Abstract
Importance
Better continuity of care is expected to improve patient outcomes and reduce health care costs, but patterns of utilization, costs, and clinical complications associated with the current patterns of care continuity have not been quantified.
Objective
To measure the association between care continuity, costs, and rates of hospitalizations, emergency department visits, and complications for Medicare beneficiaries with chronic disease.
Design
Retrospective cohort study.
Setting
Insurance claims data for a 5% sample of Medicare beneficiaries.
Participants
Medicare beneficiaries experiencing a 12-month episode of care for congestive heart failure (CHF, n=53,488), chronic obstructive pulmonary disease (COPD, n=76,520) or diabetes mellitus (DM, n=166,654) in 2008–2009.
Main outcomes and measures
Hospitalizations, emergency department visits, complications, costs of care associated with the Bice-Boxerman Continuity of Care (COC) Index, a measure of the outpatient continuity of care related to conditions of interest.
Results
The mean COC index for CHF was 0.55 (standard deviation [SD] 0.31), for COPD 0.60 (SD 0.34), and for DM 0.50 (SD 0.32). After multivariable adjustment, higher levels of continuity were associated with lower odds of inpatient hospitalization (odds ratios [OR] for a 0.1 increase in COC were 0.94 [95% CI, 0.93–0.95] for CHF, 0.95 [95% CI, 0.94–0.96] for COPD, and 0.95 [95% CI, 0.95–0.96] for DM), lower odds of emergency department visits (ORs were 0.92 [95% CI, 0.91,0.92] for CHF, 0.93 [95% CI, 0.92–0.93] for COPD, and 0.94 [95% CI, 0.93–0.94] for DM), and lower odds of complications (OR range, 0.92–0.96 across the three complication types and three conditions; all p<0.0001). For every 0.1 increase in the COC index, episode costs of care were 4.7% lower for CHF (95% CI, 4.4%–5.0%), 6.3% lower for COPD (95% CI, 6.0%–6.5%), and 5.1% lower for DM (95% CI, 5.0%–5.2%) in adjusted analyses.
Conclusions and Relevance
Modest differences in care continuity for Medicare beneficiaries are associated with sizable differences in costs, utilization, and complications.
INTRODUCTION
Patients, especially those with chronic illnesses, frequently experience a health care system in which care is poorly coordinated.1 They see many different health care providers working across multiple clinical venues.2,3 Communication among these providers is often suboptimal, and poor coordination has been shown to be widespread, with adverse impacts on health care costs, patient outcomes, and experiences with care.1
Care coordination has been identified as a priority area by the National Priorities Partnership and the Institute of Medicine.4,5 New models of patient care coupled with new provider payment mechanisms - including bundled payment, accountable care organizations, and patient-centered medical homes - are expected to achieve reductions in costs and increases in quality through improved care coordination.2,6–9 However, the potential impact of new care models, and the areas in which improvements in care coordination are likely to have the largest effects, are poorly understood. While previous studies have shown that patients with a close, continuous relationship with a specific physician were more likely to receive recommended care, many programs that aim to improve care coordination have not had the desired impacts on costs and quality.10,11
The objective of this study was to measure the difference in costs associated with variations in one aspect of coordination—care continuity—during episodes of care for patients with congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), and diabetes mellitus (DM).12
METHODS
Setting and Participants
We conducted a retrospective cohort study of the association between continuity of care as measured by the Bice-Boxerman continuity of care index and the costs for Medicare beneficiaries with chronic disease. We further examined whether continuity is associated with rates of hospitalizations, emergency department visits, and complications as each may be contributors to cost and potential poor outcomes.
The sample included Medicare beneficiaries with a diagnosis of CHF, COPD, and/or DM identified using a Medicare claims files for a 5% random sample of Medicare fee-for-service beneficiaries from 2008 and 2009. Beneficiaries were eligible for inclusion in our sample if they were over 65 years old at the start of 2008 and continuously enrolled in fee-for-service parts A and B Medicare coverage for the two years. For this sample of beneficiaries, we identified episodes of care for each of the three chronic conditions, with each episode triggered by a physician professional service for one of a set of predefined ICD-9 diagnosis codes at any point during 2008.13 Using this approach we identified 98,850 CHF episodes, 147,708 COPD episodes, and 281,584 DM episodes (Appendix Table 1). Because our measurement window was limited to two years, each person could have only a single episode for each condition; however, a single patient with comorbities could have up to three episodes (one for each condition).
Patients were excluded if they had an in-hospital death, left the hospital against medical advice, or had a medical exclusion (e.g., cardiac arrest, HIV, cancer, suicide, end stage renal disease) during the episode. Claims were excluded from episodes if they were irrelevant to the chronic condition (e.g., surgical procedures for which the chronic condition was a comorbidity rather than the primary reason for the procedure). These exclusions accounted for 38% of CHF episodes, 36% of COPD episodes, and 31% of DM episodes. We further excluded an additional 8% of CHF episodes, 12% of COPD episodes, and 10% of DM episodes with <2 outpatient evaluation and management visits (as defined below) due to the inability to construct continuity measures for these individuals. After exclusions, the final analytic cohort included 241,722 unique patients, of whom 53,488 had CHF, 76,520 had COPD, and 166,654 had DM.
Measurement of Continuity
To measure care continuity, we used the Bice-Boxerman Continuity of Care (COC) index,14 a commonly used measure of continuity.15 The COC index reflects the relative share of all of a patient’s visits during the year that are billed by distinct providers and/or practices; the index ranges from 0 (each visit involved a different provider than all other visits) to 1 (all visits were billed by a single provider).
In constructing the COC index, we included evaluation and management (E&M) visits that occurred in the outpatient setting, defined as Berenson-Eggers Type of Service codes M1A, M1B, M4A, M4B, M5C, M5D, M6. Only a single E&M visit per day for each patient-provider dyad was counted (visits on the same day to different providers were counted). Visits that were related to complications, hospitalizations, or emergency department visits were excluded from our calculation of the COC index. In addition, we counted only visits to those clinicians that were most likely to be involved in outpatient management for each of the three conditions. For CHF, this included primary care providers (PCPs - general practitioners, family practitioners, internal medicine without subspecialty training, and nurse practitioners), cardiologists, and pulmonologists. For COPD, we included PCPs and pulmonologists; for DM, we included PCPs, cardiologists, endocrinologists, podiatrists, and ophthalmologists. With the exception of general practitioners, each specialty class of provider accounted for more than 2% of outpatient E&M visits, and the included providers accounted for 90.6% of total visits for CHF, 89.6% for COPD, and 86.0% for DM. In the main analysis, we constructed the COC index using counts of visits to unique individual providers. In sensitivity analyses, we constructed the COC index using counts of visits to unique practice groups. We defined practice groups using the tax identification number listed on Medicare claims.
Measurement of utilization and complications
For each 365-day episode, we examined whether the patient had at least one inpatient hospitalization and/or emergency department visit. We also measured the incidence of complications that were categorized as related to the primary condition (CHF, COPD, or DM), related to comorbidities, or related to patient safety (Appendix Table 2). For each clinical condition, complications were defined based on Agency for Healthcare Research and Quality Clinical Classification Software.13 Three clinicians independently reviewed and rated the extent to which each complication was likely to be sensitive to care continuity for each clinical condition (see Appendix Table 3 for ratings and details). Complications that were rated as potentially sensitive to continuity and were observed in at least 1% of patients were included in our analyses. We excluded complications that occurred during inpatient stays or emergency department visits from complication rates in order to create mutually exclusive study outcomes.
We measured the total Medicare Part A and B costs of care associated with each episode by summing the Medicare and beneficiary payment amounts from all episode-related claims. We similarly measured costs of hospitalizations, emergency department visits, and each type of complication.
Covariates
Patient age, gender, and Census region were determined from beneficiary enrollment files. Risk adjustment was performed using the 2008 CMS-Hierarchical Condition Categories (HCC), which calculate a beneficiaries’ expected Medicare expenditure on the basis of diagnosis codes in claims, age, gender, Medicaid status, and reason for Medicare entitlement.16 Zip code median income was used as a proxy for socioeconomic status. Medicaid enrollment was included as a measure of socioeconomic status and/or disability. We adjusted for the number of visits to adjust for any residual association between utilization (possibly due to unmeasured severity) and the COC index that may persist, although the index also accounts for the volume of visits. Because having a primary care physician has been associated with higher quality and lower costs, we created a dummy variable indicating whether the patient had at least one visit to a PCP during the episode.17
Statistical Analysis
For each condition, we calculated descriptive statistics summarizing patient and episode characteristics, the COC index, and complications among patients. Bivariate analyses were used to examine the association between patient characteristics and the COC index. We constructed separate multivariable logistic regression models for each condition and with each type of event as the dependent variable (hospitalization, emergency department visit, category of complication, and specific complication) and the COC index as an independent variable, adjusting for the relevant covariates. To test the association between the COC index and total episode costs, we used generalized linear regression models with gamma variance distribution and log link function.18 We used two-part models to test the association between the COC index and the costs of hospitalizations, emergency department visits, and complications. Two-part models were chosen due to the high concentration of beneficiaries with zero costs in these categories in the study population.18 The first part of the model was a logistic regression model predicting the incidence of each type of event, as described above. The second part of the model was a generalized linear regression model with gamma variance distribution and log link function estimating complication costs, with the model estimated only for the population of patients that had each type of event. We used the results of the two models to calculate predicted costs for each beneficiary, including an estimate that the beneficiary had non-zero cost and, conditional on non-zero cost, the predicted amount. Specifically, we used recycled predictions from the two-part model to estimate the costs associated with a 0.1 difference in the COC index. We multiplied the predicted probability that the event occurred by the predicted costs given that the event occurred assuming the COC index was 0.4, then again assuming the COC index was 0.5. We then calculated the average predicted cost across all patients in the sample at each level of COC. We generated bootstrapped confidence intervals for the estimated differences in costs using 1,000 bootstrap samples taken from the study population with replacement.
In sensitivity analyses, we tested whether our findings were similar when we used COC calculated at the practice group level. To test whether complications lead to an increased visit rate and potentially lowered continuity, we compared the monthly rate of evaluation and management visits before and after a patient’s first complication. This calculation was limited to patients who had their first complication between months 3 and 6 of their 12 month episode.
Statistical analyses were performed using SAS software, Version 9.3 (SAS Institute Inc., Cary, NC, USA). This study was approved by the RAND Human Subjects Protection Committee and the Johns Hopkins Institutional Review Board.
Role of the Funding Source
The funder, the Aetna Foundation, provided input on the design of the study but the authors had final discretion over all aspects of the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
RESULTS
A majority of the study patients were age 75 or older and female (Table 1). The population was predominantly white (83 to 90 percent of patients with each condition). The median number of visits to a clinician during a year-long episode ranged from five (COPD) to seven (CHF). Patients with DM had the lowest mean COC index (0.50; standard deviation [SD] 0.32) compared to patients with CHF (mean 0.55, SD 0.31) and COPD (mean 0.60, SD 0.34).
Table 1.
Characteristics of Medicare beneficiaries with 12-month episodes of care for CHF, COPD, and DM in 2008–09
| CHF | COPD | DM | |
|---|---|---|---|
| Number (%) of beneficiaries, total | 53,488 (100) | 76,520 (100) | 166,654 (100) |
| Number (%) of beneficiaries, by age | |||
| 65–74 | 15,985 (30) | 33,418 (44) | 80,262 (48) |
| 75–84 | 21,851 (41) | 30,382 (40) | 64,136 (38) |
| 85+ | 15,652 (30) | 12,720 (17) | 22,256 (13) |
| Number (%) of beneficiaries, by gender | |||
| Female | 28,653 (54) | 41,685 (54) | 88,565 (53) |
| Male | 24,835 (46) | 34,835 (46) | 78,089 (47) |
| Number (%) of beneficiaries, by race/ethnicity | |||
| Unknown | 47 (0) | 46 (0) | 128 (0) |
| White | 46,405 (87) | 68,637 (90) | 137,648 (83) |
| Black | 4999 (9) | 5001 (7) | 18,700 (11) |
| Other | 449 (1) | 673 (1) | 2873 (2) |
| Asian | 489 (1) | 706 (1) | 2769 (2) |
| Hispanic | 896 (2) | 1109 (1) | 3681 (2) |
| North American Native | 203 (0) | 348 (0) | 855 (1) |
| Number (%) of beneficiaries, by Census region | |||
| Midwest | 13,606 (25) | 19,269 (25.18) | 40,164 (24) |
| Northeast | 11,075 (21) | 14,314 (19) | 34,131 (21) |
| Other | 228 (0) | 238 (0) | 1299 (1) |
| South | 21,025 (39) | 32,109 (41.96) | 66,934 (40) |
| West | 7554 (14) | 10,590 (14) | 24,123 (14) |
| Number (%) of beneficiaries, by metro status | |||
| Does not live in MSA | 12,674 (24) | 18,781 (25) | 35,904 (22) |
| Lives in MSA | 40,577 (76) | 57,484 (75) | 129,406 (78) |
| Unknown/other | 228 (0) | 255 (0) | 1344 (1) |
| Number (%) of beneficiaries with an episode for >1 condition (DM, CHF, COPD) | 31,071 (58%) | 32,485 (42%) | 39,225 (24%) |
| Number (%) of beneficiaries, by Medicaid enrollment | |||
| Enrolled | 9987 (19) | 13,009 (17) | 25,738 (15) |
| Not enrolled | 43,501 (81) | 63,511 (83) | 140,916 (85) |
| Number (%) of beneficiaries, by median ZCTA Household Income, 2000 ($) (IQR) | 39,864 (34,778, 47,603) | 39,685 (34,623, 46,956) | 40,029 (35,042, 48,397) |
| Median HCC Score (IQR) | 0.7 (0.5, 1.3) | 0.6 (0.5, 0.9) | 0.5 (0.4, 0.7) |
| Median number of E&M visits per episode (IQR)* | 7 (4, 11) | 5 (3, 8) | 6 (4, 10) |
| Number (%) of beneficiaries, by PCP visit | |||
| Yes | 48,677 (91) | 71,493 (93) | 152,714 (92) |
| No | 4811 (9) | 5027 (7) | 13,940 (8) |
| Mean Bice-Boxerman Continuity of Care Index (SD) | 0.55 (0.31) | 0.60 (0.34) | 0.50 (0.32) |
visits to PCPs and Frequent Providers
ZCTA = zip code tabulation area. IQR = interquartile range. E&M = evaluation and management. PCP = primary care provider. MSA = metropolitan statistical area. DM = diabetes mellitus. CHF = congestive heart failure. COPD = chronic obstructive pulmonary disease. SD = standard deviation. HCC = hierarchical condition category.
Between 4% (DM) and 10% (CHF) of patients had at least one hospitalization during the episode of care (Table 2). Emergency department utilization was common (range, 27% to 45% of beneficiaries across the three conditions). Over half of all patients with each condition had a complication related to a comorbidity (range, 50% to 67%). Complications related to the primary condition and patient safety issues were less common, but still substantial among patients (range, 9% to 41% for complications related to the primary condition, 15% to 25% for patient safety issues). The incidence and costs of specific complications varied by condition (Appendix Table 4).
Table 2.
Incidence and cost of complications for Medicare beneficiaries during 12-month episodes of care, by condition, 2008–09
| CHF | COPD | DM | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Percent with complication | Mean per patient cost^ | SD | Percent with complication | Mean per patient cost^ | SD | Percent with complication | Mean per patient cost^ | SD | |
| Inpatient hospitalization | 10 | 2,744 | 19,050 | 7 | 2,475 | 6,462 | 4 | 2,895 | 11,613 |
| ED visit | 45 | 793 | 1,061 | 36 | 763 | 998 | 27 | 717 | 957 |
| Complications related to index condition* | 17 | 339 | 571 | 41 | 658 | 1,312 | 9 | 447 | 1,413 |
| Complications related to comorbidity | 67 | 1,134 | 2,107 | 55 | 959 | 1,590 | 50 | 715 | 1,296 |
| Complications related to patient safety | 25 | 772 | 11,013 | 19 | 514 | 1,343 | 15 | 699 | 4,513 |
limited to specific complications that were highly rated by expert panel, had >=1% prevalence for the episode, and were not included in inpatient or ED visits
Mean costs are calculated for patients who experienced at least 1 of each type of event/complication
SD=standard deviation. CHF = congestive heart failure. COPD = chronic obstructive pulmonary disease. ED = emergency department.
Table 3 shows the unadjusted association between patient characteristics and the COC index. The differences in the COC index by age group, gender, race/ethnicity, Census region, median household income, Medicaid enrollment, and HCC score were generally small but statistically significant. Overall, patients with the highest HCC scores tended to have lower COC indices; the difference in the COC index between patients in the highest quartile of HCC scores (indicating highest health risk) vs. the lowest quartile was −0.03 for CHF and −0.07 for COPD and DM (all p<0.0001). There were larger differences in the COC index between patients with higher numbers of physician visits; the difference between the highest and lowest quartiles of visits was −0.14 for CHF, −0.17 for COPD, and −0.21 for DM (all p<0.0001). The small number of patients without a PCP visit (<10%) had higher mean COC index than those with a PCP visit.
Table 3.
Associations between the Continuity of Care (COC) Index and characteristics of Medicare beneficiaries with 12-month episodes of care for CHF, COPD, and DM in 2008–09
| CHF | COPD | DM | |
|---|---|---|---|
|
| |||
| COC Index mean (SD) | COC Index mean (SD) | COC Index mean (SD) | |
| Total | 0.55 0.31) | 0.60 (0.34) | 0.50 (0.32) |
| Age | |||
| 65–74 | 0.53 (0.32) | 0.61 (0.34) | 0.51 (0.33) |
| 75–84 | 0.54 (0.31) | 0.60 (0.34) | 0.48 (0.31) |
| 85+ | 0.59 (0.32) | 0.62 (0.34) | 0.49 (0.30) |
| Gender | |||
| Female | 0.56 (0.31) | 0.61 (0.34) | 0.50 (0.31) |
| Male | 0.53 (0.31) | 0.60 (0.34) | 0.50 (0.32) |
| Race/ethnicity | |||
| Unknown | 0.56 (0.29) | 0.61 (0.34) | 0.46 (0.31) |
| White | 0.54 (0.31) | 0.60 (0.34) | 0.50 (0.31) |
| Black | 0.57 (0.33) | 0.69 (0.34) | 0.51 (0.32) |
| Other | 0.60 (0.32) | 0.67 (0.33) | 0.53 (0.32) |
| Asian | 0.62 (0.32) | 0.68 (0.33) | 0.55 (0.32) |
| Hispanic | 0.57 (0.31) | 0.62 (0.33) | 0.51 (0.32) |
| North American native | 0.51 (0.32) | 0.52 (0.37) | 0.47 (0.45) |
| Census regions | |||
| West | 0.57 (0.32) | 0.62 (0.34) | 0.51 (0.32) |
| Northeast | 0.55 (0.31) | 0.60 (0.34) | 0.47 (0.31) |
| South | 0.54 (0.31) | 0.60 (0.34) | 0.50 (0.32) |
| Midwest | 0.55 (0.32) | 0.61 (0.34) | 0.50 (0.32) |
| Other | 0.57 (0.32) | 0.54 (0.33) | 0.49 (0.33) |
| HCC Score | |||
| Q1 (lowest) | 0.54 (32) | 0.62 (0.35) | 0.53 (0.33) |
| Q2 | 0.57 (0.32) | 0.62 (0.34) | 0.50 (0.31) |
| Q3 | 0.57 (0.32) | 0.63 (0.34) | 0.50 (0.31) |
| Q4 (highest) | 0.51 (0.30) | 0.55 (0.33) | 0.46 (0.30) |
| Median Household Income | |||
| Q1 (lowest) | 0.57 (0.32) | 0.62 (0.34) | 0.52 (0.32) |
| Q2 | 0.55 (0.32) | 0.61 (0.34) | 0.50 (0.32) |
| Q3 | 0.53 (0.31) | 0.59 (0.34) | 0.49 (0.31) |
| Q4 (highest) | 0.54 (0.31) | 0.60 (0.34) | 0.48 (0.31) |
| Total Visits* | |||
| Q1 (lowest) | 0.63 (0.40) | 0.69 (0.41) | 0.60 (0.40) |
| Q2 | 0.55 (0.30) | 0.63 (0.33) | 0.51 (0.28) |
| Q3 | 0.51 (0.26) | 0. 57 (0.30) | 0.44 (0.24) |
| Q4 (highest) | 0.49 (0.26) | 0.52 (0.27) | 0.39 (0.23) |
| PCP visit | |||
| Yes | 0.53 (0.30) | 0.59 (0.34) | 0.49 (0.31) |
| No | 0.76 (0.33) | 0.76 (0.34) | 0.56 (0.36) |
| Medicaid enrollment | |||
| Enrolled | 0.59 (0.32) | 0.60 (0.34) | 0.49 (0.32) |
| Not enrolled | 0.54 (0.31) | 0.63 (0.34) | 0.52 (0.32) |
visits to PCPs and frequent providers included in the COC analysis
PCP = primary care provider. DM = diabetes mellitus. CHF = congestive heart failure. COPD = chronic obstructive pulmonary disease. SD = standard deviation. HCC = hierarchical condition category.
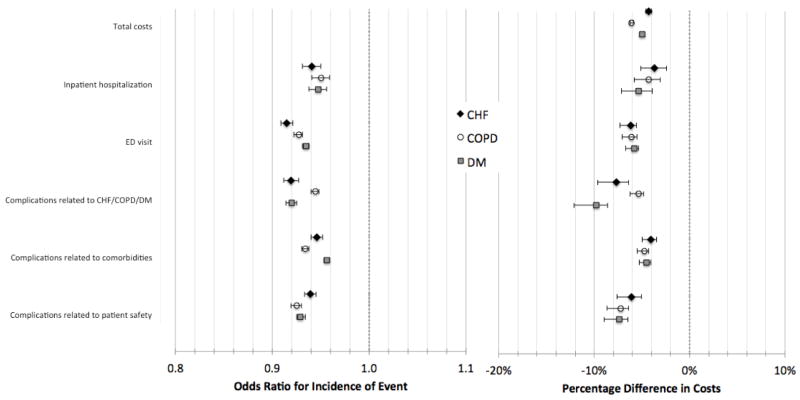
Figure 1 shows the association between the COC index and health care utilization and outcomes after adjustment. For each condition, higher levels of COC were associated with lower odds of inpatient hospitalization (odds ratios [OR] were 0.94 [95% CI, 0.93–0.95] for CHF, 0.95 [95% CI, 0.94–0.96] for COPD, and 0.95 [95% CI, 0.95–0.96] for DM) and lower odds of emergency department visits (ORs were 0.92 [95% CI, 0.91,0.92] for CHF, 0.93 [95% CI, 0.92–0.93] for COPD, and 0.94 [95% CI, 0.93–0.94] for DM). Higher levels of COC were also associated with lower odds of complications in three categories: those related to the primary condition, those related to comorbidity, and those related to patient safety (OR range, 0.92 to 0.96 across the three complication types and three conditions; all p<0.0001). For each condition, some of the specific complications were statistically significantly associated with the COC index, with odds ratios ranging between 0.87 and 0.99 (Appendix Tables 5 to 7).
Figure 1.
Odds of incidence of hospitalizations, emergency department visits, and complications (left) and percentage change in costs (right) associated with a 0.1 increase in the Bice-Boxerman Continuity of Care (COC) Index.
Note: Medicare beneficiaries with CHF, COPD, and DM for 12-month episodes of care in 2008–09. Incidence reflects the odds ratio using logistic regression models. Cost models show the change in the COC Index change from 0.4 and 0.5. Total episode cost ratios derived from generalized linear regression models with gamma variance distribution and log link function. Cost ratios for hospitalizations, ED visits, and complications derived from two-part models with bootstrapped confidence intervals. All results are adjusted for patient age, gender, Census region, HCC, median zip code income, Medicaid enrollment, number of visits, and whether patient had visit to PCP. Error bars represent 95% confidence intervals.
For total episode costs, a 0.1 increase in the COC index was associated with 4.7% lower costs for CHF (95% CI, 4.4%–5.0%), 6.3% lower costs for COPD (95% CI, 6.0%–6.5%), and 5.1% lower costs for DM (95% CI, 5.0%–5.2%). For a CHF patient with median total costs of $1,437, this translates into a $66 decrease (95% CI, $62–$70). With median costs of $1,062 for patients with COPD, a 0.1 increase in the COC index was associated with $64 lower costs (95% CI, $62–$67); with $1047 in median costs for DM, costs were $52 lower (95% CI, $51–$53). In estimates from two-part models (Figure 1), we found that a 0.1 increase in COC was associated with statistically significantly lower costs for hospitalizations (4.6% to 6.1% lower across the three conditions), emergency department visits (5.8% to 6.2% lower), and complications (4.1% to 9.8% lower).
In sensitivity analyses, using continuity calculated at the practice group level revealed qualitatively similar results with respect to the main outcomes (Appendix Tables 5 to 7). In tests of differences in utilization following a patient’s first complication during an episode, we found that the mean rate of visits per 30 days was slightly lower in the post-complication period compared with the pre-complication period (for CHF, mean number of visits per 30 days was 0.7 post-complication vs. 0.9 pre-complication; for COPD, 0.5 vs. 0.7; for DM, 0.7 vs. 0.8; see Appendix Table 8).
DISCUSSION
For Medicare beneficiaries with each of three chronic diseases (DM, COPD, and CHF), we found a consistent association between higher levels of care continuity, lower rates of utilization of hospital and emergency department visits, lower complication rates, and lower episode costs. A 0.1 unit increase in the COC index (which ranges from 0 to 1) was associated with a difference of between 4.7% to 6.3% lower costs across the three conditions.
Although it has been used frequently in health services research, the Bice-Boxerman Continuity of Care index may be unfamiliar to many readers and difficult to interpret. Assuming the number of visits in a year is constant, an increase in the COC index can be achieved by either involving fewer providers in a patient’s care or by concentrating the visits among fewer providers. For example, among patients with 7 total visits—the median in the CHF sample—moving from 3 to 2 providers or increasing the number of visits with a primary care provider from 4 to 5 visits can increase the COC index by 0.1. All of the potential COC indices possible for a patient with 7 visits are listed in Appendix Table 9. In our sample, a difference of 0.1 in the COC index corresponds to 0.3 standard deviations in COC, a difference that would generally be interpreted as a “small” effect size.
The finding that a higher continuity score is associated with lower complication rates may also be difficult to interpret given the cross-sectional design. It is possible that underlying processes of care that may be affected by continuity (e.g. the flow of information across providers and care settings) may be relevant for many different types of complications.19 However, it is also plausible that patients with complications see more physicians. We tried to exclude this possibility by examining the average number of visits before and after a patient’s initial complication. Measuring processes of care that may be sensitive to information continuity - for example, repeated laboratory or radiologic testing by multiple providers - may be an important next step in examining potential mechanisms as well as drivers of cost.
Because they are derived from health insurance claims data, the measures of care continuity and complications used in this study may be useful in tracking aspects of care that are sensitive to continuity involving large populations.20 Physician organizations and insurers may use such measures to target specific patients who are at increased risk for complications and high costs. Disadvantages of the COC measure include the lack of detailed information about communication across providers and the lack of direct guidance about the optimal modifications to current care delivery that can improve coordination. Care coordination is a multidimensional construct and the COC Index reflects only one aspect of coordination.20
Episodes of care may be an important framework for studying the role of care coordination in health care delivery.2 Yearlong episodes of care for chronic conditions help standardize comparisons across patients; they suggest specific providers and encounters that are associated with the chronic condition and which may be used to measure coordination; and they define aspects of health care utilization and complications that may be most sensitive to differences in coordination.
Our results are subject to limitations. First, the analyses focus on adults over age 65 enrolled in fee-for-service Medicare and may not be generalizable to younger populations and those with other types of health insurance. Second, like all claims-based analyses, the risk adjustment model lacks clinical detail that may be associated with both our measures of continuity and study outcomes. Unmeasured severity of illness could be a confounder. Third, our analyses are cross-sectional and therefore do not address causality. Adverse health outcomes may lead to patterns of care, such as increased visit rates, that reduce the COC Index. However, we found that mean visit rates decreased following complications in our sample. Fourth, we used COC at the provider- and practice group-levels as our measure of continuity. We have found that other commonly used measures of continuity (e.g. SECON,21 usual provider of care22) are highly correlated with one another and thus unlikely to significantly alter our findings.23 However, as discussed above, all claims-based continuity measures share certain limitations. Fourth, our results showed a counterintuitive finding that the group of patients without a PCP had a lower COC index score on average. This group without a PCP comprises a relatively small proportion of each sample (<10%). Patients with no PCP and CHF had higher HCC scores (1.1 vs. 1.0), but not among the DM and COPD populations. Patients with no PCP visit had lower total visit counts than patients with at least one PCP visit (mean total visit count for patients with no PCP visit vs. with >0 PCP visits: CHF, 8.9 vs. 5.2; COPD, 6.7 vs. 4.2; DM, 7.7 vs. 5.2.). Consistent with the prior literature, patients with a PCP tended to have lower costs after adjustment for other factors. We speculate that not having a PCP is a marker for other differences between patients with and without PCPs that we were unable to adjust for using claims data. Our findings of the association between the COC index and costs, hospital and ED utilization, and complications were not sensitive to exclusion of the PCP indicator from the model. Fifth, complications were classified into different types; however, these categories are unlikely to be mutually exclusive. Sixth, our analysis excluded patients with <2 outpatient evaluation and management visits. Seventh, our analysis did not include pharmaceutical utilization and costs.
While improving care continuity and realizing the associated benefits has, in practice, proved challenging,10 our results suggest the potential importance of care continuity and underscore the potential benefits that may be achievable through programs that improve continuity. With changes in health care delivery and payment, it will be necessary to measure whether these reforms have an impact on continuity and whether this, in turn, reduces health care utilization, the rate of complications, and costs of care.
Supplementary Material
Acknowledgments
This work was supported by the Aetna Foundation. Dr. Pollack’s salary is supported by the National Cancer Institute (NCI) and Office of Behavioral and Social Sciences (K07 CA151910). The Aetna Foundation provided input on the design of the study but the authors had final discretion over all aspects of the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Dr. Hussey had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We would like to thank Claude Setodji for his statistical advice.
Footnotes
This paper was presented at the Society for General Internal Medicine Annual Meeting in Denver, Colorado on April 25th, 2013.
All authors report no conflicts of interest.
References
- 1.Bodenheimer T. Coordinating care--a perilous journey through the health care system. N Engl J Med. 2008 Mar 6;358(10):1064–1071. doi: 10.1056/NEJMhpr0706165. [DOI] [PubMed] [Google Scholar]
- 2.Hussey PS, Sorbero ME, Mehrotra A, Liu H, Damberg CL. Episode-Based Performance Measurement And Payment: Making It A Reality. Health Affairs. 2009 Sep 1;28(5):1406–1417. doi: 10.1377/hlthaff.28.5.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. New England Journal of Medicine. 2007 Mar 15;356(11):1130–1139. doi: 10.1056/NEJMsa063979. [DOI] [PubMed] [Google Scholar]
- 4.National Priorities Partnership. [Accessed February 24, 2010];National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. 2008 http://www.nationalprioritiespartnership.org/uploadedFiles/NPP/08-253-NQFReportLo%5B6%5D.pdf.
- 5.Institute of Medicine. Priority areas for national action: transforming health care quality. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 6.Miller HD. From Volume To Value: Better Ways To Pay For Health Care. Health Aff. 2009 Sep 1;28(5):1418–1428. doi: 10.1377/hlthaff.28.5.1418. 2009. [DOI] [PubMed] [Google Scholar]
- 7.Rittenhouse DR, Shortell SM, Fisher ES. Primary Care and Accountable Care -- Two Essential Elements of Delivery-System Reform. N Engl J Med. 2009 Dec 10;361(24):2301–2303. doi: 10.1056/NEJMp0909327. 2009. [DOI] [PubMed] [Google Scholar]
- 8.Fisher ES, McClellan MB, Bertko J, et al. Fostering Accountable Health Care: Moving Forward In Medicare. Health Aff. 2009 Jan 27;2009 doi: 10.1377/hlthaff.28.2.w219. hlthaff.28.22.w219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenthal MB. Beyond Pay for Performance -- Emerging Models of Provider-Payment Reform. New England Journal of Medicine. 2008 Sep 18;359(12):1197–1200. doi: 10.1056/NEJMp0804658. 2008. [DOI] [PubMed] [Google Scholar]
- 10.Peikes D, Chen A, Schore J, Brown R. Effects of Care Coordination on Hospitalization, Quality of Care, and Health Care Expenditures Among Medicare Beneficiaries: 15 Randomized Trials. JAMA. 2009 Feb 11;301(6):603–618. doi: 10.1001/jama.2009.126. 2009. [DOI] [PubMed] [Google Scholar]
- 11.Atlas SJ, Grant RW, Ferris TG, Chang Y, Barry MJ. Patient–Physician Connectedness and Quality of Primary Care. Annals of Internal Medicine. 2009;150(5):325–335. doi: 10.7326/0003-4819-150-5-200903030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saultz JW. Defining and Measuring Interpersonal Continuity of Care. Ann Fam Med. 2003 Sep 1;1(3):134–143. doi: 10.1370/afm.23. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Brantes F, Rastogi A, Painter M. Reducing Potentially Avoidable Complications in Patients with Chronic Diseases: The Prometheus Payment Approach. Health Services Research. 2010;45(6p2):1854–1871. doi: 10.1111/j.1475-6773.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bice TW, Boxerman SB. A quantitative measure of continuity of care. Medical Care. 1977:347–349. doi: 10.1097/00005650-197704000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Jee SH, Cabana MD. Indices for continuity of care: a systematic review of the literature. Medical Care Research and Review. 2006;63(2):158. doi: 10.1177/1077558705285294. [DOI] [PubMed] [Google Scholar]
- 16.Pope G, Kautter J, Ellis R, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financing Review. 2004;25(4):119–142. [PMC free article] [PubMed] [Google Scholar]
- 17.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank quarterly. 2005;83(3):457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diehr P, Yanez D, Ash A, Hornbrook M, Lin D. Methods for analyzing health care utilization and costs. Annual Review of Public Health. 1999;20(1):125–144. doi: 10.1146/annurev.publhealth.20.1.125. [DOI] [PubMed] [Google Scholar]
- 19.McDonald K, Sundaram V, Bravata D, et al. Closing the quality gap: A critical analysis of quality improvement strategies. Volume 7 - care coordination. Rockville, MD: AHRQ; 2007. [PubMed] [Google Scholar]
- 20.McDonald K, Schultz E, Albin L, et al. Care Coordination Measures Atlas. Rockville, MD: Agency for Heathcare Research and Quality; 2010. [Google Scholar]
- 21.Steinwachs DM. Measuring provider continuity in ambulatory care: an assessment of alternative approaches. Medical Care. 1979:551–565. doi: 10.1097/00005650-197906000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Breslau N, Haug MR. Service delivery structure and continuity of care: a case study of a pediatric practice in process of reorganization. Journal of Health and Social Behavior. 1976:339–352. [PubMed] [Google Scholar]
- 23.Pollack CE, Hussey PS, Rudin RS, Fox DS, Lai J, Schneider EC. Measuring Care Coordination: A Comparison of Claims-based Methods. unpublished. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.