Abstract
Stress occurs in everyday life, but the relationship between stress and the onset or development of depression/anxiety remains unknown. Increasing evidence suggests that the impairment of antioxidant defense and the neuronal cell death are important in the process of emotional disorders. Chronic stress impairs the homeostasis of antioxidants/oxidation, which results in the aberrant stimulation of the cell cycle proteins where cGMP-PKG signaling is thought to have an inhibitory role. Phosphodiesterase 2 (PDE2) is linked to cGMP-PKG signaling and highly expressed in the limbic brain regions including hippocampus and amygdala, which may play important roles in the treatment of depression and anxiety. To address the possible effects of PDE2 inhibitors on depression-/anxiety-like behaviors and the underlying mechanisms, Bay 60-7550 (0.75, 1.5 and 3 mg/kg, i.p.) was administered 30 min before chronic stress. The results suggested that Bay 60-7550 not only restored the behavioral changes but also regulated Cu/Zn superoxide dismutase (SOD) levels differentially in hippocampus and amygdala, which were increased in the hippocampus while decreased in the amygdala. It was also significant that Bay 60-7550 regulated the abnormalities of pro- and anti-apoptotic components, such as Bax, Caspase 3 and Bcl-2, and the indicator of PKG signaling characterized by pVASPser239, in these two brain regions. The results suggested that Bay 60-7550 is able to alleviate oxidative stress and mediate part of the apoptotic machinery in neuronal cells possibly through SOD-cGMP/PKG-anti-apoptosis signaling and that inhibition of PDE2 may represent a novel therapeutic target for psychiatric disorders, such as depression and anxiety.
Keywords: Phosphodiesterase 2, Oxidative damage, Neuronal apoptosis, Cyclic GMP
1. Introduction
Modern humans experience different stressors from their daily activities, which once become excessive and prolonged, can cause psychiatric disorders. Depression and anxiety are such illnesses that are chronic, recurring and potentially life-threatening, and have been estimated to affect 31% of the US population [1]. Extensive research has been conducted to reveal multiple neural substrates and mechanisms that contribute to the etiology of depression and anxiety, among which the imbalance between oxidation and antioxidant defense system, as well as apoptotic events that occur among neuronal cells have gained attention.
It is well known that chronic stress induces oxidative stress, possibly through activation of HPA axis followed by overproduction of stress hormones such as glucocorticoids and glutamate, and several inflammatory reactions involving TNF-α and IL-1β [2,3]. Among the various organs, the brain is the most susceptible to oxidative stress due to its relatively high consumption of oxygen, high iron content, fatty acids peroxidation, and low antioxidant capacity [2]. Therefore brain oxidative stress is now known as a key mechanism in the pathology of brain disorders such as depression and anxiety. In fact, several studies have shown that chronic restraint stress was able to remarkably induce oxidative damage to the brain, as evidenced by the increased levels of reactive oxygen species (ROS) and lowered levels of antioxidant components [4,5].
Apoptosis has also been proposed to be a mechanism contributing to stress-related mood disorders both in humans and animal models [6]. Cell death often occurs among certain populations of neurons as a result of chronic stress, in which case antidepressants show the ability to oppose the effects and promote neuroprotection. The apoptotic process is generally controlled by proapoptotic (Bax) and antiapoptotic (Bcl-2) proteins [7]. However, the data concerning the level of antiapoptotic protein Bcl-2 so far are contradictory: some reported a decrease [8] while others reported an increase [9] of Bcl-2 expression after chronic stress.
Increasing evidence indicates that cyclic adenosine monophosphate (cAMP)- or cyclic guanosine monophosphate (cGMP)-mediated signaling appears to participate in neuronal modulation related to depression and anxiety [10,11]. As an enzyme family that mainly hydrolyzes these cyclic nucleotides, phosphodiesterases (PDEs) have been pointed out in several reviews regarding their possible involvement in stress-related emotional/cognitive disorders [11,12], but detailed mechanisms remain to be investigated. Indeed, chronic stress impairs the homeostasis of antioxidants/oxidation, which results in the aberrant stimulation of the cell cycle proteins on which cAMP/cGMP-dependent signaling is thought to have an inhibitory role. Inhibition of PDEs can increase intracellular cAMP and/or cGMP and affect the downstream signaling [12,13]. Some PDE inhibitors, such as those of PDE4 and PDE5, have been known for their neuroprotective effects against emotion and cognitive disorders, but are also known for their side effects [10,14]. PDE2, as a relatively new player in this field, catalyzes both cGMP and cAMP and is found in brain regions such as hippocampus and amygdala that are essential components of the neural circuitry mediating psychiatric disorders [15]. Moreover, PDE2 is also found in the adrenal glands [16], which is an integral part of the HPA axis, thereby making PDE2 a likely candidate in controlling stress-induced emotionality and depressive behaviors [17].
The present study was designed to evaluate the long-term effects of a specific PDE2 inhibitor Bay 60-7550 on chronic unpredictable stress (CUS)-induced depression- and anxiety-like behaviors. To further examine underlying mechanism, possible antioxidant and anti-apoptotic actions of Bay 60-7550 were also investigated.
2. Materials and Methods
2.1 Animals
Male ICR mice, 12-16 weeks of age and weighing 25-30 g were used (Harlan, Indianapolis, IN) for all the experiments. Rodent chow and tap water were freely available. Mice were kept in a temperature-controlled room under standard laboratory conditions, with a 12 h light/12 h dark cycle (lights on at 6:00 a.m.). All experiments were carried out according to the “NIH Guide for the Care and Use of Laboratory Animals” (revised 2011) and were approved by the Institutional Animal Care and Use Committee.
2.2 Drugs and treatments
Bay 60-7550 (97% purity) was purchased from Cayman Chemical Company Inc (Chicago, IL), and dissolved in 10% DMSO (Fisher Scientific, Fair Lawn, NJ). The positive control drugs desipramine and diazepam were purchased from Sigma Aldrich (St. Louis, MO). Mice were given Bay 60-7550 at 0.75, 1.5 and 3 mg/kg, desipramine at 10 mg/kg, or diazepam at 1.5 mg/kg, once daily via intraperitoneal injections (i.p.) in a volume of 10 ml/kg body weight 30 min before stress every day. Selection of Bay 60-7550’s working dose was based on our previous studies with minor modifications [17,18]. Control animals received vehicle only. Behavioral testing was done 24 hrs after the last stress event. Hippocampus and amygdala were dissected from brains immediately after behavioral testing and stored at -80°C until analyses were carried out.
2.3 Chronic unpredictable stress (CUS) procedure
The CUS paradigm exposed mice to two of eight different stressors daily (forced swim, restraint stress, overnight lights, cage tilting, cold stress, food/water deprivation etc., as shown in Tab. 1) for ten consecutive days as described previously [19-21]. This protocol has been shown to cause significant changes characteristic of depressive/anxiogenic behavior, as well as a number of associated cellular and neurochemical changes. Control groups also were handled every day but not subjected to the stressors. 24 h after the last stress, each animal was subjected to two behavioral tests in two consecutive days, one each for depression and anxiety behaviors (as shown in Tab 1). Brain samples from each mouse were collected after behavioral tests for mRNA or protein level analyses to achieve n=10.
Table 1.
Chronic unpredictable stress (CUS) protocol
| Days | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stressor 1 |
Cage tilting (6 h) |
Switching cages (6 h) |
Swim stress (12°C, 5 min) |
Cage tilting (6 h) |
Food/water deprivation (6 h) |
Swim stress (12°C, 5 min) |
Cold room (4 °C, 15 min) |
Humid sawdust (6 h) |
Isolation (6 h) |
Food/water deprivation (6 h) |
FST or TST |
EPM or MBT |
|
| ||||||||||||
| Stressor 2 |
Lights on (overni ght) |
Cold room (4 °C, 15 min) |
Humid sawdust (6 h) |
Restrain t (60 min) |
Humid sawdust (overnight) |
Isolation (overnight) |
Cage tilting (6 h) |
Swim stress (12°C, 5 min) |
Restraint (60 min) |
Lights on (overnight) |
||
2.5 Tail suspension test
The tail-suspension test (TST) for depressive behavior in mice was carried out as described previously [22]. Mice were suspended from a stand arm by a 1.9 × 15 cm with masking tape loop attached to the end of their tail, leaving the last 1 cm of tail exposed. Mice were observed over a 6-min duration and the time spent immobile during the last 4-min period was recorded.
2.4 Forced swimming test
The forced swimming test (FST) was carried out similarly to that described elsewhere [23]. Briefly, mice were individually placed in glass cylinders (height: 25 cm; diameter: 10 cm; containing 10 cm depth of water at 24 ± 1 °C) for 6 min. A mouse was determined to be immobile when there were only small movements necessary to keep its head above water. The duration of immobility was recorded during the last 4 min of the 6-min testing period.
2.6 Elevated plus-maze test
Behavior in the elevated plus-maze test (EPM) was assessed as described previously [17,24]. The EPM (San Diego Instruments, San Diego, CA) consisted of two open arms (30 cm × 5 cm) and two closed arms (30 ×5 × 15 cm) that extended from a central platform (5 cm × 5 cm). The entire maze was elevated 40 cm above the floor. During the 5-min of free exploration, the number of entries into and the time spent in open and closed arms were recorded. An entry was defined as all four paws in an arm.
2.7 Marble burying test
Behavior in the Marble burying test (MBT) was assessed as described previously [25]. Briefly, group-housed mice were individually placed in transparent propylene cages that were identical to their home cages (28 cm × 16 cm × 12 cm) containing 5 cm layer of sawdust and 24 clean glass marbles (1.5 cm in diameter) equally spaced along the wall. No food or water was present. 30 min later, animals were removed, and the number of marbles at least two-third buried in the sawdust was recorded [26,27].
2.8 Real-time PCR for Blcl-2, Bax and Caspase 3
Total RNA was isolated from the hippocampus and amygdala using the TRiZOL reagent (TriZOL®Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. This was followed by the reverse transcription of 0.5 μg total RNA into cDNA using High Capacity cDNA archive kit (Applied Biosystems, Foster City, CA). PCR reactions were performed in duplicate wells, using iCycler Real-Time PCR machine (Bio-Rad, Hercules, CA, USA). After cDNA synthesis, a PCR mixture containing 50% v/v per sample of SYBR Green (iQ SYBR Green Supermix reagent, Bio-Rad, Hercules, CA, USA) was tested with specific primers for Bcl-2, Bax, Caspase 3 and β-actin. PCR products were amplified followed by melt curve analysis and gel electrophoresis to verify specificity and purity of product. All the data were normalized to the housekeeping gene β-actin.
2.9 Immuno-blot analyses for Cu/Zn SOD, p-VASPser239 and apoptotic markers
Aliquots of supernatant from hippocampus and amygdala (40 μg protein/well) were separated using 10% SDS-PAGE as described previously [17]; prestained protein molecular markers were run in parallel according to the specific molecular weight of the detected protein. Proteins from the gels were then transferred to PVDF membranes and incubated with primary antibodies overnight at 4 °C (anti-Cu/Zn SOD, anti-pVASPser239, anti-Bcl-2, anti-Bax, anti-Caspase 3 and anti-β-actin, all diluted 1:1,000). After 3 washes with TBST, the blots were incubated with the secondary goat anti-mouse or anti-rabbit antibodies (LI-COR Biosciences, Lincoln, NE) for 1 h at room temperature, and detected for bands using LI-COR infrared imaging system. Labeled protein bands were compared within individual gels/blots and expressed as percent of control density.
2.10 Statistical analysis
The data are expressed as means ± SEM. Comparisons among different groups were analyzed by Student’s t test between vehicle-treated non-stressed controls and vehicle-treated stressed groups or one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests among vehicle-treated stressed and drug-treated stressed groups. A p value of less than 0.05 was considered significant.
3. Results
3.1 CUS-induced depression- and anxiety-like behaviors were prevented by PDE2 inhibition
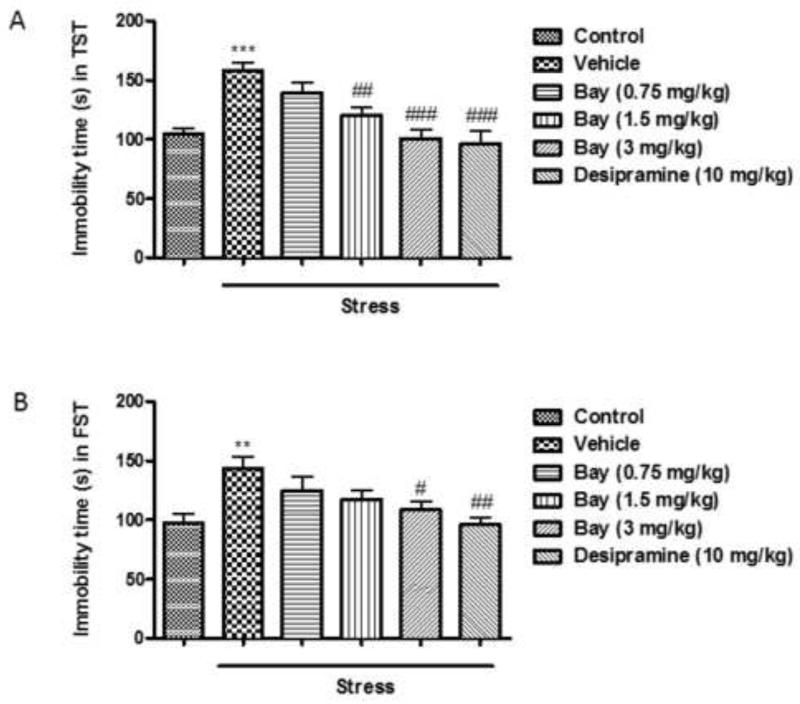
The antidepressant-like effects of Bay 60-7550 were investigated by the TST (Fig. 1A) and FST (Fig. 1B). CUS induced significant increases of immobility time of mice in both tests (p<0.001 and p<0.01, respectively), which were prevented by pretreatment with high doses of Bay 60-7550, i.e. 1.5 and 3 mg/kg in the TST (F(4,45)=15.05, p<0.01 and p<0.001 v.s. vehicle-treated non-stressed groups) and 3 mg/kg in the FST (F(4,45)=8.304, p<0.05); The classical antidepressant desipramine exhibited similar antidepressant-like effects at 10 mg/kg (F(4,45)=15.05, p<0.001 in TST and F(4,45)=8.304, p<0.01 in FST).
Fig. 1.
CUS-induced depressive behaviors. Mice were subjected to CUS or not, and stressed animals were administered vehicle, Bay 60-7550 (0.75, 1.5 and 3 mg/kg), or desipramine (10mg/kg). A) The immobility time in mouse tail suspension test (TST). B) The immobility time in mouse forced swim test (FST). Values are means ± S.E.M. with 10 mice in each group. **p<0.01 vs. non-stressed control group and ***p<0.001 vs. non-stressed control group. #p<0.05, ##p<0.01 and ###p<0.001 vs. vehicle-treated CUS group.
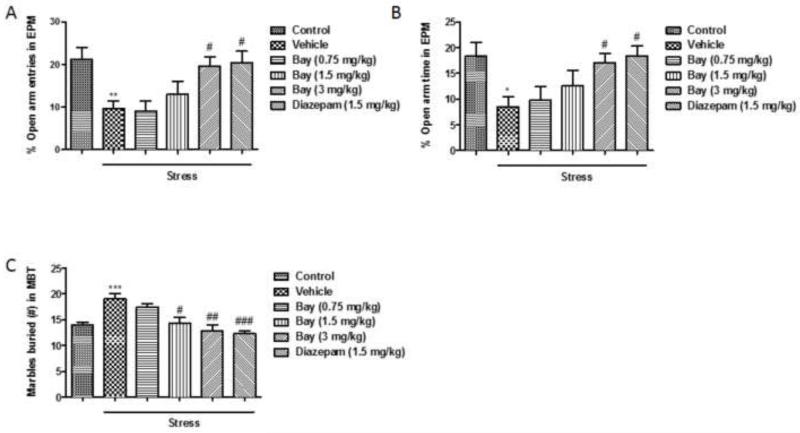
The role of PDE2 in regulation of anxiety-like behaviors was assessed using EPM and MBT. In the EPM, CUS decreased both percent of entries and time spent in open arms (p<0.01 and p<0.05, respectively), and mice treated with Bay 60-7550 at 3 mg/kg (F(4,45)=9.013, p<0.05 in % open arm entries and F(4,45)=7.29, p<0.05 in % open arm time) or diazepam at 1.5 mg/kg (F(4,45)=9.013, p<0.05 in % open arm entries and F(4,45)=7.29, p<0.05 in % open arm time) prior to CUS exhibited a significant increase in these parameters compared to the stressed group (Fig. 2A & 2B). Numbers of total entries into both closed and open arms do not differ significantly among any of the groups. Consistently, in the MBT, CUS significantly increased the number of marbles buried (p<0.05), and this increase was prevented by treatment with Bay 60-7550 at 1.5 and 3 mg/kg (F (4,45)=20.35, p<0.05 and p<0.01, respectively) or diazepam at 1.5 mg/kg (F (4,45)=20.35, p<0.001) (Fig. 2C).
Figure 2.
CUS-induced anxious behaviors. Mice were subjected to CUS or not, and stressed animals were administered vehicle, Bay 60-7550 (0.75, 1.5 and 3 mg/kg), or diazepam (1.5 mg/kg). A) % open arm entries in the elevated plus maze test (EPM). B) % time spent in open arms in the elevated plus maze test (EPM). C) Numbers of marbles buried in the marble burying test (MBT). Values are means ± S.E.M. with 10 mice in each group. *p<0.05, **p<0.01 and ***p<0.001 vs. non-stressed control group. #p<0.05, ##p<0.01 and ###p<0.001 vs. vehicle-treated CUS group.
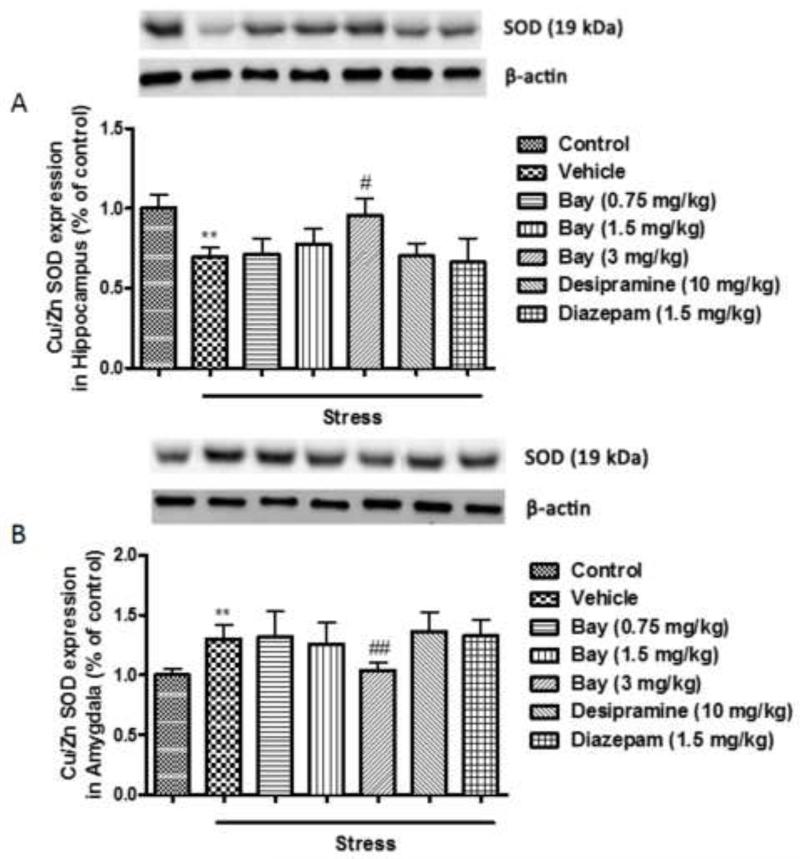
3.2 Effects of CUS and Bay 60-7550 on the expression of Cu/Zn SOD in the hippocampus and amygdala
In order to determine whether CUS-induced depression- and anxiety-like behaviors were associated with oxidative stress in hippocampus and amygdala, two brain regions that are considered to play critical roles in regulating such behaviors, Cu/Zn SOD levels were measured in different groups of mice, i.e. the non-stressed controls and the CUS mice that were pretreated with vehicle, Bay 60-7550 (0.75, 1.5 and 3 mg/kg), the classical antidepressant desipramine (10 mg/kg) and anxiolytic diazepam (1.5 mg/kg). In the hippocampus, CUS induced about a 25% decrease in the Cu/Zn SOD level, compared to non-stressed control mice (p<0.01). This change was reversed in mice pretreated with 3 mg/kg Bay 60-7550 (F(5,54)=2.719, p<0.05), but not with desipramine or diazepam (Fig. 3A). By contrast, mice exposed to CUS exhibited a significant increase in the expression of Cu/Zn SOD in the amygdala (p<0.01); this also was prevented by pretreatment with the highest dose of Bay 60-7550 at 3 mg/kg (F(5,54)=5.078, p<0.01), but still not with desipramine or diazepam (Fig. 3B).
Figure 3.
Effects of Bay 60-7550 on Cu/Zn SOD protein expression in control and CUS-exposed mice, in hippocampus and amygdala. Mice were subjected to CUS or not, and stressed animals were administered vehicle, Bay 60-7550 (0.75, 1.5 and 3 mg/kg), desipramine (10mg/kg) or diazepam (1.5 mg/kg). A) Expression of Cu/Zn SOD in hippocampus. B) Expression of Cu/Zn SOD in amygdala. Values are means ± S.E.M. with 10 mice in each group. **p<0.01 vs. non-stressed control group. #p<0.05 and ##p<0.01 vs. vehicle-treated CUS group.
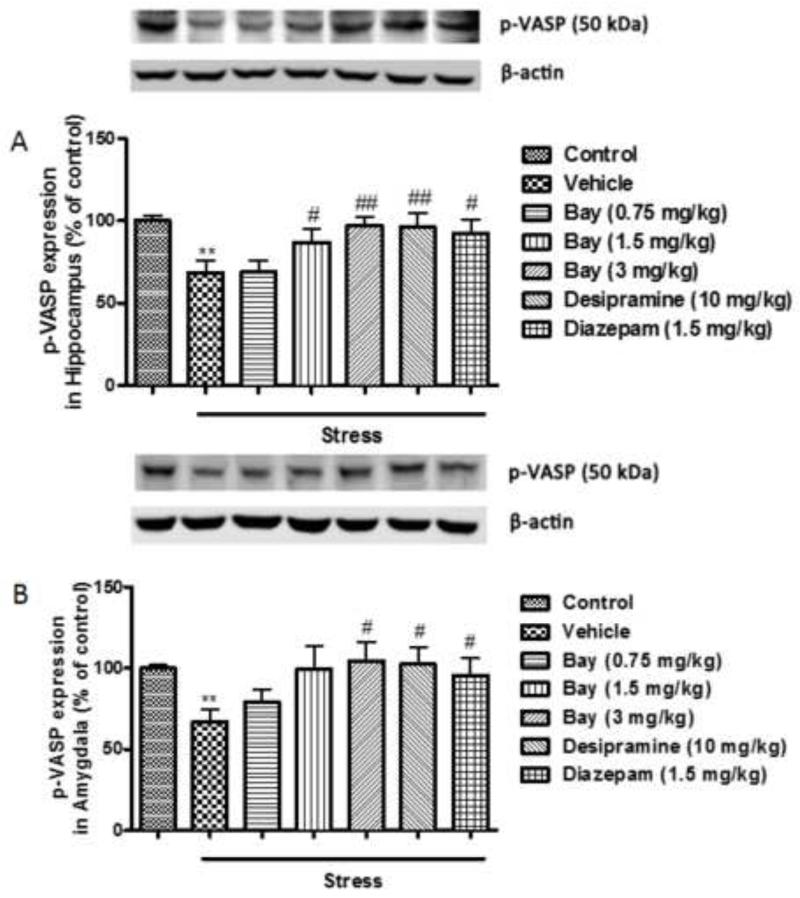
3.3 Effects of CUS and Bay 60-7550 on the expression of p-VASPser239 in the hippocampus and amygdala
Bay 60-7550 is known to increase cGMP signaling in primary neurons in our previous study [28]. In order to confirm the findings in vivo, expression of p-VASPser239, an indicator of cGMP-PKG signaling, was measured in the present study. In the hippocampus, expression of p-VASPser239 was reduced in the CUS-exposed mice (p<0.01); this was reversed by the pretreatment with Bay 60-7550 at 1.5 and 3 mg/kg in the hippocampus (F(5,54)=11.074, p<0.05 and p<0.01, respectively), desipramine and diazepam (F(5,54)=11.074, p<0.01 and p<0.05, respectively) (Fig. 4A). Similarly, in the amygdala, CUS led to a decreased expression of p-VASPser239 (p<0.01) and this change was completely prevented by Bay 60-7550 at 3 mg/kg (F(5,54)=9.684, p<0.05), desipramine and diazepam (F(5,54)=9.684, p<0.05 and p<0.05, respectively) (Fig. 4B).
Figure 4.
Effects of Bay 60-7550 on p-VASPSer239 protein expression in control and CUS-exposed mice, in hippocampus and amygdala. Mice were subjected to CUS or not, and stressed animals were administered vehicle, Bay 60-7550 (0.75, 1.5 and 3 mg/kg), desipramine (10mg/kg) or diazepam (1.5 mg/kg). A) Expression of p-VASPSer239 in hippocampus. B) Expression of p-VASPSer239 in amygdala. Values are means ± S.E.M. with 10 mice in each group. **p<0.01 vs. non-stressed control group. #p<0.05 and ##p<0.01 vs. vehicle-treated CUS group.
3.4 Effects of CUS and Bay 60-7550 on the expression of apoptotic markers in the hippocampus and amygdala
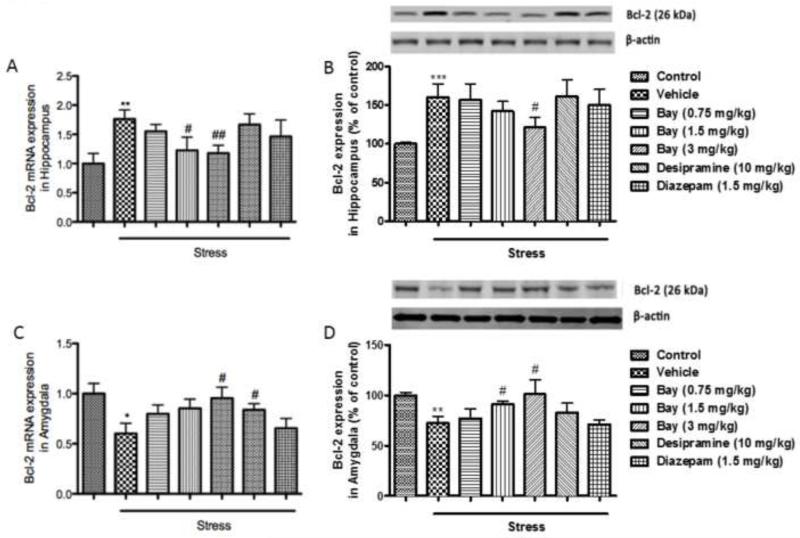
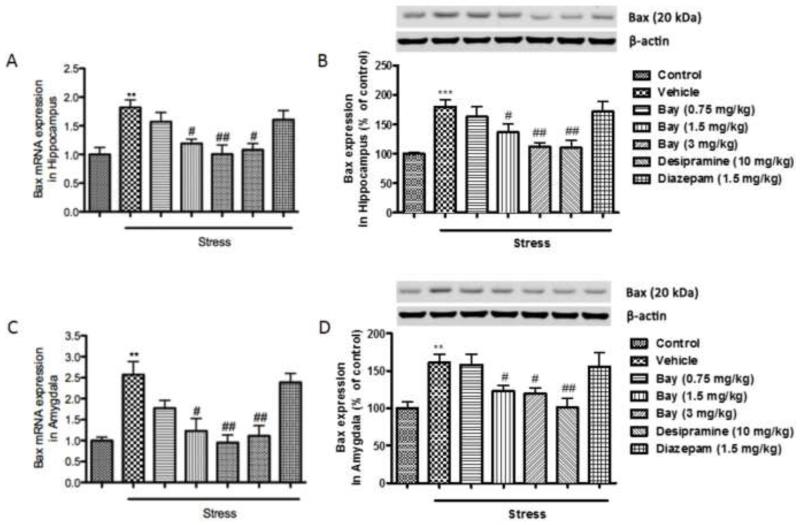
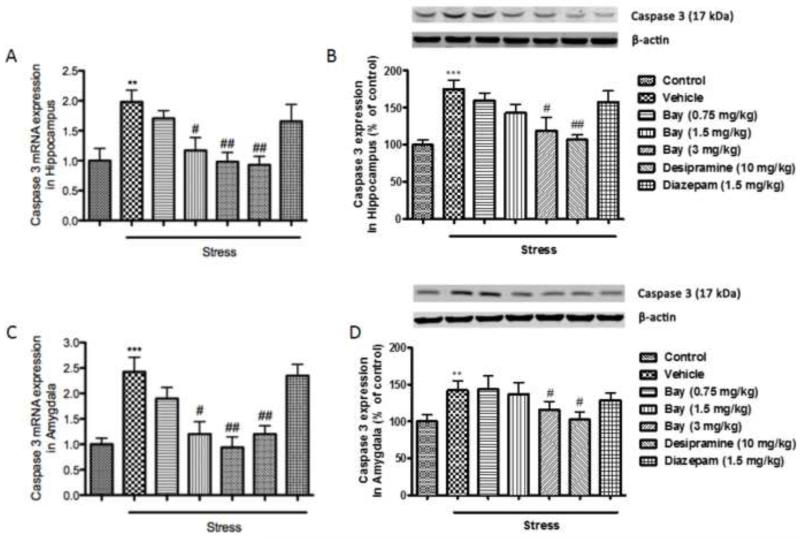
Since depressive- and anxiety-like behaviors induced by CUS are often accompanied by neuronal cell death, which is indicated by changes of series of anti- and pro-apoptotic markers, the expression of Bcl-2, Bax, and Caspase 3 at both the transcriptional and translational levels (mRNAs and proteins) were measured in the absence or presence of Bay 60-7550. Surprisingly in the hippocampus, mRNA and protein expressions of Bcl-2, a known anti-apoptotic marker was significantly increased after CUS exposure (p<0.01 for mRNA levels and p<0.001 for protein levels) (Fig 5A and 5B). In the amygdala, on the other hand, both mRNA expression and protein levels of Bcl-2 were significantly decreased following CUS exposure (p<0.05 for mRNA levels and p<0.01 for protein levels) (Fig. 5C and 5D). Pretreatment with 1.5 and 3 mg/kg of Bay 60-7550 prevented the mRNA changes, and 3 mg/kg of Bay 60-7550 prevented the protein changes in the hippocampus (F(5,54)=9.156, p<0.05 and p<0.01 for mRNA levels and F(5,54))=3.118, p<0.05 for protein levels). In the amygdala, 3 mg/kg of Bay 60-7550 prevented the mRNA changes, and 1.5 and 3 mg/kg of Bay 60-7550 prevented the protein changes (F(5,54)=5.237, p<0.05 for mRNA levels and F(5,54))=6.094, p<0.05 and p<0.05 for protein levels). The expressions of the 2 pro-apoptotic markers, Bax and Caspase 3, were significantly elevated in both hippocampus and amygdala after CUS exposure, at the mRNA and protein levels (p<0.01 for Bax mRNA and p<0.001 for Bax protein in the hippocampus; p<0.01 for Bax mRNA and p<0.01 for Bax protein in the amygdala; p<0.01 for Caspase 3 mRNA and p<0.001 for Caspase 3 protein in the hippocampus; p<0.001 for Caspase 3 mRNA and p<0.01 for Caspase 3 protein in the amygdala) (Fig. 6 and 7). These changes were also reversed by treatment with Bay 60-7550 at 1.5 and/or 3 mg/kg (F(5,54)=10.198 for Bax mRNA and F(5,54)=11.963 for Bax protein in the hippocampus; F(5,54)=12.356 for Bax mRNA and F(5,54)=7.985 for Bax protein in the amygdala; F(5,54)=13.014 for Caspase 3 mRNA and F(5,54)=5.906 for Caspase protein in the hippocampus; F(5,54)=8.174 for Caspase 3 mRNA and F(5,54)=5.665 for Caspase 3 protein in the amygdala). However, out of the two positive drugs, only desipramine was able to reverse the changes in mRNA level of Bcl-2 in the amygdala, and both mRNA and protein levels of Bax and Caspase 3 in the hippocampus and amygdala.
Figure 5.
Effects of Bay 60-7550 on Bcl-2 mRNA and protein expression in control and CUS-exposed mice, in hippocampus and amygdala. Mice were subjected to CUS or not, and stressed animals were administered vehicle, Bay 60-7550 (0.75, 1.5 and 3 mg/kg), desipramine (10mg/kg) or diazepam (1.5 mg/kg). A) mRNA expression of Bcl-2 in hippocampus. B) Protein expression of Bcl-2 in hippocampus. C) mRNA expression of Bcl-2 in amygdala. D) Protein expression of Bcl-2 in amygdala. Values are means ± S.E.M. with 10 mice in each group. *p<0.05, **p<0.01 and ***p<0.001 vs. non-stressed control group. #p<0.05 and ##p<0.01 vs. vehicle-treated CUS group.
Figure 6.
Effects of Bay 60-7550 on Bax mRNA and protein expression in control and CUS-exposed mice, in hippocampus and amygdala. Mice were subjected to CUS or not, and stressed animals were administered vehicle, Bay 60-7550 (0.75, 1.5 and 3 mg/kg), desipramine (10mg/kg) or diazepam (1.5 mg/kg). A) mRNA expression of Bax in hippocampus. B) Protein expression of Bax in hippocampus. C) mRNA expression of Bax in amygdala. D) Protein expression of Bax in amygdala. Values are means ± S.E.M. with 10 mice in each group. **p<0.01 and ***p<0.001 vs. non-stressed control group. #p<0.05 and ##p<0.01 vs. vehicle-treated CUS group.
Figure 7.
Effects of Bay 60-7550 on Caspase 3 mRNA and protein expression in control and CUS-exposed mice, in hippocampus and amygdala. Mice were subjected to CUS or not, and stressed animals were administered vehicle, Bay 60-7550 (0.75, 1.5 and 3 mg/kg), desipramine (10mg/kg) or diazepam (1.5 mg/kg). A) mRNA expression of Caspase 3 in hippocampus. B) Protein expression of Caspase 3 in hippocampus. C) mRNA expression of Caspase 3 in amygdala. D) Protein expression of Caspase 3 in amygdala. Values are means ± S.E.M. with 10 mice in each group. **p<0.01 and ***p<0.001 vs. non-stressed control group. #p<0.05 and ##p<0.01 vs. vehicle-treated CUS group.
4. Discussion
In the present study, chronic treatment with Bay 60-7550 at 0.75, 1.5 and 3 mg/kg dose-dependently produced antidepressant- and anxiolytic-like effects that were comparable to the classical antidepressant and anxiolytic, desipramine and diazepam, respectively. These effects may be due to Bay 60-7550’s ability to regulate the parameters involving oxidative/antioxidant defense and pro-/anti-apoptosis processes, as characterized by superoxide dismutase (Cu/Zn SOD), Bcl-2, Bax and Caspase 3 expression in the hippocampus and amygdala. Bay 60-7550 also reversed the reduced phospho-VASPser239, an indicator of PKG signaling, induced by chronic stress, suggesting the involvement of cGMP/PKG signaling in Bay 60-7550’s actions.
Disruption of the Hypothalamus-Pituitary-Adrenal (HPA) axis, as well as structural and pathological alterations in the limbic brain regions caused by chronic stress, may contribute to depressive symptoms including state of despair and loss of coping behaviors [29]. As an important element in the development of depression, anxiety is often characterized by excessive fear and reluctance to explore a novel environment. These behavioral deficits can be reversed by antidepressant treatments [30]. Previous studies suggested that inhibitors of PDEs, such as PDE4 and PDE5, ameliorate stress-related depression- and anxiety-like behaviors through regulating the cAMP or cGMP signaling [31,32]. PDE2 is enriched in the limbic brain regions and adrenal cortex, which play important roles in regulating the negative feedback inhibition of HPA axis in stress-induced disorders [33]. In the current study, the effects of Bay 60-7550 on stress-induced depressive-like behaviors were measured in 2 behavioral assays, the TST and the FST. Both tests present non-escapable stressful situation to the animals, and are sensitive to antidepressant treatment thus providing validation for drug efficacy. Similar to desipramine, pretreatment with Bay 60-7550 prior to CUS reduced the immobility time in the tail suspension test and forced swim test. The anxiolytic-like effects of Bay 60-7550 were investigated using the EPM and MBT, which showed that Bay 60-7550, as well as diazepam, prevented the onset of anxious behaviors that were seen in the stressed animals, such as decreased open arm entries in the EPM and increased numbers of marbles buried in the MBT.
Given that the brain has one of the highest mass-specific oxygen consumption rates in the body, small imbalances in oxidative damage and antioxidant defense mechanisms during CUS may contribute to the above-mentioned behavioral deficits. Cu/Zn SOD is a key antioxidant enzyme involved in superoxide detoxification in cellular metabolism [34]. Many brain regions, especially those in the limbic system, such as hippocampus and amygdala, act in concert to mediate the symptoms of depression and anxiety [35], and are thus good locations for observations of these antioxidant factors. Indeed, insufficient expression of Cu/Zn SOD in the hippocampus are often seen in stressed animals [36-38]. A similar decrease in Cu/Zn SOD expression was observed in the hippocampus of the present stressed mice, which was reversed by pretreatment with Bay 60-7550, but not by desipramine or diazepam. Due to the anatomical and functional connections between the hippocampus and the amygdala, the neural activity of hippocampus is largely modulated by the amygdala, which plays a key role in anxiety and emotional responses to stress [39], therefore Cu/Zn SOD expression in the amygdala was also examined in the current study. Surprisingly, a significant increase in Cu/Zn SOD in the amygdala was found after stress in the present study, which was also prevented by Bay 60-7550. It was possible that compensatory adaptation was triggered when physiological conditions are disturbed by oxidative stress, and Bay 60-7550 restores such a disturbance. Whittle and the co-authors reported a similar elevation of Mn SOD in mouse amygdala, in response to chronic Mg-restricted diet, which is used to generate depression-like behaviors [40]. Indeed, increasing evidence also supports the differential response of hippocampus and amygdala toward stress. For example, in patients suffering from multiple depression episodes, hippocampal volume decreases from the first episode while the amygdala is enlarged in the beginning and shrinks in recurrent episodes [41]. Therefore, our findings may suggest compensatory adaptation in mouse amygdala toward stress, and its regulation of hippocampal functions.
Previous studies suggested that inhibition of a cGMP specific PDE, i.e. PDE5, protects against reactive oxygen species-induced toxicity in cultured neurons [42,43]. The effects are blocked by several inhibitors of cGMP-dependent protein kinase (PKG), such as KT5823 and GKIP [42,44,45], confirming the role of the cGMP-PKG pathway in alleviating oxidative stress. Bay 60-7550, a highly selective PDE2 inhibitor, was shown to protect neurons against glucocorticoids and oxidative stress exposure through the regulation of cGMP/PKG signaling [17,46]. This led us to examine whether this neuronal protective effect of Bay 60-7550 also had effect against CUS-induced depression- and anxiety-like behaviors. The present study confirmed that Bay 60-7550 regulated the hippocampal and amygdaloid SOD expression, which were accompanied by elevation of p-VASPser239, an index of cGMP-PKG signaling. Desipramien and diazepam were also able to reverse the changes of p-VASPser239 induced by CUS, agreeing with several previous findings stating that neuroprotective effects of desipramine and diazepam involved NO-cGMP signaling [47,48].
Although Bay 60-7550 may mediate its long-term therapeutic effects by increasing cGMP signaling to counteract oxidative stress, research has shown that apoptotic machinery also plays a role in the effects of PDE inhibitors on stress-related disorders [49]. Generally, CUS-induced oxidative damage to the brain is characterized by neuronal apoptosis, a programmed cell death that is critically controlled by the balance between pro- and anti-apoptotic proteins [7]. Bcl-2 and Bax are both from the Bcl-2 family, the former belonging to the pro-survival subfamily and the latter belonging to the pro-apoptotic family. Upon translocation from cytosol to mitochondria, Bax promotes the release of apoptogenic factors such as cytochrome c, which in turn stimulates initiator caspases, leading to the activation of effector caspases such as Caspase 3, a main executor of the apoptotic process [8,50]. Possible neuronal apoptosis in both hippocampus and amygdala in the study were suggested by increased expression of Bax and Caspase 3 after CUS. However, the results also revealed a clear regional specificity in the regulation of Bcl-2 levels by stress, i.e., a significant increase in the hippocampus and decrease in the amygdala, both of which were prevented by pretreatment with Bay 60-7550. While previous work has suggested stress-induced decrease in Bcl-2 expression in human and rodent brains due to different stress protocols and conditions [51], the increase of hippocampal Bcl-2 in this study may be explained by the triggering of a neuroprotective pathway that aimed to promote neuroregeneration [52]. Similar increases in Bcl-2 expression in hippocampus from aged rats, or after chronic isolation have been reported [53,54]. Another explanation may be that the concomitant increase of the anti-apoptotic Bcl-2 and the pro-apoptotic Bax and Caspase 3, which are usually inhibited by Bcl-2, indicates that factors other than Bcl-2, such as p53 that target the mitochondrial pathway for inducing apoptosis, might be playing a predominant role in activating Bax and subsequently Caspase 3 in the hippocampus [55]. Indeed, our present discovery revealed adaptive changes of Bcl-2 directed toward protecting hippocampal neurons against oxidative stress [56]. The increased expression of Bcl-2 in the hippocampus can be seen as another form of self-defense during stress, which is comparable to the increase of SOD in the amygdala. Since these abnormalities of apoptotic proteins were all prevented by pretreatment with Bay 60-7550, it is possible that the antidepressant- and anxiolytic-like effects of this PDE2 inhibitor are, at least in part, through inhibition of the neuronal cell apoptosis.
In conclusion, the present study demonstrated that chronic stress induces oxidative damage to the brain, which may contribute to neuronal apoptosis, as well as depressive- and anxiety-like behaviors. Bay 60-7550 exhibited neuroprotective effects by restoring the antioxidative status, apoptotic protein expression, and cGMP-PKG signaling close to normal levels. The alterations for oxidative/antioxidant and subsequent pro- and anti-apoptotic factors observed in the presence of PDE2 inhibition could open new avenues for the development of innovative treatment of stress-related disorders.
Highlights.
Bay60-7550 exhibits antidepressant- and anxiolytic-like effects in mouse behaviors.
Increased cGMP signaling may contribute to the protective effects of Bay60-7550.
Bay60-7550 is able to antagonize the oxidative damage produced by chronic stress.
Another possible mechanism of Bay60-7550’s function is its anti-apoptotic effects.
Acknowledgements
This work was supported by research grants from the National Institute of Health to Dr. J.M. O’Donnell (No. 5RC1MH088480-02) and the Science and Technology Development Foundation of Huai’an to Dr. L.S. Ding (China, No. HG201213).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kessler RC, Angermeyer M, Anthony JC, DE Graaf R, Demyttenaere K, Gasquet I, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–76. [PMC free article] [PubMed] [Google Scholar]
- [2].Madrigal JLM, García-Bueno B, Caso JR, Pérez-Nievas BG, Leza JC. Stress-induced oxidative changes in brain. CNS Neurol Disord Drug Targets. 2006;5:561–8. doi: 10.2174/187152706778559327. [DOI] [PubMed] [Google Scholar]
- [3].Tell G, Gustincich S. Redox state, oxidative stress, and molecular mechanisms of protective and toxic effects of bilirubin on cells. Curr Pharm Des. 2009;15:2908–14. doi: 10.2174/138161209789058174. [DOI] [PubMed] [Google Scholar]
- [4].Lee W-Y, Jang S-W, Lee J-S, Kim Y-H, Kim H-G, Han J-M, et al. Uwhangchungsimwon, a traditional herbal medicine, protects brain against oxidative injury via modulation of hypothalamus-pituitary-adrenal (HPA) response in a chronic restraint mice model. J Ethnopharmacol. 2014;151:461–9. doi: 10.1016/j.jep.2013.10.066. [DOI] [PubMed] [Google Scholar]
- [5].Kwon D-H, Kim B-S, Chang H, Kim Y-I, Jo SA, Leem Y-H. Exercise ameliorates cognition impairment due to restraint stress-induced oxidative insult and reduced BDNF level. Biochem Biophys Res Commun. 2013;434:245–51. doi: 10.1016/j.bbrc.2013.02.111. [DOI] [PubMed] [Google Scholar]
- [6].McKernan DP, Dinan TG, Cryan JF. “Killing the Blues”: a role for cellular suicide (apoptosis) in depression and the antidepressant response? Prog Neurobiol. 2009;88:246–63. doi: 10.1016/j.pneurobio.2009.04.006. [DOI] [PubMed] [Google Scholar]
- [7].Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- [8].Lindsten T, Zong W-X, Thompson CB. Defining the role of the Bcl-2 family of proteins in the nervous system. Neuroscientist. 2005;11:10–5. doi: 10.1177/1073858404269267. [DOI] [PubMed] [Google Scholar]
- [9].Bravo JA, Díaz-Veliz G, Mora S, Ulloa JL, Berthoud VM, Morales P, et al. Desipramine prevents stress-induced changes in depressive-like behavior and hippocampal markers of neuroprotection. Behav Pharmacol. 2009;20:273–85. doi: 10.1097/FBP.0b013e32832c70d9. [DOI] [PubMed] [Google Scholar]
- [10].Li Y-F, Cheng Y-F, Huang Y, Conti M, Wilson SP, O’Donnell JM, et al. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J Neurosci. 2011;31:172–83. doi: 10.1523/JNEUROSCI.5236-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reneerkens OAH, Rutten K, Steinbusch HWM, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl) 2009;202:419–43. doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Halene TB, Siegel SJ. PDE inhibitors in psychiatry--future options for dementia, depression and schizophrenia? Drug Discov Today. 2007;12:870–8. doi: 10.1016/j.drudis.2007.07.023. [DOI] [PubMed] [Google Scholar]
- [13].Dlaboga D, Hajjhussein H, O’Donnell JM. Chronic haloperidol and clozapine produce different patterns of effects on phosphodiesterase-1B, -4B, and -10A expression in rat striatum. Neuropharmacology. 2008;54:745–54. doi: 10.1016/j.neuropharm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- [14].Rundfeldt C, Socala K, Wlaź P. The atypical anxiolytic drug, tofisopam, selectively blocks phosphodiesterase isoenzymes and is active in the mouse model of negative symptoms of psychosis. J Neural Transm. 2010;117:1319–25. doi: 10.1007/s00702-010-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Van Staveren WCG, Steinbusch HWM, Markerink-Van Ittersum M, Repaske DR, Goy MF, Kotera J, et al. mRNA expression patterns of the cGMP-hydrolyzing phosphodiesterases types 2, 5, and 9 during development of the rat brain. J Comp Neurol. 2003;467:566–80. doi: 10.1002/cne.10955. [DOI] [PubMed] [Google Scholar]
- [16].Nikolaev VO, Gambaryan S, Engelhardt S, Walter U, Lohse MJ. Real-time monitoring of the PDE2 activity of live cells: hormone-stimulated cAMP hydrolysis is faster than hormone-stimulated cAMP synthesis. J Biol Chem. 2005;280:1716–9. doi: 10.1074/jbc.C400505200. [DOI] [PubMed] [Google Scholar]
- [17].Masood A, Nadeem A, Mustafa SJ, O’Donnell JM. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther. 2008;326:369–79. doi: 10.1124/jpet.108.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xu Y, Pan J, Chen L, Zhang C, Sun J, Li J, et al. Phosphodiesterase-2 inhibitor reverses corticosterone-induced neurotoxicity and related behavioural changes via cGMP/PKG dependent pathway. Int J Neuropsychopharmacol. 2013;16:835–47. doi: 10.1017/S146114571200065X. [DOI] [PubMed] [Google Scholar]
- [19].Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–34. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- [20].Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–52. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- [21].Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, et al. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- [23].Porsolt RD, Bertin A, Jalfre M. “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol. 1978;51:291–4. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- [24].Masood A, Banerjee B, Vijayan VK, Ray A. Modulation of stress-induced neurobehavioral changes by nitric oxide in rats. Eur J Pharmacol. 2003;458:135–9. doi: 10.1016/s0014-2999(02)02688-2. [DOI] [PubMed] [Google Scholar]
- [25].Tsuji M, Takeda H, Matsumiya T. Different effects of 5-HT1A receptor agonists and benzodiazepine anxiolytics on the emotional state of naive and stressed mice: a study using the hole-board test. Psychopharmacology (Berl) 2000;152:157–66. doi: 10.1007/s002130000514. [DOI] [PubMed] [Google Scholar]
- [26].Skalisz LL, Beijamini V, Andreatini R. Effect of Hypericum perforatum on marble-burying by mice. Phytother Res. 2004;18:399–402. doi: 10.1002/ptr.1450. [DOI] [PubMed] [Google Scholar]
- [27].Dekeyne A, Mannoury la, Cour C, Gobert A, Brocco M, Lejeune F, Serres F, et al. S32006, a novel 5-HT2C receptor antagonist displaying broad-based antidepressant and anxiolytic properties in rodent models. Psychopharmacology (Berl) 2008;199:549–68. doi: 10.1007/s00213-008-1177-9. [DOI] [PubMed] [Google Scholar]
- [28].Masood A, Huang Y, Hajjhussein H, Xiao L, Li H, Wang W, et al. Anxiolytic effects of phosphodiesterase-2 inhibitors associated with increased cGMP signaling. J Pharmacol Exp Ther. 2009;331:690–9. doi: 10.1124/jpet.109.156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Alfonso J, Frasch AC, Flugge G. Chronic stress, depression and antidepressants: effects on gene transcription in the hippocampus. Rev Neurosci. 2005;16:43–56. doi: 10.1515/revneuro.2005.16.1.43. [DOI] [PubMed] [Google Scholar]
- [30].Xu Y, Ku B, Cui L, Li X, Barish P a, Foster TC, et al. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007;1162:9–18. doi: 10.1016/j.brainres.2007.05.071. [DOI] [PubMed] [Google Scholar]
- [31].Li Y-F, Huang Y, Amsdell SL, Xiao L, O’Donnell JM, Zhang H-T. Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology. 2009;34:2404–19. doi: 10.1038/npp.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matsushita H, Matsuzaki M, Han X-J, Nishiki T-I, Ohmori I, Michiue H, et al. Antidepressant-like effect of sildenafil through oxytocin-dependent cyclic AMP response element-binding protein phosphorylation. Neuroscience. 2012;200:13–8. doi: 10.1016/j.neuroscience.2011.11.001. [DOI] [PubMed] [Google Scholar]
- [33].Rosman GJ, Martins TJ, Sonnenburg WK, Beavo JA, Ferguson K, Loughney K. Isolation and characterization of human cDNAs encoding a cGMP-stimulated 3′,5′-cyclic nucleotide phosphodiesterase. Gene. 1997;191:89–95. doi: 10.1016/s0378-1119(97)00046-2. [DOI] [PubMed] [Google Scholar]
- [34].Peluffo H, Acarin L, Faiz M, Castellano B, Gonzalez B. Cu/Zn superoxide dismutase expression in the postnatal rat brain following an excitotoxic injury. J Neuroinflammation. 2005;2:12. doi: 10.1186/1742-2094-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McIntosh LJ, Hong KE, Sapolsky RM. Glucocorticoids may alter antioxidant enzyme capacity in the brain: baseline studies. Brain Res. 1998;791:209–14. doi: 10.1016/s0006-8993(98)00115-2. [DOI] [PubMed] [Google Scholar]
- [36].Grundmann O, Lv Y, Kelber O, Butterweck V. Mechanism of St. John’s wort extract (STW3-VI) during chronic restraint stress is mediated by the interrelationship of the immune, oxidative defense, and neuroendocrine system. Neuropharmacology. 58:767–73. doi: 10.1016/j.neuropharm.2009.12.014. n.d. [DOI] [PubMed] [Google Scholar]
- [37].Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F, et al. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int. 54:358–62. doi: 10.1016/j.neuint.2009.01.001. n.d. [DOI] [PubMed] [Google Scholar]
- [38].Liu W, Zhou C. Corticosterone reduces brain mitochondrial function and expression of mitofusin, BDNF in depression-like rodents regardless of exercise preconditioning. Psychoneuroendocrinology. 2012;37:1057–70. doi: 10.1016/j.psyneuen.2011.12.003. [DOI] [PubMed] [Google Scholar]
- [39].Kirby ED, Friedman AR, Covarrubias D, Ying C, Sun WG, Goosens KA, et al. Basolateral amygdala regulation of adult hippocampal neurogenesis and fear-related activation of newborn neurons. Mol Psychiatry. 2012;17:527–36. doi: 10.1038/mp.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Whittle N, Li L, Chen W-Q, Yang J-W, Sartori SB, Lubec G, et al. Changes in brain protein expression are linked to magnesium restriction-induced depression-like behavior. Amino Acids. 2011;40:1231–48. doi: 10.1007/s00726-010-0758-1. [DOI] [PubMed] [Google Scholar]
- [41].Tanti A, Belzung C. Open questions in current models of antidepressant action. Br J Pharmacol. 2010;159:1187–200. doi: 10.1111/j.1476-5381.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nakamizo T, Kawamata J, Yoshida K, Kawai Y, Kanki R, Sawada H, et al. Phosphodiesterase inhibitors are neuroprotective to cultured spinal motor neurons. J Neurosci Res. 2003;71:485–95. doi: 10.1002/jnr.10483. [DOI] [PubMed] [Google Scholar]
- [43].Pifarré P, Prado J, Giralt M, Molinero A, Hidalgo J, Garcia A. Cyclic GMP phosphodiesterase inhibition alters the glial inflammatory response, reduces oxidative stress and cell death and increases angiogenesis following focal brain injury. J Neurochem. 2010;112:807–17. doi: 10.1111/j.1471-4159.2009.06518.x. [DOI] [PubMed] [Google Scholar]
- [44].Mattson MP, Guo ZH, Geiger JD. Secreted form of amyloid precursor protein enhances basal glucose and glutamate transport and protects against oxidative impairment of glucose and glutamate transport in synaptosomes by a cyclic GMP-mediated mechanism. J Neurochem. 1999;73:532–7. doi: 10.1046/j.1471-4159.1999.0730532.x. [DOI] [PubMed] [Google Scholar]
- [45].Urushitani M, Inoue R, Nakamizo T, Sawada H, Shibasaki H, Shimohama S. Neuroprotective effect of cyclic GMP against radical-induced toxicity in cultured spinal motor neurons. J Neurosci Res. 2000;61:443–8. doi: 10.1002/1097-4547(20000815)61:4<443::AID-JNR11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- [46].Boess FG, Hendrix M, van der Staay F-J, Erb C, Schreiber R, van Staveren W, et al. Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology. 2004;47:1081–92. doi: 10.1016/j.neuropharm.2004.07.040. [DOI] [PubMed] [Google Scholar]
- [47].Jiménez-Velázquez G, López-Muñoz FJ, Fernández-Guasti A. Participation of the GABA/benzodiazepine receptor and the NO-cyclicGMP pathway in the “antinociceptive-like effects” of diazepam. Pharmacol Biochem Behav. 2008;91:128–33. doi: 10.1016/j.pbb.2008.06.021. [DOI] [PubMed] [Google Scholar]
- [48].Gaur V, Kumar A. Protective effect of desipramine, venlafaxine and trazodone against experimental animal model of transient global ischemia: possible involvement of NO-cGMP pathway. Brain Res. 2010;1353:204–12. doi: 10.1016/j.brainres.2010.07.004. [DOI] [PubMed] [Google Scholar]
- [49].Aquilano K, Baldelli S, Cardaci S, Rotilio G, Ciriolo MR. Nitric oxide is the primary mediator of cytotoxicity induced by GSH depletion in neuronal cells. J Cell Sci. 2011;124:1043–54. doi: 10.1242/jcs.077149. [DOI] [PubMed] [Google Scholar]
- [50].Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem. 2004;90:1281–9. doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- [51].Agrawal M, Kumar V, Kashyap MP, Khanna VK, Randhawa GS, Pant AB. Ischemic insult induced apoptotic changes in PC12 cells: protection by trans resveratrol. Eur J Pharmacol. 2011;666:5–11. doi: 10.1016/j.ejphar.2011.05.015. [DOI] [PubMed] [Google Scholar]
- [52].Chen DF, Schneider GE, Martinou JC, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385:434–9. doi: 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- [53].Kaufmann JA, Bickford PC, Taglialatela G. Oxidative-stress-dependent up-regulation of Bcl-2 expression in the central nervous system of aged Fisher-344 rats. J Neurochem. 2001;76:1099–108. doi: 10.1046/j.1471-4159.2001.00118.x. [DOI] [PubMed] [Google Scholar]
- [54].Djordjevic A, Djordjevic J, Elaković I, Adzic M, Matić G, Radojcic MB. Fluoxetine affects hippocampal plasticity, apoptosis and depressive-like behavior of chronically isolated rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:92–100. doi: 10.1016/j.pnpbp.2011.10.006. [DOI] [PubMed] [Google Scholar]
- [55].Sharma DR, Sunkaria A, Bal A, Bhutia YD, Vijayaraghavan R, Flora SJS, et al. Neurobehavioral impairments, generation of oxidative stress and release of pro-apoptotic factors after chronic exposure to sulphur mustard in mouse brain. Toxicol Appl Pharmacol. 2009;240:208–18. doi: 10.1016/j.taap.2009.06.015. [DOI] [PubMed] [Google Scholar]
- [56].Park JR, Hockenbery DM. BCL-2, a novel regulator of apoptosis. J Cell Biochem. 1996;60:12–7. doi: 10.1002/(sici)1097-4644(19960101)60:1<12::aid-jcb3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]