Abstract
Purpose
This pilot study examined if the combination of exercise training and reducing sedentary time (ST) results in greater changes to health markers than either intervention alone.
Methods
Fifty-seven overweight/obese participants (19M/39F) (mean ± SD; age 43.6 ± 9.9 y, BMI 35.1 ± 4.6 kg/m2) completed the 12-week study and were randomly assigned to 1) EX: exercise 5-days/week for 40-minutes/session at moderate intensity; 2) rST: reduce ST and increase non-exercise physical activity; 3) EX-rST: combination of EX and rST and 4) CON: maintain behavior. Fasting lipids, blood pressure (BP), VO2 peak, BMI and 2-hr oral glucose tolerance tests were completed pre- and post-intervention.
Results
EX and EX-rST increased VO2 peak by ~10% and decreased systolic BP (both p<0.001). BMI decreased by −3.3% (95% CI: −4.6 to −1.9%) for EX-rST and −2.2% (−3.5 to 0.0%) for EX. EX-rST significantly increased C-ISI by 17.8% (2.8 to 32.8%) and decreased insulin area-under-the-curve by 19.4% (−31.4 to −7.3%). No other groups improved in insulin action variables. rST group decreased ST by 7% (~50 min/day), however BP was the only health-related outcome that improved.
Conclusions
EX and EX-rST improved VO2 peak and BMI providing further evidence that moderate intensity exercise is beneficial. The within-group analysis provides preliminary evidence that exercising and reducing ST may result in improvements in metabolic biomarkers that are not seen with exercise alone, though between group differences did not reach statistical significance. Future studies, with larger samples, should examine health-related outcomes resulting from greater reductions in ST over longer intervention periods.
Keywords: sitting, training, cardiometabolic risk factors, insulin resistance
Introduction
There is consensus that engaging in moderate to vigorous physical activity (MVPA) decreases risk of type 2 diabetes and cardiovascular disease (CVD) (PAGAC 2008). However, when individuals begin exercise training they may compensate by decreasing their physical activity throughout the rest of the day, including activities of daily living, maintaining posture, and ambulation (Garland et al. 2010; King et al. 2007; Levine et al. 2000; Melanson et al. 2013). If compensation occurs, the magnitude of weight loss and improvements in health following exercise training may be attenuated (Di Blasio et al. 2012; Manthou et al. 2009). As non-exercise physical activity decreases, there is an increase in time spent in sedentary behaviors (awake, sitting/lying with low levels of EE (<1.5 METs)) (Garland et al. 2010; King et al. 2007; Levine et al. 2000; Melanson et al. 2013; Sedentary Behavior Research Network, 2012). Healy et al. (2008) showed time spent in sedentary behaviors had a detrimental dose-response relationship with waist circumference and 2-hr plasma glucose among individuals meeting public health recommendations for MVPA. This evidence supports the notion that reducing sedentary time in addition to exercise training may add to the benefits of exercise training. However, this evidence is primarily observational in which MVPA is statistically controlled, rather than directly modified (Grøntved and Hu 2011; Healy et al. 2011; Matthews et al. 2012; Thorp et al. 2010). Thus, cause (decreasing sedentary time) and effect (improving health outcomes) cannot be determined.
Despite public health initiatives, the majority of the population does not meet current recommendations for MVPA (Troiano et al. 2007). Since sedentary behaviors are highly prevalent and associated with an increased risk of obesity, chronic disease and mortality (Grøntved and Hu 2011; Healy et al. 2011; Matthews et al. 2012), it has been suggested that reducing sedentary time without changing MVPA may be a more achievable target to improve health. There are observational data supporting this but experimental data examining the health impact of changing sedentary behavior is limited to animal studies (Zderic and Hamilton 2006) or 1-day interventions in humans (Dunstan et al. 2012; Peddie et al. 2013; Stephens et al. 2011). There are limited data on the health effects of changing sedentary behavior (Bassett et al. 2010). The limited data are in studies using treadmill workstations, which may not be feasible for all individuals (Alkhajah et al. 2012; John et al. 2011; Koepp et al. 2013).
To improve our understanding of the consequences of sedentary behaviors on human health, we performed a study that manipulated both sedentary time and MVPA during a 12-week intervention. Specifically, we examined changes in cardiorespiratory fitness (CRF), insulin sensitivity, blood lipids, and body composition in four intervention groups: 1) non-exercise control (CON), 2) sedentary time reduction group (rST), 3) exercise training group (EX) or 4) exercise training group plus sedentary time reduction (EX-rST). We hypothesized that an intervention combining exercise training with reducing sedentary time will cause greater changes to health markers than either intervention alone. This intervention is both novel and timely, given the paucity of experimental evidence directly examining the impact of reducing sedentary time on health.
Materials and methods
Eligibility criteria
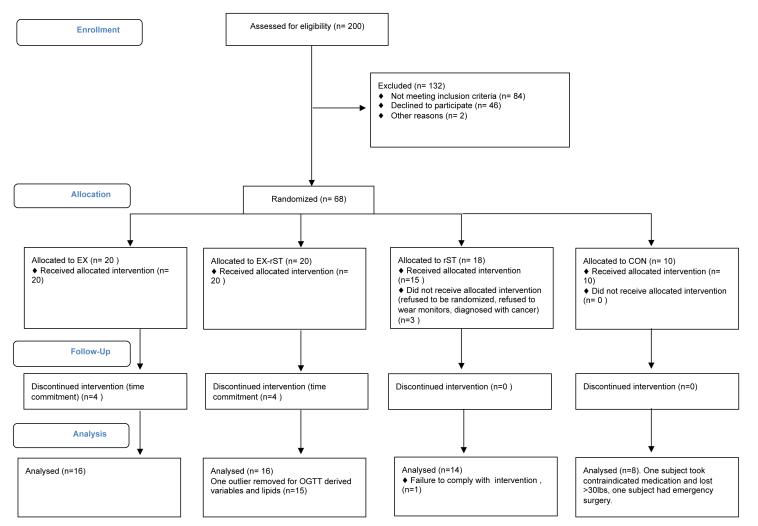
Fifty-seven individuals completed the pre-and post-intervention measures. Screening, randomization and reasons for withdrawal are shown in Figure 1. Potential participants were recruited from Amherst, MA and the surrounding area from March 2010 to May 2011. Eligible participants were 20-60 years of age, non-exercising (< 3 days/week for < 20 minutes per session) and employed in an inactive occupation (self-report >75% day at work was spent sedentary). Exclusion criteria included major orthopedic limitations, life-threatening illness (e.g., terminal cancer), or any condition for which a physician would not recommend exercise. Participants were excluded if they had weight loss surgery within the last year or were taking medication for type II diabetes (e.g. metformin) or beta-blocker medication for high blood pressure. Participants read and signed an informed consent document approved by the University of Massachusetts Institutional Review Board. Eligible participants were those at increased risk of cardiovascular disease and satisfied two of the following three criteria: 1) Prehypertensive: resting blood pressure between 125-160 mmHg systolic and/or 85-100 mmHg diastolic, 2) overweight/obese: body mass index (BMI) between 25 and 45 kg·m−2, 3) high central adiposity: as defined by elevated natural waist circumference (> 102 cm [males] > 88cm [females]), a surrogate measure of visceral fat. Of the 103 participants who signed informed consent documents, 33 were ineligible based on the criteria above, and the remaining 70 were scheduled for study visits (Figure 1).
Figure 1.
Flow- chart of participant recruitment and enrollment.
Study design and group assignment
This study was a four-arm intervention study and is registered as clinical trial at www.clinicaltrials.gov (NCT 01580930). This is not a randomized controlled trial, as control participants were able to re-enroll as exercise participants after completing the control period, with random assignment to EX or EX-rST. Participants were assigned to groups that were blocked on age (<40 or >40), sex (M/F) and BMI (<35 and >35 kg/m2). For example, the first female was assigned to EX, the second female to EX-rST and so on. We a priori decided the control group would have 10 participants and that the exercise groups, the primary comparison of interest, would have 15 participants with complete data (power analysis below). Therefore, if an exercise participant dropped out the next eligible subject was used as a replacement. Participants were assigned by a researcher (SKK) to one of four groups (described below) and were notified of group assignment immediately after completion of the VO2 peak test. Figure 1 shows the flow chart of participant recruitment and enrollment process.
Exercise group (EX)
Participants exercised 5-days per week, for 12 weeks. Exercise training intensity was prescribed as a percentage of heart rate reserve (HRR = HRmax–HRrest) and each exercise session lasted for 40 minutes (a total of 200 minutes per week). Exercise training was performed on a treadmill (3 of 5 sessions per week), stationary cycle ergometer or Arctrainer™ (Cybex, Medway, MA). Week 1: 40-50% of HRR for 30 min/session; Week 2: 50-60% of HRR for 35 min/session; Weeks 3-6: 50-60% of HRR for 40 min/session; Weeks 7-12: 55-65% of HRR for 40 min/session. All exercise sessions were supervised and exercise intensity was monitored throughout the session using heart rate (Polar RS400, Polar USA) and rating of perceived exertion.
Sedentary time reduction group (rST)
Participants were provided home, work, and discretionary time strategies to increase their non-exercise physical activity and decrease sedentary time, and was modeled from a pilot intervention (Kozey Keadle et al. 2012). At the beginning of the intervention period, a trained researcher (SKK) discussed home, work, and discretionary time strategies to increase their non-exercise physical activity (e.g., standing during all commercials, taking a 5-minute movement break each hour at work) and counseled participants on the benefits of reducing sedentary time. They were instructed not to participate in exercise, but to accumulate non-exercise physical activity throughout the day in small bouts of activity. After the initial delivery of the intervention, participants met with a trained research assistant weekly (SKK or AH). Each meeting followed a standard format where participants were asked to identify the following; a) successful strategies they used in the previous week to reduce sedentary time, b) barriers or challenges they faced, c) times of the day or days of the week that were particularly challenging, and d) strategies to overcome barriers.
There were no known tools that could simultaneously monitor sitting time and provide immediate feedback to participants during the intervention. Thus, to facilitate compliance and encourage self-monitoring of behavior participants wore an Omron pedometer daily (HJ720-ITC, Omron Healthcare, Bannockburn, Illinois). They were provided a weekly step goal that was a modest increase based on the participant’s baseline steps·day−1. If the participant was taking <5000 steps·day−1 at baseline, the step goal was increased by 10%. Once the participant attained >5000 steps·day−1, the step goal was increased by 5% for the subsequent week. Adherence to the step goal was reviewed at the weekly meeting with researchers and participants. Following the weeks that the participants wore the activPAL™ (baseline, and weeks 3, 6, and 9) the participants reviewed the daily printout of sedentary time and identified problematic times of day.
Exercise and sedentary time reduction group (EX-rST)
The EX-rST received the identical exercise training dose (i.e., duration, intensity, and frequency) as the EX group. In addition, participants received the rST strategies to reduce sedentary time and increase non-exercise physical activity.
Control group (CON)
Participants in the control group were asked to maintain their current level of activity for the 12-week study period. They completed the physical activity measurement protocols at the same time-points as the other groups (discussed below). Participants in the control group were offered the opportunity to be randomized into one of the two exercise groups following the post-intervention measures.
Measurement of physical activity and sedentary behavior
Participants wore an activPAL™ (PAL Technologies, Glasgow, Scotland) activity monitor for seven days at baseline and weeks three, six, nine, and twelve of the intervention period. Details of the monitoring protocol are published elsewhere (Kozey Keadle et al. 2013). Multiple physical activity and sedentary behavior measures, including average time spent sedentary, steps·day−1, MVPA-total (total minutes > 3 METs), MVPA-guideline (minutes of MVPA that were accumulated in 10-minute bouts), MET-hrs, and break-rate were determined for each time-point. Sedentary time is expressed as a % of waking hours.
Measurement of diet
Diet was measured using an Automated Self-administered 24-hour Dietary Recall (ASA24™) (http://riskfactor.cancer.gov/tools/instruments/asa24/). The format and design of ASA24 are based on a modified version of the interviewer-administered Automated Multiple Pass Method (AMPM) 24-hour recall developed by the U.S. Department of Agriculture (USDA). AMPM uses multi-level food probes to assess food types and amounts. AMPM was adapted to enable the development of a computer-based self-administered 24-hour recall. Participants completed the ASA24 the morning of their baseline oral glucose tolerance test (OGTT) and at weeks 4, 8 and 12 of the training period to assess confounding effects of changes in diet over the course of the training period. Participants were reminded of their dietary intake from the day prior to the baseline OGTT and asked to eat the same items the day before their post-OGTT. They were instructed to maintain their diet throughout the study.
Outcomemeasures
Cardiorespiratory fitness (CRF)
A VO2 peak test was used to assess CRF. Participants completed a brief (~2 minute) habituation period on the treadmill and were asked to choose a walking speed that was brisk but comfortable. They were then fitted with the metabolic measurement system (True- Max2400 Metabolic Measurement System, Parvomedics, Salt Lake City, UT) and completed an incremental, graded exercise test to maximal voluntary exhaustion. For males >45 y and females > 55 y a physician was present during the protocol. The treadmill speed was the participant chosen walking speed and treadmill grade was increased every 2-min until the participant could no longer continue the test. Gas-exchange variables were recorded every 30 seconds. Volume and gas calibrations were conducted on the metabolic measurement system before each test. Standard criteria for achievement of maximal exertion were used including RER >1.1, a plateau in VO2 despite an increase in work, and HR within 15 beats of age-predicted maximum. The post-intervention VO2 peak test was performed within 72 hours of the last exercise session for EX and EX-rST. The VO2 peak was performed within 72 hours of the end of the 12-week intervention period for the rST and CON participants.
Body weight and body composition
Body weight (measured to the nearest 0.1 kg) and height (measured to the nearest 0.1 cm) were measured while participants wore a thin layer of clothing and no shoes using a calibrated floor scale/stadiometer. BMI was calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured at the level of the imbilicus using a plastic tape measure to the nearest 0.1cm. Two measurements were taken and the average of the two measures was used. Participants also completed a Dual X-Ray Absorptiometry Test (DEXA; GE/Lunar Corp., Madison, WI) to estimate total percent fat (TBF%) and visceral fat mass. For all groups, these measures were done within 48-hours of the OGTT pre-intervention. Post-intervention they was completed within 48 hours of the last exercise training session for EX and EX-rST. The OGTT was performed within 48 hours of the end of the 12-week intervention period for the rST and CON participants.
Blood pressure
Blood pressure was measured manually following a minimum of 10 minutes of quiet sitting. It was measured twice with a 1-min break between measures. If the values were not within 5mmHg, a third measure was taken. Blood pressure measurements were done at the initial screening visit for all groups, Post-intervention it was completed within 24 hours of the last exercise training session for EX and EX-rST and within 24 hours of the end of the 12-week intervention period for the rST and CON participants
Blood lipids
Fasting blood samples were collected in syringes and then transferred to vacutainers containing EDTA. All samples were immediately centrifuged, and the plasma was transferred to cryogenic vials and frozen at −80° C until analysis. All samples were run in duplicate and pre/post samples were run concurrently. Triglyceride, total cholesterol and HDL were measured using the MICRO-STAT multi-assay analyzer. For participants in EX and EX-rST, the fasting lipids were measured 20-24 hours following the last exercise session. The fasting lipids were measured within 24 hours of the end of the 12-week intervention period for the rST and CON participants.
Insulin action
Following the baseline period, subjects reported to the laboratory after an overnight fast. A catheter was inserted into a forearm vein, and a resting blood sample was taken. Subjects ingested a 75-g glucose solution (Sun Dex, Fisher Healthcare, Houston, TX) and blood samples were collected at 30, 60, 90 and 120 minutes while subjects rested in a seated position. Samples for analysis of glucose were collected in syringes and then transferred to vacutainers containing sodium fluoride. Samples for insulin analysis were transferred to vacutainers containing EDTA. All samples were immediately centrifuged, and the plasma was transferred to cryogenic vials and frozen at −80° C until analysis. For participants in EX and EX-rST, the post-intervention OGTT was scheduled 20-24 hours following the last exercise session. The OGTT was performed within 24 hours of the end of the 12-week intervention period for the rST and CON participants.
Glucose concentrations were determined using a MICRO-STAT Multi-Assay Analyzer (GM7 Analyzer, Analox Inrstuments, Lunenberg, MA). Insulin concentration was measured using radioimmunoassay (Millipore, Billerica, MA). All samples were run in duplicate and pre/post samples were run concurrently. The composite insulin-sensitivity index (C-ISI) was used to estimate insulin sensitivity from the OGTT (Matsuda and DeFronzo 1999). This index uses a two-term equation to account for insulin sensitivity of the hepatic and peripheral tissue ((10,000/√ [FPG * FPI] * [G * I]), where FPG is fasting plasma glucose (mmol·L−1), FPI is fasting plasma insulin (μU·mL−1), G is mean glucose concentration (mmol·L−1), and I is mean insulin concentration (μU·mL−1) during the OGTT. Glucose and insulin area under the curve were calculated using the trapezoid method. In addition, FPG, FPI, and 2-hour glucose and insulin values were assessed.
Statistical Evaluation
All statistical analyses were performed using R-software packages (www.r-project.org). Significance level was set at p<0.05. An analysis of variance was used to estimate the sample size and power. The test is a two- sample comparison across people of the pre-training minus post-training effects within people. Assuming an alpha of 0.05, a 10% difference between the groups and, conservatively, 75% reliability of the pre- and post- measurements within a person, the power is 90% with 15 subjects per group. We used information on the variability in VO2 peak from Tjonna et al. (2008) as inputs to these power calculations. Pre-to-post intervention effects for each group and outcome measure were evaluated using linear mixed models. We used a per-protocol analysis that included those who had both pre-and-post measurements. The outcome measures were evaluated on a log scale when the size of the change was significantly correlated with the baseline level. That was true for all outcomes except percent body fat and the two cardiorespiratory fitness measures. Changes in the difference scores between groups were tested with analysis of covariance models where the post-intervention measurement was regressed against the pre-intervention measurement and the group (Vickers 2001) . For the activity and inactivity variables, the model described above was used in combination with code to account for multiple days of observation per week. For all outcome measures, values are reported as a mean percent change (95% confidence interval).
Results
Adherence to exercise training protocol
The adherence to the exercise training protocol was excellent. Participants completed over 99% of the prescribed exercise dose (sessions and duration). At week 12, participants were at 96% of the target HR (65% of HRR) on average and only one participant was below 95%, (HR at 91% of the target). For all sessions in week 12, the average HR was within 5 bpm of the target HR (65% of HRR). Similar results were seen for weeks 3, 6, and 9.
Summary of physical activity and sedentary time changes
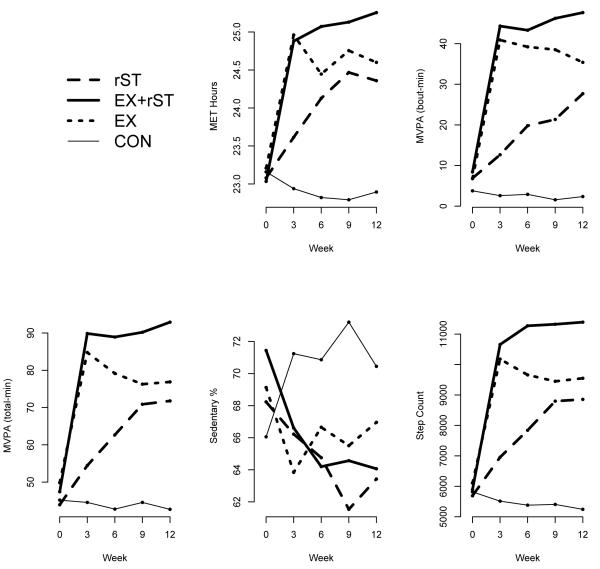
The participant characteristics for each group are shown in Table 1. There were no differences between groups in age, BMI, VO2 peak or any activity/sedentary behavior variable at baseline. Changes in physical activity and sedentary time for all measurement time-points are shown in Figure 2 and described in detail in Kozey Keadle et al. (2013). Table 2 shows the specific changes between baseline and week 12. The EX-rST group significantly increased time in MVPA-guideline by 39.2 (32.2 to 46.2) min/day, non-exercise MVPA-total by 27.8 (20.5 to 35.2) min/day and decreased total day sedentary time by 10.3% (70 min/day per day assuming a 16-hr waking day). Participants in the EX group increased MVPA-guideline 28.6 (22.1 to 35.2) min/day, as expected but did not significantly increase non-exercise MVPA (−0.4 (−5.5 to 4.7) min/day), or decrease sedentary time −3.0% (~20 min assuming 16-hr waking day). The rST group reduced sedentary time by 7.0 % (~48 min assuming 16-hr waking day) and increased MVPA-total 27.9 (20.5 to 35.2) min/day. Notably, MVPA-total, MET-hrs, steps, percent stepping, and sedentary time were not different between EX and rST during weeks six, nine and twelve, even when exercise time for EX was included. CON significantly increased sedentary time by 6.5% and standing time significantly decreased.
Table 1.
Participant Characteristics by Group
| EX | EX-rST | rST | CON | |||||
|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | mean | SD | |
| Age (y) | 43.9 | 9.7 | 42.4 | 10.7 | 44.5 | 9.5 | 42.7 | 10.1 |
| BMI (kg/m2) | 35.2 | 5.3 | 35 | 4.2 | 34.8 | 4.3 | 35.3 | 5.2 |
| VO2 peak (ml/kg/min) | 26 | 4.6 | 24.3 | 5.1 | 24.4 | 4.8 | 24.5 | 3.1 |
| Systolic BP (mmHg) | 124.8 | 10.6 | 122.6 | 7.9 | 128.5 | 11 | 132.1 | 7.3 |
| Diastolic BP (mmHg) | 77.3 | 8.9 | 78.8 | 6.8 | 84.2 | 7.1 | 80.9 | 9.1 |
Note: There were no significant differences between groups at p<0.05.
Figure 2.
Changes in physical activity and sedentary time over the intervention period. EX-rST is exercise and sedentary time reductions (n=16), rST is sedentary time reductions (n=14), EX is exercise only (n=16), CON is control (n=8)
Table 2.
Baseline and week 12 values for physical activity and sedentary behavior by group
| EX | EX-rST | rST | CON | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week | mean | SD | mean | SD | mean | SD | mean | SD | Between group differences |
|
|
Percent Sedentary
(%) |
base | 69.1 | 7.8 | 71.4 | 7.4 | 68.2 | 8.0 | 66.1 | 7.1 | ns |
| twelve | 67 | 5.2 | 64.1* | 5.6 | 63.4* | 6.2 | 70.4* | 5.4 | EX-rST < EX; rST, EX- rST, EX < CON |
|
|
| ||||||||||
|
Percent Standing
(%) |
base | 22 | 6.2 | 20.2 | 6.6 | 23 | 5.9 | 25 | 5.4 | ns |
| twelve | 21.2 | 4.8 | 22.2 | 4.4 | 25 | 4.9 | 21.4* | 4.5 | rST, EX-rST > CON | |
|
| ||||||||||
| Steps per day | base | 6108 | 2138 | 5892 | 2576 | 5689 | 2491 | 5818 | 1513 | ns |
| twelve | 9548* | 1792 | 11392* | 1828 | 8855* | 1528 | 5243 | 1194 | EX-rST > rST, EX; EX- rST, EX, rST > CON |
|
|
| ||||||||||
|
Break-rate
(breaks·sed.hour−1) |
base | 4.6 | 1.6 | 4.4 | 1.6 | 4.6 | 1.4 | 4.9 | 1.7 | ns |
| twelve | 4.3 | 0.8 | 4.9* | 0.8 | 5.1 | 1.0 | 4.6 | 0.9 | EX-rST > EX | |
|
| ||||||||||
| MET-hrs | base | 23.2 | 1 | 23 | 1 | 23.1 | 1.1 | 23.2 | 0.8 | ns |
| twelve | 24.6* | 0.8 | 25.3* | 0.8 | 24.4* | 0.7 | 22.9 | 0.6 | EX-rST> rST, EX, CON; EX, rST > CON |
|
|
| ||||||||||
|
MVPA-Guideline
(min per day) |
base | 6.8 | 10.9 | 8.4 | 18.1 | 6.9 | 15 | 3.8 | 3.6 | ns |
| twelve | 35.4* | 14.3 | 47.6* | 14.6 | 27.7* | 11.2 | 2.4 | 4.7 | EX-rST > rST, CON; EX, rST > CON |
|
|
| ||||||||||
|
MVPA-total
(min per day) |
base | 49.9 | 16.5 | 47.4 | 21.7 | 43.9 | 20.1 | 45.2 | 12.6 | ns |
| twelve | 76.9* | 15.8 | 92.9* | 16.5 | 71.8* | 12.9 | 42.7 | 9.8 | EX-rST > rST, EX, CON; rST, EX > CON |
|
Note: Values are based on activPAL estimates of active and sedentary behaviors. Break-rate refers to sit-to-stand transitions per hour of sedentary time. MVPA-guideline refers to physical activity >3 METs that was accumulated in 10-minute bouts, allowing for a 2-minute interruption. MVPA-total is the sum of time spent >3METs, based on 1-second epochs.
indicate statistical significance between baseline and week 12 within groups at P<0.05. Between group differences are also based on P<0.05. EX-rST is exercise and sedentary time reductions (n=16), rST is sedentary time reductions (n=14), EX is exercise only (n=16), CON is control (n=8)
Diet
There were no significant differences in caloric intake between groups at baseline. The CON significantly increased Kcal/day at week three compared to baseline and the EX-rST group reported significantly high Kcal/day in week 8 compared to baseline (p<0.05). There were no other significant differences in Kcal/day within or between groups.
Outcome Measures
CRF
Results are shown in Table 3. Both EX and EX-rST significantly improved CRF when expressed as ml/kg/min and L/min (p<0.01). There were no significant changes in VO2 peak for rST or CON (Table 3). EX and EX-rST had a significantly greater change in CRF than CON and rST, when VO2 peak was expressed in ml/kg/min and L/min.
Table 3.
Pre and Post intervention values for outcome measures
| Group | Pre | Post | Percent change | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | average | Lower 95% CI |
Upper 95% CI |
Within-group P-value |
||
| VO2 peak | CON | 23.5 | 2.3 | 23.2 | 3.6 | −1.2 | −7.9 | 5.6 | ns |
| ml/kg/min | rST | 24.6 | 5.0 | 24.8 | 5.4 | 0.7 | −2.4 | 3.8 | ns |
| EX | 26.1 | 4.7 | 28.6 | 5.2 | 9.3 † | 4.5 | 14.2 | < 0.001 | |
| EX-rST | 24.3 | 5.1 | 27.2 | 6.2 | 11.8 † | 7.0 | 16.7 | < 0.001 | |
| VO2 peak | CON | 2.3 | 0.4 | 2.2 | 0.5 | −1.2 | −8.2 | 5.7 | ns |
| L/min | rST | 2.5 | 0.8 | 2.5 | 0.8 | 0.3 | −2.6 | 3.2 | ns |
| EX | 2.6 | 0.6 | 2.7 | 0.7 | 7.2 † | 2.6 | 11.8 | < 0.01 | |
| EX-rST | 2.5 | 0.7 | 2.6 | 0.7 | 6.9 † | 1.9 | 11.9 | <0.01 | |
| BMI | CON | 34.8 | 5.3 | 34.6 | 4.8 | −0.4 | −2.1 | 1.4 | ns |
| rST | 34.9 | 4.4 | 34.9 | 4.1 | 0.0 | −1.3 | 1.2 | ns | |
| EX | 34.9 | 5.3 | 34.1 | 4.9 | −2.2 ‡ | −3.5 | −0.9 | < 0.01 | |
| EX-rST | 35.0 | 4.2 | 33.9 | 4.4 | −3.3 † | −4.7 | −1.9 | < 0.01 | |
| Total Body Fat | CON | 45.9 | 5.9 | 46.6 | 6.2 | 1.6 | −0.5 | 3.8 | ns |
| (%) | rST | 45.3 | 5.8 | 45.1 | 6.5 | −0.5 | −2.8 | 1.8 | ns |
| EX | 44.9 | 6.3 | 43.9 | 6.2 | −2.2 | −4.0 | −0.4 | 0.02 | |
| EX-rST | 44.4 | 8.0 | 43.0 | 8.0 | −3.2 ‡ | −6.8 | 0.4 | 0.08 | |
| Weight | CON | 96.3 | 14.1 | 95.9 | 13.1 | −0.3 | −2.0 | 1.5 | ns |
| (Kg) | rST | 101.2 | 15.2 | 101.2 | 14.7 | 0.1 | −1.1 | 1.2 | ns |
| EX | 97.7 | 17.5 | 95.4 | 16.5 | −2.2 † | −3.4 | −1.0 | <0.01 | |
| EX-rST | 100.1 | 14.5 | 96.7 | 14.3 | −3.5 † | −4.8 | −2.2 | <0.01 | |
| Systolic BP | CON | 133.8 | 7.2 | 127.9 | 9.5 | −4.6 | −9.4 | 0.3 | ns |
| mmHg | rST | 127.3 | 10.4 | 122.6 | 11.8 | −3.8 | −7.4 | −0.3 | 0.04 |
| EX | 124.9 | 11.0 | 117.9 | 8.4 | −5.7 | −10.9 | −0.4 | 0.04 | |
| EX-rST | 122.6 | 7.9 | 116.7 | 8.3 | −5.0 | −9.8 | −0.1 | 0.04 | |
| Diastolic BP | CON | 80.1 | 10.0 | 78.3 | 6.3 | −1.9 | −13.1 | 9.4 | ns |
| mmHg | rST | 82.9 | 5.3 | 78.9 | 6.9 | −5.1 | −9.6 | −0.6 | 0.03 |
| EX | 78.2 | 8.4 | 76.9 | 8.5 | −1.8 | −7.4 | 3.9 | ns | |
| EX-rST | 78.8 | 6.8 | 75.3 | 8.3 | −4.9 | −11.2 | 1.4 | ns | |
| Total | CON | 4.2 | 0.7 | 4.5 | 0.8 | 5.7 | −11.7 | 23.1 | ns |
| Cholesterol | rST | 5.0 | 0.8 | 4.9 | 0.6 | −1.0 | −7.0 | 5.1 | ns |
| mmol/L | EX | 4.4 | 1.0 | 4.6 | 0.7 | 5.5 | −4.7 | 15.7 | ns |
| EX-rST | 4.6 | 1.3 | 4.4 | 1.0 | −2.1 | −11.5 | 7.3 | ns | |
| HDL | CON | 1.5 | 0.6 | 1.7 | 0.7 | 8.7 | −24.7 | 42.0 | ns |
| Cholesterol | rST | 1.8 | 0.5 | 1.7 | 0.3 | −1.2 | −12.5 | 10.1 | ns |
| mmol/L | EX | 1.7 | 0.5 | 1.7 | 0.4 | 0.9 | −14.9 | 16.7 | ns |
| EX-rST | 1.7 | 0.6 | 1.6 | 0.5 | −0.8 | −22.7 | 21.1 | ns | |
| Triglycerides | CON | 1.8 | 0.7 | 1.9 | 1.3 | −19.6 | −74.8 | 35.7 | ns |
| mmol/L | rST | 1.9 | 1.4 | 2.1 | 1.5 | 16.9 | −1.5 | 35.2 | 0.07 |
| EX | 1.7 | 1.0 | 1.4 | 0.9 | −30.2* | −55.8 | −4.7 | 0.02 | |
| EX-rST | 2.1 | 1.3 | 1.7 | 0.9 | −19.7 | −43.0 | 3.8 | 0.09 | |
| C-ISI | CON | 2.0 | 1.4 | 2.1 | 1.0 | 13.6 | −18.7 | 46.0 | ns |
| rST | 2.2 | 1.2 | 2.1 | 0.9 | −0.4 | −26.2 | 25.3 | ns | |
| EX | 2.4 | 1.1 | 2.8 | 1.6 | 12.0 | −4.6 | 28.6 | ns | |
| EX-rST | 2.0 | 1.1 | 2.3 | 1.1 | 17.8 | 2.8 | 32.8 | 0.02 | |
| Fasting insulin | CON | 19.3 | 10.4 | 16.5 | 12.3 | −17.4 | −54.6 | 19.7 | ns |
| μIU/mL | rST | 23.8 | 16.2 | 20.2 | 14.8 | −16.5 | −34.2 | 1.1 | <0.1 |
| EX | 18.2 | 11.3 | 16.2 | 9.8 | −11.5 | −32.8 | 9.8 | ns | |
| EX-rST | 20.7 | 11.4 | 17.5 | 7.1 | −10.6 | −27.6 | 6.4 | ns | |
| 2-hr Insulin | CON | 164.9 | 157.3 | 151.5 | 105.2 | 4.1 | −53.5 | 61.6 | ns |
| μIU/mL | rST | 78.1 | 47.0 | 86.1 | 21.1 | 22.5 | −11.5 | 56.4 | ns |
| EX | 101.4 | 52.7 | 72.4 | 46.8 | −44.0* | −92.5 | 4.6 | 0.07 | |
| EX-rST | 134.6 | 84.8 | 104.1 | 95.6 | −36.3* | −62.8 | −9.8 | <0.01 | |
| Insulin AUC | CON | 17080.2 | 13274.8 | 16302.4 | 11419.1 | 0.6 | −25.7 | 26.9 | ns |
| μIU/mL | rST | 12073.6 | 5601.0 | 12447.9 | 4591.6 | 6.3 | −17.7 | 30.2 | ns |
| EX | 12992.5 | 4468.2 | 12212.4 | 4828.9 | −8.9 | −21.6 | 3.8 | ns | |
| EX-rST | 14987.5 | 8288.3 | 12620.7 | 8197.4 | −19.4* | −31.4 | −7.3 | <0.01 | |
| Fasting Glucose | CON | 104.2 | 15.8 | 98.3 | 12.1 | −5.4 | −12.3 | 1.6 | ns |
| mmol/L | rST | 103.7 | 9.9 | 104.7 | 14.1 | 0.5 | −3.7 | 4.7 | ns |
| EX | 95.9 | 9.6 | 97.7 | 9.7 | 1.9 | −2.4 | 6.2 | ns | |
| EX-rST | 102.0 | 17.3 | 102.1 | 12.2 | 0.5 | −3.6 | 4.6 | ns | |
| 2-Hr glucose | CON | 149.3 | 37.9 | 142.6 | 31.6 | −3.7 | −20.7 | 13.2 | ns |
| mmol/L | rST | 117.7 | 35.7 | 144.2 | 41.8 | 21.6 | 5.8 | 37.4 | 0.01 |
| EX | 121.2 | 26.2 | 116.7 | 20.6 | −3.3* | −15.9 | 9.4 | ns | |
| EX-rST | 139.8 | 44.6 | 126.0 | 40.6 | −11.3* | −25.1 | 2.5 | 0.1 | |
| Glucose AUC | CON | 19825.3 | 2803.2 | 18694.7 | 2918.6 | −6.1 | −15.5 | 3.3 | ns |
| mmol/L | rST | 17333.3 | 3354.3 | 18704.2 | 3824.6 | 7.5 | −3.9 | 18.9 | ns |
| EX | 17092.5 | 3566.5 | 16751.3 | 3071.2 | −1.6 | −7.5 | 4.4 | ns | |
| EX-rST | 18037.9 | 4669.2 | 17452.4 | 4003.0 | −3.1 | −11.3 | 5.0 | ns | |
Note: All values were analyzed on the log scale except VO2 peak (ml/kg/min and L/min) and body fat (%) EX-rST is exercise and sedentary time reductions (n=16), rST is sedentary time reductions (n=14), EX is exercise only (n=16), CON is control (n=8)
ns indicates p-value is greater than 0.1.
is significantly different than CON at p < 0.05;
is significantly different than rST;
is significantly different than both CON and rST.
Body composition
BMI was significantly decreased in both EX and EX-rST (both p< 0.01) (Table 3). There were no changes in BMI for rST or CON. EX and EX-rST had a significantly decreased BMI compared to CON and rST; no other group differences were significant. Similar trends were observed for TBF%, with a decrease of −2.2% (95% CI) ( −4.0 to −0.4)% in the EX group (p<0.05) and −3.2% (−6.8 to 0.4)% in EX+rST. rST −0.46% (−2.8 to 1.8)% and CON 1.6% (−0.5 to 3.8)% had lower changes than EX-rST.
Blood pressure and lipids
Systolic blood pressure significantly decreased in all intervention groups. (Table 3). Diastolic blood pressure significantly decreased in rST. There were no significant within-group or between-group differences in total cholesterol or HDL (Table 3). Triglycerides decreased significantly for EX (−30.2 %(−55.8 to −4.7)%).
Insulin action
The pre-and post- intervention changes in insulin action are shown in Table 3. The primary outcome measure for insulin action was C-ISI, which improved in the EX-rST group (p<0.05). The change in C-ISI was not statistically significant for the EX group, and there were no significant between group differences in the percent change for C-ISI. Insulin AUC significantly decreased for EX-rST and did not significantly decrease in EX, rST or CON. The EX-rST insulin AUC improved significantly more than rST and no other between group differences were significant. There were no significant changes in fasting insulin pre-to-post-intervention or between groups. Insulin concentration at 2-hours significantly decreased for EX-rST while the change for EX, rST or CON was not significant There were no significant changes in glucose AUC or fasting glucose pre-to-post intervention or between groups. Surprisingly, rST had a significant increase in 2-hr glucose.
Discussion
This pilot study explored the combined and independent effects of exercise training and reducing sedentary time on cardiometabolic health markers. As expected, exercise training (with and without sedentary time reductions) resulted in improvements in VO2 peak, body weight, body composition, total body fat, and systolic blood pressure. Contrary to our hypothesis, combining exercise training with reductions in sedentary time did not result in greater improvements in markers of cardiometabolic health compared to exercise training only. Individuals who reduced sedentary time (mean 7.0% decrease, ~50 min/day), but did not participate in exercise training, did not improve the majority of health outcomes. Despite no between group differences, we did see significant within-group improvements in metabolic outcomes in the combined exercise training/reduction in sedentary time group but these within-group effects were not observed in the other groups. This provides preliminary indications of differences in training responses that warrant follow-up in larger studies.
The group who exercised and also reduced sedentary time (EX-rST) significantly improved CISI, 2-hr insulin and insulin AUC, while exercise only (EX) and sedentary time reduction groups (rST) did not. Our results showing no change in C-ISI and insulin AUC among the EX group participants are consistent with previous studies that used OGTTs to estimate insulin sensitivity (Kang et al. 1996; Seals et al. 1984). Data comparing different amounts of exercise and insulin sensitivity are sparse and are confounded by differences in training protocols and methods used to determine insulin sensitivity. For example, in the STRRIDE trial the moderate intensity group improved insulin sensitivity by ~80%, which is higher than the ~20% increase seen in the current study. However, STRRIDE participants trained for 6-months and used an intravenous glucose tolerance test (IVGTT) that sampled plasma glucose and insulin 25-times over a 3-hour period to estimate insulin sensitivity (Houmard et al. 2003). Studies using IVGTT or the hyperinsulinemic euglycemic clamp have reported greater increases in insulin sensitivity (Boulé et al. 2005; Mayer-Davis et al. 1998; Oshida et al. 1989) than training studies using OGTT following moderate-intensity training (Kang et al. 1996; Seals et al. 1984). The finding that the EX-rST improved these metabolic outcomes pre/post appears to be in contrast to the fact that there was no statistically significant difference in C-ISI, 2-hr insulin and insulin AUC between groups. We offer two potential interpretations of our apparent opposing within group and between group findings.
A plausible explanation is that the study was underpowered to detect between-group differences in metabolic outcomes. This study was designed as a pilot examination of the effect of non-exercise physical activity on responsiveness to exercise training. While the field of sedentary behavior and health has grown rapidly, prior to this study there were no experimental studies examining the combined effects of exercise and reducing sedentary time on health outcomes. As a result, the expected changed of sedentary time on any health markers, including CRF, was difficult to estimate. We decided to base the power analysis for this novel intervention on the detection of a 10% difference in CRF between groups based on previous exercise studies comparing doses of exercise on CRF (Tjonna et al. 2008). Since the study commenced, few studies have examined the effects of sedentary time or light-intensity activity on CRF in adults. McGuire and Ross (2011) showed that incidental physical activity, which included light-intensity physical activity and sporadic MVPA (< 10-min bouts) was positively associated with CRF (McGuire and Ross 2011). Pettee et al. (2009) showed TV viewing was negatively associated with CRF. We did see a statistically significant difference between EX and EX-rST compared to rST and CON in CRF, but no statistically significant difference between EX and EX-rST, both groups significantly improved and there was no trend observed for a difference in response between these groups. Our hypothesis that there would be a difference in CRF was not supported, and potential reasons for this are discussed below. For the cardiometabolic variables, there were difference in the pre/post within-group responses to the OGTT, with EX-rST having improvements that the other groups did not.
Cardiometabolic response to exercise training is highly variable (Bouchard et al. 2012; Melanson et al. 2013; Thomas et al. 2012), which increases the sample needed to detect a significant difference between groups. It is also likely that cardiometablic response to reductions in sedentary time is highly variable, but this has yet to be studied. Further, in the current study, we observed large variability in the effect of the intervention on sedentary time among rST (range: −25.2 to +12.0%) and EX-rST (range −35.6 to +19.9%). Ideally, the EX participants would not have changed their sedentary time outside training and EX-rST participants would have uniformly decreased sedentary time by an additional 2 hours (for example) per day. However, that was not what we observed. We were able to uniformly prescribe, monitor and verify the exercise training sessions across individuals, but because sedentary behaviors are ubiquitous, it was impossible to observe participants throughout the entire day to ensure they decreased sedentary time. Further, there were individual differences in behavior outside training among the EX group. Approximately half of participants in the EX group compensated by decreasing their non-exercise activity and increasing sedentary time (for details see Kozey Keadle et al. 2013). Thus, both EX and EX-rST participants had variable activity outside training. These individual differences in behavior are an added challenge sedentary behavior researchers must reconcile in order to come to firm conclusions about the effect of “reducing sedentary time”.
Despite the large variability in activity and sedentary behavior outside of training, a strength of this study was that we quantified habitual behavior at multiple time-points during the intervention using a validated activity monitor (Kozey Keadle et al. 2011). The majority of exercise training studies quantify exercise volume in the training sessions but do not measure what participants are doing during the remainder of their day. The variability in behavior across groups and even within the EX group highlights the need for objective monitoring of physical activity and sedentary behavior during any interventions examining the effects of exercise or sedentary time. It also supports the work of Di Blasio et al. (2012), who suggested some individuals may require additional intervention when beginning an exercise training program to ensure behavioral compensation does not occur. Taken together, the observed variability in response to the intervention and the known variability in metabolic response to exercise training support the notion that a larger sample is needed to detect between-group differences. The preliminary finding that the EX-rST group improved their response to the OGTT pre/post intervention is novel and should be examined in future studies. Moving forward, this study can guide sample size requirements for larger trials to explore the combined effects of reducing sedentary time, exercise training and cardiometabolic health.
A second possibility is that there is no additional benefit of reducing sedentary time when an individual begins to exercise. This is not consistent with experimental studies in rodents, where it was reported that exercise and sedentary behavior influence different metabolic pathways (Hamilton et al. 2007). In humans, acute experimental studies have consistently shown metabolic benefits to standing/light intensity movement and breaks from sitting time (Dunstan et al. 2012; Stephens et al. 2011). However, few studies in humans have examined combined exercise and sedentary time changes, and most studies used models of sedentary time that are not representative of free-living behavior. For example, Stephens et al. (2011) showed that, even when controlling for energy balance, one day of sitting decreased insulin sensitivity by 18% compared to a day with high amounts of low-intensity activity and little sitting. However, the high-sit condition was 16-consecutive hours of sitting, which is much higher than even the most sedentary free-living adult (Matthews et al. 2008) and was over 10 hours greater than the low-sit condition. In our sample, the magnitude of change for the rST group was modest (7.0%, equivalent to approximately 50 minutes over a 16-hr waking day) and there were large individual differences (−25.6 to 12.0%). While the experimental work has provided important insights to physiologic mechanisms, more models that are reflective of real-life sedentary behavior are needed. Experimental models should also be expanded to include direct comparisons of the effect of sedentary time changes independent of and in addition to exercise.
It is important to note that our results for change in sedentary time are consistent with other studies that showed a modest reduction following an intervention (Gardiner et al. 2011; Kozey Keadle et al. 2012; Otten et al. 2009). Inducing greater changes may require environmental intervention such as standing workstations, which have been shown in quasi-experimental studies to improve cardiometabolic outcomes (Alkhajah et al. 2012; John et al. 2011; Koepp et al. 2013), but have not, to our knowledge, been examined in a randomized intervention trial, nor in combination with exercise training. Before conclusions regarding sedentary behavior and health benefits can be made, further research is needed that induces greater changes in sedentary time over a longer time period. It is also important to determine how feasible and acceptable it is for participants to maintain these changes over time.
The EX and EX-rST groups had comparable increases in CRF (9.3% and 11.8% respectively), indicating a similar physiological training effect for these groups. This magnitude of change in CRF is comparable to other training studies. For example, two studies with moderate-intensity exercise training programs reported increases in CRF of 6.3% and 12% after 6-months of training (Seals et al. 1984; Slentz et al. 2007). The EX and EX-rST also had similar reductions in BMI, TBF%, body weight and SBP indicating the reduction in sedentary time did not enhance these outcomes. None of the groups had significant changes in total cholesterol or HDL cholesterol, which is also consistent with the findings from the STRRIDE trial (Kraus et al. 2002). In the current study, triglycerides decreased in EX, the decrease in EX-rST was marginally significant (p=0.09) and the rST group actually had an increase in triglycerides (p=0.07). These findings are surprising given a series of studies in rodents showing that sedentary behavior decreases lipoprotein lipase (LPL), an enzyme that regulates triglyceride uptake, HDL production and glucose uptake (Hamilton et al. 2007; Hamilton et al. 2004). However, Peddie et al. (2013) recently reported that, compared to a prolonged sitting period, breaking-up sitting time was not associated with a improvements in post-prandial triglyceride levels while a single bout of exercise did result in significant improvements. Interestingly, they observed greater changes in insulin and glucose with regular activity breaks compared to a single bout of exercise and prolonged sitting, which is also consistent with our data and suggests the benefits of sedentary time reductions are specific to glycemic variables in humans (Peddie et al. 2013). Additional research is needed to understand how sedentary behavior and exercise interact to affect LPL and triglycerides levels in humans (Peddie et al. 2012).
Another important finding was that MVPA total-min, MET-hrs, steps·day−1, and sedentary time were not different between EX and rST during weeks 6, 9 and 12, even when exercise training time was included. However, the EX group improved body composition and CRF while the rST group did not. Previous cross-sectional research has shown that incidental physical activity, such as what was prescribed to the rST group, is associated with CRF among overweight/obese adults (McGuire and Ross 2011). They showed that sporadic MVPA was driving the associations with CRF, while light-intensity activity was not independently associated with CRF (McGuire and Ross 2011). Considering these data and results from the current study, it appears that incidental physical activity may be sufficient to maintain, but not to improve, aerobic fitness. In addition, the improvements in aerobic fitness may also be specific to activity intensity; the training intensity in EX groups was sustained and likely higher than the intensity of activity in the rST group.
This study also has limitations. The measure of diet that was used is validated (Arab et al. 2011) but has error associated with it and the measures were only obtained at a few time-points. It is possible there were variations in menstrual cycle that may have affected the metabolic outcomes for the females in the sample, though it is likely this affected all groups equally as subjects were randomly assigned. As discussed above, the sample size was small and potentially underpowered for detecting between-group differences for metabolic outcomes. Lastly, the study sample included overweight/obese, non-exercising individuals who had sedentary occupations and were at risk for CVD. Therefore, our results cannot be generalized to other groups.
Conclusions
This study showed that reducing sedentary time, without exercise training, by an average of 50 min per day was not sufficient to elicit benefits on risk factors for type 2 diabetes and CVD. Future studies should examine the health outcomes resulting from larger decreases in sedentary time over a longer study period. The two groups who exercised saw improvements in VO2 peak and body composition, adding to the body of evidence showing moderate intensity exercise is beneficial for health. The EX-rST also showed improvements in insulin sensitivity and insulin AUC, while the EX group did not; however there were no significant differences in response between groups. Thus, we cannot conclude that reducing sedentary time in addition to exercise training results in significantly greater improvements than traditional exercise training alone. This study provide preliminary evidence that reducing sedentary time may enhance the metabolic benefits of exercise training, and this should be explored in future studies. We highlighted some of the challenges in conducting these trials and the importance of measuring non-exercise physical activity throughout the intervention period. Large, long-term, randomized controlled trials are needed to examine the impact of reducing sedentary time, both with and without exercise training, on health outcomes.
Acknowledgements
The authors would like to thank the study participants and the dedicated graduate and undergraduate research assistants and trainers. We would like to thank Carolyn Kuzontkoski and Anna Kenseth for their assistance with data collection.
This study was funded by NIH RC1HL099557
Sarah Kozey Keadle is supported by the National Cancer Institute through the Cancer Prevention Fellowship Program
Disclosure of funding: NIH RC1HL099557
Footnotes
Conflict of Interest: The authors have no conflicts of interest to report
References
- Alkhajah TA, Reeves MM, Eakin EG, Winkler EAH, Owen N, Healy GN. Sit–Stand Workstations: A pilot intervention to reduce office sitting time. Am. J. Prev. Med. 2012;43(3):298–303. doi: 10.1016/j.amepre.2012.05.027. PMID: 22898123. [DOI] [PubMed] [Google Scholar]
- Arab L, Tseng CH, Ang A, Jardack P. Validity of a multipass, web-based, 24-hour self-administered recall for assessment of total energy intake in blacks and whites. Am. J. Epi. 2011;174(11):1256–1265. doi: 10.1093/aje/kwr224. PMID: 22021561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DR, Freedson P, Kozey S. Medical hazards of prolonged sitting. Exerc. Sport. Sci. Rev. 2010;38(3):101–102. doi: 10.1097/JES.0b013e3181e373ee. PMID: 20577056. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Blair SN, Church TS, Earnest CP, HAGBERG JM, Häkkinen K, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PloS One. 2012;7(5):e37887. doi: 10.1371/journal.pone.0037887. doi:10.1371/journal.pone.0037887. PMID: 22666405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulé NG, Weisnagel SJ, Lakka TA, Tremblay A, Bergman RN, Rankinen T, et al. Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care. 2005;28(1):108–114. doi: 10.2337/diacare.28.1.108. PMID: 15616242. [DOI] [PubMed] [Google Scholar]
- Di Blasio A, Ripari P, Bucci I, Di Donato F, Izzicupo P, D’Angelo E, et al. Walking training in postmenopause: effects on both spontaneous physical activity and training-induced body adaptations. Menopause. 2012;19(1):23–32. doi: 10.1097/gme.0b013e318223e6b3. PMID: 21993080. [DOI] [PubMed] [Google Scholar]
- Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35(5):976–983. doi: 10.2337/dc11-1931. PMID: 22374636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner PA, Eakin EG, Healy GN, Owen N. Feasibility of Reducing Older Adults’ Sedentary Time. Am. J. Prev. Med. 2011;41(2):174–177. doi: 10.1016/j.amepre.2011.03.020. PMID: 21767725. [DOI] [PubMed] [Google Scholar]
- Garland T, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J. Exp. Biol. 2010;214(Pt 2):206–229. doi: 10.1242/jeb.048397. PMID: 21177942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305(23):2448–2455. doi: 10.1001/jama.2011.812. PMID: 21673296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MT, Hamilton DG, Zderic TW. Exercise physiology versus inactivity physiology: an essential concept for understanding lipoprotein lipase regulation. Exerc. Sport. Sci. Rev. 2004;32(4):161–166. doi: 10.1097/00003677-200410000-00007. PMID: 15604935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655–2667. doi: 10.2337/db07-0882. PMID: 17827399. [DOI] [PubMed] [Google Scholar]
- Healy GN, Dunstan DW, Salmon JO, Shaw JE, Zimmet PZ, Owen N. Television Time and Continuous Metabolic Risk in Physically Active Adults. Med. Sci. Sport. Exerc. 2008;40(4):639–645. doi: 10.1249/MSS.0b013e3181607421. PMID: 18317383. [DOI] [PubMed] [Google Scholar]
- Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Europ. Heart. J. 2011;32(5):590–597. doi: 10.1093/eurheartj/ehq451. PMID: 21224291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J. Appl. Phys. 2003;96(1):101–106. doi: 10.1152/japplphysiol.00707.2003. PMID: 12972442. [DOI] [PubMed] [Google Scholar]
- John D, Thompson DL, Raynor H, Bielak K. Treadmill Workstations: A Worksite Physical Activity Intervention in Overweight and Obese Office Workers. J. Phys. Act. Health. 2011;8(8):1034–1043. doi: 10.1123/jpah.8.8.1034. PMID: 22039122. [DOI] [PubMed] [Google Scholar]
- Kang J, Robertson RJ, Hagberg JM, Kelley DE, Goss FL, DaSilva SG, et al. Effect of exercise intensity on glucose and insulin metabolism in obese individuals and obese NIDDM patients. Diabetes Care. 1996;19(4):341–349. doi: 10.2337/diacare.19.4.341. PMID: 8729157. [DOI] [PubMed] [Google Scholar]
- King NA, Caudwell P, Hopkins M, Byrne NM, Colley R, Hills AP, et al. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity. 2007;15(6):1373–1383. doi: 10.1038/oby.2007.164. PMID: 17557973. [DOI] [PubMed] [Google Scholar]
- Koepp GA, Manohar CU, McCrady-Spitzer SK, Ben-Ner A, Hamann DJ, Runge CF, Levine JA. Treadmill desks: A 1-year prospective trial. Obesity. 2013;21(4):705–711. doi: 10.1002/oby.20121. PMID: 23417995. [DOI] [PubMed] [Google Scholar]
- Kozey Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med. Sci. Sport. Exerc. 2011;43(8):1561–1567. doi: 10.1249/MSS.0b013e31820ce174. PMID: 21233777. [DOI] [PubMed] [Google Scholar]
- Kozey Keadle S, Libertine A, Staudenmayer J, Freedson P. The Feasibility of Reducing and Measuring Sedentary Time among Overweight, Non-Exercising Office Workers. J. Obes. 2012:282303. doi: 10.1155/2012/282303. 2012. PMID: 22175004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozey Keadle S, Staudenmayer J, Hickey A, Lyden K, Braun B, Freedson PS. Changes in sedentary time and non-exercise activity in response to an exercise training and/or lifestyle intervention. J. Phys. Act. Health. doi: 10.1123/jpah.2012-0340. E pub: Oct 31 2013. PMID: 24184493. [DOI] [PubMed] [Google Scholar]
- Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002;347(19):1483–1492. doi: 10.1056/NEJMoa020194. PMID: 12421890. [DOI] [PubMed] [Google Scholar]
- Levine JA, Schleusner SJ, Jensen MD. Energy expenditure of nonexercise activity. Am. J. Clin. Nutr. 2000;72(6):1451–1454. doi: 10.1093/ajcn/72.6.1451. PMID: 11101470. [DOI] [PubMed] [Google Scholar]
- Manthou E, Gill JMR, Wright A, Malkova D. Behavioural Compensatory Adjustments to Exercise Training In Overweight Women. Med. Sci. Sports Exerc. 2009;42(6):1121–1128. doi: 10.1249/MSS.0b013e3181c524b7. PMID: 19997033. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. doi: 10.2337/diacare.22.9.1462. PMID: 10480510. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am. J. Epi. 2008;167(7):875–881. doi: 10.1093/aje/kwm390. doi:10.1093/aje/kwm390. PMID: 18303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CE, George SM, Moore SC, Bowles HR, Blair A, Park Y, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am. J. Clin. Nutr. 2012;95(2):437–445. doi: 10.3945/ajcn.111.019620. PMID: 22218159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Davis EJ, D’Agostino R, Karter AJ, Haffner SM, Rewers MJ, Saad M, Bergman RN. Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA. 1998;279(9):669–674. doi: 10.1001/jama.279.9.669. PMID: 9496984. [DOI] [PubMed] [Google Scholar]
- McGuire KA, Ross R. Incidental physical activity is positively associated with cardiorespiratory fitness. Med. Sci. Sport. Exerc. 2011;43(11):2189–2194. doi: 10.1249/MSS.0b013e31821e4ff2. PMID: 21502894. [DOI] [PubMed] [Google Scholar]
- Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA. Resistance to Exercise-Induced Weight Loss: Compensatory Behavioral Adaptations. Med. Sci. Sports. Exerc. 2013;45(8):1600–1609. doi: 10.1249/MSS.0b013e31828ba942. PMID: 23470300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshida Y, Yamanouchi K, Hayamizu S, Sato Y. Long-term mild jogging increases insulin action despite no influence on body mass index or VO2 max. J. Appl. Phys. 1989;66(5):2206–2210. doi: 10.1152/jappl.1989.66.5.2206. PMID: 2663816. [DOI] [PubMed] [Google Scholar]
- Otten JJ, Jones KE, Littenberg B, Harvey-Berino J. Effects of television viewing reduction on energy intake and expenditure in overweight and obese adults: a randomized controlled trial. Arch. Int. Med. 2009;169(22):2109–2115. doi: 10.1001/archinternmed.2009.430. PMID: 20008695. [DOI] [PubMed] [Google Scholar]
- Peddie MC, Bone JL, Rehrer NJ, Skeaff CM, Gray AR, Perry TL. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am, J. Clin. Nutr. 2013;98(2):358–366. doi: 10.3945/ajcn.112.051763. PMID: 23803893. [DOI] [PubMed] [Google Scholar]
- Peddie MC, Rehrer NJ, Perry TL. Physical activity and postprandial lipidemia: are energy expenditure and lipoprotein lipase activity the real modulators of the positive effect? Prog. Lipid Res. 2012;51(1):11–22. doi: 10.1016/j.plipres.2011.11.002. PMID: 22123195. [DOI] [PubMed] [Google Scholar]
- Pettee KK, Ham SA, Macera CA, Ainsworth BE. The reliability of a survey question on television viewing and associations with health risk factors in US adults. Obesity. 2009;17(3):487–93. doi: 10.1038/oby.2008.554. PMID: 19238138. [DOI] [PubMed] [Google Scholar]
- Physical Activity Guidelines Committee Report 2008 www.health.gov/paguidelines/report.
- R Core Development Team 2013 www.R-project.org.
- Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Effects of endurance training on glucose tolerance and plasma lipid levels in older men and women. JAMA. 1984;252(5):645–649. PMID: 6376837. [PubMed] [Google Scholar]
- Sedentary Behavior Research Network Letter to the Editor: Standardized use of the terms “sedentary” and ‘sedentary behaviours’. Appl. Physiol. Nutr. Metab. 2012;37:540–542. doi: 10.1139/h2012-024. PMID: 22540258. [DOI] [PubMed] [Google Scholar]
- Slentz CA, Houmard JA, Johnson JL, Bateman LA, Tanner CJ, McCartney JS, et al. Inactivity, exercise training and detraining, and plasma lipoproteins. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl. Phys. 2007;103(2):432–442. doi: 10.1152/japplphysiol.01314.2006. PMID: 17395756. [DOI] [PubMed] [Google Scholar]
- Stephens BR, Granados K, Zderic TW, Hamilton MT, Braun B. Effects of 1 day of inactivity on insulin action in healthy men and women: interaction with energy intake. Metabolism. 2011;60(7):941–949. doi: 10.1016/j.metabol.2010.08.014. PMID: 21067784. [DOI] [PubMed] [Google Scholar]
- Thorp AA, Healy GN, Owen N, Salmon J, Ball K, Shaw JE, et al. Deleterious Associations of Sitting Time and Television Viewing Time With Cardiometabolic Risk Biomarkers: Australian Diabetes, Obesity and Lifestyle (AusDiab) study 2004-2005. Diabetes Care. 2010;33(2):327–334. doi: 10.2337/dc09-0493. PMID: 19918003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Bouchard C, Church T, Slentz C, Kraus WE, Redman LM, et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes. Revs. 2012;13(10):835–847. doi: 10.1111/j.1467-789X.2012.01012.x. doi:10.1111/j.1467-789X.2012.01012.x. PMID: 22681398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjønna AE, Lee SJ, Rognmo Ø , Stølen TO, Bye A, Haram PM, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;22(118(4)):346–54. doi: 10.1161/CIRCULATIONAHA.108.772822. PMID: 18606913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical Activity in the United States Measured by Accelerometer. Med. Sci. Sports Exerc. 2007;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. PMID: 18091006. [DOI] [PubMed] [Google Scholar]
- Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001;1:6. doi: 10.1186/1471-2288-1-6. PMID: 11459516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zderic TW, Hamilton MT. Physical inactivity amplifies the sensitivity of skeletal muscle to the lipid-induced downregulation of lipoprotein lipase activity. J. Appl. Phys. 2006;100(1):249–257. doi: 10.1152/japplphysiol.00925.2005. PMID: 16195388. [DOI] [PubMed] [Google Scholar]