Abstract
The binding of Ca2+ to troponin C (TnC) in the troponin complex is a critical step regulating the thin filament, the actin-myosin interaction and cardiac contraction. Phosphorylation of the troponin complex is a key regulatory mechanism to match cardiac contraction to demand. Here we demonstrate phosphorylation of the troponin I (TnI) subunit is simultaneously increased at Ser-150 and Ser-23/24 during in vivo myocardial ischemia. Myocardial ischemia decreases intracellular pH resulting in depressed binding of Ca2+ to TnC and impaired contraction. To determine the pathological relevance of simultaneous TnI phosphorylation we measured individual TnI Ser-150 (S150D), Ser-23/24 (S23/24D) and combined (S23/24/150D) pseudo-phosphorylation effects on thin filament regulation at acidic pH similar to that in myocardial ischemia. Results demonstrate that while acidic pH decreased thin filament Ca2+ binding to TnC regardless of TnI composition, TnI S150D attenuated this decrease rendering it similar to non-phosphorylated TnI at normal pH. The dissociation of Ca2+ from TnC was unaltered by pH such that TnI S150D remained slow, S23/24D remained accelerated and the combined S23/24/150D remained accelerated. This effect of the combined TnI Ser-150 and Ser-23/24 pseudo-phosphorylation to maintain Ca2+ binding while accelerating Ca2+ dissociation represents the first post-translational modification of troponin by phosphorylation to both accelerate thin filament deactivation and maintain Ca2+ sensitive activation. These data suggest TnI Ser-150 phosphorylation attenuation of the pH-dependent decrease in Ca2+ sensitivity and its combination with Ser-23/24 phosphorylation to maintain accelerated thin filament deactivation may impart an adaptive role to preserve contraction during acidic ischemia pH without slowing relaxation.
Keywords: Cardiac troponin I, thin filament deactivation, acidosis, phosphorylation
1. Introduction
Contraction of the heart results from the Ca2+ regulated interaction of myosin with the actin thin filament. The troponin (Tn) complex is an essential molecular switch that regulates this interaction of myosin with the thin filament. The binding of Ca2+ to the troponin C (TnC) subunit of the Tn complex is the initiating step in activation of the thin filament to allow myocardial contraction while the disassociation of Ca2+ from TnC is essential to relaxation (for review see [1, 2]). Tn post-translational modification is a key cardiac mechanism to modulate this Ca2+ sensitive activation of the thin filament and therefore helps match myocyte contraction to meet altered cardiac demand [3, 4]. The β-adrenergic signaling pathway is a significant physiological mechanism utilized by the heart to match myocyte contraction to demand [3–5]. While the β-adrenergic signaling pathway modulates a number of cellular processes, protein kinase A (PKA) mediated troponin I (TnI) Ser-23/24 phosphorylation is a primary myofilament target of this signaling [6–8]. In the normal heart, basal TnI Ser-23/24 phosphorylation comprises approximately 40% of the total TnI with increases or decreases of this phosphorylation central to the modulation of cardiac function [6, 7, 9–11]. This phosphorylation of TnI at Ser-23/24 functions to alter contraction by desensitizing the myofilament to Ca2+. Thus, while TnI Ser-23/24 phosphorylation decreases submaximal Ca2+-induced force production, desensitization also induces an acceleration in the rate of muscle relaxation necessary to allow adequate diastolic filling and maintained cardiac output during elevated heart rates [6, 7, 12].
In various pathological states of the heart, contractile dysfunction results in an impaired ability of the heart to meet demand. One such pathological state occurs during cardiac ischemia when a reduction in blood flow to the heart results in decreased ATP production and diminished contractile function [13]. While reduced blood flow affects a number of cardiac processes, a hallmark of ischemia is a decrease in intracellular pH. During global cardiac ischemia the intracellular pH of the cardiomyocyte can drop from 7.0 to ~ 6.2 [14]. This decrease in pH directly affects cellular processes including an altered Ca2+ regulation of the thin filament. Ischemia induced acidosis decreases the Ca2+ sensitive activation of the thin filament contributing to decreased myocardial force production and exacerbating hypoxia-induced contractile dysfunction [15].
Recently we characterized the AMP-activated protein kinase (AMPK) phosphorylation of TnI at Ser-150 and its effect on TnI Ser-23/24 phosphorylation function. Our findings, and those of others, demonstrate that TnI Ser-150 phosphorylation increases myofilament Ca2+-sensitive force production and blunts TnI PKA-dependent desensitization of skinned fibers and the reconstituted thin filament [16, 17]. Consistent with the AMPK phosphorylation of TnI Ser-150, Oliveira et al. demonstrated an elevation of TnI Ser-150 phosphorylation during the increased cardiac demand of ex vivo cardiac ischemia [17]. To date, the effects of TnI Ser-150 phosphorylation on the depressed cardiac contractile function that occurs during myocardial ischemia and how Ser-150 interacts with TnI Ser-23/24 PKA phosphorylation are unknown.
In the current study we sought to investigate the integrated role of TnI Ser-23/24 and Ser-150 phosphorylation combination on cardiac thin filament contractile regulation under acidic conditions similar to those occurring in ischemia. Towards this end we quantified changes in TnI Ser-150 and Ser-23/24 phosphorylation following in vivo myocardial ischemia and investigated the combined effects of TnI pseudo-phosphorylation on thin filament regulation at acidic pH. Our findings demonstrate myocardial ischemia increases both TnI Ser-150 and Ser-23/24 phosphorylation. We demonstrate TnI Ser-150 pseudo-phosphorylation in isolation blunts pH mediated thin filament Ca2+ desensitization while its combination blunts Ser-23/24 Ca2+ desensitization with a minimal effect on Ser-23/24 induced acceleration of thin filament Ca2+ disassociation. These data support a role for ischemia-induced TnI Ser-150 and Ser-23/24 phosphorylation to maintain Ca2+ regulated force production while accelerating relaxation. The concurrent phosphorylation of TnI Ser-150 and Ser-23/24 may therefore play an adaptive role in sustaining cardiac contraction during the acidic conditions of an ischemic event without delaying relaxation.
2. Materials and Methods
2.1 In vivo myocardial ischemia
In vivo left ventricular myocardial infarction was achieved via left coronary ligation in C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine) at 4 months of age as previously done [18]. Briefly, mice were anesthetized with ketamine (55 mg/kg) plus xylazine (15 mg/kg). Animals were intubated and ventilated (tidal volume 250 μl, 150 breath/min) with a mouse respirator (687, Harvard Apparatus). Body temperature was maintained at 37°C using a heating blanket (TC-1000, CWE). Through a left thoracotomy, we ligated the left coronary artery 1 to 2 mm below the border of the left atrial appendage. Ischemia was confirmed by pallor distal to the occlusion and by ST elevation on ECG. At 30 minutes after ligation, the heart was removed, the left ventricular free wall quickly dissected free and flash-frozen in liquid nitrogen. All animal protocols and procedures were performed in accordance with National Institutes of Health guidelines and approved by the Institutional Laboratory Animal Care and Use Committee at The Ohio State University.
2.2 Protein electrophoresis and Western blot
Myofibrils from sham or ischemic left ventricle free wall were solubilized in denaturing buffer (2% SDS, 0.1% bromophenol blue, 10% glycerol and 50 mM Tris-HCl, pH 6.8), heated for five minutes at 80°C and clarified by centrifugation for five minutes. SDS-PAGE and western blot were carried out as previously described [19]. Briefly, cardiac TnI Ser-150 phosphorylation was quantified by incubation with a custom rabbit anti-phosphorylated TnI Ser-150 antibody that we previously demonstrated as specific to the detection of TnI only when phosphorylated at Ser-150 [16]. The Ser-150 phosphorylation antibody was followed by incubation with a Dylight fluorescent secondary antibody (Jackson ImmunoResearch Laboratories, Inc.) and visualized on a Typhoon 9410 imager (GE Healthcare). Subsequent quantification of total cardiac TnI was conducted by re-probing the same membrane with a mouse anti-cardiac TnI antibody (Fitzgerald; clone C5) visualized as above. Sequential development using different primary/secondary combinations allows for quantification of phosphorylated and total TnI species in the same membrane [19, 20]. The phosphorylation of TnI Ser-23 and Ser-24 was quantified by incubation with the anti-phosphorylated TnI Ser-23/24 antibody (Cell Signaling Technology, Inc.), detected and visualized as above.
2.3 cDNA constructs
All cardiac TnI residue numbers presented in this manuscript are presented according to the native human sequence including the first methionine. The human cardiac TnI Ser-150 to Asp (S150D), Ser-23/24 to Asp (S23/24D) and Ser-23/24/150 to Asp (S23/24/150D) pseudo-phosphorylation mutant cDNA were generated by site-directed mutagenesis (Quick Change II kit, Agilent) according to the manufacturer’s direction and resultant constructs were verified by DNA sequencing as previously described [16].
2.4 Proteins
The individual recombinant human cardiac Tn subunits were expressed in E. coli and purified to homogeneity as previously described [21]. Tn used in Ca2+ binding experiments consisted of native human cardiac TnT, cardiac TnI and cardiac T53C labeled 2-(4′-iodoacetamidoanilo)naphthalene-6-sulfonic acid (IAANS) TnC containing the C35/84S mutations. Cardiac Tn complexes were reconstituted by sequential dialysis as previously described. Thin filaments were reconstituted by sequential incubation of actin first with a stoichiometric amount of tropomyosin followed by incubation with Tn as previously described [16].
2.5 Measurement of Tn steady-state Ca2+ binding to TnC
Steady-state Ca2+ binding to TnC in the thin filament or isolated Tn was measured at 15°C in 150 mM KCl, 3 mM MgCl2, 2 mM EGTA and 200 mM MOPS as previously described [16]. Briefly, Tn or thin filaments containing various TnI pseudo-phosphorylations were reconstituted at pH 7.0 or pH 6.5 and the change in IAANS labeled T53C TnC (C35/84S) fluorescence was monitored at various free [Ca2+] as indicative of Ca2+ binding to TnC.
2.6 Measurement of thin filament and Tn Ca2+ dissociation from TnC
Isolated Tn or reconstituted thin filaments were prepared at either pH 7.0 or 6.5. Following reconstitution, 0.2 mM Ca2+ was added to saturate Tn or the thin filament. Ca2+ saturated thin filament or Tn was rapidly mixed with EGTA buffer and Ca2+ dissociation was monitored by the change in IAANS fluorescence at 15°C as previously described [22, 23].
2.7 Measurement of myosin S1 dissociation from the thin filament
Reconstituted thin filaments were prepared as previously described at either pH 7.0 or 6.5. Following reconstitution EGTA was added to 5 mM and myosin S1 added at a ratio of 7:4 (actin:myosin S1). Rigor bound myosin S1 thin filaments were then rapidly mixed with ATP (2 mM final) and the dissociation of myosin monitored by the change in IAANS fluorescence at 15°C as previously described [22, 23].
2.8 Data processing and statistical analysis
Steady-state Ca2+ binding plots were fit to the Hill equation to determine 50% maximal binding. Ca2+ dissociation data were fit using a program (by P. J. King, Applied Photophysics Ltd.) utilizing the nonlinear Levenberg-Marquardt algorithm where each Ca2+ dissociation event represents an average of at least three separate experiments. Results of Ca2+ binding and Ca2+ dissociation were compared by 2-way ANOVA with Tukey’s post-hoc test. In vivo sham vs. ischemic cardiac TnI Ser-150 and Ser-23/24 phosphorylation was compared by Student’s t-Test. P<0.05 was considered statistically significant. Data are presented as mean ± SEM.
3. Results
3.1 In vivo myocardial ischemia increases both TnI Ser-150 and Ser-23/24 phosphorylation
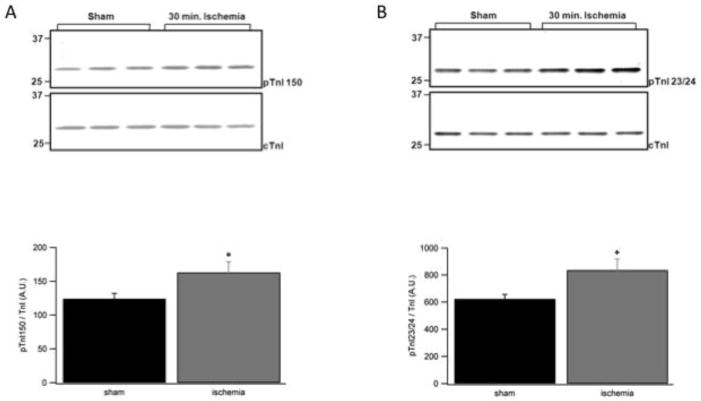
While the phosphorylation of TnI Ser-150 is low in the normal, non-stressed heart, its phosphorylation becomes elevated during myocardial ischemia [17]. We previously demonstrated that TnI Ser-150 pseudo-phosphorylation increases Ca2+ sensitivity and blunts TnI S23/24D Ca2+ desensitization [16]. To investigate when the combination of TnI Ser-150 and Ser-23/24 phosphorylation would be relevant to modulate cardiovascular function we determined TnI phosphorylation levels during myocardial ischemia. Western blot of left ventricular tissue from 6 mice subjected to in vivo ischemia for 30 minutes demonstrates the phosphorylation of TnI Ser-150 increased 31% and TnI Ser-23/24 phosphorylation increased 34% compared to that of 7 sham mice (Fig. 1). This simultaneous increase in both phosphorylations demonstrates their significance to cardiac contractile function during the pathological state of myocardial ischemia.
Fig. 1. Western blot of TnI Ser-150 and Ser-23/24 phosphorylation in ischemia cardiac tissue.
Myofilament preparations of left ventricular free wall from mice that underwent 30 minutes of in vivo left coronary artery occlusion or sham operation were subjected to SDS-PAGE and subsequent western blot to determine the level of TnI Ser-150 (pTnI 150) and Ser-23/24 (pTnI 23/24) phosphorylation following regional ischemia. (A) Representative western blot and relative quantitation of TnI Ser-150 phosphorylation with a pTnI 150 specific antibody or total cardiac TnI antibody (cTnI). (B) Representative western blot and relative quantitation of TnI Ser-23/24 phosphorylation with a pTnI 23/24 specific antibody or total cardiac TnI antibody (cTnI). n = 7 ischemic and 7 sham ventricles, each run in triplicate. *P<0.05 vs. sham.
3.2 TnI Ser-150 and Ser-23/24 phosphorylation alters the acidic effects of thin filament regulation
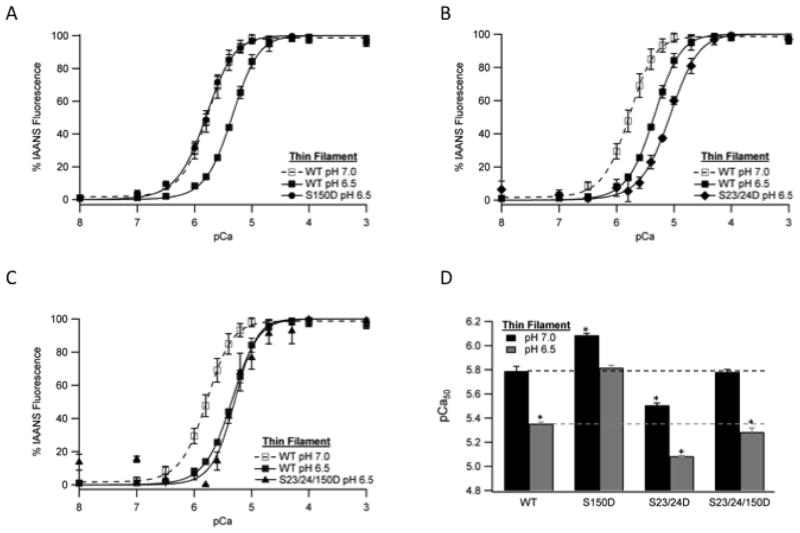
It is well established that an acidic environment, such as that which occurs in the cardiomyocyte during myocardial ischemia, depresses Ca2+-dependent force production at the level of the myofilament [15]. To determine the effect of Tn containing TnI Ser-150 phosphorylation, Ser-23/24 phosphorylation, and their combination on myofilament regulation at ischemic pH we measured TnC Ca2+ binding at normal (7.0) and ischemic (6.5) pH in the reconstituted thin filament. As demonstrated in Figure 2, lowering the pH to 6.5 decreased Ca2+ binding to TnC in all thin filament groups compared to their Ca2+ binding at normal pH. Additionally, the Tn type also affected Ca2+ binding at pH 6.5 such that thin filaments containing TnI S150D remained sensitized, TnI S23/24D were desensitized and TnI S23/24/150D were similar to WT (Fig. 2 and Table 1). Importantly, the effect of acidic pH to decrease Ca2+ binding in filaments containing TnI S150D was blunted by 39% compared to WT filaments. Therefore the combined effect of pH with TnI S150D resulted in a pCa50 at pH 6.5 that was not different from that of WT filaments at pH 7.0 (Fig. 2D). Similar effects of TnI S150D on Ca2+ binding compared to WT were observed at the intermediate acidic pH of 6.8 although the magnitude of the pH effect was decreased (data not shown).
Fig. 2. The effect of TnI phosphorylation on thin filament steady-state Ca2+ binding at ischemic pH.
The change in IAANS fluorescence following the addition of varied free Ca2+ amounts in thin filaments containing IAANS labeled TnC and non-phosphorylated TnI at pH 7.0 (WT, open square) or pH 6.5 (WT, closed square) vs. pseudo-phosphorylated TnI Ser-150 filaments (S150D, closed circle) (A), pseudo-phosphorylated TnI Ser-23/24 filaments (S23/24D, closed diamond) (B) or pseudo-phosphorylated TnI Ser-23/24/150 filaments (S23/24/150D, closed triangle) (C). Comparison of thin filament pCa50 at pH 7.0 (black) and pH 6.5 (dark gray) (D). *P<0.05 vs. WT.
Table 1. Steady-state Ca2+ binding to TnC in reconstituted thin filaments containing WT, S150D, S23/24D, or S23/24/150D Tn at pH 6.5 or pH 7.0.
Values are mean ± SEM. pCa50, Ca2+ concentration at 50% activation; n, number of thin filaments in each group
| Thin Filament | pH 6.5
|
pH 7.0
|
|||
|---|---|---|---|---|---|
| pCa50 | n | pCa50 | n | ΔpCa50 | |
| Tn WT | 5.35±0.01 *(W) | 3 | 5.79±0.04 | 3 | 0.44 |
| Tn S150D | 5.82±0.04 *(P, SS) | 3 | 6.09±0.01 *(W, P, SS) | 3 | 0.27 |
| Tn S23/24D | 5.09±0.01 *(W, P, PP, S) | 4 | 5.51±0.02 *(W, P, S) | 3 | 0.42 |
| Tn S23/24/150D | 5.28±0.04 *(W, PP, S, SS) | 3 | 5.78±0.02 *(P, S, SS) | 3 | 0.50 |
Two-way ANOVA demonstrates a significant interaction of pH and TnI phosphorylation type.
, P<0.05; W, significantly different vs. WT pH 7.0; P, significantly different vs. WT pH 6.5; PP, significantly different vs. same pseudo-phosphorylation pH 7.0; S, significantly different vs. S150D at same pH; SS, significantly different vs. S23/24D at same pH.
3.3 The phosphorylation of TnI Ser-150 and Ser-23/24 affect function through different thin filament interactions
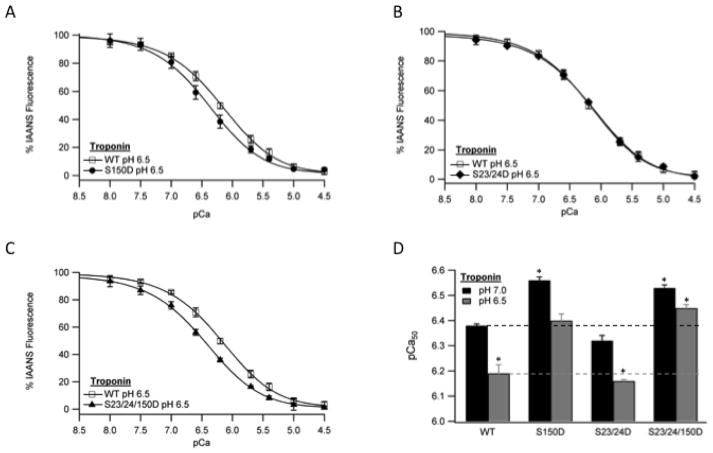
To investigate the mechanism by which TnI Ser-150 phosphorylation blunts pH mediated Ca2+ desensitization, we employed a reductionist approach. To minimize the interactions within the thin filament responsible for the effects of TnI phosphorylation, we determined Ca2+ binding to TnC in isolated Tn devoid of actin and tropomyosin interactions at pH 6.5. Isolated Tn containing S150D increased Ca2+ binding to TnC compared to WT Tn (Fig. 3A and Table 2) preserving the TnI S150D function observed in reconstituted thin filaments and skinned fibers [16]. Unexpectedly, isolated Tn containing TnI S23/24D did not alter TnC Ca2+ binding compared to WT (Fig. 3B and Table 2) differing from the previously observed effect of TnI Ser-23/24 phosphorylation in the reconstituted thin filament [16]. The effect of S150D to increase Ca2+ binding was retained when combined with S23/24D such that TnI S23/24/150D Tn Ca2+ binding was not different from that of S150D (Fig. 3C and Table 2). These findings demonstrate TnI Ser-150 and Ser-23/24 phosphorylation function through different Tn interactions at acidic pH. To determine if the TnI Ser-150 phosphorylation difference in Tn Ca2+ binding resulted from decreased pH or the phosphorylation itself, Tn Ca2+ measurements were repeated at normal pH. While elevating the pH to 7.0 increased Ca2+ binding of all Tn’s, Ca2+ binding to TnC in the isolated Tn containing TnI S150D and S23/24/150D remained increased and TnI S23/24D was not affected (Fig. 3D and Table 2). Thus the phosphorylation of TnI Ser-150 and Ser-23/24 is responsible for alteration of thin filament Ca2+ regulation through different Tn interactions.
Fig. 3. The effect of TnI phosphorylation on isolated Tn steady-state Ca2+ binding at ischemic pH.
The change in IAANS fluorescence following the addition of varied free Ca2+ amounts in isolated recombinant human Tn containing IAANS labeled TnC of non-phosphorylated TnI (WT, open square) vs. pseudo-phosphorylated TnI Ser-150 (S150D, closed circle) (A), pseudo-phosphorylated TnI Ser-23/24 Tn (S23/24D, closed diamond) (B) or pseudo-phosphorylated TnI Ser-23/24/150 Tn (S23/24/150D, closed triangle) (C). Comparison of pCa50 between WT, S150D, S23/24D, and S23/24/150D isolated Tn at pH 7.0 (black) and pH 6.5 (dark gray) (D). *P<0.05 vs. WT.
Table 2. Steady-state Ca2+ binding to TnC in isolated Tn containing WT, S150D, S23/24D, or S23/24/150D TnI at pH 6.5 or pH 7.0.
Values are mean ± SEM. pCa50, Ca2+ concentration at 50% activation; n, number of Tn in each group
| Tn | pH 6.5
|
pH 7.0
|
||
|---|---|---|---|---|
| pCa50 | n | pCa50 | n | |
| TnI WT | 6.19±0.03 *(W) | 3 | 6.38±0.01 | 3 |
| TnI S150D | 6.40±0.03 *(P, PP, SS) | 3 | 6.56±0.01 *(W, P, SS) | 3 |
| TnI S23/24D | 6.16±0.01 *(W, PP, S) | 3 | 6.32±0.02 *(P, S) | 3 |
| TnI S23/24/150D | 6.45±0.01 *(P, SS) | 3 | 6.53±0.01 *(W, P, SS) | 3 |
Two-way ANOVA demonstrates a significant interaction of pH and TnI phosphorylation type.
, P<0.05; W, significantly different vs. WT pH 7.0; P, significantly different vs. WT pH 6.5; PP, significantly different vs. same pseudo-phosphorylation pH 7.0; S, significantly different vs. S150D at same pH; SS, significantly different vs. S23/24D at same pH.
3.4 The combination of TnI Ser-150 with Ser-23/24 phosphorylation retains accelerated thin filament deactivation
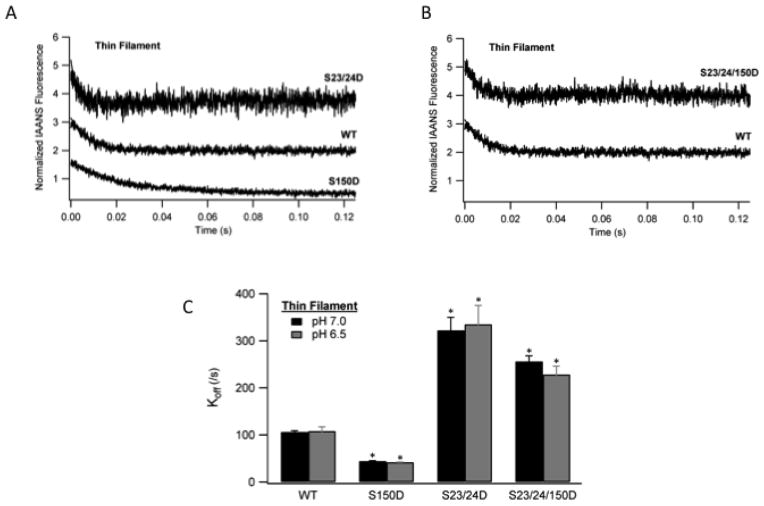
The rate at which Ca2+ can be removed from TnC represents a critical event in thin filament deactivation and myocardial relaxation [2]. To investigate the effect of TnI Ser-150 phosphorylation on the Ca2+ component of thin filament deactivation, we measured Ca2+ dissociation from TnC in the thin filament at acidic pH. The rate of Ca2+ dissociation was determined by monitoring the change in IAANS-labeled TnC fluorescence following rapid mixing with EGTA by stopped-flow fluorimetry [23]. Consistent with our previous Ca2+ sensitivity findings [16], thin filaments containing TnI S150D slowed the rate of Ca2+ dissociation to half of WT filaments while S23/24D filaments accelerated Ca2+ dissociation 3.1-fold compared to WT (Fig. 4A; Table 3). We next sought to investigate whether crosstalk between TnI Ser-150 and Ser-23/24 phosphorylation would affect TnC Ca2+ dissociation. Upon combination, TnI S23/24/150D retained a 2.1-fold accelerated Ca2+ dissociation rate compared to that of WT (Fig. 4B; Table 3).. Thus, while the Ca2+ dissociation of the combined TnI S23/24/150D was marginally slowed by the presence of S150D compared to that of TnI S23/24D alone, this combined S23/24/15D disassociation was still dramatically faster than that of WT. These findings demonstrate the functional outcome of TnI Ser-150 phosphorylation to modulate the Ca2+ regulated component of thin filament deactivation at acidic pH is dependent upon its combination with TnI Ser-23/24 phosphorylation.
Fig. 4. The effect of TnI phosphorylation on thin filaments Ca2+ dissociation kinetics at ischemic pH.
Ca2+ saturated thin filaments were rapidly mixed with EGTA and Ca2+ dissociation monitored as the change in IAANS fluorescence. Representative stopped-flow Ca2+ dissociation traces from thin filaments containing non-phosphorylated WT TnI (WT) vs. pseudo-phosphorylated TnI Ser-150 (S150D) and pseudo-phosphorylated TnI Ser-23/24 (S23/24D) filaments (A) or pseudo-phosphorylated TnI Ser-23/24/150D (S23/24/150D) filaments (B). Traces demonstrating the change in fluorescence over time are normalized and staggered for clarity. Comparison of the Ca2+ dissociation rates (koff) from WT, S150D, S23/24D, and S23/24/150D containing thin filaments at pH 7.0 (black) and pH 6.5 (dark gray) (C). *P<0.05 vs. WT.
Table 3. Ca2+ dissociation from TnC in reconstituted thin filaments containing WT, S150D, S23/24D, or S23/24/150D Tn at pH 6.5 or pH 7.0.
Values are mean ± SEM. Koff, rate of Ca2+ removal from TnC per second; n, number of thin filaments in each group
| Thin Filament | pH 6.5
|
pH 7.0
|
||
|---|---|---|---|---|
| Koff (/s) | n | Koff (/s) | n | |
| Tn WT | 108.48±8.98 | 6 | 106.42±2.57 | 9 |
| Tn S150D | 42.13±0.27 *(SS) | 6 | 44.80±0.28 *(W, SS) | 16 |
| Tn S23/24D | 335.23±39.86 *(W, P, S) | 6 | 332.50±27.45 *(W, P, S) | 8 |
| Tn S23/24/150D | 228.68±17.69 *(W, P, S, SS) | 9 | 256.48±10.78 *(W, P, S) | 16 |
Two-way ANOVA demonstrates no significant interaction of pH and TnI phosphorylation type.
, P<0.05; W, significantly different vs. WT pH 7.0; P, significantly different vs. WT pH 6.5; PP, significantly different vs. same pseudo-phosphorylation pH 7.0; S, significantly different vs. S150D at same pH; SS, significantly different vs. S23/24D at same pH.
To determine if this unique effect of combined TnI S150D with S23/24D resulted from a pH effect or was an intrinsic property of phosphorylation itself we determined the rate of Ca2+ dissociation from TnC at normal pH. The results in Figure 4 demonstrate that increasing the pH to 7.0 did not affect the Ca2+ dissociation rate of thin filaments containing any Tn compared to that at pH 6.5. Additionally, thin filaments containing TnI S23/24/150D retained a 2.4-fold accelerated Ca2+ dissociation rate compared to WT at pH 7.0 (Fig. 4C; Table 3). The similarly increased rate of TnI S23/24/150D thin filament Ca2+ dissociation at both pH 6.5 and 7.0 demonstrates this increased Ca2+ dissociation is dependent upon the phosphorylation of TnI and is not a pH-dependent effect.
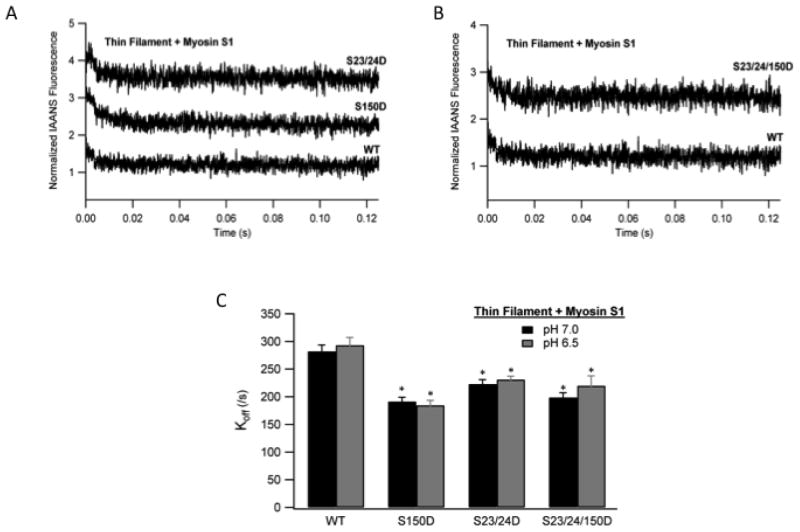
In addition to Ca2+ dissociation from TnC, deactivation of the thin filament in the muscle also requires myosin dissociation from the actin filament. To investigate the effect of TnI Ser-150 phosphorylation on the myosin component of thin filament deactivation, we measured myosin dissociation from actin in the thin filament at acidic pH. The rate of myosin S1 dissociation was determined by monitoring the change in IAANS-labeled TnC fluorescence following rapid mixing with ATP by stopped-flow fluorimetry [23]. Thin filaments reconstituted with Tn containing either TnI S150D alone, TnI S23/24D alone or the combination of both all marginally slowed the rate of myosin S1 dissociation compared to WT by about 25% (Fig. 5; Table 4). Since both the individual and combined TnI phosphorylations similarly decrease myosin S1 dissociation at pH 6.5 we sought to determine if this change in S1 dissociation resulted from the phosphorylation of TnI or the acidic pH. Repetition of the myosin S1 dissociation rate measurements at pH 7.0 demonstrates the dissociation of S1 from thin filaments containing TnI S150D, S23/24D or their combination were not different from those at pH 6.5 (Fig. 5C; Table 4). These data demonstrate the phosphorylation of TnI at Ser-150, Ser-23/24 or their combination similarly functions to marginally slow the myosin component of thin filament deactivation regardless of pH.
Fig. 5. The effect of TnI phosphorylation on Myosin S1 dissociation kinetics from thin filaments at ischemic pH.
Ca2+ free thin filaments containing rigor bound myosin S1 were rapidly mixed with ATP and the dissociation of myosin S1 monitored as the change in IAANS TnC fluorescence. Representative stopped-flow traces from myosin S1 bound thin filaments containing WT TnI (WT) vs. pseudo-phosphorylated TnI Ser-150 (S150D) and pseudo-phosphorylated TnI Ser-23/24 (S23/24D) filaments (A) or pseudo-phosphorylated TnI Ser-23/24/150D (S23/24/150D) filaments (B). Traces demonstrating the change in fluorescence over time are normalized and staggered for clarity. Comparison of the myosin S1 dissociation rates (koff) from WT, S150D, S23/24D, and S23/24/150D containing thin filaments at pH 7.0 (black) and pH 6.5 (dark gray). (C). *P<0.05 vs. WT.
Table 4. Myosin S1 dissociation from actin in reconstituted thin filaments containing WT, S150D, S23/24D, or S23/24/150D Tn at pH 6.5 or pH 7.0.
Values are mean ± SEM. Koff, rate of myosin S1 removal from actin per second; n, number of thin filaments in each group
| Thin Filament + Myosin s1 | pH 6.5
|
pH 7.0
|
||
|---|---|---|---|---|
| Koff (/s) | n | Koff (/s) | n | |
| Tn WT | 293.31.17±13.88 | 9 | 282.17±11.20 | 18 |
| Tn S150D | 184.52±8.79 *(W, P) | 9 | 191.48±7.55 *(W, P) | 18 |
| Tn S23/24D | 231.18±5.98 *(W, P) | 9 | 223.02±7.84 *(W, P) | 18 |
| Tn S23/24/150D | 219.95±9.95 *(W, P) | 8 | 198.78±8.83 *(W, P) | 18 |
Two-way ANOVA demonstrates no significant interaction of pH and TnI phosphorylation type.
, P<0.05; W, significantly different vs. WT pH 7.0; P, significantly different vs. WT pH 6.5; PP, significantly different vs. same pseudo-phosphorylation pH 7.0; S, significantly different vs. S150D at same pH; SS, significantly different vs. S23/24D at same pH.
4. Discussion
The goal of this study was to determine the integrated role of TnI Ser-150 and Ser-23/24 phosphorylation on thin filament regulation in an acidic environment similar to that of ischemia. The major findings from our study demonstrate: 1) The phosphorylation of TnI Ser-150 and Ser-23/24 are concurrently increased in response to myocardial ischemia. 2) TnI Ser-150 phosphorylation blunts the pH-dependent decrease in Ca2+ binding to TnC to sustain Ca2+ sensitivity at pH 6.5 identical to WT at pH 7.0. 3) Tn phosphorylation regulation of the thin filament is dependent upon whether TnI phosphorylation occurs alone or in combination. In isolation TnI Ser-150 phosphorylation increases thin filament Ca2+ sensitivity and slows Ca2+ dissociation while isolated TnI Ser-23/24 phosphorylation decreases Ca2+ sensitivity and accelerates Ca2+ dissociation. Upon the combination of TnI Ser-150 with Ser-23/24 phosphorylation thin filament Ca2+ sensitivity remains similar to wild-type while Ca2+ dissociation is greatly accelerated.
4.1 Myocardial ischemia simultaneously increases phosphorylation of both TnI Ser-150 and Ser-23/24
The observed simultaneous increase of both TnI Ser-150 and Ser-23/24 phosphorylation in the presence of myocardial ischemia demonstrates the significance of these phosphorylations to modulate myofilament contraction during cardiac stress. TnI Ser-150 is normally present at low amounts in the unstressed heart and it is increased 31% in response to myocardial ischemia (Fig. 1), indicating a modulatory role [17]. Conversely, approximately 40% of TnI is basally phosphorylated at both Ser-23 and 24 [9]. While TnI Ser-23/24 was previously reported to be decreased by approximately 30% in the ischemic, non-perfused rat papillary muscle [24, 25], we demonstrate ischemia increases TnI Ser-23/24 phosphorylation by 34% in the mouse left ventricular free wall containing both perfused and ischemic tissue. A limitation of this study is that we did not directly determine TnI phosphorylation in ischemic and perfused regions of the left ventricular free wall. However, our findings suggest TnI Ser-23/24 phosphorylation may be differentially regulated in ischemic versus remote perfused tissue during myocardial infarction to invoke regional modulation of cardiac contraction. Regardless, the ischemia-induced increase in TnI Ser-150 phosphorylation in the presence of high levels of TnI Ser-23/24 phosphorylation demonstrates a modulatory role of TnI Ser-150 phosphorylation, both in isolation and when combined with Ser-23/24 on the same TnI molecule.
4.2 TnI Ser-150 phosphorylation differentially modulates thin filament regulation
In the normal perfused heart, TnI Ser-150 phosphorylation is low and its functional impact is therefore minimal [16, 17]. During myocardial ischemia, decreased oxygen availability leads to an impaired metabolism, ATP depletion and acidosis [26]. In addition to a number of effects, ischemia-induced acidosis directly depresses myofilament Ca2+ regulated force production [15] while ATP depletion activates AMPK to phosphorylate TnI at Ser-150 [16, 17, 27]. Previously we demonstrated TnI Ser-150 phosphorylation increases Ca2+ sensitive force production at normal pH [16]; however, its role during acidosis when its phosphorylation is elevated was unknown. We now demonstrate TnI S150D in isolation blunts the effect of acidosis-induced Ca2+ desensitization. Thus ischemia-induced elevation of TnI Ser-150 phosphorylation results in enhanced Ca2+ activation of the thin filament at pH 6.5 that is not different from that of WT filaments at pH 7.0 (Fig. 2). TnI Ser-150 phosphorylation in isolation therefore functions to oppose acidosis-induced myofilament desensitization.
With the high basal level of Ser-23/24 phosphorylation it is likely that ischemia-induced Ser-150 phosphorylation will occur in combination with phosphorylated Ser-23/24 on the same TnI molecule. Our lab and others have demonstrated myofilament post-translational modifications, including TnI Ser-150 phosphorylation, can modulate the contractile effects of TnI Ser-23/24 phosphorylation [16, 28–30]. Our current work expands upon these previous findings demonstrating the ischemia induced pH effect on Ca2+ desensitization is dependent upon whether TnI Ser-150 and Ser-23/24 phosphorylation occurs alone or in combination. By itself TnI S150D blunts pH-dependent Ca2+ desensitization while S23/24D by itself enhances pH desensitization; however, their combination results in a Ca2+ sensitivity similar to WT at acidic pH (Fig. 2). While these studies were conducted in thin filaments containing 100% pseudo-phosphorylated TnI, we previously demonstrated similar results at pH 7.0 upon the combination of TnI Ser-150 with Ser-23/24 at sub-maximal phosphate incorporation levels [16]. Based upon this previous finding we speculate that the ischemia elevated combination of TnI Ser-150 with Ser-23/24 phosphorylation will result in a similar Ca2+ sensitivity effect to that of the 100% pseudo-phosphorylated TnI combinations. In the future it will be important to determine if ischemia preferentially induces TnI Ser-150 phosphorylation alone or in combination with Ser-23/24 phosphorylation.
The binding of Ca2+ to TnC is a necessary event in contraction; however, the heart functions such that the activating intracellular myocyte Ca2+ concentration is constantly changing and never reaches equilibrium. Ca2+ binding to TnC and activation of the thin filament is therefore dependent upon constantly changing amounts of Ca2+ such that Ca2+ binding to TnC is not in steady-state. Equilibrium, steady-state Ca2+ binding measurements therefore provide only a partial picture of the force contraction processes in the heart. In addition, deactivation of the thin filament and relaxation requires the dissociation of Ca2+ from TnC. We therefore determined the effect of TnI Ser-150 and Ser-23/24 ischemia-induced TnI phosphorylation on TnC Ca2+ dissociation kinetics at acidic pH. Based upon the understanding that TnI S23/24D desensitizes and S150D sensitizes the thin filament to Ca2+ one would expect TnI S23/24D to accelerate Ca2+ disassociation and S150D to slow disassociation compared to WT, which is exactly what we observed (Fig. 4). This finding that TnI S150D significantly slows Ca2+ dissociation is supported by previous FRET findings at normal pH [31]. However, the combination of S23/24/150D on the same TnI molecule retained wild-type like Ca2+ sensitivity but exhibited significantly accelerated Ca2+ dissociation compared to wild-type (Figs. 2 and 4). Thus, while TnI S150D alone dramatically slowed disassociation to half of WT, the combination of S150 with S23/24 phosphorylation (S23/24/150D) was only marginally slowed from that of S23/24D alone such that it remained 2.1-fold accelerated compared to WT. Interestingly, TnI phosphorylation had a negligible effect on the myosin S1 disassociation component of thin filament deactivation with all 3 phosphorylation combinations minimally slowing S1 disassociation by about 25% of wild-type. Consistent with these phosphorylation data, we have also reported similar differential effects of TnI and TnT disease mutations on Ca2+ sensitivity and dissociation rates [22, 32]. The combination effect of TnI Ser-150 and Ser-23/24 phosphorylation on dissociation was not the result of a pH effect but rather resulted from combination of the phosphate residues as demonstrated by the identical dissociation rates of TnI S23/24/150D at both pH 6.5 and 7.0 (Fig. 5). This represents the first demonstration of Tn phosphorylation to both maintain Ca2+ regulated thin filament activation and accelerate thin filament deactivation. We speculate this combination of TnI Ser-150 with Ser-23/24 phosphorylation is significant to contractile function during ischemia by maintaining Ca2+-dependent force while accelerating relaxation.
4.3 TnI Ser-150 and Ser-23/24 structure-function
The balance between TnI regulatory inhibitory region binding to actin and switch peptide binding to TnC is a critical determinant of thin filament activation upon Ca2+ binding to TnC [1]. TnI Ser-150 phosphorylation directly alters TnI regulatory region binding [31, 33]; however, the mechanism for how its combination with Ser-23/24 phosphorylation differentially affects Ca2+ sensitivity and dissociation is unknown. The finding that TnI S150D alters Ca2+ binding in isolated Tn devoid of actin and tropomyosin interactions but TnI S23/24D does not (Fig 3) demonstrates TnI Ser-150 phosphorylation functions through Tn interactions not present in TnI phosphorylated at Ser-23/24. Thus the effect of TnI Ser-23/24 phosphorylation to alter Ca2+ sensitivity and dissociation further requires additional actin and/or tropomyosin interactions. Previous reports support Tn modification differences in Ca2+ binding to TnC in thin filament versus isolated Tn [32]. The lack of such a regulatory effect in isolated Tn containing TnI S23/24D demonstrates Ca2+ regulation of the thin filament can be modulated through more than one Tn interaction and these regulatory interactions are different between Ser-150 and Ser-23/24 phosphorylation. Different mechanisms of function are further demonstrated by the similar Ca2+ sensitivity of Tn S23/24/150D to S150D. Future definition of the protein structure-function interactions responsible for the functional effects of these phosphorylations will be beneficial to targeted drug design modulation of Ca2+ sensitivity and/or dissociation.
4.4 Functional consequences of ischemia-induced TnI phosphorylation
The elevated cardiac demand evoked during an ischemic event results in a response by the myocardium to increase Ca2+-sensitive force development [34]. While a number of factors likely contribute to this increase, TnI represents a prime effector of this sensitization. Genetic manipulation has demonstrated TnI as a significant molecule to modulate cardiac contraction in response to ischemia. Mutation of the cardiac TnI Ala-164 residue to its skeletal His counterpart confers contractile resistance to the ischemic effects of cardiac acidosis [35]. In addition to genetic manipulation, myocardial ischemia itself directly induces post-translational modification of TnI to modulate the ischemic contractile response. Ischemia was previously shown to induce proteolysis of the 17 TnI C-terminal amino acids resulting in increased Ca2+ sensitivity and slowed myofibril relaxation kinetics [34, 36–39]. Our data now demonstrate myocardial ischemia also causes the post-translational phosphorylation of TnI Ser-150 that further contributes to an additional mechanism of ischemia increased Ca2+ sensitivity (Fig. 2). While expression of the Ca2+ sensitized slow skeletal TnI has been shown to be protective during ischemia [40], it is currently unknown if direct Ca2+ sensitization of contraction alone is sufficient to improve cardiac function and/or long-term protection from myocardial ischemia. Future experiments are necessary to establish the ultimate role of integrated ischemia induced TnI post-translational modifications and their significance to survival following myocardial ischemia.
5. Conclusions
Taken as a whole, we demonstrate that TnI Ser-150 and Ser-23/24 phosphorylation function through varied Tn interactions contributing to different thin filament regulatory modulation. While isolated TnI Ser-150 phosphorylation blunts the ischemic pH mediated Ca2+ desensitization, the combination of these phosphorylations in the same TnI molecule represents the first demonstration of Tn phosphorylation to maintain Ca2+ sensitivity and accelerate Ca2+ dissociation. We propose ischemia-induced TnI Ser-150 phosphorylation alters cardiac contraction during myocardial ischemia to improve recovery from ischemia.
Highlights.
Myocardial ischemia increases TnI Ser-150 and Ser-23/24 phosphorylation.
TnI Ser-150 phosphorylation blunts pH-mediated Ca2+ desensitization.
Combination of phosphorylations maintains Ca2+ sensitivity in acidosis.
Combination of phosphorylations accelerates deactivation in acidosis.
Combined TnI phosphorylations may play an adaptive role in ischemia.
The abbreviations used are
- AMPK
AMP activated protein kinase
- PKA
protein kinase A
- Tn
troponin
- Tn
troponin
- TnI
troponin I
- TnT
troponin T
- TnC
troponin C
- IAANS
2-(4′-iodoacetamidoanilo)naphthalene-6-sulfonic acid
Footnotes
Support for this work was obtained from T32 007692. B.J.B (HL114940) and J.P.D (HL091986) are supported by grants from the NIH.
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 2.Davis JP, Tikunova SB. Ca(2+) exchange with troponin C and cardiac muscle dynamics. Cardiovasc Res. 2008;77:619–26. doi: 10.1093/cvr/cvm098. [DOI] [PubMed] [Google Scholar]
- 3.Solaro RJ. Multiplex kinase signaling modifies cardiac function at the level of sarcomeric proteins. J Biol Chem. 2008;283:26829–33. doi: 10.1074/jbc.R800037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solaro RJ. Modulation of Cardiac Myofilament Activity by Protein Phosphorylation: Comprehensive Physiology. 2011. [Google Scholar]
- 5.Ramirez-Correa GA, Cortassa S, Stanley B, Gao WD, Murphy AM. Calcium sensitivity, force frequency relationship and cardiac troponin I: critical role of PKA and PKC phosphorylation sites. J Mol Cell Cardiol. 2010;48:943–53. doi: 10.1016/j.yjmcc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kranias EG, Solaro RJ. Phosphorylation of troponin I and phospholamban during catecholamine stimulation of rabbit heart. Nature. 1982;298:182–4. doi: 10.1038/298182a0. [DOI] [PubMed] [Google Scholar]
- 7.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Pena JR, Wolska BM. Troponin I phosphorylation plays an important role in the relaxant effect of beta-adrenergic stimulation in mouse hearts. Cardiovasc Res. 2004;61:756–63. doi: 10.1016/j.cardiores.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Ayaz-Guner S, Zhang J, Li L, Walker JW, Ge Y. In vivo phosphorylation site mapping in mouse cardiac troponin I by high resolution top-down electron capture dissociation mass spectrometry: Ser22/23 are the only sites basally phosphorylated. Biochemistry. 2009;48:8161–70. doi: 10.1021/bi900739f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamdani N, de Waard M, Messer AE, Boontje NM, Kooij V, van Dijk S, et al. Myofilament dysfunction in cardiac disease from mice to men. J Muscle Res Cell Motil. 2008;29:189–201. doi: 10.1007/s10974-008-9160-y. [DOI] [PubMed] [Google Scholar]
- 11.Messer AE, Jacques AM, Marston SB. Troponin phosphorylation and regulatory function in human heart muscle: dephosphorylation of Ser23/24 on troponin I could account for the contractile defect in end-stage heart failure. J Mol Cell Cardiol. 2007;42:247–59. doi: 10.1016/j.yjmcc.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Pi Y, Zhang D, Kemnitz KR, Wang H, Walker JW. Protein kinase C and A sites on troponin I regulate myofilament Ca2+ sensitivity and ATPase activity in the mouse myocardium. J Physiol. 2003;552:845–57. doi: 10.1113/jphysiol.2003.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol. 2009;46:318–31. doi: 10.1016/j.yjmcc.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Garlick PB, Radda GK, Seeley PJ. Studies of acidosis in the ischaemic heart by phosphorus nuclear magnetic resonance. Biochem J. 1979;184:547–54. doi: 10.1042/bj1840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solaro RJ, el-Saleh SC, Kentish JC. Ca2+, pH and the regulation of cardiac myofilament force and ATPase activity. Mol Cell Biochem. 1989;89:163–7. doi: 10.1007/BF00220770. [DOI] [PubMed] [Google Scholar]
- 16.Nixon BR, Thawornkaiwong A, Jin J, Brundage EA, Little SC, Davis JP, et al. AMP-activated protein kinase phosphorylates cardiac troponin I at Ser-150 to increase myofilament calcium sensitivity and blunt PKA-dependent function. J Biol Chem. 2012;287:19136–47. doi: 10.1074/jbc.M111.323048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira SM, Zhang YH, Solis RS, Isackson H, Bellahcene M, Yavari A, et al. AMP-activated protein kinase phosphorylates cardiac troponin I and alters contractility of murine ventricular myocytes. Circ Res. 2012;110:1192–201. doi: 10.1161/CIRCRESAHA.111.259952. [DOI] [PubMed] [Google Scholar]
- 18.Mital R, Zhang W, Cai M, Huttinger ZM, Goodman LA, Wheeler DG, et al. Antioxidant network expression abrogates oxidative posttranslational modifications in mice. Am J Physiol Heart Circ Physiol. 2011;300:H1960–70. doi: 10.1152/ajpheart.01285.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biesiadecki BJ, Tachampa K, Yuan C, Jin JP, de Tombe PP, Solaro RJ. Removal of the cardiac troponin I N-terminal extension improves cardiac function in aged mice. J Biol Chem. 2010;285:19688–98. doi: 10.1074/jbc.M109.086892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monasky MM, Biesiadecki BJ, Janssen PM. Increased phosphorylation of tropomyosin, troponin I, and myosin light chain-2 after stretch in rabbit ventricular myocardium under physiological conditions. J Mol Cell Cardiol. 2010;48:1023–8. doi: 10.1016/j.yjmcc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biesiadecki BJ, Schneider KL, Yu ZB, Chong SM, Jin JP. An R111C polymorphism in wild turkey cardiac troponin I accompanying the dilated cardiomyopathy-related abnormal splicing variant of cardiac troponin T with potentially compensatory effects. J Biol Chem. 2004;279:13825–32. doi: 10.1074/jbc.M314225200. [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Lee RS, Biesiadecki BJ, Tikunova SB, Davis JP. Engineered troponin C constructs correct disease-related cardiac myofilament calcium sensitivity. J Biol Chem. 2012;287:20027–36. doi: 10.1074/jbc.M111.334953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little SC, Biesiadecki BJ, Kilic A, Higgins RS, Janssen PM, Davis JP. The rates of Ca2+ dissociation and cross-bridge detachment from ventricular myofibrils as reported by a fluorescent cardiac troponin C. J Biol Chem. 2012;287:27930–40. doi: 10.1074/jbc.M111.337295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han YS, Ogut O. Regulation of fibre contraction in a rat model of myocardial ischemia. PLoS One. 2010;5:e9528. doi: 10.1371/journal.pone.0009528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han YS, Ogut O. Force relaxation and thin filament protein phosphorylation during acute myocardial ischemia. Cytoskeleton (Hoboken) 2011;68:18–31. doi: 10.1002/cm.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennings RB, Hawkins HK, Lowe JE, Hill ML, Klotman S, Reimer KA. Relation between high energy phosphate and lethal injury in myocardial ischemia in the dog. Am J Pathol. 1978;92:187–214. [PMC free article] [PubMed] [Google Scholar]
- 27.Sancho Solis R, Ge Y, Walker JW. A preferred AMPK phosphorylation site adjacent to the inhibitory loop of cardiac and skeletal troponin I. Protein Sci. 2011;20:894–907. doi: 10.1002/pro.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biesiadecki BJ, Kobayashi T, Walker JS, Solaro RJ, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res. 2007;100:1486–93. doi: 10.1161/01.RES.0000267744.92677.7f. [DOI] [PubMed] [Google Scholar]
- 29.Kooij V, Saes M, Jaquet K, Zaremba R, Foster DB, Murphy AM, et al. Effect of troponin I Ser23/24 phosphorylation on Ca2+-sensitivity in human myocardium depends on the phosphorylation background. J Mol Cell Cardiol. 2010;48:954–63. doi: 10.1016/j.yjmcc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Y, Schmidtmann A, Redlich A, Westerdorf B, Jaquet K, Thieleczek R. Effects of phosphorylation and mutation R145G on human cardiac troponin I function. Biochemistry. 2001;40:14593–602. doi: 10.1021/bi0115232. [DOI] [PubMed] [Google Scholar]
- 31.Ouyang Y, Mamidi R, Jayasundar JJ, Chandra M, Dong WJ. Structural and kinetic effects of PAK3 phosphorylation mimic of cTnI(S151E) on the cTnC-cTnI interaction in the cardiac thin filament. J Mol Biol. 2010;400:1036–45. doi: 10.1016/j.jmb.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B, Tikunova SB, Kline KP, Siddiqui JK, Davis JP. Disease-related cardiac troponins alter thin filament Ca2+ association and dissociation rates. PLoS One. 2012;7:e38259. doi: 10.1371/journal.pone.0038259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li MX, Wang X, Lindhout DA, Buscemi N, Van Eyk JE, Sykes BD. Phosphorylation and mutation of human cardiac troponin I deferentially destabilize the interaction of the functional regions of troponin I with troponin C. Biochemistry. 2003;42:14460–8. doi: 10.1021/bi035408y. [DOI] [PubMed] [Google Scholar]
- 34.Van Eyk JE, Powers F, Law W, Larue C, Hodges RS, Solaro RJ. Breakdown and release of myofilament proteins during ischemia and ischemia/reperfusion in rat hearts: identification of degradation products and effects on the pCa-force relation. Circ Res. 1998;82:261–71. doi: 10.1161/01.res.82.2.261. [DOI] [PubMed] [Google Scholar]
- 35.Day SM, Westfall MV, Fomicheva EV, Hoyer K, Yasuda S, La Cross NC, et al. Histidine button engineered into cardiac troponin I protects the ischemic and failing heart. Nat Med. 2006;12:181–9. doi: 10.1038/nm1346. [DOI] [PubMed] [Google Scholar]
- 36.Foster DB, Noguchi T, VanBuren P, Murphy AM, Van Eyk JE. C-terminal truncation of cardiac troponin I causes divergent effects on ATPase and force: implications for the pathophysiology of myocardial stunning. Circ Res. 2003;93:917–24. doi: 10.1161/01.RES.0000099889.35340.6F. [DOI] [PubMed] [Google Scholar]
- 37.McDonough JL, Arrell DK, Van Eyk JE. Troponin I degradation and covalent complex formation accompanies myocardial ischemia/reperfusion injury. Circ Res. 1999;84:9–20. doi: 10.1161/01.res.84.1.9. [DOI] [PubMed] [Google Scholar]
- 38.Narolska NA, Piroddi N, Belus A, Boontje NM, Scellini B, Deppermann S, et al. Impaired diastolic function after exchange of endogenous troponin I with C-terminal truncated troponin I in human cardiac muscle. Circ Res. 2006;99:1012–20. doi: 10.1161/01.RES.0000248753.30340.af. [DOI] [PubMed] [Google Scholar]
- 39.Tachampa K, Kobayashi T, Wang H, Martin AF, Biesiadecki BJ, Solaro RJ, et al. Increased cross-bridge cycling kinetics after exchange of C-terminal truncated troponin I in skinned rat cardiac muscle. J Biol Chem. 2008;283:15114–21. doi: 10.1074/jbc.M801636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arteaga GM, Warren CM, Milutinovic S, Martin AF, Solaro RJ. Specific enhancement of sarcomeric response to Ca2+ protects murine myocardium against ischemia-reperfusion dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H2183–92. doi: 10.1152/ajpheart.00520.2005. [DOI] [PubMed] [Google Scholar]