Abstract
Magnetic resonance elastography (MRE) has been successfully implemented in the assessment of diffuse liver diseases. Currently, MRE is the most accurate noninvasive technique for detection and staging of liver fibrosis with a potential to replace liver biopsy. Magnetic resonance elastography is able to differentiate isolated fatty liver disease from steatohepatitis with or without fibrosis. Potential clinical applications include the differentiation of benign and malignant focal liver masses and the assessment of treatment response in diffuse liver diseases.
Keywords: magnetic resonance elastography, clinical applications, diffuse liver diseases, liver fibrosis, focal lesions, treatment response
For hundreds of years, palpation has been a simple technique for assessing tissue stiffness during physical examination. With palpation, physicians are usually able to differentiate soft normal tissues from pathological tissues that are typically stiffer or harder. However, this clinical method is most suitable for superficial structures and organs. Deep-seated organs, like liver, and lesions within them, are difficult to palpate entirely except at surgery. It is well known to surgeons that normal liver is soft, like abdominal fat, whereas cirrhotic liver and malignant liver tumors are stiffer or hard. Elastography techniques that assess mechanical properties of tissues are now available and can provide stiffness information of deep-seated tissues including the liver. The techniques currently available for use in assessment of chronic liver diseases include ultrasound-based transient elastography, acoustic radiation force impulse imaging, and magnetic resonance elastography (MRE).
Chronic liver diseases, irrespective of etiology, lead to liver fibrosis, and continued damage to the liver progresses to cirrhosis with its associated complications. Traditionally, liver biopsy has been the gold standard for assessment of chronic liver diseases. However, its invasive nature, risk of complications, sampling errors, and interobserver variability limit the use of liver biopsy for assessment of liver fibrosis and cirrhosis.1–5 Serum markers for liver fibrosis are useful to differentiate advanced fibrosis and cirrhosis but are not sufficiently accurate for differentiating earlier stages of fibrosis.6,7 Elastography techniques can demonstrate increased stiffness in chronic liver diseases that correlates with the severity of fibrosis and are useful for noninvasively assessing the liver fibrosis.
Magnetic resonance elastography,8 a magnetic resonance imaging (MRI)–based technique, can evaluate mechanical properties of tissues in vivo. The most successful clinical application of MRE has been in the evaluation of chronic liver diseases. Magnetic resonance elastography is now available at several leading medical centers and is an integral part of noninvasive assessment of chronic liver disorders at some centers. In this review, we will briefly describe the technique of MRE of liver and the clinical applications of MRE in liver diseases.
MRE OF THE LIVER TECHNIQUE
Magnetic resonance elastography can be easily incorporated into a routine liver MRI protocol with only a few minutes added to the total scan time. Magnetic resonance elastography of the liver essentially involves 3 steps: (1) generating mechanical shear waves within the liver; (2) imaging the propagating shear waves in the liver using a special MRI sequence with motion-encoding gradients; and (3) processing the information in the wave images with an inversion algorithm to generate quantitative maps of mechanical properties.
The MRE setup needs additional hardware: (1) an active acoustic driver, (2) a 7.6-m-long plastic (polyvinyl chloride) tube that connects the active driver to a passive pneumatic driver and transmits acoustic waves produced by the active driver, and (3) a passive pneumatic driver that is placed in contact with the patient and delivers the acoustic mechanical waves (Fig. 1). The passive pneumatic driver is a 19-cm plastic disc with a drum membrane that is placed against the right lower chest wall and upper abdominal wall, with its center at the level of xiphisternum (Fig. 2). This location is chosen so that the largest part of the liver is directly under the passive driver. The passive driver is held in place with an elastic strap to ensure continued contact with body wall and prevent migration of the driver. The passive driver can be easily placed between the patient and the surface receiver coils used for liver imaging. The active driver is synced to the MRE sequence from the scanner as previously described.9 Typically, 2 to 4 slices through the widest cross section of the liver are obtained.
FIGURE 1.
Setup for clinical liver MRE examination. The active driver is placed outside the scanner room. The active driver is connected via a long plastic tube to the passive driver placed over the liver.
FIGURE 2.
Schematic diagram showing the placement of the passive driver for liver MRE. The driver is positioned at the level of xiphisternum.
A continuous 60-Hz acoustic vibration is used for MRE of the liver. The vibrations produced in the tissues with MRE are very low energy and well tolerated. The amplitude of vibrations is maintained within European Union directive limits.10
The wave images are processed automatically by an inversion algorithm11 that is installed on the scanner. The inversion algorithm generates elastogram/stiffness maps that depict stiffness of the tissues in the section. The inversion algorithm reports a confidence map based on the correlation coefficients of polynomial fits, and a threshold value of 0.95 is used to differentiate between regions of reliable and less reliable shear wave data.
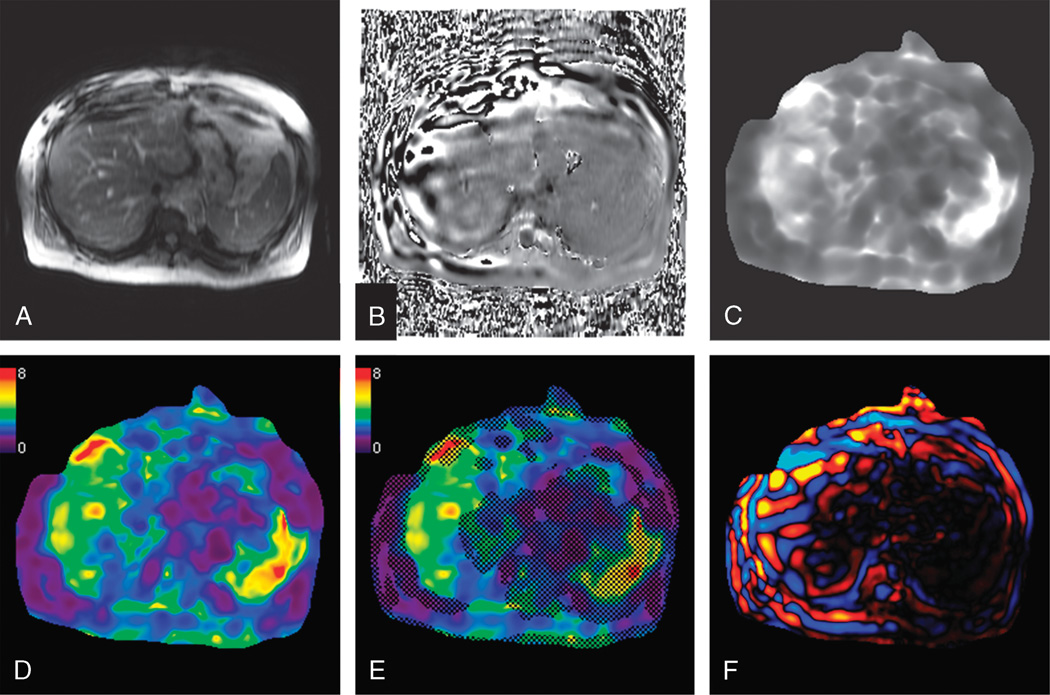
The MRE sequence typically produces a set of images (Fig. 3) that includes a magnitude image, a phase contrast image, a stiffness map in gray scale on which measurements can be performed, a color stiffness map with a scale of 0 to 8 kPa, a confidence map that outlines the areas with sufficient displacement to obtain valid measurements, and a color wave image. The stiffness maps depict shear stiffness in units of kilopascals. Regions of interest (ROI) are placed on the stiffness maps to obtain stiffness values. The ROI can be oval, circular, or geographical and should avoid liver edges, vessels greater than 3 mm, fissures, and areas of pulsation artifacts and wave interference. Generally, the left lobe is avoided because it is subject to motion caused by cardiac pulsations. To ensure a larger volume of liver being sampled, geographical ROIs are preferred. In our clinical practice, we report a mean stiffness value obtained by averaging the stiffness values from 4 slices. Larger volumes of liver may be assessed with 3-dimensional MRE, and this is currently under evaluation. Mean stiffness of livers can also be calculated using an automated segmentation algorithm.12
FIGURE 3.
Magnetic resonance elastography of the liver in a patient with NASH. Images from a single MRE slice show the magnitude (A) and phase (B) images from the MRE sequence. Processed images from the inversion algorithm includes a gray-scale stiffness map (C), color stiffness map with a 0- to 8-kPa scale (D), confidence map (E) with hatched out areas representing less valid areas for measurement, and a color wave image (F). The mean stiffness of the liver was 4.1 kPa.
Studies have demonstrated that MRE of the liver has excellent reproducibility13–15 and repeatability,16–18 with high interobserver agreement.13,14,19
Performing a Clinical Liver MRE
Liver MRE is performed in a supine position. To reproduce the slice positions consistently, MRE is usually performed in end-expiration; however, it can also be obtained in inspiration in patients who are unable to hold their breath well in end-expiration. Liver MRE can be performed in most patients, with the exception of contraindications to an MRI study like a cardiac pacemaker and severe claustrophobia. MRE of the liver has been successfully performed in pediatric subjects,20 obese patients, patients with ascites and anatomical variants,9 and posttransplant recipients.21 MRE of the liver is generally performed in fasting status because postprandial status may result in higher stiffness in patients with chronic liver disease.22 It is therefore important to perform follow-up MRE examinations of the liver in similar conditions for valid comparisons.
Normal Liver Stiffness
The normal liver is soft, and the mean stiffness values reported in the literature ranges from 2.05 to 2.44 kPa, and the range of normal liver stiffness is between 1.54 and 2.87 kPa.14,15,17,23–26 The reasons for this range of liver stiffness are not well understood but may be dependent on age, sex, body mass index, diet, and ethnicity. Studies on normal healthy volunteer populations to date do not report any correlation with age, sex, and body mass index.14,15 Large population studies are needed to establish any possible relation between ethnicity and liver stiffness. The liver stiffness measured with MRE is not affected by the presence of isolated fatty change only.13,15,23 In a normal liver, stiffness does not change significantly after a meal challenge,17,22 whereas a fibrotic liver may show significant changes.22
CLINICAL APPLICATIONS OF MRE
Detection of Liver Fibrosis
Inflammation of the portal tracts and necroinflammatory activity are the predominant abnormalities in the early stages of chronic liver diseases, and fibrosis follows as a healing response. In most chronic liver diseases, inflammation and fibrosis develop around the portal triads that progress to form bridging septa across portal tracts and/or central veins. In advanced fibrosis, islands of regenerative parenchyma are surrounded by bands of fibrosis of variable thickness, giving rise to the typical nodular appearance of cirrhosis. The degree of fibrosis is variable in chronic liver diseases from different etiologies. 27–30 Liver histology is the gold standard for staging of liver fibrosis. Several pathologic staging systems for liver fibrosis exist, and the most widely used is the METAVIR system.31 The METAVIR system was initially developed for staging in hepatitis C but later was expanded for staging of additional conditions secondary to other chronic infective etiologies. This histopathological staging system evaluates architectural change and provides a semiquantitative assessment of fibrosis. Briefly, fibrosis is staged as follows: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with rare septa; F3, numerous septa without cirrhosis; F4, cirrhosis. Fibrosis staging systems are limited by sampling errors and interobserver variability.4,5 Histologic staging does not precisely measure the degree or amount of fibrosis and therefore may not be suitable for monitoring therapies.32
With MRE, it is possible to detect elevated stiffness caused by liver fibrosis even when there are no morphological changes or anatomical features of liver fibrosis/cirrhosis on conventional imaging techniques (Fig. 4).33,34 This is an important advantage of MRE because livers with mild to moderate fibrosis often have normal appearances on ultrasound, computed tomography, and MRI, and detection of liver fibrosis with MRE may help in early treatment of chronic liver diseases.
FIGURE 4.
Axial T2-weighted (A, D) and postcontrast T1-weighted MR images (B, E) and stiffness maps (C, F) obtained with MRE in a patient with chronic hepatitis C (top row) and another patient with alpha-1-antitrypsin deficiency (bottom row). The physical examination and liver function tests were normal in both patients. T2-weighted and postcontrast MRI images do not show any significant abnormalities to suggest chronic liver disease. Magnetic resonance elastography revealed a normal liver stiffness of 2.3 kPa in the patient with chronic hepatitis C and an elevated stiffness of 3.3 kPa in the patient with alpha-1-antitrypsin deficiency, consistent with mild fibrosis.
Several studies have established MRE as an accurate technique for diagnosis of hepatic fibrosis and for differentiating fibrotic livers from normal livers.14,23,24 MRE can distinguish fibrotic livers (≥F1) from normal and/or F0 stage livers with 80% to 98% sensitivity, 90% to 100% specificity, and 0.89 to 0.99 accuracy.19,23,24,35–37 Livers solely with chronic inflammation may have a higher stiffness than normal livers, and the stiffness values overlap those with mild fibrosis.23,24,35,36 Correlation with serum liver enzyme levels is useful in conditions when liver stiffness is only mildly elevated. Similarly, acute flare episodes in chronic viral hepatitis may result in a higher liver stiffness. Follow-up MRE studies after normalization of serum liver enzyme levels may be useful for confirmation of liver fibrosis.
The cutoff stiffness value for differentiating normal and/or F0 livers from hepatic fibrosis ranges from 2.4 kPa to 2.93 kPa in published studies.23,24,35,36 Possible reasons for this variability are inclusion of patients with different etiologies and combining normal and F0 livers as one group. Preliminary studies with single etiologies like chronic hepatitis B,19 chronic hepatitis C,37 nonalcoholic steatohepatitis (NASH),38 alcohol,39 and Gaucher disease40 are now available, and the reported cutoff values may be useful for application in those specific etiologies.
Some conditions not associated with liver fibrosis can cause increased liver stiffness. These include acute hepatitis from any cause, acute biliary obstruction, and passive congestion caused by congestive cardiac failure or raised central venous pressure. These conditions often can be diagnosed with clinical features and laboratory tests, and MRE is rarely required in the evaluation of these conditions. However, MRE of the liver can be performed when the acute conditions have resolved and an underlying chronic liver disease is suspected. Knowledge of the presence of confounding conditions is useful in the interpretation of MRE in patients without any chronic liver diseases.
Staging of Liver Fibrosis
The stage of liver fibrosis influences the management of chronic liver diseases. Differentiation of significant liver fibrosis (≥F2) is important because treatment is often indicated to prevent progression of disease depending on an individual patient’s profile and other markers of disease.41–43 Most cirrhotic patients, if not indicated for antifibrotic treatment, benefit from surveillance for complications such as portal hypertension and development of hepatocellular carcinoma.44
Liver stiffness measured with MRE increases systematically with increasing stages of fibrosis (Fig. 5).19,23,24,35–37 The sensitivity, specificity, and accuracy of MRE in differentiating significant fibrosis (≥F2) from F0 to F1 stages are 86% to 100%, 85% to 100%, and 94% to 99%, respectively.19,23,35–37,45 The positive predictive value is 97% to 100%. The cutoff values reported in these studies range from 2.5 kPa to 4.9 kPa, with most reporting greater than 3 kPa. Differentiation of cirrhosis from lesser degrees of fibrosis has 100% sensitivity, 86% to 100% specificity, and an accuracy ranging from 98%to 100%.19,23,24,35–37,45,46 The cutoff values are more than 4.13 kPa. Overall, MRE has a high positive predictive value for ruling in significant fibrosis and a high negative predictive value for ruling out cirrhosis that is useful in clinical decision making.
FIGURE 5.
Representative MRE images from different patients with chronic hepatitis C and biopsy-proven fibrosis stages F0 through F4. Each column represents different patients. The top row images are magnitude images, with corresponding wave images (middle row) and color stiffness maps (bottom row). The color scale for shear stiffness in kilopascals is on the right. Mean ± SD values of liver stiffness are at the bottom of the column.
The misclassifications of fibrosis stages with MRE are generally in the lower stages of fibrosis: F0 and F1, and F1 to F2.19,36 The misclassifications are probably minimized when a single etiology is studied.19 Uncommonly, when MRE stiffness values and clinical and laboratory findings do not match, it is useful to perform a liver biopsy for clinical decisions.
Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease spectrum consists of diffuse fatty change or isolated fatty liver (IFL) only, steatohepatitis, and steatohepatitis with fibrosis. Isolated fatty liver does not affect liver stiffness.19,23 Both steatohepatitis and fibrosis cause an increase in liver stiffness, and it is important to distinguish both these conditions from IFL for clinical management. In one study, Chen et al38 demonstrated that IFL can be differentiated from steatohepatitis ± fibrosis, with 94% sensitivity, 73% specificity, and an accuracy of 0.93 using a cutoff value of 2.74 kPa. Another recent study47 showed that MRE is useful in detecting advanced fibrosis (F3-F4) in nonalcoholic fatty liver disease, with 85% sensitivity, 93% specificity, and an accuracy of 0.954 using a cutoff value of 4.15 kPa. The distinction between IFL and NASH with or without fibrosis (Fig. 6) is clinically important because patients with NASH have a risk of progression to fibrosis and cirrhosis. The use of MRE in this particular chronic liver disease will have a significant role as obesity-related chronic liver disease is a major health burden in Western countries and an emerging health care problem in lesser-developed countries.
FIGURE 6.
Examples of steatosis only, steatohepatitis, and steatohepatitis with fibrosis. Magnetic resonance elastography magnitude images (top row) and stiffness maps (bottom row) in patients with biopsy-proven steatosis only (first column), steatohepatitis (second column), and steatohepatitis with grade 1 fibrosis (third column). The mean liver stiffness was 2.1, 3.6, and 4.3 kPa, respectively.
Portal Hypertension
Spleen stiffness correlates with liver stiffness and, in patients with liver fibrosis, spleen stiffness increases in parallel with increasing liver stiffness.48–50 Spleen stiffness correlates with splenic size, platelet count, and presence of esophageal varices. A splenic stiffness greater than 10.5 kPa is predictive of esophageal varices (Fig. 7), suggesting splenic stiffness evaluation as a noninvasive method to assess portal hypertension. In an animal model, Nedredal et al51 showed excellent correlation (r2 = 0.86, P < 0.01) between spleen stiffness and direct hepatic venous pressure gradient. Results from these preliminary studies provide motivation for future studies on the role of assessment of splenic stiffness with MRE in chronic liver diseases and portal hypertension.
FIGURE 7.
Contrast-enhanced T1-weighted image (B) and stiffness map (B) in chronic hepatitis C. The mean liver stiffness was 6.5 kPa, and the spleen stiffness was 15.7 kPa. The liver is nodular with an enlarged left lobe. Nodular liver, splenomegaly (*), and esophageal varices (arrow) are consistent with cirrhosis with portal hypertension.
Focal Liver Lesions
Focal liver lesions (Fig. 8) can have variable stiffness depending on their tissue components. Malignant tumors tend to have a higher stiffness as compared with benign tumors and normal tissues. Preliminary studies have shown that malignant liver tumors are stiffer than benign liver tumors and normal liver.52,53 A stiffness value greater than 5 kPa accurately differentiated malignant liver tumors from benign liver tumors.52 MRE is a promising technique for characterization of focal liver lesion, and further studies are required to establish the role of MRE in evaluation of focal liver lesions.
FIGURE 8.
Magnetic resonance elastography of focal liver lesions. Non–contrast-enhanced (top row) and contrast-enhanced (middle row) T1-weighted images and stiffness maps (bottom row) of hepatic adenoma (first column), focal nodular hyperplasia (second column), hepatocellular carcinoma (third column), and intrahepatic cholangiocarcinoma (fourth column). The tumors are outlined by dotted lines in the stiffness maps. Benign tumors are softer than malignant tumors. The stiffness of hepatic adenoma is 2.8 kPa and that of focal nodular hyperplasia is 3.1 kPa. The mean stiffness of hepatocellular carcinoma is 7 kPa (K). Note the surrounding stiff cirrhotic liver parenchyma. Cholangiocarcinomas are much stiffer and have a stiffness of 12 kPa (L) in the example illustrated.
Assessment of Treatment Response
Fibrosis of the liver is reversible when the etiology of the chronic liver disease is specifically treated.54,55 Liver stiffness with MRE correlates with the fibrotic content of the liver.56 MRE may therefore be able to demonstrate changes in liver stiffness that reflects changes in liver fibrotic content. In our clinical experience, MRE demonstrates changes in liver stiffness in patients showing a response to treatment (Figs. 9, 10) as well as in those who show progression of disease (Fig. 11). Larger studies demonstrating the use of MRE in the assessment of treatment response are awaited.
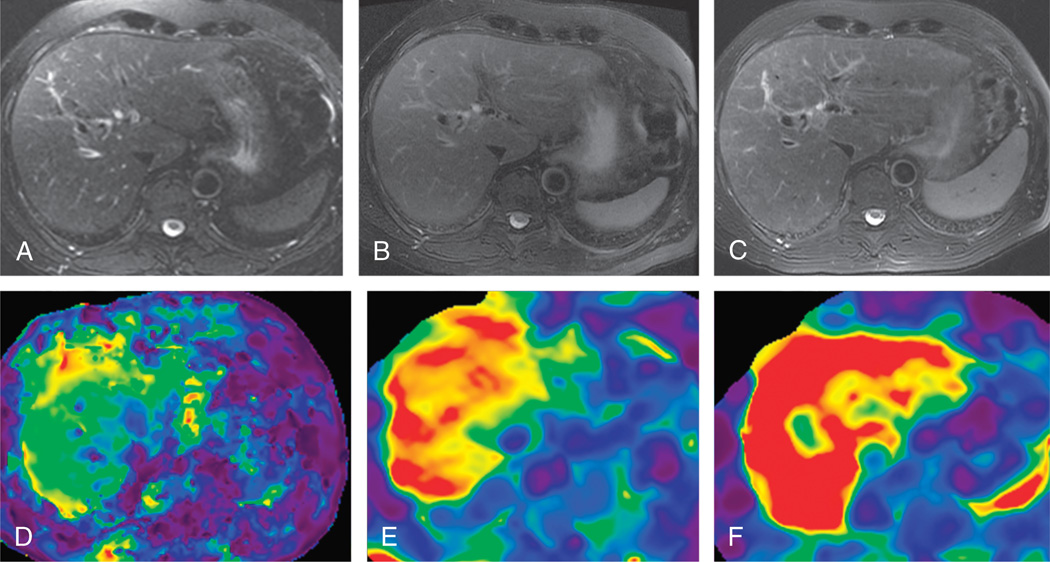
FIGURE 9.
A 60-year-old man with chronic hepatitis C. Contrast-enhanced T1-weighted images (top row) and stiffness maps (bottom row) at baseline (A, C) and 3 years after antiviral treatment (B, D). The baseline liver stiffness was 4.2 kPa, which was reduced to 2.8 kPa at follow-up, suggestive of response to treatment. The serum liver enzyme levels were normal both at baseline and at follow-up.
FIGURE 10.
Biopsy-proven NASH. Baseline MRI with in- (A) and opposed-phase (B) images showed an estimated hepatic fat signal fraction of 30% and a mean liver stiffness of 3.2 kPa with MRE (C). Three years later and with about 7-lb weight loss, MRI (D, E) shows that the estimated hepatic fat signal fraction was reduced to 4% and a mean liver stiffness of 2.5 kPa.
FIGURE 11.
Progression of chronic liver disease. A 60-year-old man with chronic hepatitis C at presentation showed a mean liver stiffness of 4.1 kPa. After initiation of antiviral treatment, a year later, the liver stiffness increased to 6.3 kPa (B, E), and 3 years later (C, F), there was progression of chronic liver disease with increased stiffness to 8.5 kPa. Note that there are no gross morphologic changes of liver fibrosis in the liver at 1 year, but the left lobe is enlarged and there is mild spleen enlargement at 3 years, suggesting progression of disease.
Other Applications
Magnetic resonance elastography of the liver is also useful for detection of liver fibrosis secondary to hepatotoxic drugs, such as methotrexate used for psoriasis.57 Other emerging clinical indications include evaluation of liver stiffness in post-Fontan procedure patients.58
LIMITATIONS OF MRE
Magnetic resonance elastography of the liver, as any other abdominal MRI sequence, is a breath-hold sequence and subject to artifacts caused by inadequate breath hold. Signal may be poor in moderate to severe iron overload, leading to failed liver MREs. Special MRE sequences with low echo times that are designed to obtain increased signal from the liver are developed for use in iron overload patients,59 and this may be useful in most patients with iron overload.
FUTURE DEVELOPMENTS
Magnetic resonance elastography as a technique can be refined further to optimize image quality and improve resolution. Three-dimensional acquisitions are promising for characterization of focal lesions and estimating overall liver fibrosis burden. Discrimination of liver stiffness caused by inflammation, edema, passive congestion, and fibrosis may be possible by applying detailed mechanical models to the postprocessing of the wave information.
CONCLUSIONS
Magnetic resonance elastography is an established technique for detection and staging of liver fibrosis and is useful in assessment of treatment response and clinical follow-up of chronic liver diseases. Potential clinical applications are still emerging and include evaluation of focal liver lesions and assessment of treatment response to antifibrotic treatments.
REFERENCES
- 1.Piccinino F, Sagnelli E, Pasquale G, et al. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–173. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 2.Castéra L, Nègre I, Samii K, et al. Pain experienced during percutaneous liver biopsy. Hepatology. 1999;30:1529–1530. doi: 10.1002/hep.510300624. [DOI] [PubMed] [Google Scholar]
- 3.Maharaj B, Maharaj R, Leary W, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–525. doi: 10.1016/s0140-6736(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 4.Rousselet MC, Michalak S, Dupré F, et al. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology. 2005;41:257–264. doi: 10.1002/hep.20535. [DOI] [PubMed] [Google Scholar]
- 5.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Castera L. Invasive and noninvasive methods for the assessment of fibrosis and disease progression in chronic liver disease. Best Pract Res Clin Gastroenterol. 2011;25:291–303. doi: 10.1016/j.bpg.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Afdhal N, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160–1174. doi: 10.1111/j.1572-0241.2004.30110.x. [DOI] [PubMed] [Google Scholar]
- 8.Muthupillai R, Lomas D, Rossman P, et al. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 9.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544–555. doi: 10.1002/jmri.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehman EC, Rossman PJ, Kruse SA, et al. Vibration safety limits for magnetic resonance elastography. Phys Med Biol. 2008;53:925–935. doi: 10.1088/0031-9155/53/4/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manduca A, Oliphant TE, Dresner MA, et al. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Med Image Anal. 2001;5:237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 12.Dzyubak B, Glaser K, Yin M, et al. Automated liver stiffness measurements with magnetic resonance elastography. J Magn Reson Imaging. 2013;38:371–379. doi: 10.1002/jmri.23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DH, Lee JM, Han JK, et al. MR elastography of healthy liver parenchyma: Normal value and reliability of the liver stiffness value measurement. J Magn Reson Imaging. 2013;38:1215–1223. doi: 10.1002/jmri.23958. [DOI] [PubMed] [Google Scholar]
- 14.Lee YJ, Lee JM, Lee JE, et al. MR elastography for noninvasive assessment of hepatic fibrosis: reproducibility of the examination and reproducibility and repeatability of the liver stiffness value measurement. J Magn Reson Imaging. 2013 doi: 10.1002/jmri.24147. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Venkatesh SK, Wang G, Teo LLS, et al. Magnetic resonance elastography of liver in healthy Asians: normal liver stiffness quantification and reproducibility assessment. J Magn Reson Imaging. 2013 doi: 10.1002/jmri.24084. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Hines CDG, Bley TA, Lindstrom MJ, et al. Repeatability of magnetic resonance elastography for quantification of hepatic stiffness. J Magn Reson Imaging. 2010;31:725–731. doi: 10.1002/jmri.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hines CDG, Lindstrom MJ, Varma AK, et al. Effects of postprandial state and mesenteric blood flow on the repeatability of MR elastography in asymptomatic subjects. J Magn Reson Imaging. 2011;33:239–244. doi: 10.1002/jmri.22354. [DOI] [PubMed] [Google Scholar]
- 18.Shire NJ, Yin M, Chen J, et al. Test-retest repeatability of MR elastography for noninvasive liver fibrosis assessment in hepatitis C. J Magn Reson Imaging. 2011;34:947–955. doi: 10.1002/jmri.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venkatesh SK, Wang G, Lim SG, et al. Magnetic resonance elastography for the detection and staging of liver fibrosis in chronic hepatitis B. Eur Radiol. 2013 doi: 10.1007/s00330-013-2978-8. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Binkovitz LA, El-Youssef M, Glaser KJ, et al. Pediatric MR elastography of hepatic fibrosis: principles, technique and early clinical experience. Pediatr Radiol. 2012;42:402–409. doi: 10.1007/s00247-011-2298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee VS, Miller FH, Omary RA, et al. Magnetic resonance elastography and biomarkers to assess fibrosis from recurrent hepatitis C in liver transplant recipients. Transplantation. 2011;92:581–586. doi: 10.1097/TP.0b013e31822805fa. [DOI] [PubMed] [Google Scholar]
- 22.Yin M, Talwalkar JA, Glaser KJ, et al. Dynamic postprandial hepatic stiffness augmentation assessed with MR elastography in patients with chronic liver disease. AJR Am J Roentgenol. 2011;197:64–70. doi: 10.2214/AJR.10.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207–1213. e2. doi: 10.1016/j.cgh.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim BH, Lee JM, Lee YJ, et al. MR elastography for noninvasive assessment of hepatic fibrosis: experience from a tertiary center in Asia. J Magn Reson Imaging. 2011;34:1110–1116. doi: 10.1002/jmri.22723. [DOI] [PubMed] [Google Scholar]
- 25.Herzka DA, Kotys MS, Sinkus R, et al. Magnetic resonance elastography in the liver at 3 Tesla using a second harmonic approach. Magn Reson Med. 2009;62:284–291. doi: 10.1002/mrm.21956. [DOI] [PubMed] [Google Scholar]
- 26.Mannelli L, Godfrey E, Graves MJ, et al. Magnetic resonance elastography: feasibility of liver stiffness measurements in healthy volunteers at 3T. Clin Radiol. 2012;67:258–262. doi: 10.1016/j.crad.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 27.Vizzotto L, Vertemati M, Gambacorta M, et al. Analysis of histological and immunohistochemical patterns of the liver in posthepatitic and alcoholic cirrhosis by computerized morphometry. Mod Pathol. 2002;15:798–806. doi: 10.1097/01.MP.0000024365.92937.5E. [DOI] [PubMed] [Google Scholar]
- 28.Kage M, Shimamatu K, Nakashima E, et al. Long-term evolution of fibrosis from chronic hepatitis to cirrhosis in patients with hepatitis C: morphometric analysis of repeated biopsies. Hepatology. 1997;25:1028–1031. doi: 10.1002/hep.510250439. [DOI] [PubMed] [Google Scholar]
- 29.Yano M, Kumada H, Kage M, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334–1340. doi: 10.1002/hep.510230607. [DOI] [PubMed] [Google Scholar]
- 30.Marcellin P, Ziol M, Bedossa P, et al. Noninvasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242–247. doi: 10.1111/j.1478-3231.2008.01802.x. [DOI] [PubMed] [Google Scholar]
- 31.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien MJ, Keating NM, Elderiny S, et al. An assessment of digital image analysis to measure fibrosis in liver biopsy specimens of patients with chronic hepatitis C. Am J Clin Pathol. 2000;114:712–718. doi: 10.1309/D7AU-EYW7-4B6C-K08Y. [DOI] [PubMed] [Google Scholar]
- 33.Venkatesh SK, Takahashi N, Glocker JF, et al. Noninvasive Diagnosis of Liver Fibrosis: Conventional MR Imaging Findings Versus MR Elastography. Proceedings of the 17th annual meeting of ISMRM, Toronto 2008 (abstract 2613) Toronto, Ontario, Canada: ISMRM; 2008. [Google Scholar]
- 34.Rustogi R, Horowitz J, Harmath C, et al. Accuracy of MR elastography and anatomic MR imaging features in the diagnosis of severe hepatic fibrosis and cirrhosis. J Magn Reson Imaging. 2012;35:1356–1364. doi: 10.1002/jmri.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huwart L, Sempoux C, Salameh N, et al. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology. 2007;245:458–466. doi: 10.1148/radiol.2452061673. [DOI] [PubMed] [Google Scholar]
- 36.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa S, Motosugi U, Ichikawa T, et al. Magnetic resonance elastography for staging liver fibrosis in chronic hepatitis C. Magn Reson Med Sci. 2012;11:291–297. doi: 10.2463/mrms.11.291. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Talwalkar JA, Yin M, et al. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749–756. doi: 10.1148/radiol.11101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bensamoun SF, Leclerc GE, Debernard L, et al. Cutoff values for alcoholic liver fibrosis using magnetic resonance elastography technique. Alcohol Clin Exp Res. 2013;37:811–817. doi: 10.1111/acer.12025. [DOI] [PubMed] [Google Scholar]
- 40.Bohte AE, van Dussen L, Akkerman EM, et al. Liver fibrosis in type I Gaucher disease: magnetic resonance imaging, transient elastography and parameters of iron storage. PLoS ONE. 2013;8:e57507. doi: 10.1371/journal.pone.0057507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liver EAFTSOT. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 43.Ghany MG, Nelson DR, Strader DB, et al. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–1444. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez SM, Crespo G, Navasa M, et al. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53:325–335. doi: 10.1002/hep.24013. [DOI] [PubMed] [Google Scholar]
- 45.Huwart L, Peeters F, Sinkus R, et al. Liver fibrosis: non-invasive assessment with MR elastography. NMR Biomed. 2006;19:173–179. doi: 10.1002/nbm.1030. [DOI] [PubMed] [Google Scholar]
- 46.Huwart L, Salameh N, ter Beek LC, et al. MR elastography of liver fibrosis: preliminary results comparing spin-echo and echo-planar imaging. European Radiology. 2008;18:2535–2541. doi: 10.1007/s00330-008-1051-5. [DOI] [PubMed] [Google Scholar]
- 47.Kim D, Kim WR, Talwalkar JA, et al. Advanced Fibrosis in Nonalcoholic Fatty Liver Disease: Noninvasive Assessment with MR Elastography. Radiology. 2013;268:411–419. doi: 10.1148/radiol.13121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talwalkar JA, Yin M, Venkatesh SK, et al. Feasibility of in vivo MR elastographic splenic stiffness measurements in the assessment of portal hypertension. American Journal of Roentgenology. 2009;193:122–127. doi: 10.2214/AJR.07.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirsch S, Guo J, Reiter R, et al. MR elastography of the liver and the spleen using a piezoelectric driver, single-shot wave-field acquisition, and multifrequency dual parameter reconstruction. Magn Reson Med. 2013 doi: 10.1002/mrm.24674. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 50.Yin M, Kolipaka A, Woodrum DA, et al. Hepatic and splenic stiffness augmentation assessed with MR elastography in an in vivo porcine portal hypertension model. J Magn Reson Imaging. 2013;38:809–815. doi: 10.1002/jmri.24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nedredal GI, Yin M, McKenzie T, et al. Portal hypertension correlates with splenic stiffness as measured with MR elastography. J Magn Reson Imaging. 2011;34:79–87. doi: 10.1002/jmri.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Venkatesh SK, Yin M, Glockner JF, et al. MR elastography of liver tumors: preliminary results. AJR Am J Roentgenol. 2008;190:1534–1540. doi: 10.2214/AJR.07.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garteiser P, Doblas S, Daire JL, et al. MR elastography of liver tumours: value of viscoelastic properties for tumour characterisation. Eur Radiol. 2012;22:2169–2177. doi: 10.1007/s00330-012-2474-6. [DOI] [PubMed] [Google Scholar]
- 54.Friedman S, Bansal M. Reversal of hepatic fibrosis—fact or fantasy? Hepatology. 2006;43:S82–S88. doi: 10.1002/hep.20974. [DOI] [PubMed] [Google Scholar]
- 55.Dixon JB, Bhathal PS, Hughes NR, et al. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–1654. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 56.Venkatesh SK, Tai D, Wee A, et al. Comparison of Liver Stiffness With MRE and Fibrosis Quantification With Fibro-C-Index in Chronic Hepatitis B Patients. Proceedings of the 19th Annual ISMRM Conference (abstract 2934) Montreal, Quebec, Canada: ISMRM; 2011. [Google Scholar]
- 57.Hoganson DD, Chen J, Ehman RL, et al. Magnetic resonance elastography for liver fibrosis in methotrexate treatment. Open J Rheumatol Autoimmun Dis. 2012;2:6–13. doi: 10.4236/ojra.2012.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serai SD, Wallihan DB, Venkatesh Sk, et al. MR Elastography of the Liver in Patients Status Post-Fontan Procedure: A Pilot Investigation. Proceedings of the 21st Annual ISMRM Conference (abstract 3361) Salt Lake City, UT: ISMRM; 2013. [Google Scholar]
- 59.Mariappan YK, Venkatesh SK, Glaser KJ, et al. MR Elastography of Liver With Iron Overload: Development, Evaluation and Preliminary Clinical Experience With Improved Spin Echo and Spin Echo EPI Sequences. Proceedings of the 21st annual ISMRM conference (abstract 985) Salt Lake City, UT: ISMRM; 2013. [Google Scholar]