated with these drugs in mice may preclude their utility in PSACH.
Fig. 7.
Valproate, PBA, and Li reduce intracellular retention of D469del-COMP, chondrocyte loss, and chondrocyte inflammation in D469del-COMP hind limbs. Tibias from P28 mice were collected and analyzed using H&E and TUNEL staining, human COMP and YM1 immunostaining. Valproate, PBA, and Li was administered from birth to P28. The D469del-COMP growth plate was disorganized (E) compared to control (A). Valproate and PBA treatment improved growth plate organization (I, M), The Li-treated D469del-COMP growth plate was more disorganized and appeared to have fewer cells (Q). Intracellular retention of D469del-COMP is decreased by drug treatments (J, N, and R) compared to untreated D469del-COMP mice (F). TUNEL and YM1 immunostaining is reduced by valproate, PBA, and Li therapy at 28 days (K, O, and S compared to G, and L, P, T compared to H). Bar = 100 μm.
Discussion
This study was undertaken to identify when, how and what contributes to the D469del-COMP chondrocyte pathology in order to test treatment modalities. By modulating D469del-COMP expression, we show that the growth plate can tolerate approximately 2 weeks (which translates to 4 years in humans) of postnatal expression of D469del-COMP before induction of chondrocyte death. Thereafter, all of the growth plate chondrocytes are compromised and there is widespread cell death. Inflammatory-related proteins are present in the growth plate and articular cartilages during the same period and exercise exacerbates inflammation. Elimination of CHOP, a central player in the D469del-COMP intracellular retention, reduces the inflammation in the joint cartilages. These results indicate that there is a window of treatment opportunity. Although administration of Li, PBA, and valproate reduced ER stress, skeletal growth and viability were negatively impacted in the treated mice.
The diagnosis of PSACH occurs around 2 years of age when decreased linear bone growth becomes apparent and an abnormal waddling gait develops.(3,30) Human skeletal growth spans approximately 16 years compared to mouse skeletal growth that is essentially complete by 8 weeks.(31) Therefore, 1 week in mouse skeletal growth roughly equates to 2 years in humans (CDC data: http://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf). A shown in Fig. 1, loss of long bone growth was progressive from P7 to P28 in the D469del-COMP mice. At P21, substantial growth plate chondrocyte death and widespread intracellular retention of COMP is maximal.(14) Reductions in the skull and limbs were evident by P7 at the same age that reductions in the whole skeleton were observed (Fig. 1 B, panels 1 and 2). Slowing of linear growth (P7) precedes extensive loss of chondrocytes (P21). These findings suggest that, at diagnosis (around age 2 years), most growth plate chondrocytes are viable and that there may be a treatment window in children from 2 to 6 years of age in which viable growth plate chondrocytes may be salvaged by therapeutic intervention. However, once most growth plate chondrocytes are depleted, treatments will likely have little effect on long bone growth.
To better understand the pathology, we evaluated the temporal expression of D469del-COMP on growth plate chondrocyte survival to assess the duration of D469del-COMP expression necessary to induce chondrocyte death. Importantly, we found that 1 week of postnatal expression was not sufficient to induce chondrocyte death. However, 2 weeks of continuous postnatal D469del-COMP expression triggered chondrocyte death (Fig. 2). Interestingly, inflammatory-related mRNAs in the D469del-COMP mice were largely unchanged until P21 and this correlates with the expression studies, which indicate that 14 days postnatal expression is necessary to evoke growth plate chondrocyte death (Figs. 2 and 6, panel 1). This suggests that the duration of D46gdel-COMP expression is an important factor in chondrocyte death, and that earlier intervention, especially at diagnosis, may yield a better outcome.
Pain is a significant complication in PSACH beginning in early childhood and is typically attributed to abnormal articular cartilage and joints as well as joint erosion,(3) To better understand the potential etiology of pain, we evaluated inflammatory markers from P1 to P28 in the growth plate and articular cartilages. Although we did not find elevations in the traditional inflammatory transcripts (TNFα, C-reactive protein (CRP), IL6, and IL1),(14) a group of inflammatory-related mRNAs (IL16 and IL18), eosinophil-related mRNAs (EAR6 and EPX), chemokine receptor CCL5, CCR5, and the CCR5 ligand were all elevated (Figs. 3 and 6, panel 1). Immunostaining for EPX and YM1 was increased in the D469del-COMP growth plates compared to controls (Figs. 3 and 4). Both EPX and YM1 are associated with tissue remodeling. EPX, a peroxidase secreted from eosinophils, plays a role in pathogen control and has been implicated in tissue remodeling and inflammation associated with asthma.(32,33) YM1 is a chemoattractant for eosinophils secreted by macrophages and has been shown to play a role in breakdown of glycosaminoglycans, a major component of cartilage.(34,35) Additionally, studies have shown that both CCR5 and CCL5 are expressed by chondrocytes,(36,37) immunostaining showed increased CCR5 expression in articular cartilages of the D469del-COMP mice, and likewise the ligand CCL5 mRNA was 1.4× elevated at P28 (Fig. 3).
During the inflammatory peak in the D469del-COMP growth plate both IL16 and IL18 expression was elevated and immunostaining confirmed the presence of these two proinflammatory proteins (Fig. 6, panel 1; Figs. 3 and 4). IL18 induces inflammatory processes in chondrocytes and increased levels of IL18 in plasma and synovial fluid correlated with joint destruction.(22–24) IL18 stimulates TNFα expression and TNFα, a well-characterized component of inflammatory cascades.(38) IL16 is a proinflammatory cytokine that has pleiotropic effects including stimulating IL1β, IL6, and TNFα(39) and attracting eosinophils in cooperation with CCR5.(40,41) This suggests that IL16 in combination with CCR5 and CCL5 increased eosinophil-related mRNAs in the D469del-COMP growth plate.
Importantly, IL1 and TNFα, induced by IL16 and IL18, are increased in the D469del·COMP growth plate despite the lack of increase in mRNA signal (Fig. 4). This finding is significant because it indicates that PSACH inflammation may be treated by well-characterized therapeutics and suggests a framework for understanding the role of inflammation in this dwarfing condition. Interestingly, the increase in SNCA, a marker for chronic inflammation, indicates that the inflammatory process in the D469del-COMP mice is persistent.(16–19)
The presence of these proteins suggests that inflammation in D469del-COMP mouse cartilage involves IL18, IL1, TNFα, and eosinophil processes and perhaps tissue remodeling that may favor cartilage degradation.(32–35) Therefore, pain in PSACH may also be attributed to an inflammation process that has important clinical implications. Children with PSACH report painful joints, a symptom that is poorly documented and inadequately treated. These novel finding suggest that more rigorous treatment approach to reduce inflammation processes should be considered.
In support of this suggestion, we have shown that CHOP plays a key role in D469del-COMP retention and that loss of CHOP alleviates intracellular retention and premature chondrocyte death.(14) We asked whether the loss of CHOP and intracellular retention affected the levels of inflammatory markers in the growth palate and articular cartilages. Indeed, there was a marked decrease in many inflammation-related transcripts (HDC, IRF9, IL12A, IL16, ILRN, IL6RA, IL18R1, OSM, EAR2, EAR3, EAR4, EAR6, EAR12, EPX, CD52, and SPIC) that were previously elevated (Fig. 6, panel 1). Ablating CHOP expression in mice reduces body size and therefore we were unable to evaluate bone growth in CHOP−/− D469del-COMP mice.(42) SNCA mRNA was decreased in the CHOP−/− D469del-COMP growth plate, which also showed little intracellular retention.(14) SNCA insoluble aggregates are associated with several disorders including Parkinson disease and multiple system atrophy,(43,44) and are found in Alzheimer amyloid plaques.(45) The results from our study suggest that intracellular retention of D469del-COMP stimulates a chronic inflammatory process that may contribute to growth plate chondrocyte death and PSACH joint pain and that SNCA may be expressed in response to protein aggregation in PSACH similar to its role in neurological diseases.(43–45)
Because CHOP clearly plays a central role in the D469del-pathology, we tested ER stress reduction drugs in an effort to increase chondrocyte viability and improve long bone growth. Although administration of these drugs diminished the PSACH chondrocyte phenotype, there were significant side effects and long bone growth was not improved. Although these drugs may not be suitable for PSACH therapy, the results indicate that the PSACH chondrocyte pathology can be reduced by pharmacological modalities and suggest that other therapeutics be evaluated to identify those that are effective and have fewer negative side effects.
Using our D469del-COMP mouse, we show that inflammation and ROS play a significant role in PSACH growth plate chondrocyte pathology and that the pathology can be reduced. Here, for the first time, we have defined a time period spanning 2 to 6 years when PSACH growth plate chondrocytes may be preserved by interventions that (1) reduce D469del-COMP expression/intracellular retention and/or (2) reduce inflammation in growth plate and articular chondrocytes. These findings provide the foundation for the development of a therapy for PSACH.
Acknowledgments
This work was supported by grants from NIH (#5R01 AR057117-03), Shriners Hospital for Children grant, and the Leah Lewis Foundation. We thank Melissa Conner, Seenya Vincent, and Wei Lu for technical assistance.
Footnotes
Disclosures All authors state that they have no conflicts of interest.
References
- 1.Maroteaux P, Lamy M. Pseudo-achondroplastic forms of spondyloepiphyseal dysplasias] Presse Med. 1959;67(10):383–6. French. [PubMed] [Google Scholar]
- 2.Cooper RR, Ponseti IV, Maynard JA. Pseudoachondroplastic dwarfism. A rough-surfaced endoplasmic reticulum storage disorder.****** J Bone Joint Surg Am. 1973;55(3):475–84. [PubMed] [Google Scholar]
- 3.Unger S, Hecht JT. Pseudoachondroplasia and multiple epiphyseal dysplasia: new etiologic developments. Am J Med Genet. 2001;106(4):244–50. [PubMed] [Google Scholar]
- 4.McKeand J, Rotta J, Hecht JT. Natural history study of pseudoachondroplasia. Am J Med Genet. 1996;63(2):406–10. doi: 10.1002/(SICI)1096-8628(19960517)63:2<406::AID-AJMG16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Briggs MD, Hoffman SM, King LM, et al. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10(3):330–6. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- 6.Hecht JT, Nelson LD, Crowder E, et al. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet. 1995;10(3):325–9. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- 7.Hecht JT, Makitie O, Hayes E, et al. Chondrocyte cell death and intracellular distribution of COMP and type IX collagen in the pseudoachondroplasia growth plate. J Orthop Res. 2004;22(4):759–67. doi: 10.1016/j.orthres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Hecht JT, Montufar-Solis O, Decker G, Lawler J, Daniels K, Duke PJ. Retention of cartilage oligomeric matrix protein (COMP) and cell death in redifferentiated pseudoachondroplasia chondrocytes. Matrix Biol. 1998;17(8–9):625–33. doi: 10.1016/s0945-053x(98)90113-5. [DOI] [PubMed] [Google Scholar]
- 9.Posey KL, Veerisetty AC, Liu P, et al. An inducible cartilage oligomeric matrix protein mouse model recapitulates human pseudoachondroplasia phenotype. Am J Pathol. 2009;175(4):1555–63. doi: 10.2353/ajpath.2009.090184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posey KL, Yang Y, Veerisetty AC, Sharan SK, Hecht JT. Model systems for studying skeletal dysplasias caused by TSP-5/COMP mutations. Cell Mol Life Sci. 2008;65(5):687–99. doi: 10.1007/s00018-007-7485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deere M, Sanford T, Ferguson HL, Daniels K, Hecht JT. Identification of twelve mutations in cartilage oligomeric matrix protein (COMP) in patients with pseudoachondroplasla. Am J Med Genet. 1998;80(5):510–3. doi: 10.1002/(sici)1096-8628(19981228)80:5<510::aid-ajmg14>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Deere M, Sanford T, Francomano CA, Daniels K, Hecht JT. Identification of nine novel mutations in cartilage oligomeric matrix protein in patients with pseudoachondroplasia and multiple epiphyseal dysplasia. Am J Med Genet. 1999;85(5):486–90. doi: 10.1002/(sici)1096-8628(19990827)85:5<486::aid-ajmg10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Briggs MD, Mortier GR, Cole WG, et al. Diverse mutations in the gene for cartilage oligomeric matrix protein in the pseudoachondroplasia-multiple epiphyseal dysplasia disease spectrum. Am J Hum Genet. 1998;62(2):311–9. doi: 10.1086/301713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Posey KL, Coustry F, Veerisetty AC, Liu P, Alcorn JL, Hecht JT. Chop (Ddit3) is essential for D469del-COMP retention and cell death in chondrocytes in an inducible transgenic mouse model of pseudoachondroplasia. Am J Pathol. 2012;180(2):727–37. doi: 10.1016/j.ajpath.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao J, Chen S, Yang T, et al. Osteosclerosis owing to Notch gain of function is solely Rbpj-dependent. J Bone Miner Res. 2010;25(10):2175–83. doi: 10.1002/jbmr.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sferruzzi-Perri AN, Macpherson AM, Roberts CT, Robertson SA. Csf2 null mutation alters placental gene expression and trophoblast glycogen cell and giant cell abundance in mice. Biol Reprod. 2009;81(1):207–21. doi: 10.1095/biolreprod.108.073312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soreq L, Ben-Shaul V, Israel Z, Bergman H, Soreq H. Meta-analysis of genetic and environmental Parkinson’s disease models reveals a common role of mitochondrial protection pathways. Neurobiol Dis. 2012;45(3):1018–30. doi: 10.1016/j.nbd.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Couch V, Alvarez-Erviti L, Sibson NR, Wood MJ, Anthony DC. The acute inflammatory response to intranlgral alpha-synuclein differs significantly from intranigraol lipopolysaccharide and is exacerbated by peripheral inflammation. J Neuroinflammation. 2011;8:166. doi: 10.1186/1742-2094-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee EJ, Woo MS, Moon PG, et al. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol. 2010;185(1):615–23. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- 20.Moore KA, Hollien J. The unfolded protein response in secretory cell function. Ann Rev Genet. 2012;46:165–83. doi: 10.1146/annurev-genet-110711-155644. [DOI] [PubMed] [Google Scholar]
- 21.Inoue H, Hiraoka K, Hoshino T, et al. High levels of serum IL-18 promote cartilage loss through suppression of aggrecan synthesis. Bone. 2008;42(6):1102–10. doi: 10.1016/j.bone.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Jiang JM, Yang DH, Wang FL, Mao ZX. Determination of the concentrations of interleukin-18 and other cytokines in the synovial fluid in patients with osteoarthritis] Nan Fang Yi Ke Da Xue Xue Bao. 2009;29(4):729–31. [PubMed] [Google Scholar]
- 23.Fu Z, Liu P, Yang D, et al. Interleukin-18-induced inflammatory responses in synoviocytes and chondrocytes from osteoarthritic patients. Int J Mol Med. 2012;30(4):805–10. doi: 10.3892/ijmm.2012.1073. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Xu D, Long L, Deng X, Tao R, Huang G. Correlation between plasma, synovial fluid and articular cartilage Interleukin-18 with radiographic severity In 33 patients with osteoarthritis of the knee. Clin Exp Med. doi: 10.1007/s10238-013-0251-8. Forthcoming. Epub 2013 Aug 20. DOI:10.1007/s10238-013-0251-8. [DOI] [PubMed] [Google Scholar]
- 25.Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12(7):982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao L, Sun X, Xu L, Young LT, Wang JF. Mood stabilizing drug lithium increases expression of endoplasmic reticulum stress proteins in primary cultured rat cerebral cortical cells. Life Sci. 2006;78(12):1317–23. doi: 10.1016/j.lfs.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Hiroi T, Wei H, Hough C, Leeds P, Chuang OM. Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2. Pharmacogenomics J. 2005;5(2):102–11. doi: 10.1038/sj.tpj.6500296. [DOI] [PubMed] [Google Scholar]
- 28.Woody JN, London WL, Wilbanks GD., Jr Lithium toxicity in a newborn. Pediatrics. 1971;47(1):94–6. [PubMed] [Google Scholar]
- 29.Tunnessen WW, Jr, Hertz CG. Toxic effects of lithium in newborn infants: a commentary. J Pediatr. 1972;81(4):804–7. doi: 10.1016/s0022-3476(72)80111-2. [DOI] [PubMed] [Google Scholar]
- 30.Posey KL, Hayes E, Haynes R, Hecht JT. Role of TSP-5/COMP in pseudoachondroplasia. Int J Biochem Cell Biol. 2004;36(6):1005–12. doi: 10.1016/j.biocel.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Efstratiadis A. Genetics of mouse growth. Int J Dev Biol. 1998;42(7):955–76. [PubMed] [Google Scholar]
- 32.Walsh ER, Thakar J, Stokes K, Huang F, Albert R, August A. Computational and experimental analysis reveals a requirement for eosinophil-derived IL-13 for the development of allergic airway responses in C57BL/6 mice. J Immunol. 2011;186(5):2936–49. doi: 10.4049/jimmunol.1001148. [DOI] [PubMed] [Google Scholar]
- 33.Blumenthal MN. Genetics of asthma and allergy. Allergy Asthma Proc. 2000;21(1):55–9. doi: 10.2500/108854100778249042. [DOI] [PubMed] [Google Scholar]
- 34.Chang NC, Hung SI, Hwa KY, et al. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J Biol Chem. 2001;276(20):17497–506. doi: 10.1074/jbc.M010417200. [DOI] [PubMed] [Google Scholar]
- 35.Harbord M, Novelli M, Canas B, et al. Ym1 is a neutrophil granule protein that crystallizes in p47phox-deficient mice. J Biol Chem. 2002;277(7):5468–75. doi: 10.1074/jbc.M110635200. [DOI] [PubMed] [Google Scholar]
- 36.Borzi RM, Mazzetti I, Cattini L, Uguccioni M, Baggiolini M, Facchini A. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum. 2000;43(8):1734–41. doi: 10.1002/1529-0131(200008)43:8<1734::AID-ANR9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 37.Hsu YH, Hsieh MS, Liang YC, et al. Production of the chemokine eotaxin-1 in osteoarthritis and its role in cartilage degradation. J Cell Biochem. 2004;93(5):929–39. doi: 10.1002/jcb.20239. [DOI] [PubMed] [Google Scholar]
- 38.Matsui K, Tsutsui H, Nakanishi K. Pathophysiological roles for IL-18 in inflammatory arthritis. Expert Opin Ther Targets. 2003;7(6):701–24. doi: 10.1517/14728222.7.6.701. [DOI] [PubMed] [Google Scholar]
- 39.Mathy NL, Scheuer W, Lanzendorfer M, et al. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology. 2000;100(1):63–9. doi: 10.1046/j.1365-2567.2000.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynch EA, Heijens CA, Horst NF, Center DM, Cruikshank WW. Cutting edge: IL-16/CD4 preferentially induces Th1 cell migration: requirement of CCR5. J Immunol. 2003;171(10):4965–8. doi: 10.4049/jimmunol.171.10.4965. [DOI] [PubMed] [Google Scholar]
- 41.Ferland C, Flamand N, Davoine F, Chakir J, Laviolette M. IL-16 activates plasminogen-plasmin system and promotes human eosinophil migration into extracellular matrix via CCR3-chemokine-mediated Signaling and by modulating CD4 eosinophil expression. J Immunol. 2004;173(7):4417–24. doi: 10.4049/jimmunol.173.7.4417. [DOI] [PubMed] [Google Scholar]
- 42.Pereira RC, Stadmeyer L, Marciniak SJ, Ron O, Canalis E. C/EBP homologous protein is necessary for normal osteoblastic function. J Cell Biochem. 2006;97(3):633–40. doi: 10.1002/jcb.20660. [DOI] [PubMed] [Google Scholar]
- 43.Arima K, Ueda K, Sunohara N, et al. NACP/alpha-synuciein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol. 1998;96(5):439–44. doi: 10.1007/s004010050917. [DOI] [PubMed] [Google Scholar]
- 44.Arima K, Ueda K, Sunohara N, et al. Immunoelectron-microscopic demonstration of NACP/alpha-synuciein-epitopes on the filamentous component of Lewy bodies in Parkinson’s disease and in dementia with Lewy bodies. Brain Res. 1998;808(1):93–100. doi: 10.1016/s0006-8993(98)00734-3. [DOI] [PubMed] [Google Scholar]
- 45.Ueda K, Fukushima H, Masliah E, et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S.A. 1993;90(23):11282–6. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]