Twenty-five years ago a case-control study published in the Journal of the American Medical Association (Uhlmann, Larson, Rees, Koepsell, & Duckert, 1989) first reported that hearing impairment in older adults was strongly and independently associated with the likelihood of having dementia—raising the intriguing hypothesis that age-related hearing impairment may contribute to dementia risk. Surprisingly, in the ensuing years, little research has been conducted to further explore this association despite the high prevalence of hearing impairment in older adults. Progress in this field has been hindered by the lack of interaction among disciplines. For example, while auditory scientists and audiologists/ENTs have expertise in understanding how to measure and address hearing impairment, they have had limited opportunities to work with researchers who study cognitive decline and dementia. Likewise, epidemiologists and cognitive scientists who have investigated the risks for cognitive decline and dementia have been unfamiliar with how to measure hearing and to integrate these tests into studies. Underlying much of this divide is the fact that age-related hearing loss is a condition that will affect nearly every person in society—giving rise to the perception that hearing loss is likely to be an inevitable, and hence mostly inconsequential, part of aging.
Over the last four years, however, growing epidemiologic and clinical research studies have suggested otherwise. Longitudinal studies of community-dwelling older adults have demonstrated that hearing impairment is independently associated with a 30-40% rate of accelerated cognitive decline (Lin et al., 2013) (on both auditory and non-auditory cognitive tests) and with a substantially increased risk of incident all-cause dementia (Gallacher et al., 2012; Lin, Metter, et al., 2011). Compared to individuals with normal hearing, those individuals with a mild, moderate, and severe hearing impairment, respectively, had a 2-, 3-, and 5-fold increased risk of incident all-cause dementia over >10 years of follow-up (Lin, Metter, et al., 2011). Neuroimaging studies have also demonstrated independent associations of hearing impairment with reduced cortical volumes in the auditory cortex (Peelle, Troiani, Grossman, & Wingfield, 2011) and accelerated rates of lateral temporal lobe and whole brain atrophy (Lin et al., 2014).
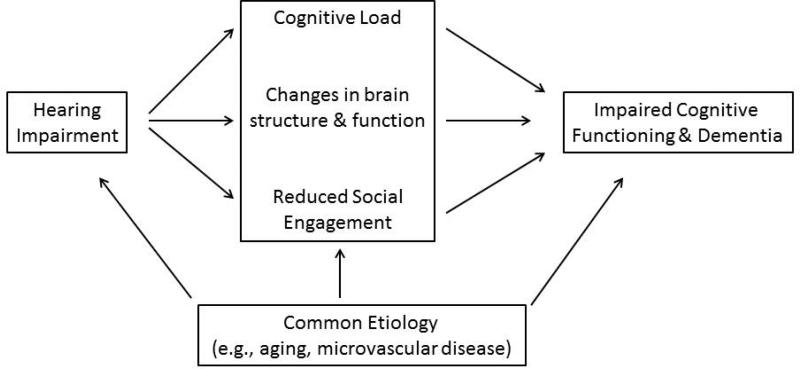
Investigating the potential mechanisms (Figure) which underlie these associations begins with an understanding that age-related hearing loss reflects progressive damage to cochlear structures from aging and other factors (e.g., noise, vascular risk factors) that result in poorer encoding of sound by the cochlea. Common factors that could underlie a simple correlation between hearing and cognition include: age, vascular risk factors (e.g., diabetes, smoking), or social factors (e.g., education). In contrast, mechanistic pathways through which hearing impairment could contribute to poorer cognitive functioning include the effect of hearing impairment on cognitive load, brain structure, and decreased social engagement.
Figure 1.
Conceptual model of the association of hearing impairment with cognitive functioning and dementia.
The effect of poor peripheral encoding of sound by an impaired cochlea is demonstrated by studies in which under conditions where the auditory signal is degraded, greater cognitive resources are required for auditory perceptual processing to the detriment of other cognitive processes such as working memory (Rabbitt, 1968; Tun, McCoy, & Wingfield, 2009). Importantly, for an individual with hearing impairment, such a cognitive load would be a “dual task” that is always present (hearing and auditory processing are evolutionarily-evolved processes that remain constantly active (Horowitz, 2012)) and could, therefore, affect an individual's performance in usual activities and cognitive tasks (among the criteria for the diagnosis of dementia).
Hearing impairment may also constitute a “second hit” on the brain and thereby adversely affect cognitive performance and increase the risk of dementia by adding to brain pathology resulting from other disorders (e.g., amyloid-beta accumulation, neurofibrillary tangles, and microvascular disease). For example, data from animal models (Groschel, Gotze, Ernst, & Basta, 2010; Kakigi, Hirakawa, Harel, Mount, & Harrison, 2000; Schwaber, Garraghty, & Kaas, 1993) and humans (Peelle et al., 2011) show that impoverished auditory signals and reduced stimulation from the impaired cochlea may precipitate changes in cortical reorganization and brain morphometry. Additionally, a recent neuroimaging study (Lin et al., 2014) demonstrated that individuals with hearing impairment have accelerated rates of whole brain atrophy as well as specific volume declines in the right superior, middle, and inferior temporal gyri over a mean 6.4 years of follow-up. These temporal regions are intriguing because they are important not only for spoken language processing (Peelle, 2012), but also for semantic memory, sensory integration, and are involved in the early stages of mild cognitive impairment or early Alzheimer disease (Chetelat et al., 2005).
While numerous risk factors (e.g, diabetes, physical activity) have been studied in relation to cognitive decline and dementia over the last several decades (Daviglus et al., 2011), hearing impairment is unique in likely being the least studied factor, yet the one with potentially the greatest public health impact, if the hypothesized mechanisms linking hearing and cognition are discovered to be amenable to hearing rehabilitative therapies. The prevalence of hearing impairment doubles with each age decade such that nearly two-thirds of adults 70 years and older have a meaningful hearing loss that affects daily communication (Lin, Niparko, & Ferrucci, 2011). Concurrently, rates of hearing aid use remain <25% in the U.S. and other industrialized countries. However, while it is possible that hearing rehabilitative therapies and devices could plausibly lessen cognitive load (Sarampalis, Kalluri, Edwards, & Hafter, 2009), provide increased auditory stimulation, and promote social engagement, there has been no research that has investigated whether such therapies could actually reduce the risk of cognitive decline and dementia. Results from observational epidemiologic studied have demonstrated trends for a protective effect of hearing aid use, but such results remain difficult to interpret. Individuals with hearing impairment who choose to use hearing aids and other technologies are likely to be healthier and of higher socioeconomic status (creating a positive bias of seeing a protective effect) but at the same time are also likely to have more severe hearing problems (leading to a negative bias) than individuals with hearing impairment who don't use hearing aids.
Key challenges going forward pertain to both broader research as well as clinical issues. The proposed conceptual model (Figure) serves as a starting point from which to initiate more focused studies examining the impact of hearing impairment on cognitive functioning and brain structure/function particularly in the context of other established dementia risk factors. Currently, few epidemiologic or clinical studies of cognition and dementia include assessments of objective audiometric function despite growing evidence of the substantial role of hearing in cognitive outcomes. A prior study of hearing and incident dementia (Lin, Metter, et al., 2011) estimated the attributable risk of dementia associated with hearing impairment to be 36%. While this estimate assumes causality and may be inflated, the possibility of even slightly reducing the proportion of risk associated with hearing loss is important given the high prevalence of hearing impairment in older adults. Ongoing and future studies of cognitive aging and dementia should clearly incorporate objective assessment of hearing function into research protocols (i.e., pure tone audiometry). However, while further observational studies are warranted, one study of key importance will be to conduct a randomized clinical trial of current best-practices hearing loss treatment (counseling/education, provision of hearing aid and other assistive devices) versus watchful waiting in a large cohort of older adults with untreated hearing impairment. A substantial advantage of such an RCT is that a well-designed and carefully planned trial can definitively answer the critical public health question at hand (“Does treating hearing loss reduce the risk of dementia”) while also providing the data to carefully explore the various mechanistic pathways that underlie this association.
Another challenge pertains to current clinical care. For the clinician confronted with an older adult patient in clinic, hearing impairment has generally been an afterthought in the face of more pressing clinical issues, particularly given the relative complexity of managing and addressing hearing impairment in older adults. As a result, many clinicians have adopted a primarily reactive rather than proactive approach toward hearing loss (i.e., only addressing the issue if the patient is insistent on bringing it to the clinician's attention). This approach is unfortunate and requires reconsideration, particularly given that most patients won't understand the importance of hearing unless spurred by the clinician. While definitive evidence of the effects of hearing treatment on dementia is years away, the benefits of early screening and management of hearing loss are likely significant and without risk. Importantly, challenges currently exist in developing affordable and accessible approaches toward hearing health care, but these issues are increasingly coming to the forefront and being addressed at the national level (Institute of Medicine, 2014).
Over the next forty years, prevalence rates of dementia are projected to double every twenty years because of the aging of the world population (Alzheimer's Disease International, 2010). The potential public health impact of hearing loss in the context of dementia is substantial given the high worldwide prevalence of hearing loss in older adults and the ready availability of existing hearing rehabilitative interventions which remain risk-free and underutilized. Concerted, interdisciplinary efforts bringing together hearing and mental health specialists to investigate and address hearing loss in the context of brain and cognitive aging are urgently needed – a message to which we all need to listen.
Acknowledgments
This work was supported in by part by NIH K23DC011279 (F.L), the Eleanor Schwartz Charitable Foundation (F.L.), a Triological Society/American College of Surgeons Clinician Scientist Award (F.L.), and NIA P50-AG005146 (M.A.)
Footnotes
Disclosures: Dr. Lin reports being on the scientific advisory board of Pfizer and Autifony Therapeutics, a consultant to Cochlear Ltd, and a speaker for Med El and Amplifon. Dr. Albert reports being a consultant to Agenebio, Genentech, Eisai and Eli Lilly.
References
- Alzheimer's Disease I. World Alzheimer Report 2010. London: 2010. [Google Scholar]
- Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, Baron JC. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27(4):934–946. doi: 10.1016/j.neuroimage.2005.05.015. doi: S1053-8119(05)00327-7 [pii] 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Daviglus ML, Plassman BL, Pirzada A, Bell CC, Bowen PE, Burke JR, Williams JW., Jr. Risk factors and preventive interventions for Alzheimer disease: state of the science. Arch Neurol. 2011;68(9):1185–1190. doi: 10.1001/archneurol.2011.100. doi: archneurol.2011.100 [pii] 10.1001/archneurol.2011.100. [DOI] [PubMed] [Google Scholar]
- Gallacher J, Ilubaera V, Ben-Shlomo Y, Bayer A, Fish M, Babisch W, Elwood P. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79(15):1583–1590. doi: 10.1212/WNL.0b013e31826e263d. doi: WNL.0b013e31826e263d [pii] 10.1212/WNL.0b013e31826e263d. [DOI] [PubMed] [Google Scholar]
- Groschel M, Gotze R, Ernst A, Basta D. Differential impact of temporary and permanent noise-induced hearing loss on neuronal cell density in the mouse central auditory pathway. J Neurotrauma. 2010;27(8):1499–1507. doi: 10.1089/neu.2009.1246. doi: 10.1089/neu.2009.1246. [DOI] [PubMed] [Google Scholar]
- Horowitz SS. The Universal Sense. Bloomsbury USA; New York: 2012. [Google Scholar]
- Institute of Medicine. IOM/NRC Workshop on Hearing Loss and Healthy Aging 2014 Retrieved from http://www.iom.edu/hearingloss-aging.
- Kakigi A, Hirakawa H, Harel N, Mount RJ, Harrison RV. Tonotopic mapping in auditory cortex of the adult chinchilla with amikacin-induced cochlear lesions. Audiology. 2000;39(3):153–160. doi: 10.3109/00206090009073068. [DOI] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, An Y, Goh JO, Doshi J, Metter EJ, Resnick SM. Association of hearing impairment with brain volume changes in older adults. Neuroimage. 2014 doi: 10.1016/j.neuroimage.2013.12.059. doi: 10.1016/j.neuroimage.2013.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214–220. doi: 10.1001/archneurol.2010.362. doi: 68/2/214 [pii] 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Niparko JK, Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med. 2011;171(20):1851–1852. doi: 10.1001/archinternmed.2011.506. doi: 171/20/1851 [pii] 10.1001/archinternmed.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Yaffe K, Xia J, Xue QL, Harris TB, Purchase-Helzner EL, Simonsick E. Hearing loss and cognitive decline among older adults. JAMA Intern Med. 2013 doi: 10.1001/jamainternmed.2013.1868. Epublished January 21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE. The hemispheric lateralization of speech processing depends on what “speech” is: a hierarchical perspective. Front Hum Neurosci. 2012;6:309. doi: 10.3389/fnhum.2012.00309. doi: 10.3389/fnhum.2012.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011;31(35):12638–12643. doi: 10.1523/JNEUROSCI.2559-11.2011. doi: 31/35/12638 [pii] 10.1523/JNEUROSCI.2559-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt PM. Channel-capacity, intelligibility and immediate memory. Q.J.Exp.Psychol. 1968;20(3):241–248. doi: 10.1080/14640746808400158. [DOI] [PubMed] [Google Scholar]
- Sarampalis A, Kalluri S, Edwards B, Hafter E. Objective measures of listening effort: effects of background noise and noise reduction. J Speech Lang Hear Res. 2009;52(5):1230–1240. doi: 10.1044/1092-4388(2009/08-0111). doi: 1092-4388_2009_08-0111 [pii] 10.1044/1092-4388(2009/08-0111. [DOI] [PubMed] [Google Scholar]
- Schwaber MK, Garraghty PE, Kaas JH. Neuroplasticity of the adult primate auditory cortex following cochlear hearing loss. Am J Otol. 1993;14(3):252–258. [PubMed] [Google Scholar]
- Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol.Aging. 2009;24(3):761–766. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261(13):1916–1919. [PubMed] [Google Scholar]