Abstract
Alterations and injury to glomerular podocytes play a key role in the initiation and early progression of diabetic kidney disease. Multiple factors in the diabetic milieu cause abnormalities in podocyte signaling that lead to podocyte foot process effacement, hypertrophy, detachment, loss and death. Alterations in insulin action and mTOR activation have been well documented to lead to pathology. For example, reduced insulin action directly leads to albuminuria, increased glomerular matrix accumulation, thickening of the glomerular basement membrane, podocyte apoptosis and glomerulosclerosis. In addition, the podocyte generates factors that alter signaling in other glomerular cells. Prominent among these is VEGF-A which plays a complex role in maintaining glomerular endothelium viability but causes endothelial cell pathology when generated at too high a level. Finally, circulating vascular factors, such as activated protein C have a profound effect on podocyte stability and survival. This cytoprotective factor is critical for podocyte health and its deficiency promotes podocyte injury and apoptosis. Thus, the podocyte sits in the center of a network of paracrine and hormonal signaling systems that in health keep the podocyte adaptable and viable, but in diabetes can lead to pathologic changes, detachment and death. This podocyte injury is a critical determinant of the progression of diabetic kidney disease.
Keywords: insulin, glucose, diabetes, glomerulus, mammalian target of rapamycin
Alterations and injury to glomerular podocytes play a key role in the initiation and early progression of diabetic kidney disease. While the development and progression of diabetic changes in the renal glomerulus clearly involve all resident cells as well as several passers-by such as macrophages, podocyte abnormalities are among the first manifestations to be detected morphologically in humans and animal models with diabetic kidney disease (DKD)1-3. In humans, reduction in podocyte number predicts future progression of nephropathy1,4 and in animal models podocyte loss can directly lead to glomerulosclerosis5, one of the hallmarks of DKD. In addition, the damaged podocyte may contribute to nephropathy via alterations in its expression of paracrine factors that impact other glomerular cells. Therefore, it is imperative to understand the metabolic and vascular factors, as well as, the signaling abnormalities that lead to podocyte dysfunction, damage and loss in early DKD in order to be able to develop effective treatments or preventive strategies that could forestall this process and thereby prevent progression of nephropathy.
Podocyte injury in diabetes is manifested morphologically by foot process effacement, hypertrophy, detachment, loss and death (not necessarily in that order)1,3,6,7. Studies suggest that diabetic podocytes become less stably anchored to the underlying glomerular capillary basement membrane and therefore can be more easily dislodged into the urinary space, in part due to reduction in alpha3beta1 integrin expression resulting from hemodynamic-induced stretch and TGF-β signaling8. In addition, podocyte loss may occur via apoptotic cell death as has also been demonstrated in several animal models of diabetes9,10.
The podocyte acts as a signaling pericyte11 to the glomerular endothelium and elsewhere in the glomerulus. Pericytes are contractile cells that support and wrap themselves around capillaries; they are widely distributed throughout the body and have different actions depending on their location. The podocyte receives signals from the endothelium and from circulating vascular factors12. It is now clear that there are a number of signaling pathways, some of which are interrelated in the podocyte that may be important and relevant in DKD. Potential molecular causes of the various alterations that lead to podocyte loss will be examined in the remainder of this review.
Podocyte Signaling Pathways in DKD
Insulin signaling in the podocyte
Insulin is a small 6 KD molecule that is freely accessible to the podocyte when released into the circulation from the pancreas. Insulin can rapidly signal to the podocyte13 after a meal to trigger a number of potentially beneficial homeostatic responses. These include the rapid absorption of glucose through translocation of glucose transporters to the plasma membrane of the cell, remodelling of its actin cytoskeleton13, and incorporation of potassium14 and calcium channels15 into the plasma membrane. Together these responses allow the cell a readily accessible energy source (glucose) and the ability to contract and remodel the cytoskeleton. These varied responses may allow podocytes to physiologically respond to the increased glomerular pressure and filtration that occurs after a meal.
There is accumulating evidence that glomerular and renal function is impaired when the podocyte is rendered insulin resistant. Specific deletion of the insulin receptor (IR) from the podocyte transgenically causes a number of features of DKD including albuminuria, increased glomerular matrix accumulation, thickening of the glomerular basement membrane, podocyte apoptosis and glomerulosclerosis, all occurring in a completely normoglycemic environment16. This suggests that a loss of podocyte insulin sensitivity could have an important effect on the development of DKD. Data from diabetic rodents support a loss of podocyte and glomerular insulin signaling in the early stages of DKD17. Indeed Mima, et al.18 have shown in models of both type 1 and type 2 diabetes that glomerular insulin signaling is lost early in disease progression. Conversely, insulin signaling in the tubular compartment, which controls ion reabsorption from the filtrate, as well as blood pressure and systemic glucose control19 are not affected. Compelling evidence from human studies shows that systemic insulin resistance is associated with proteinuric kidney disease in non-diabetic patients20 and that, in the setting of diabetes, insulin resistance predicts those patients who will develop nephropathy in both type 121-23 and type 2 diabetes24.
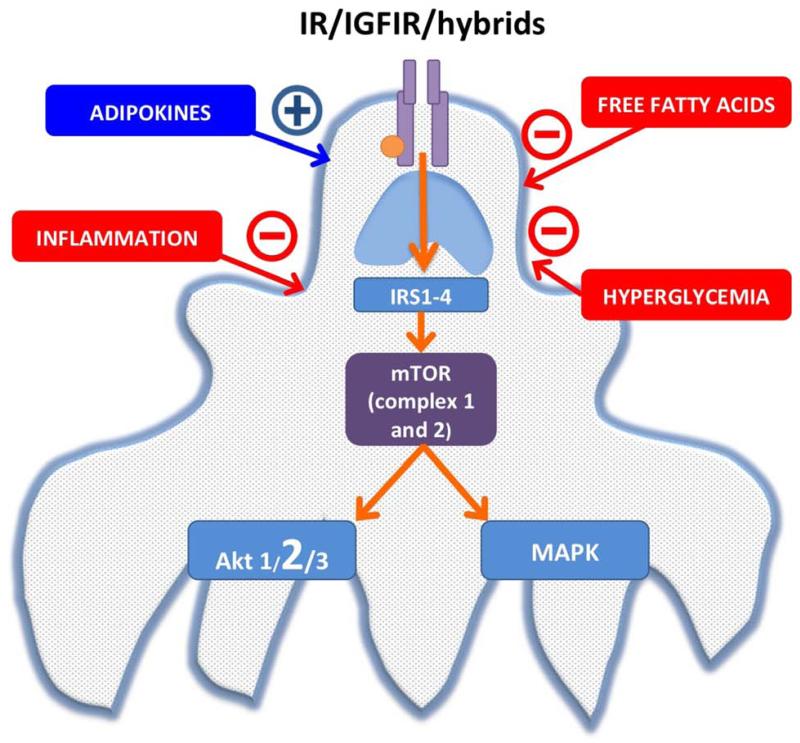
A number of other systemic changes that occur in early type 1 and type 2 diabetes can directly inhibit insulin signaling in the podocyte. These include exposure of podocytes to high glucose, which directly abrogates insulin signaling by increasing the molecule Src homology-2 domain-containing phosphatase-1 (SHP-1) which binds to the IR and prevents downstream signaling25. There is also evidence that insulin receptor substrate I (IRS-I) protein is ubiquinated more rapidly in the presence of high glucose in the glomerulus which leads to increased proteosomal degradation of this important downstream signaling molecule and therefore insulin resistance18. Activated innate immune responses and inflammation also may disrupt podocyte signaling in early DKD26. It has recently been shown that early glomerular inflammation, activation of the innate immune system and podocyte insulin resistance in diabetes are linked. The innate immune system is controlled by pattern recognition receptors which bind pathogen-associated molecules. Nucleotide-binding oligomerization domain containing 2 (NOD2) is an intracellular pattern recognition receptor that is responsible for immune activation following recognition of the bacterial cell wall component muramyl dipeptide (MDP). In a set of elegant experiments Du, et al.27 have shown that NOD2 is increased in animal models and in humans with DKD. Their studies have further shown that the MDP-NOD2 complex within podocytes abrogates insulin signaling. Moreover, mice with deletion of NOD2 are protected from DKD27. Finally, free fatty acids such as palmitate that are elevated in early diabetes and the metabolic syndrome also can directly impair podocyte insulin responsiveness28. In summary there is accumulating evidence that a number of systemic changes which occur early in diabetes can impair podocyte insulin sensitivity and signaling which in turn contributes to the development of DKD (Fig 1).
Figure 1.
Insulin/mTOR signaling pathway and also the extrinsic modifiers that are present in diabetes.
Insulin like growth factors (IGFs) in the podocyte
IGFs are structurally related to insulin and can signal through the same family of receptors as insulin via the IR, the IGF-I receptor (IGF-IR), and hybrid IR/IGF-IR receptors. IGFs have an increased affinity for the IGF-IR, whereas insulin has an increased affinity for the IR. Podocytes are the only glomerular cells that clearly produce IGF-I and IGF-II and both of these podocyte hormones signal in an autocrine manner. IGF stimulation of the podocyte causes different biological effects when compared to those resulting from insulin action even though they stimulate the same signaling pathways29. The IGF system appears to be more important for cell survival, whereas insulin stimulates glucose uptake and rapid actin remodeling.16,29 In contrast to insulin that is released into the circulation and rapidly and directly binds with its receptors, the IGFs are regulated in a much more complicated manner. These hormones are produced by the liver and also other tissues in the body and bind to a network of regulating proteins including the insulin like growth factor binding proteins (IGFBP), which control their delivery to the surrounding cell types29. In the setting of diabetes there is evidence that systemic IGF production decreases30,31 but that renal production may increase32. There are also a variety of alterations in the renal IGFBP system in diabetes33. It is therefore unclear how local delivery of biologically active IGF to the podocyte changes in diabetes34 and if this is important in modulating disease progression. However, the survival properties of these hormones on the podocyte would intuitively seem to be beneficial in this setting.
Adipokine signaling may modulate insulin action in the podocyte
Reduction in the adipokines and adiponectin, has also been shown to inhibit insulin signaling and podocyte function in DKD. Levels of adiponectin are decreased in obesity and linked to cellular insulin resistance35 and albuminuria36. The adiponectin knockout mouse model develops albuminuria with pathologic changes that are focused in the podocyte37. Specifically, these mice develop increased albuminuria and fusion of podocyte foot processes. When treated with adiponectin, the mice show reduced albuminuria, diminished podocyte foot process effacement, enhanced glomerular AMP kinase activation, and reduced urinary and glomerular markers of oxidant stress suggesting that maintenance of normal levels of adiponectin in obesity and diabetes could in turn help maintain normal signaling and normal podocyte function. However, it is unclear which, if any, of these adiponectin effects are due to maintenance of normal insulin signaling.
Mammalian target of rapamycin (mTOR) signaling and the podocyte
The mTOR pathway is important for cellular sensing of nutrient and growth factor levels, as well as cellular stress. In response to these inputs, mTOR signaling coordinates an array of cellular responses including increased protein synthesis, removal of intracellular organelle debris (autophagy), growth and survival. mTOR participates in two distinct complexes, mTORC1 and mTORC2. mTORC1 has several downstream effects but its primary role is to enhance protein synthesis via phosphorylation of two important downstream regulators, S6 kinase and 4-Elongation Factor Binding Protein-1. mTORC2 on the other hand, primarily enhances Akt activity by phosphorylating serine 47338. The importance of the mTOR pathway in the podocyte has been clearly demonstrated through the development of a number of sophisticated transgenic mouse models that have examined different aspects of this pathway39-41. Activation of mTORC1 specifically in podocytes by genetic deletion of an upstream negative regulator leads to many changes of DKD including albuminuria, glomerular basement membrane widening, podocyte loss, mesangial expansion and glomerular mesangial accumulation of fibronectin and collagen IV. All of these alterations can be at least partly prevented by treatment with the mTORC1 inhibitor, rapamycin, as well as by a podocyte-specific reduction in mTORC1 expression via heterozygous deletion of raptor, a specific component of mTORC1. Rapamycin treatment also reduces glomerular disease in the type 2 diabetic db/db model of DKD39. Although these studies indicate that targeting mTORC1 for therapy of DKD is attractive, the necessity of maintaining mTOR activity at a normal level was underlined by the finding that elimination of raptor expression in podocytes resulted in significant proteinuria41. These studies have clearly shown that modulation of mTOR activity in the podocyte in either direction can result in pathogenic changes.
The insulin/IGF and mTOR pathways are closely associated. Insulin/IGF activates mTORC1 through AKT signaling and conversely mTORC2 inhibits insulin signaling. The close relationship of these pathways may explain why overexpressing or depleting specific insulin regulatable glucose transporters in the podocyte42,43 do not result in glucose toxicity or glucose debt effects in the podocyte as they seem to in other glomerular cells such as the mesangial cell44. Indeed, podocyte-specific overexpression of the glucose transporter, GLUT1, in podocytes leads to reduced nephropathy in animal models of diabetes42 while conversely deletion of GLUT4 from podocytes also reduces nephropathy even though it stimulates podocyte hypertrophy via an mTOR-independent mechanism43. While the complexities of the interactions between insulin signaling, mTOR activation and glucose uptake and glucose transporter expression remain to be elucidated, it seems likely that a balance of nutrient and growth factor stimulation of this cell is critical for its function. This is perhaps not surprising as the podocyte needs biological strategies to survive for a prolonged time in vivo as a terminally differentiated cell.
A recent study found that Akt2 which functions in the PI3K signaling pathway is critically important for podocyte, glomerular and renal survival in a number of rodent and human models of chronic kidney disease (CKD)45. This molecule is activated by both insulin and also the mTOR2 complex45. In low GFR glomerular stress situations it is up-regulated in the podocyte and when mTOR is inhibited genetically or pharmacologically with rapamycin it accelerates kidney damage. Assessing Akt2 in the setting of DKD will be critical especially in light of the evidence that mTOR in the podocyte is activated in a detrimental way in diabetes and that treatment with an mTOR inhibitor may be beneficial39. These recent findings indicate that such treatment could also be detrimental since mTORC2, as well as mTORC1, can be inhibited by long-term rapamycin treatment and the kidney protective effect of Akt2 could be eliminated.
Vitamin D signaling in the podocyte
There is strong evidence that the vitamin D receptor is highly expressed in podocytes in culture and in vivo46. It also appears that vitamin D treatment protects podocytes from injury in non-diabetic47 and diabetic animal models46,48,49 and may work synergistically with renin-angiotensin-aldosterone system (RAAS) blockade to further reduce injury in diabetes. The VITAL study, a randomized controlled clinical trial, showed that vitamin D therapy modestly reduced proteinuria in type 2 diabetic patients on RAAS blockade with progressive nephropathy50. A more recent mouse study found that podocyte-specific expression of a human vitamin D receptor reduced albuminuria, podocyte loss and other signs of nephropathy in streptozotocin diabetes and, importantly, rescued the nephropathic phenotype found in vitamin D receptor knockout mice51. Although the signaling mechanisms responsible for the podocyte protective effects are not completely clear there is evidence that vitamin D treatment may prevent podocyte apoptosis by inhibition of p38 MAP kinsase signaling, PI3 kinase, Akt51, as well as, RAAS signaling52.
Estrogen mediated signaling and the diabetic podocyte
There is relatively convincing evidence that female gender, at least in premenopausal women, protects against progression of non-diabetic glomerular diseases53. However, there is less evidence that female gender is protective in DKD54. Nonetheless, a number of reports suggest that podocyte estrogen receptor signaling may be protective in DKD. Estrogen effects are mediated by two distinct estrogen receptor subtypes, ERá and ERâ and both receptors are present in podocytes54. Estradiol has been shown to stimulate ERá protein expression as well as to reduce podocyte apopotosis after puromycin aminonucleoside exposure55. There is less compelling evidence that ER signaling is protective in diabetic models or humans. However it has recently been reported that a genetic variant found in the FinnDiane cohort is associated with ESRD in type 1 diabetic females56. This is located on 2q31.1 and is potentially a variant in SP3 which is a transcription factor that interacts with the estogen receptor alpha. Furthermore a recent rodent study suggests that estradiol administration prevents podocyte apoptosis in a diabetic mouse model57 possibly by Rac1-mediated stabilization of F-actin58. However, because the studies were performed in an inadequate model of diabetic nephropathy, the C57BL/6 db/db mouse, these results will need to be verified in other more robust models and in humans with DKD.
Vascular factors, the podocyte and DKD
Vascular endothelial growth factor-A (VEGF-A)
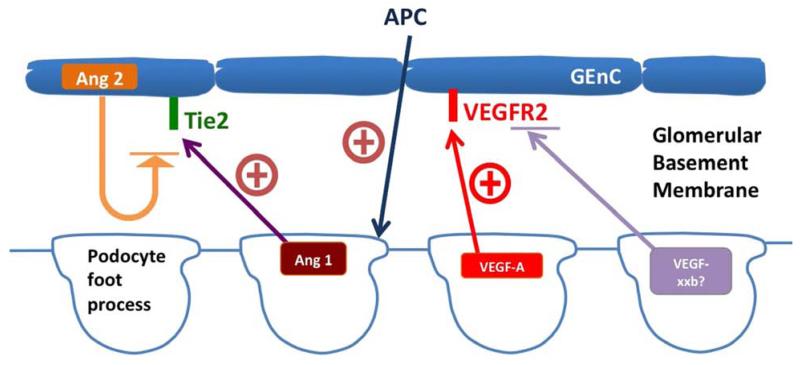
The podocyte both generates and responds to a number of angiogenic growth factors. The best known is vascular endothelial growth factor A (VEGF-A). This molecule is produced in great quantities by the podocyte in the glomerulus. It does not cause neovascularization as it does in the eye59 or in neoplastic tissue60 but is important for maintaining the integrity of the glomerular filtration barrier as well as a survival factor for the podocyte61. It does this by signaling through the VEGF-Receptor-2 (VEGFR2) which is most abundantly expressed in the glomerular endothelium. Podocyte specific VEGF-A excess or deficiency in development62 or maturity63 causes glomerular damage. Too much VEGF-A induces endothelial growth and swelling, a condition known as endotheliosis, whereas inadequate VEGF-A release results in endothelial damage and apoptosis leading to glomerulosclerosis64. As is the case for mTOR, VEGF-A levels and signaling need to be regulated closely to maintain healthy glomerular structure and function. In diabetes there is evidence that glomerular VEGF-A levels can be both elevated65,66 or reduced67 and this may be related to the duration of the disease. The human data suggest that VEGF-A expression may be elevated early in DKD and then probably goes down below normal levels as the disease progresses. Therapeutic strategies to elevate68 and suppress local VEGF-A expression69 have both been shown to be beneficial depending on experimental conditions. Going forward it will be interesting if we can delineate how to regulate this signaling pathway in a beneficial manner in DKD. Recently there has also been a link made between the production of VEGF-A and the insulin signaling pathway in the podocyte70. Similar to retinal pigment epithelial cells in the eye, insulin is able to control the release of VEGF-A. It is possible that VEGF-A production may be at least partially governed by insulin sensitivity of the podocyte.
An interesting development in this field is the proposition that as well as pro-angiogenic VEGF isoforms there are complementary antagonistic anti-angiogenic forms that have been generated through splicing variants of the VEGF mRNA causing the final 6 amino acids of the molecule to be altered. These are called VEGFxxb isoforms71. This is currently a controversial field and it is unclear if these forms exist in all species72,73. However, if these isoforms are present they could represent another method of manipulating this system in the setting of diabetes.
Angiopoietins and the podocyte
Another class of angiogenic growth factors in the glomerulus are the angiopoietins, Ang-1 and Ang-2. Manipulating these molecules has shown some therapeutic promise in DKD. Ang-1 is produced by the podocyte and signals through the Tie-2 receptor which is found on the glomerular endothelium. Ang-2 is released from endothelial cells and acts as an autocrine competitive inhibitor of Tie-274. Encouragingly, there are several reports that demonstrate that Ang-1 production either by podocytes74,75 or mesangial cells74, or via exogenous administration76 can slow DKD in rodent models. Moreover, altering the Ang-1/Ang-2 ratio in favor of Ang-2 has been shown to be detrimental in DKD77 suggesting this pathway is important.
Activated protein C
In addition to producing angiogenic factors the podocyte is responsive to circulating factors that are altered by endothelial dysfunction in diabetes. The vascular factor that most clearly affects podocyte behaviour in diabetes is the anti-thrombotic agent, activated protein C (APC). The circulating zymogen, protein C, binds endothelial thrombomodulin and is activated by thrombin to APC. APC is a serine protease that proteolytically inactivates coagulation Factors V and VIII; however, it has several effects independent of its coagulation regulatory role. It has been shown to be cytoprotective, anti-apoptotic and have anti-inflammatory effects78,79. Glomerular APC formation, which is regulated by endothelial thrombomodulin, is reduced in diabetic mice and APC deficiency promotes podocyte injury and apoptosis at least in part via reduced activation of its receptors, PAR1 and 378, and downstream signaling via the pro-oxidant factor, p66Shc80_ENREF_79. Restoration of APC levels in diabetic mice reverses enhanced oxidative stress as well as podocyte loss, both hallmarks of early DKD.
In summary, the podocyte is an important source of a number of angiogenic growth factors that signal to the glomerular endothelium. These factors change in diabetes and may be able to be therapeutically modifiable (Fig 2). The podocyte also responds to circulating factors, such as APC, insulin and adipokines and very likely to yet unknown factors from the endothelium. All of these factors may impact podocyte signaling and how podocytes respond to metabolic stress thus heightening podocyte susceptibility to injury.
Figure 2.
Podocyte and VEGF/angiopoietins and regulation in diabetes
Clinical Summary.
Podocyte pathology is central to the progression of diabetic kidney disease.
Signaling abnormalities within the podocyte (e.g., changes insulin action and mTOR signaling) are central to the development of podocyte pathology.
The podocyte secretes paracrine factors, such as VEGF-A, that alter endothelial cell function and morphology in diabetes.
Cytoprotective vascular factors such as activated protein C are reduced in diabetes which promote podocyte injury and death.
Acknowledgements
Neither author has financial or other conflicts relevant to this review. RJC is supported by the Medical Research Council (MR/K010492/1) and Kidney Research UK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997 Jan 15;99(2):342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steffes MW, Schmidt D, McCrery R, Basgen JM. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001 Jun;59(6):2104–2113. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 3.Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte Detachment and Reduced Glomerular Capillary Endothelial Fenestration in Human Type 1 Diabetic Nephropathy. Diabetes. 2007 May 29; doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 4.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999 Nov;42(11):1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 5.Wharram BL, Goyal M, Wiggins JE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005 Oct;16(10):2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 6.Weil EJ, Lemley KV, Mason CC, et al. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney international. 2012 Nov;82(9):1010–1017. doi: 10.1038/ki.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petermann AT, Pippin J, Krofft R, et al. Viable podocytes detach in experimental diabetic nephropathy: potential mechanism underlying glomerulosclerosis. Nephron Exp Nephrol. 2004;98(4):e114–123. doi: 10.1159/000081555. [DOI] [PubMed] [Google Scholar]
- 8.Dessapt C, Baradez MO, Hayward A, et al. Mechanical forces and TGF{beta}1 reduce podocyte adhesion through {alpha}3{beta}1 integrin downregulation. Nephrol. Dial. Transplant. 2009 May 6; doi: 10.1093/ndt/gfp204. 2009:gfp204. [DOI] [PubMed] [Google Scholar]
- 9.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006 Jan;55(1):225–233. [PubMed] [Google Scholar]
- 10.Menini S, Iacobini C, Oddi G, et al. Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia. 2007 Dec;50(12):2591–2599. doi: 10.1007/s00125-007-0821-y. [DOI] [PubMed] [Google Scholar]
- 11.Eremina V, Baelde HJ, Quaggin SE. Role of the VEGF--a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol. 2007;106(2):p32–37. doi: 10.1159/000101798. [DOI] [PubMed] [Google Scholar]
- 12.Yuen DA, Stead BE, Zhang Y, et al. eNOS deficiency predisposes podocytes to injury in diabetes. Journal of the American Society of Nephrology: JASN. 2012 Nov;23(11):1810–1823. doi: 10.1681/ASN.2011121170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coward RJ, Welsh GI, Yang J, et al. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005 Nov;54(11):3095–3102. doi: 10.2337/diabetes.54.11.3095. [DOI] [PubMed] [Google Scholar]
- 14.Kim EY, Dryer SE. Effects of insulin and high glucose on mobilization of Slo1 BKCa channels in podocytes. J Cell Physiol. 2010 Dec 6; doi: 10.1002/jcp.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim EY, Anderson M, Dryer SE. Insulin increases surface expression of TRPC6 channels in podocytes: Role of NADPH oxidases and reactive oxygen species. Am J Physiol Renal Physiol. 2011 Oct 26; doi: 10.1152/ajprenal.00423.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welsh GI, Hale LJ, Eremina V, et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010 Oct 6;12(4):329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tejada T, Catanuto P, Ijaz A, et al. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008 Jun;73(12):1385–1393. doi: 10.1038/ki.2008.109. [DOI] [PubMed] [Google Scholar]
- 18.Mima A, Ohshiro Y, Kitada M, et al. Glomerular-specific protein kinase C-beta-induced insulin receptor substrate-1 dysfunction and insulin resistance in rat models of diabetes and obesity. Kidney Int. 2011 Apr;79(8):883–896. doi: 10.1038/ki.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari S, Singh RS, Li L, et al. Deletion of the insulin receptor in the proximal tubule promotes hyperglycemia. Journal of the American Society of Nephrology: JASN. 2013 Jul;24(8):1209–1214. doi: 10.1681/ASN.2012060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mykkanen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998 May;47(5):793–800. doi: 10.2337/diabetes.47.5.793. [DOI] [PubMed] [Google Scholar]
- 21.Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int. 2002 Sep;62(3):963–970. doi: 10.1046/j.1523-1755.2002.00507.x. [DOI] [PubMed] [Google Scholar]
- 22.Yip J, Mattock MB, Morocutti A, Sethi M, Trevisan R, Viberti G. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993 Oct 9;342(8876):883–887. doi: 10.1016/0140-6736(93)91943-g. [DOI] [PubMed] [Google Scholar]
- 23.Ekstrand AV, Groop PH, Gronhagen-Riska C. Insulin resistance precedes microalbuminuria in patients with insulin-dependent diabetes mellitus. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 1998 Dec;13(12):3079–3083. doi: 10.1093/ndt/13.12.3079. [DOI] [PubMed] [Google Scholar]
- 24.Parvanova AI, Trevisan R, Iliev IP, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006 May;55(5):1456–1462. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 25.Drapeau N, Lizotte F, Denhez B, Guay A, Kennedy CR, Geraldes P. Expression of SHP-1 induced by hyperglycemia prevents insulin actions in podocytes. American journal of physiology. Endocrinology and metabolism. 2013 Jun 1;304(11):E1188–1198. doi: 10.1152/ajpendo.00560.2012. [DOI] [PubMed] [Google Scholar]
- 26.Mora C, Navarro JF. Inflammation and diabetic nephropathy. Current diabetes reports. 2006 Dec;6(6):463–468. doi: 10.1007/s11892-006-0080-1. [DOI] [PubMed] [Google Scholar]
- 27.Du P, Fan B, Han H, et al. NOD2 promotes renal injury by exacerbating inflammation and podocyte insulin resistance in diabetic nephropathy. Kidney international. 2013 Aug;84(2):265–276. doi: 10.1038/ki.2013.113. [DOI] [PubMed] [Google Scholar]
- 28.Lennon R, Pons D, Sabin MA, et al. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dial Transplant. 2009 Nov;24(11):3288–3296. doi: 10.1093/ndt/gfp302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hale LJ, Welsh GI, Perks CM, et al. Insulin-like growth factor-II is produced by, signals to and is an important survival factor for the mature podocyte in man and mouse. The Journal of pathology. 2013 May;230(1):95–106. doi: 10.1002/path.4165. [DOI] [PubMed] [Google Scholar]
- 30.Amin R, Schultz C, Ong K, et al. Low IGF-I and elevated testosterone during puberty in subjects with type 1 diabetes developing microalbuminuria in comparison to normoalbuminuric control subjects: the Oxford Regional Prospective Study. Diabetes Care. 2003 May;26(5):1456–1461. doi: 10.2337/diacare.26.5.1456. [DOI] [PubMed] [Google Scholar]
- 31.Teppala S, Shankar A. Association between serum IGF-1 and diabetes among U.S. adults. Diabetes Care. 2010 Oct;33(10):2257–2259. doi: 10.2337/dc10-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segev Y, Landau D, Marbach M, Shehadeh N, Flyvbjerg A, Phillip M. Renal hypertrophy in hyperglycemic non-obese diabetic mice is associated with persistent renal accumulation of insulin-like growth factor I. Journal of the American Society of Nephrology: JASN. 1997 Mar;8(3):436–444. doi: 10.1681/ASN.V83436. [DOI] [PubMed] [Google Scholar]
- 33.Rajpathak SN, Gunter MJ, Wylie-Rosett J, et al. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab Res Rev. 2009 Jan;25(1):3–12. doi: 10.1002/dmrr.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasylyeva TL, Ferry RJ., Jr. Novel roles of the IGF-IGFBP axis in etiopathophysiology of diabetic nephropathy. Diabetes Res Clin Pract. 2007 May;76(2):177–186. doi: 10.1016/j.diabres.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. The Journal of clinical investigation. 2006 Jul;116(7):1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyvis K, Verrijken A, Wouters K, Van Gaal L. Plasma adiponectin level is inversely correlated with albuminuria in overweight and obese nondiabetic individuals. Metabolism: clinical and experimental. 2013 Nov;62(11):1570–1576. doi: 10.1016/j.metabol.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 37.Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008 May;118(5):1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004 Jul 27;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 39.Inoki K, Mori H, Wang J, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011 Jun;121(6):2181–2196. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cina DP, Onay T, Paltoo A, et al. Inhibition of MTOR Disrupts Autophagic Flux in Podocytes. J Am Soc Nephrol. 2011 Dec 22; doi: 10.1681/ASN.2011070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godel M, Hartleben B, Herbach N, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011 Jun;121(6):2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Schin M, Saha J, et al. Podocyte-specific overexpression of GLUT1 surprisingly reduces mesangial matrix expansion in diabetic nephropathy in mice. Am J Physiol Renal Physiol. 2010 Jul;299(1):F91–98. doi: 10.1152/ajprenal.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guzman J, Jauregui AN, Merscher-Gomez S, et al. Podocyte-specific GLUT4 deficient mice have fewer and larger podocytes and are protected from diabetic nephropathy. Diabetes. 2013 Oct 7; doi: 10.2337/db13-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Heilig K, Saunders T, et al. Transgenic overexpression of GLUT1 in mouse glomeruli produces renal disease resembling diabetic glomerulosclerosis. Am J Physiol Renal Physiol. 2010 Jul;299(1):F99–F111. doi: 10.1152/ajprenal.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canaud G, Bienaime F, Viau A, et al. AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nature medicine. 2013 Sep 22; doi: 10.1038/nm.3313. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Zhou J, Minto AW, et al. Altered vitamin D metabolism in type II diabetic mouse glomeruli may provide protection from diabetic nephropathy. Kidney Int. 2006 Sep;70(5):882–891. doi: 10.1038/sj.ki.5001624. [DOI] [PubMed] [Google Scholar]
- 47.Kuhlmann A, Haas CS, Gross ML, et al. 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol. 2004 Mar;286(3):F526–533. doi: 10.1152/ajprenal.00316.2003. [DOI] [PubMed] [Google Scholar]
- 48.Deb DK, Sun T, Wong KE, et al. Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney international. 2010 Jun;77(11):1000–1009. doi: 10.1038/ki.2010.22. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Deb DK, Kong J, et al. Long-term therapeutic effect of vitamin D analog doxercalciferol on diabetic nephropathy: strong synergism with AT1 receptor antagonist. American journal of physiology. 2009 Sep;297(3):F791–801. doi: 10.1152/ajprenal.00247.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010 Nov 6;376(9752):1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Deb DK, Zhang Z, et al. Vitamin D receptor signaling in podocytes protects against diabetic nephropathy. J Am Soc Nephrol. 2012 Dec;23(12):1977–1986. doi: 10.1681/ASN.2012040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li YC. Podocytes as target of vitamin D. Curr Diabetes Rev. 2011 Jan;7(1):35–40. doi: 10.2174/157339911794273964. [DOI] [PubMed] [Google Scholar]
- 53.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000 Feb;11(2):319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 54.Doublier S, Lupia E, Catanuto P, Elliot SJ. Estrogens and progression of diabetic kidney damage. Curr Diabetes Rev. 2011 Jan;7(1):28–34. doi: 10.2174/157339911794273982. [DOI] [PubMed] [Google Scholar]
- 55.Kummer S, Jeruschke S, Wegerich LV, et al. Estrogen receptor alpha expression in podocytes mediates protection against apoptosis in-vitro and in-vivo. PLoS ONE. 2011;6(11):e27457. doi: 10.1371/journal.pone.0027457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandholm N, McKnight AJ, Salem RM, et al. Chromosome 2q31.1 associates with ESRD in women with type 1 diabetes. Journal of the American Society of Nephrology: JASN. 2013 Oct;24(10):1537–1543. doi: 10.1681/ASN.2012111122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Catanuto P, Doublier S, Lupia E, et al. 17beta-estradiol and tamoxifen upregulate estrogen receptor beta expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 2009 Jun;75(11):1194–1201. doi: 10.1038/ki.2009.69. [DOI] [PubMed] [Google Scholar]
- 58.Catanuto P, Fornoni A, Pereira-Simon S, et al. In vivo 17beta-estradiol treatment contributes to podocyte actin stabilization in female db/db mice. Endocrinology. 2012 Dec;153(12):5888–5895. doi: 10.1210/en.2012-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu M, Amano S, Miyamoto K, et al. Insulin-induced vascular endothelial growth factor expression in retina. Invest Ophthalmol Vis Sci. 1999 Dec;40(13):3281–3286. [PubMed] [Google Scholar]
- 60.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003 Jun;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 61.Foster RR, Hole R, Anderson K, et al. Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes. Am J Physiol Renal Physiol. 2003 Jun;284(6):F1263–1273. doi: 10.1152/ajprenal.00276.2002. [DOI] [PubMed] [Google Scholar]
- 62.Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003 Mar;111(5):707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008 Mar 13;358(11):1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sivaskandarajah GA, Jeansson M, Maezawa Y, Eremina V, Baelde HJ, Quaggin SE. Vegfa protects the glomerular microvasculature in diabetes. Diabetes. 2012 Nov;61(11):2958–2966. doi: 10.2337/db11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cooper ME, Vranes D, Youssef S, et al. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999 Nov;48(11):2229–2239. doi: 10.2337/diabetes.48.11.2229. [DOI] [PubMed] [Google Scholar]
- 66.Hohenstein B, Hausknecht B, Boehmer K, Riess R, Brekken RA, Hugo CP. Local VEGF activity but not VEGF expression is tightly regulated during diabetic nephropathy in man. Kidney Int. 2006 May;69(9):1654–1661. doi: 10.1038/sj.ki.5000294. [DOI] [PubMed] [Google Scholar]
- 67.Baelde HJ, Eikmans M, Lappin DW, et al. Reduction of VEGF-A and CTGF expression in diabetic nephropathy is associated with podocyte loss. Kidney Int. 2007 Apr;71(7):637–645. doi: 10.1038/sj.ki.5002101. [DOI] [PubMed] [Google Scholar]
- 68.Sivaskandarajah GA, Jeansson M, Maezawa Y, Eremina V, Baelde HJ, Quaggin SE. Vegfa Protects the Glomerular Microvasculature in Diabetes. Diabetes. 2012 Aug 13; doi: 10.2337/db11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002 Oct;51(10):3090–3094. doi: 10.2337/diabetes.51.10.3090. [DOI] [PubMed] [Google Scholar]
- 70.Hale LJ, Hurcombe J, Lay A, et al. Insulin directly stimulates VEGF-A production in the glomerular podocyte. American journal of physiology. Renal physiology. 2013 Jul;305(2):F182–188. doi: 10.1152/ajprenal.00548.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woolard J, Wang WY, Bevan HS, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer research. 2004 Nov 1;64(21):7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 72.Harris S, Craze M, Newton J, et al. Do anti-angiogenic VEGF (VEGFxxxb) isoforms exist? A cautionary tale. PLoS One. 2012;7(5):e35231. doi: 10.1371/journal.pone.0035231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bates DO, Mavrou A, Qiu Y, et al. Detection of VEGF-A(xxx)b isoforms in human tissues. PLoS One. 2013;8(7):e68399. doi: 10.1371/journal.pone.0068399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeansson M, Gawlik A, Anderson G, et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest. 2011 Jun 1;121(6):2278–2289. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dessapt-Baradez C, Woolf AS, White KE, et al. Targeted Glomerular Angiopoietin-1 Therapy for Early Diabetic Kidney Disease. Journal of the American Society of Nephrology: JASN. 2013 Sep 5; doi: 10.1681/ASN.2012121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee S, Kim W, Moon SO, et al. Renoprotective effect of COMP-angiopoietin-1 in db/db mice with type 2 diabetes. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2007 Feb;22(2):396–408. doi: 10.1093/ndt/gfl598. [DOI] [PubMed] [Google Scholar]
- 77.Davis B, Dei Cas A, Long DA, et al. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. Journal of the American Society of Nephrology: JASN. 2007 Aug;18(8):2320–2329. doi: 10.1681/ASN.2006101093. [DOI] [PubMed] [Google Scholar]
- 78.Isermann B, Vinnikov IA, Madhusudhan T, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007 Nov;13(11):1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 79.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost. 2006 Apr;32(Suppl 1):49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 80.Bock F, Shahzad K, Wang H, et al. Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc. Proceedings of the National Academy of Sciences of the United States of America. 2013 Jan 8;110(2):648–653. doi: 10.1073/pnas.1218667110. [DOI] [PMC free article] [PubMed] [Google Scholar]