Abstract
Members of the Src family of kinases (SFKs) are non-receptor tyrosine kinases involved in numerous signal transduction pathways. The catalytic, SH3 and SH2 domains are attached to the membrane-anchoring SH4 domain through the intrinsically disordered “Unique” domains, which exhibit strong sequence divergence among SFK members. In the last decade, structural and biochemical studies have begun to uncover the crucial role of the Unique domain in the regulation of SFK activity. This mini-review discusses what is known about the phosphorylation events taking place on the SFK Unique domains, and their biological relevance. The modulation by phosphorylation of biologically relevant inter- and intra- molecular interactions of Src, as well as the existence of complex phosphorylation/dephosphorylation patterns observed for the Unique domain of Src, reinforces the important functional role of the Unique domain in the regulation mechanisms of the Src kinases and, in a wider context, of intrinsically disordered regions in cellular processes.
Keywords: IDPs, IDRs, phosphorylation, unique domain, SFKs, Src
INTRODUCTION
The Src kinase family is composed of 10 proteins: Src, Frk, Lck, Lyn, Blk, Hck, Fyn, Yrk, Fgr, and Yes. Src family kinases (SFKs) are membrane-associated, non-receptor tyrosine kinases that act as important signaling intermediaries regulating a variety of outputs, such as cell proliferation, differentiation, apoptosis, migration, and metabolism. All SFKs share the same domain arrangement: a large catalytic C-terminal domain is preceded by regulatory Src 2 and 3 homology domains (SH2 and SH3, respectively) and the membrane-anchoring SH4 N-terminal region, which contain myristoylation and palmitoylation sites as well as positively charged residues. The SH3 and SH4 domains are linked by an intrinsically disordered segment of 50–90 residues, called the Unique domain (UD) because of the lack of sequence similarity among the different SFKs. However, the UD of each individual SFK member is well conserved between different organisms suggesting a more specific role than that of a simple spacer. Figure 1 shows the sequences of the UDs of the 10 SFKs, highlighting known phosphorylation sites.
FIGURE 1.
Phosphorylation and lipid binding sites in the Unique domain of SFK. Alignment of the UD sequences of the ten SFK. The alignment has been manually edited to emphasize the similarities in regions that are known to be functionally relevant in at least one SFK member. Residues highlighted in green are identified as phosphorylation sites in PhosphoSitePlus (Hornbeck et al., 2012). Conservation of phosphorylation sites was used as a reference in the alignment presented.
Efforts to find specific UD functions during the last two decades have confirmed an active role of the UD in the regulation of SFK members. In the case of Lck, the UD mediates association with the cytoplasmic tail of CD4 and CD8α through a zinc clasp structure (Kim et al., 2003). For most of the other SFK members the detailed mechanisms remain obscure. The Unique domains of Fyn and Lyn were observed to be cleaved during induction of apoptosis in intact hematopoietic cells, revealing a novel mechanism for the specific regulation of different SFKs, with important consequences for their cellular localization and activity (Luciano et al., 2001). Several SFKs contain residues in the N-terminal region that are phosphorylated and dephosphorylated in cellular processes (Joung et al., 1995; Hansen et al., 1997; Johnson et al., 2000). Moreover, swapping the Unique domains of Src and Yes interchanges the functional specificity of the two SFKs (Hoey et al., 2000; Summy et al., 2003; Werdich and Penn, 2005). The versatility and relevance of the active role of the Unique domain in Src function was confirmed by the discovery of binding by the Unique domain to different targets, such as acidic lipids, the SH3 domain, and calmodulin (Pérez et al., 2013). The amino-acid sequence of the UD region responsible for some of these interactions is conserved in Src, Fyn, and Yes. The three proteins are co-expressed in many tissues and are partially redundant, in the sense that deficiency in one of them can be compensated by the action of the other two.
The involvement of SFK signaling in growth, motility and cell survival makes them important oncology targets. Activation of SFKs is tightly regulated in healthy cells (Boggon and Eck, 2004), while the kinase activation is often deregulated in cancer, giving rise to altered cellular shape, function, and growth (Vlahovic and Crawford, 2003). Among the SFKs, Src is the most studied and the most commonly discussed in the context of cancer (Yeatman, 2004). However, there has been growing interest in the other SFKs in both normal physiological and pathological states.
The UD is an intrinsically disordered region (IDR). Intrinsically disordered proteins (IDPs) are relatively more prevalent among signaling and cancer-related proteins (Iakoucheva et al., 2002). The connection between disorder and diseases such as cancer, neurodegenerative conditions, amyloidosis, cardiovascular disease, and diabetes has been extensively explored in recent reviews (Uversky et al., 2008; Uros et al., 2009). Diseases arising from structural changes in proteins, loosely grouped as “conformational diseases” are caused not only by protein misfolding, but also by failures in post-translational modifications that result in aberrant interactions with physiological partners (Uversky et al., 2008). Remarkably, mutations in disordered regions can result in the loss of important post-translational modification sites, leading to disease (Li et al., 2010). The recognition of the crucial role of IDPs in a number of diseases has heralded a new era in the design of drugs (Chen et al., 2006).
While the catalytic and Src homology domains of SFKs have long been subjects of investigation, the study of the regulatory mechanisms involving the intrinsically disordered UD has only recently started gaining momentum. Phosphorylation events are often associated with the regulation of important functional regions. In this mini-review we discuss the current knowledge about the phosphorylation events taking place in the Unique domains of SFKs.
PHOSPHORYLATION OF THE UNIQUE DOMAIN OF Lck
The Unique domain linking the SH4 and the SH3 domain of Lck is one of the smallest of the family, with only 60 amino acids. During T-cell activation, Ser59 in the Unique N-terminal region of Lck is phosphorylated (Watts et al., 1993; Winkler et al., 1993). The Unique domain of Lck contains a proline-rich region surrounding Ser59 (56PPASP60). Joung et al. (1995) found that modifications of Ser59 in the Unique N-terminal domain of tyrosine kinase Lck regulate specificity of its SH2 domain. Later on, Ser59 was identified as a site of in vivo mitotic phosphorylation in Lck (Kesavan et al., 2002). More recently, a new target was found for Lck: the protein Nck (Vázquez, 2007), consisting of one SH2 domain and three SH3 domains, which is known to link receptor tyrosine kinases to downstream proteins, and to be active in actin polymerization. It was also reported that Nck binds in T cells to the CD3 subunit of the T-cell antigen receptor (TCR) following TCR engagement. Interestingly, the inter-molecular interaction between Lck and Nck was observed to be disrupted by phosphorylation at Ser59 within the Unique domain of Lck. Wild-type Lck (wt-Lck) and a Ser59Asp-Lck mutant transfected into Lck-deficient JCaM1.6 cells showed differences in the activation of proximal versus distal signaling events (Vázquez, 2007).
PHOSPHORYLATION OF THE UNIQUE DOMAIN OF Hck
Hematopoietic cell kinase (Hck) is a potential drug target for cancer and HIV infections. High levels of Hck are associated with drug resistance in chronic myeloid leukemia and Hck activity has been connected with HIV-1 (Tintori et al., 2013). An important insight into the activation mechanism of this SFK member was the discovery that Hck is capable of performing autophosphorylation in its UD. Autophosphorylation of the activation loop in the kinase domain is a common process for SFK activation. Autophosphorylation of recombinant Hck leads to a 20-fold increase in its specific enzymatic activity (Johnson et al., 2000). Hck was found to autophosphorylate readily to a stoichiometry of 1.3 mol of phosphate per mol of enzyme, indicating that the kinase autophosphorylated at more than one site. In particular, Johnson and collaborators discovered –- in vitro as well as in vivo–-that Hck can undergo autophosphorylation at two different sites: Tyr388, which is located at the consensus autophosphorylation site in the well-characterized activation loop of similar kinases, and Tyr29, which is located in its intrinsically disordered Unique domain. By inspecting the activities and levels of phosphorylation of recombinant Hck mutants containing either the point mutation Tyr388Phe or Tyr29Phe, they demonstrated that phosphorylation at Tyr29 makes a crucial contribution to the activation of Hck through its autophosphorylation. Regulation of the catalytic activity by phosphorylation at Tyr29 in the Unique domain of Hck suggests that autophosphorylation within the N-terminal Unique region may also be an additional mechanism of regulation of other Src family tyrosine kinases.
TYROSINE PHOSPHORYLATION IN OTHER UNIQUE DOMAINS: Lyn, Yes, Fgr, AND Frk
Interestingly, the tyrosine residue in position 32 of Lyn is located in an 8-residue-long sequence common to Lyn and Hck. EGFR phosphorylates the p56 isoform of Lyn, p56Lyn, at Tyr32, which then phosphorylates MCM7, a licensing factor critical for DNA replication. Phosphorylation at Tyr600 of MCM7 increases its association with other minichromosome maintenance complex proteins, thereby promoting DNA synthesis complex assembly and cell proliferation. Both p56Lyn Tyr32 and MCM7 Tyr600 phosphorylation are enhanced in proliferating cells and correlated with poor survival of breast cancer patients (Huang et al., 2013).
All Unique domains contain aromatic residues, a rare feature in intrinsically disordered domains. A tyrosine residue is present between positions 25 and 34 in seven SFKs, and is known to be phosphorylated in five of them: Lyn, Hck, Lck (Hornbeck et al., 2012), Yes (Ariki et al., 1997), and Frg (Oppermann et al., 2009). Phosphorylation of Tyr34 of Fgr has been observed my mass spectrometry mainly in samples from leukemia patients.
Frk is the only SFK that is not myristoylated. Although it contains three tyrosine residues in its Unique domain, phosphorylation has only been reported for Tyr46, at the interface between the Unique and SH3 domains. The modification was observed by mass spectrometry mainly in samples from lung, liver, and gastric cancer (Stokes et al., 2012).
PHOSPHORYLATION OF THE UNIQUE DOMAIN OF Fyn
Fyn is ubiquitously expressed together with Src and Yes, whereas other members of the Src family are expressed only in specific cell types. Fyn is primarily localized to the cytoplasmic leaflet of the plasma membrane, where it phosphorylates tyrosine residues on key targets involved in a variety of different signaling pathways. Fyn characterization has been mainly focused on its immune and neurological function. However, Fyn has also been recognized as an important mediator of mitogenic signaling and regulator of cell-cycle entry, growth, and proliferation, integrin-mediated interactions as well as cell adhesion and migration (Kawakami et al., 1988; Chen et al., 2001; Li et al., 2003; Kim et al., 2010). In particular, Fyn is over-expressed in several cancers, such as glioblastoma multiformae and melanoma. The role of Fyn over-expression in these systems, however, has not been well defined as yet.
Fyn presents multiple phosphorylation sites that can affect its kinase activity. Activation of Platelet-Derived Growth Factor (PDGF) β-receptor by binding to PDGF leads to activation of a member of the SFK (Alonso et al., 1995). The Unique domain of Fyn was found to be phosphorylated at Tyr28 (Hansen et al., 1997). The functional role of this phosphorylation was confirmed by the observation of significantly reduced activation following PDGF stimulation of a Fyn mutant in which Tyr28 was replaced by phenylalanine (Hansen et al., 1997). It was also proposed that the autophosphorylation of the N-terminal tyrosine residues plays a key role in the activation of Fyn, by complementing the PDGF receptor-induced phosphorylation of Tyr28.
Serine 21 within the UD of Fyn is part of a RxxS motif targeted by protein kinase A (PKA). Mutation of Ser21 to Alanine (Ser21Ala-Fyn) blocks PKA phosphorylation of Fyn and alters its tyrosine kinase activity (Yeo et al., 2011). In the same work, the authors showed that the over-expression of Ser21Ala-Fyn mutant in cells lacking Src/Yes/Fyn kinases (SYF cells) led to decreased tyrosine phosphorylation of focal adhesion kinase, resulting in reduced focal adhesion targeting, and slow lamellipodia dynamics and cell migration. These important changes in cell motility demonstrate a key role of UD phosphorylation at Ser21 in the Fyn kinase activity that controls assembly and disassembly of focal adhesions in response to signals arising from cell-extracellular matrix interactions.
Protein kinase A is a crucial component of integrin-mediated signaling pathways. Interaction of cells with their substrate or adhesion to other cells leads to activation of PKA (Whittard and Akiyama, 2001), and induce the phosphorylation of a variety of protein substrates (Walsh and Van Patten, 1994). Four SFKs (Fyn, Src, Lck, and Fgr) contain a conserved RxxS motif suggesting they can be regulated by PKA.
PHOSPHORYLATION OF THE UNIQUE DOMAIN OF Src
Src is a non-receptor protein tyrosine kinase with a key role in regulating cell-to-matrix adhesion, migration, and junctional stability (Frame, 2004). Thus, precise regulation of Src activity is critical for normal cell growth. The inactive state of Src is obtained by phosphorylated tyrosine near the C-terminus of Src (Tyr530 in mammalian Src; Tyr527 in chicken Src), which is recognized by its SH2 domain, while the SH3 domain interacts with a poly-proline motif located in the linker region between the SH2 and kinase domains; these intramolecular interactions restrict access to the kinase domain (Xu et al., 1997). Dephosphorylation of Tyr530 is followed by autophosphorylation at Tyr419, leading to full activation of the kinase. Active Src may be deactivated by rephosphorylation of Tyr530 by C-terminal Src kinase (Csk; Piwnica-Worms et al., 1987; Nada et al., 1991; Brown, 1996).
Although the phosphorylation of Src at Ser17 by PKA (cAMP-dependent protein kinase) is a well-characterized process, its biological significance remains unclear (Obara et al., 2004). Potential roles in protein–protein interactions or cellular localization have been postulated for this phosphorylation site. For instance, it has been observed that the treatment of 3T3 fibroblasts with PDGF results in the translocation of Src from the plasma membrane to the cytosol, concomitant with an increase in phosphorylation of Ser17 by PKA (Walker et al., 1993). This observation suggests that this phosphorylation could interfere with the electrostatic interactions that act to anchor Src to the lipid bilayer. PKA phosphorylation of Src at Ser17 is also required in cAMP activation of Rap1, inhibition of extracellular signal-regulated kinases, and inhibition of cell growth, although the mechanism by which this phosphorylation mediates these processes is not known (Obara et al., 2004).
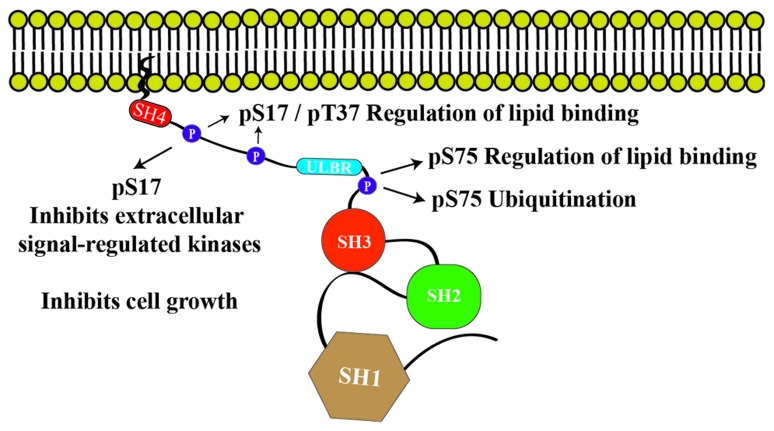
Interaction of Src with lipids is not restricted to the SH4 domain (Figure 2). The unique lipid binding region (ULBR) was discovered following NMR observations that revealed a partially structured region within the UD of Src (Pérez et al., 2009). While phosphorylation of Ser17 by PKA disturbed the interaction of the SH4 domain with lipids, phosphorylation of Thr37 and Ser75 by p25-Cdk5 decreased lipid binding by the ULBR (Pérez et al., 2009). Cross-effects were observed, suggesting a cooperative interaction of the two lipid binding regions with membranes. Conformational effects of phosphorylation at Ser17, Thr37, and Ser75 in the isolated UD are strictly local, indicating that electrostatic repulsion of the phosphorylated residues with the negatively charged lipids is the main mechanism by which lipid binding is disrupted.
FIGURE 2.
Phosphorylation sites in the UD of Src and their relationship with the two lipid-binding regions: the classical SH4 domain and the ULBR.
The ULBR major regulatory role of the UD was shown experimentally in Xenopus laevis oocytes. Mutations abolishing lipid binding by the ULBR but not affecting the SH4 domain resulted in a conditional lethal phenotype after progesterone induced maturation (Pérez et al., 2013).
Previous results had identified a functional role for Thr37 and Ser75 (Thr34 and Ser72 in chicken Src) as well as Thr46 in chicken Src (with no correspondence in humans). These residues are phosphorylated by cyclin-dependent kinase 1 (Cdk1/Cdc2) during mitosis. These phosphorylations were found to activate Src by disrupting the interaction between the SH2 domain and Tyr527/Tyr530 (chicken/human) and facilitating the dephosphorylation by protein tyrosine phosphatases (Shenoy et al., 1992; Stover et al., 1994).
In addition (or possibly related to) its effect on lipid binding, phosphorylation of Ser75 is important in other aspects of Src regulation. Mitosis-independent phosphorylation of this site was observed in neurons and in certain cancer cell lines; this phosphorylation was shown to be due to Cdk5, a widely distributed proline-directed kinase with a substrate specificity similar to Cdk1 (Kato and Maeda, 1999). Active Src is reported to be irreversibly destroyed by Cullin-5-dependent ubiquitination and proteosomal degradation (Hakak and Martin, 1999; Laszlo and Cooper, 2009). More recently, it was shown that phosphorylation at Ser75 promotes the ubiquitin-dependent degradation of Src (Pan et al., 2011). Ser75-Src phosphorylation in epithelial cells was found to depend on the activation state of Src: only active Src was phosphorylated and eventually marked for ubiquitination. Thus, Cdk5-dependent phosphorylation of Ser75 within the UD of Src represents a mechanism to restrict the availability of active Src (Pan et al., 2011). Sequence alignment suggests that serine residues are present in homologous positions of Yes and possibly Frk.
NMR and mass-spectrometry studies of human Src UD added to X. laevis egg extracts showed the phosphorylation of Ser75 and Ser69, as well as Ser 17 (Pérez et al., 2013). Phosphorylation of Ser75 and Ser69 seem to be mutually interfering in vitro. Interestingly, X. laevis Src has a glycine residue at position 69 and phosphorylation of Ser69 in human Src had only been previously detected by mass spectrometry in extracts of cancer lines HCT116 and MDA-MB-435S (Oppermann et al., 2009). It is tempting to speculate that pathological phosphorylation of Ser 69 in human cells could result in decreased phosphorylation of Ser75 and reduced degradation of active Src, leading to an oncogenic phenotype caused by Src overactivation.
The interplay between various phosphorylation sites within the UD emphasizes its role as a signaling integration hub. Further input signals, in addition to phosphorylation, include calcium-dependent interaction with calmodulin and the allosteric interaction with Src SH3 domain (Pérez et al., 2013; Figure 2). A further level of cross-talk between various phosphorylation events was observed when the time-dependent phosphorylation of the UD was studied by real-time NMR in cell extracts (Amata et al., 2013). In these experiments it was observed that the activity of a PKA-like kinase, which phosphorylates Ser17, also repressed the phosphatase(s) that catalyzed the dephosphorylation of Ser75. Thus, inhibition of PKA activity resulted in dephosphorylation of both sites. Similarly, inhibition of Cdk activity resulted in a reduction in the steady-state phosphorylation of both sites. On the other hand, addition of PKA caused a robust phosphorylation of Ser75 by preventing the action of the phosphatases that reverse the effect of Cdks. These results show that the phosphorylation state of the UD of Src represents a sensor of the kinase-phosphatase network active at any given moment in the cell. Experimental access to this information is possible by using real-time NMR techniques (Selenko et al., 2008; Theillet et al., 2012; Thongwichian and Selenko, 2012; Amata et al., 2013). In contrast to folded domains, intrinsically disordered domains, like the UD of Src, provide in vivo NMR resolution comparable to that obtainable in vitro (Kosol et al., 2013).
CONCLUDING REMARKS
The study of the UDs of SFKs, particularly that of Src, provides an example of multilevel regulation through phosphorylation of a membrane-bound intrinsically disordered domain. The occurrence of membrane tethering among IDRs is very common, although its relevance is not always recognized. The results arising from studies of UDs should stimulate further research to uncover similar mechanisms beyond SFKs. In particular, switchable internal lipid binding sites (the ULBR in the case of Src) in disordered domains anchored to membranes by a second, more stable, binding site close to the protein termini (the SH4 domain in the case of SFKs) can modulate the position of the active domains (the kinase or regulatory domains in the case of SFKs) with respect to the membrane surface. Lipid binding by the internal site can switch off the access of the active domains to target sites of membrane-anchored substrates/partners located beyond a particular distance from the membrane surface or, conversely, facilitate the interaction with sites near the membrane surface. Phosphorylation or other modifications preventing the interaction of the internal site would result in a release of the active domains, which can then reach sites located further apart from the membrane surface or disfavor the interaction with membrane-proximal sites. This represents a new compartmentalization mechanism based on the relative position of interacting sites with respect to the membrane surface. This mechanism enables modulating the functional interactions between two proteins anchored next to each other in the membrane.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by funds from Fundació Marató TV3, the Spanish Government (BIO2010-15683), the Catalan Government (2009SGR1352), and the European Union 7FP (BioNMR contract 261863, and Marie Curie Action - COFUND to Irene Amata).
REFERENCES
- Alonso G., Koegl M., Mazurenko N., Courtneidge S. A. (1995). Sequence requirements for binding of src family tyrosine kinases to acivated growth factor receptors. J. Biol. Chem. 270 9840–9848 10.1074/jbc.270.17.9840 [DOI] [PubMed] [Google Scholar]
- Amata I., Maffei M., Igea A., Gay M., Vilaseca M., Nebreda A. R., et al. (2013). Multi-phosphorylation of the intrinsically disordered unique domain of c-Src studied by in-cell and real-time NMR spectroscopy. Chembiochemistry 14 1820–1827 10.1002/cbic.201300139 [DOI] [PubMed] [Google Scholar]
- Ariki M., Tanabe O., Usui H., Hayashi H., Inoue R., Nishito Y., et al. (1997). Identification of autophosphorylation sites in c-Yes purified from rat liver plasma membranes. J. Biochem. 121 104–111 [DOI] [PubMed] [Google Scholar]
- Boggon T. J., Eck M. J. (2004). Structure and regulation of Src family kinases. Oncogene 23 7918–7927 10.1038/sj.onc.1208081 [DOI] [PubMed] [Google Scholar]
- Brown E. (1996). A signaling pathway to translational control. Cell 86 517–520 10.1016/S0092-8674(00)80125-7 [DOI] [PubMed] [Google Scholar]
- Chen J. W., Romero P., Uversky V. N., Dunker A. K. (2006). Conservation of intrinsic disorder in protein domains and families: I. A database of conserved predicted disordered regions. J. Proteome Res. 5 879–887 10.1021/pr060048x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Kim O., Yang J., Sato K., Eisenmann K. M., McCarthy J., et al. (2001). Regulation of Akt/PKB activation by tyrosine phosphorylation. J. Biol. Chem. 276 31858–31862 10.1074/jbc.C100271200 [DOI] [PubMed] [Google Scholar]
- Frame M. C. (2004). Newest findings on the oldest oncogene; how activated src does it. J. Cell Sci. 117(Pt 7) 989–998 10.1242/jcs.01111 [DOI] [PubMed] [Google Scholar]
- Hakak Y., Martin S. G. (1999). Ubiquitin-dependent degradation of active Src. Curr. Biol. 9 1039–1042 10.1016/S0960-9822(99)80453-9 [DOI] [PubMed] [Google Scholar]
- Hansen K., Alonso G., Courtneidge S. A., Rönnstrand L, Heldin C.-H. H.(1997). PDGF-induced phosphorylation of Tyr28 in the N-terminus of Fyn affects Fyn activation. Biochem. Biophys. Res. Commun. 241 355–362 10.1006/bbrc.1997.7743 [DOI] [PubMed] [Google Scholar]
- Hoey J. G., Summy J., Flynn D. C. (2000). Chimeric constructs containing the SH4/Unique domains of cYes can restrict the ability of Src527F to upregulate heme oxygenase-1 expression efficiently. Cell. Signal. 12 691–701 10.1016/S0898-6568(00)00116-9 [DOI] [PubMed] [Google Scholar]
- Hornbeck P. V., Kornhauser J. M., Tkachev S., Zhang B., Skrzypek E., Murray B., et al. (2012). PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 40 D261–D270 10.1093/nar/gkr1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. H., Huo L., Wang Y. N., Xia W., Wei Y., Chang S. S., et al. (2013). Epidermal growth factor receptor potentiates MCM7-mediated DNA replication through tyrosine phosphorylation of Lyn kinase in human cancers. Cancer Cell 23 796–810 10.1016/j.ccr.2013.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iakoucheva L. M., Brown C. J., Lawson D. J., Obradović Z., Dunker K. A. (2002). Intrinsic disorder in cell-signaling and cancer-associated proteins. J. Mol. Biol. 323 573–584 10.1016/S0022-2836(02)00969-5 [DOI] [PubMed] [Google Scholar]
- Johnson T. M., Williamson N. A., Scholz G., Jaworowski A., Wettenhall R. E., Dunn A. R., et al. (2000). Modulation of the catalytic activity of the Src family tyrosine kinase Hck by autophosphorylation at a novel site in the unique domain. J. Biol. Chem. 275 33353–33364 10.1074/jbc.M002022200 [DOI] [PubMed] [Google Scholar]
- Joung I., Kim T., Stolz L. A., Payne G., Winkler D. G., Walsh C. T., et al. (1995). Modification of Ser59 in the unique N-terminal region of tyrosine kinase p56lck regulates specificity of its Src homology 2 domain. Proc. Natl. Acad. Sci. U.S.A. 92 5778–5782 10.1073/pnas.92.13.5778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G., Maeda S. (1999). Neuron-specific phosphorylation Cdk5 kinase is responsible of c-Src at Ser75 in human for mitosis-independent Y79 retinoblastoma. J. Biochem. 126 957–961 10.1093/oxfordjournals.jbchem.a022540 [DOI] [PubMed] [Google Scholar]
- Kawakami T., Kawakami Y., Aaronson S. A., Robbins K. C. (1988). Acquisition of transforming properties by FYN, a normal SRC-related human gene. Proc. Natl. Acad. Sci. U.S.A. 85 3870–3874 10.1073/pnas.85.11.3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavan K. P., Isaacson C. C., Ashendel C. L., Geahlen R. L., Harrison M. L. (2002). Characterization of the in vivo sites of serine phosphorylation on Lck identifying serine 59 as a site of mitotic phosphorylation. J. Biol. Chem. 277 14666–14673 10.1074/jbc.M111911200 [DOI] [PubMed] [Google Scholar]
- Kim H.-J., Warren J. T., Kim S.-Y., Chappel J. C., DeSelm C. J., Ross F. P., et al. (2010). Fyn promotes proliferation, differentiation, survival and function of osteoclast lineage cells. J. Cell. Biochem. 111 1107–1113 10.1002/jcb.22841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. W., Sun Z.-Y. J., Blacklow S. C., Wagner G., Eck M. J. (2003). A zinc clasp structure tethers Lck to T cell coreceptors CD4 and CD8. Science 301 1725–1728 10.1126/science.1085643 [DOI] [PubMed] [Google Scholar]
- Kosol S., Contreras-Martos S., Cedeño C., Tompa P. (2013). Structural characterization of intrinsically disordered proteins by NMR spectroscopy. Molecules 18 10802–10828 10.3390/molecules180910802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo G. S., Cooper J. A. (2009). Restriction of Src activity by Cullin-5. Curr. Biol. 19 157–162 10.1016/j.cub.2008.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Iakoucheva L. M., Mooney S. D., Radivojac P. (2010). Loss of post-translational modification sites in disease. Pac. Symp. Biocomput. 337–347 10.1142/9789814295291_0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yang Y., Hu Y., Dang D., Regezi J., Schmidt B. L., et al. (2003). Alphavbeta6-Fyn signaling promotes oral cancer progression. J. Biol. Chem. 278 41646–41653 10.1074/jbc.M306274200 [DOI] [PubMed] [Google Scholar]
- Luciano F., Ricci J.-E., Auberger P. (2001). Cleavage of Fyn and Lyn in their N-terminal unique regions during induction of apoptosis: a new mechanism for Src kinase regulation. Oncogene 20 4935–4941 10.1038/sj.onc.1204661 [DOI] [PubMed] [Google Scholar]
- Nada S., Okada M., MacAuley A., Cooper J. A., Nakagawa H. (1991). Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature 351 69–72 10.1038/351069a0 [DOI] [PubMed] [Google Scholar]
- Obara Y., Labudda K., Dillon T. J, Stork P. J. S. (2004). PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J. Cell Sci. 117 6085–6094 10.1242/jcs.01527 [DOI] [PubMed] [Google Scholar]
- Oppermann F. S., Gnad F., Olsen J. V., Hornberger R., Greff Z., Kéri G., et al. (2009). Large-scale proteomics analysis of the human kinome. Mol. Cell. Proteomics 8 1751–1764 10.1074/mcp.M800588-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q., Qiao F., Gao C., Norman B., Optican L., Zelenka P. S. (2011). Cdk5 targets active Src for ubiquitin-dependent degradation by phosphorylating Src(S75). Cell Mol. Life. Sci 68 3425–3436 10.1007/s00018-011-0638-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez Y., Gairí M., Pons M, Bernadó P. (2009). Structural characterization of the natively unfolded N-terminal domain of human c-Src kinase: insights into the role of phosphorylation of the unique domain. J. Mol. Biol. 391 136–148 10.1016/j.jmb.2009.06.018 [DOI] [PubMed] [Google Scholar]
- Pérez Y., Maffei M., Igea A., Amata I., Gairí M., Nebreda A. R., et al. (2013). Lipid binding by the Unique and SH3 domains of c-Src suggests a new regulatory mechanism. Sci. Rep. 3 1295 10.1038/srep01295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. (1987). Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell 49 75–82 10.1016/0092-8674(87)90757-4 [DOI] [PubMed] [Google Scholar]
- Selenko P., Frueh D. P., Elsaesser S. J., Haas W., Gygi S. P., Wagner G. (2008). In situ observation of protein phosphorylation by high-resolution NMR spectroscopy. Nat. Struct. Mol. Biol. 15 321–329 10.1038/nsmb.1395 [DOI] [PubMed] [Google Scholar]
- Shenoy S., Chackalaparampil I., Bagrodia S., Lin P.-H., Shalloway D. (1992). Role of p34cdc2-mediated phosphorylations in two-step activation of pp60c-src during mitosis. Proc. Natl. Acad. Sci. U.S.A. 89 7237–7241 10.1073/pnas.89.15.7237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes M. P., Farnsworth C. L., Moritz A., Silva J. C., Jia X., Lee K. A., et al. (2012). PTMScan direct: identification and quantification of peptides from critical signaling proteins by immunoaffinity enrichment coupled with LC-MS/MS. Mol. Cell. Proteomics 11 187–201 10.1074/mcp.M111.015883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover R. D., Liebetanz J., Lydon B. N. (1994). Cdc2-mediated Modulation of pp60c-src activity. J. Biol. Chem. 269 26885–26889 [PubMed] [Google Scholar]
- Summy J. M., Qian Y., Jiang B.-H., Guappone-Koay A., Gatesman A., Shi X., et al. (2003). The SH4-Unique-SH3-SH2 domains dictate specificity in signaling that differentiate c-Yes from c-Src. J. Cell Sci. 116(Pt. 12) 2585–2598 10.1242/jcs.00466 [DOI] [PubMed] [Google Scholar]
- Theillet F.-X., Smet-Nocca C., Liokatis S., Thongwichian R., Kosten J., Yoon M.-K., et al. (2012). Cell signaling, post-translational protein modifications and NMR spectroscopy. J. Biomol. NMR 54 217–236 10.1007/s10858-012-9674-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongwichian R., Selenko P. (2012). In-cell NMR in Xenopus laevis oocytes. Methods Mol. Biol. 895 33–41 10.1007/978-1-61779-927-3_3 [DOI] [PubMed] [Google Scholar]
- Tintori C., Laurenzana I., La Rocca F., Falchi F., Carraro F., Ruiz A., et al. (2013). Identification of Hck inhibitors as hits for the development of antileukemia and anti-HIV agents. Chem. Med. Chem. 8 1353–1360 10.1002/cmdc.201300204 [DOI] [PubMed] [Google Scholar]
- Uros M., Chritopher O. J., Keith D. A., Zoran O., Uversky V. N. (2009). Unfoldomics of human genetic diseases: illustrative examples of ordered and intrinsically disordered members of the human diseasome. Protein Pept. Lett. 16 1533–1547 10.2174/092986609789839377 [DOI] [PubMed] [Google Scholar]
- Uversky V. N., Oldfield C. J., Dunker K. A. (2008). Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu. Rev. Biophys. 37 215–246 10.1146/annurev.biophys.37.032807.125924 [DOI] [PubMed] [Google Scholar]
- Vázquez M. L. (2007). Biological consequences of the Phosphorylation of Serine 59 on the Tyrosine Kinase Lck. PhD Thesis, Purdue University, West Lafayette, IN. Available at: http://search.proquest.com/docview/304827306?accountid = 28979 [Google Scholar]
- Vlahovic G., Crawford J. (2003). Activation of tyrosine kinases in cancer. Oncologist 8 531–538 10.1634/theoncologist.8-6-531 [DOI] [PubMed] [Google Scholar]
- Walker F., DeBlaquiere J., Burgess A. W. (1993). Translocation of pp60C-csrc from the plasma membrane to the cytosol after stimulation by platelet-derived growth factor. J. Biol. Chem. 268 19552–19558 [PubMed] [Google Scholar]
- Walsh A. D, Van Patten M. S. (1994). Multiple pathway signal transduction by the cAMP-dependent protein kinase. FASEB J. 8 1227–1236 [DOI] [PubMed] [Google Scholar]
- Watts J. D., Sanghera J. S., Pelech S. L., Aebersold R. (1993). Phosphorylation of serine 59 of p56lck in activated T cells. J. Biol. Chem. 268 23275–23282 [PubMed] [Google Scholar]
- Werdich X. Q., Penn J. S. (2005). Src, Fyn and Yes play differential roles in VEGF-mediated endothelial cell events. Angiogenesis 8 315–326 10.1007/s10456-005-9021-x [DOI] [PubMed] [Google Scholar]
- Whittard J. D., Akiyama S. K. (2001). Positive regulation of cell-cell and cell-substrate adhesion by protein kinase A. J. Cell Sci. 114 3265–3272 [DOI] [PubMed] [Google Scholar]
- Winkler D. G., Park I., Kim T., Payne N. S., Walsht C. T., Strominger J. L., et al. (1993). Phosphorylation Ser-42 and Ser-59 in the N-terminal region of the tyrosine kinase p56lck. Proc. Natl. Acad. Sci. U.S.A. 90 5176–5180 10.1073/pnas.90.11.5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Harrison C. S., Eck J. M. (1997). Three-dimensional structure of the tyrosine kinase c-Src. Nature 385 595–602 10.1038/385595a0 [DOI] [PubMed] [Google Scholar]
- Yeatman T. J. (2004). A renaissance for SRC. Nat. Rev. Cancer 4 470–480 10.1038/nrc1366 [DOI] [PubMed] [Google Scholar]
- Yeo M. G., Oh H. J., Cho H.-S., Chun J. S., Marcantonio E. E., Song W. K. (2011). Phosphorylation of Ser 21 in Fyn regulates its kinase activity, focal adhesion targeting, and is required for cell migration. J. Cell Physiol. 226 236–247 10.1002/jcp.22335 [DOI] [PubMed] [Google Scholar]