It is now widely recognized that dynamic coordination among groups of neurons in local and long-range circuits is critical for orchestration of behavior.1 This is especially relevant to the biological basis of psychiatric illnesses where behavior is the primary measure for determining the presence or severity of symptoms. Much of the focus in biological psychiatry has been on establishing a link between symptoms and either morphological abnormalities or altered expression of receptors or other proteins involved in neural communication. A recent line of thinking, however, posits that the mechanisms that lead to behavioral symptoms may not have a static anatomical or cellular basis but are caused by transient disruptions in the coordinated activity of ensembles of neurons.
Many models of psychiatric disorders implicitly require that behavior follow the rules of linear dynamics, where input (eg, change in dopamine availability) has a linear influence on output (eg, optimal working memory). The brain, however, is a complex system that often follows nonlinear dynamics where coordinated changes in activating or dampening components may yield diverging outcomes and trivial changes in these components can have no effect or synergize to produce catastrophic effects.1 Therefore, symptoms of these illnesses in different individuals may be driven by different activating or dampening factors that similarly disrupt how assemblies of neurons coordinate their activity in response to external events or internal representations. Without knowing the mechanisms by which these dynamic ensembles coordinate to influence behavior, the predictive power of a single genetic or environmental variable remains weak. Given this, understanding the electrophysiological basis of behaviors that are relevant to symptoms of psychiatric disorders becomes critical for linking the genetic and environmental factors that disrupt neuronal activity with the behavioral manifestations of these disruptions.
Functional imaging in humans has provided an excellent platform for understanding the nature of dynamic networks in the context of normal and abnormal behavior, especially in the case of schizophrenia.2 But to understand the mechanistic basis of these large-scale changes in distributed brain networks, it is critical to use laboratory animals where recordings can be made from individual neurons with millisecond time resolution. Herein, we describe briefly 2 examples of measuring these forms of coordination in the midbrain and prefrontal cortex of rodents.
The Dopamine Neuron: Functional Networks vs Single-Unit Activity
We start with the midbrain dopamine neuron because there has been great emphasis on the firing rate of individual dopamine neurons as a reward prediction error signal.3 Furthermore, dopamine systems have been implicated in nearly all major psychiatric illnesses. But despite convincing functional abnormalities in the dopamine system as measured by imaging methods in disorders such as schizophrenia,4 there is little evidence for static abnormalities in the dopamine system from postmortem or genetic studies. How are we then to quantitate a functional abnormality in a behaviorally relevant context in the dopamine system despite the lack of overt pathology?
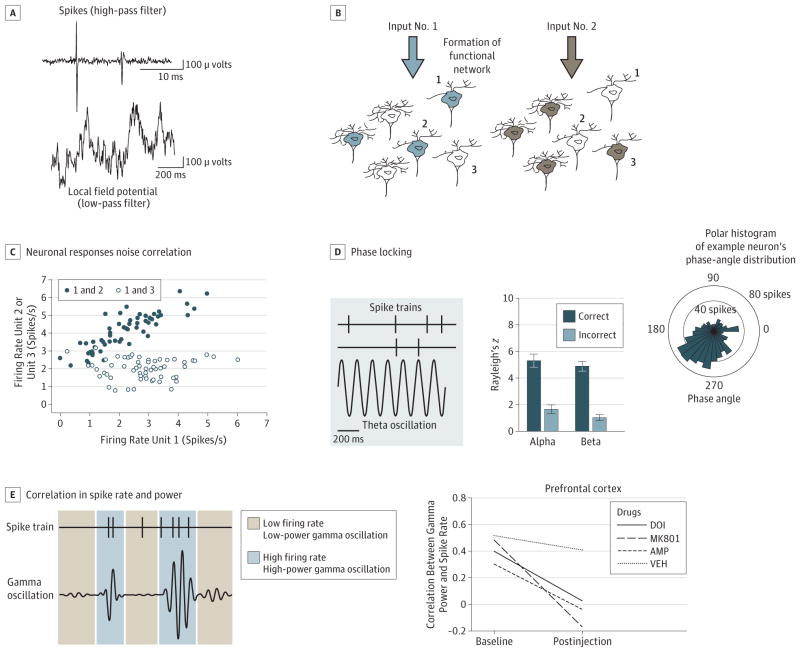
Our approach to addressing this question has been to focus on quantifying the coordinated activity of groups of neurons with the idea that dopamine dysfunction can stem from impaired coordinated activity between groups of neurons as opposed to morphological disruption of individual neurons.5 The methods we have used in this context assess correlations in activity between neuron pairs (termed noise correlations) or local field potential oscillations and neurons (Figure, A–C). These measures provide us with a “correlation structure” or index of coupling within a neuronal network.
Figure. Methods Used for Quantifying Coordinated Activity in Local and Long-range Circuits.
A, An extracellular recording electrode detects changes in voltage in the field surrounding groups of neurons. This multicomponent signal can be filtered with a high-pass filter (top) to isolate neuronal firing (known as “spikes”) or low-pass filtered to isolate the slower local field potential (LFP) oscillations (bottom). B, In different conditions, the same neurons can become part of different networks forming a transient “functional” network. The neurons in these networks can process similar or complementary types of information, and a network may dominate processing at one moment and lose prominence the next, depending on what processes are engaged. Thus, 2 networks supporting different cognitive or affective functions may comprise overlapping neurons. C, Neuronal responses vary from trial to trial. The correlation in activity between 2 neurons is termed noise correlation. The dark blue pair of neurons shows a positive linear correlation in their activity levels over these trials. The circles are blue to indicate that the neurons have formed a functional network, similar to part B. The white pair of neurons has a correlation near zero, which indicates no functional connectivity between the neurons. D, Network-wide activity may coordinate spiking activity when spikes occur at a particular angle of ongoing LFP oscillations. Left, Two spike trains and a 7-Hz oscillation. The upper spike train is strongly phase locked to the oscillation, and all spikes occur near the valley of the oscillation. In contrast, the lower spike train fires spikes that occur at opposite phase angles and is not phase locked. Middle, Magnitude of phase locking of medial prefrontal cortex neurons on trials where attention is allocated more or less optimally. On trials where a rat correctly detects the onset and location of a visual stimulus, phase locking is increased in several frequency bands compared with trials where the animal does not correctly detect the visual stimulus.6 Right, Polar histogram of an example neuron’s spike count distributed by the phase of a theta oscillation that occurred simultaneously with the neuron firing. The spikes are not distributed evenly across all phase angles but concentrated (locked) to a preferred phase angle. E, The relationship between LFP oscillations and neural activity can also be quantified by the correlation between the power of an oscillation and the firing rate of simultaneously recorded neurons. Left, Simulated correlated spiking and gamma band power. The neuron tends to fire more rapidly when the power of the gamma oscillation is higher. This correlation can reveal coupling between network input and neuronal output patterns. Right, Psychotomimetic drugs decorrelate spikes from gamma power in the prefrontal cortex.7
We find that the noise correlation between dopamine neurons increases in strength throughout associative learning (ie, learning that an event predicts an outcome), and the pattern of this change is different when the outcome is rewarding or aversive. Similarly, phase locking of neurons with theta oscillations is modulated by associative learning.5 Moreover, when contingencies change (ie, when the cue that predicted a rewarding outcome now predicts an aversive outcome) the correlation structure tracks the change, suggesting that dopamine neurons flexibly organize as a functional network in response to changing environmental contingencies. Similar coordinated activity has been reported in the hippocampus and pre-frontal cortex,8 and within and between cortical areas. It also is predictive of behavioral outcome9 (Figure, D).
These analyses provide a dynamic measure of functional connectivity at the level of individual neurons, which complements large-scale functional connectivity and connectomics assessed using functional magnetic resonance imaging.2 Dopamine-related dysfunction in cognitive or affective disorders may be caused by disruptions in correlated activity of groups of midbrain neurons as opposed to anatomical or structural abnormalities in individual neurons. This suggestion can be further explored in animal models of the disorder.
Disrupted Coordinated Activity in the Prefrontal Cortex During Manipulations Relevant to Schizophrenia
Although evaluating the impact of a manipulation on cortical pyramidal or γ-aminobutyric acid interneuron activity in animal models of schizophrenia has mechanistic value, we found recently that measures of single-neuron activity or local field potential in behaving animals produce different responses in different animal models of schizophrenia. For example, 3 psychotomimetic compounds produce divergent effects on cortical firing rate and local field potential oscillations.6 Local field potential oscillations generally reflect network-wide dendritic processing and when the analysis is extended to examining neuronal interactions, we find that these compounds decouple spike rate from cortical network oscillations, selectively in the gamma range (Figure, E). Effectively, psychotomimetic compounds may influence behavior by decorrelating, or disconnecting, input to a neuronal network from the output of the network. While the neuronal basis of these disruptions remains to be determined, these measures may lead to a better understanding of the coordinated neuronal activity underlying the behavioral symptoms of psychiatric disorders and provide translational fingerprints for validating animal models at a physiological level.
Implication for Future Research
Developing mechanistically based and clinically relevant dynamic network models is critical for improving our understanding of the biological basis of psychiatric disorders. While evolving psychiatric genetic findings are providing the field with a wealth of potential animal models,7 their detailed phenotypic characterization will take enormous time and resources and may in the end be unproductive if the traditional phenotypes are irrelevant to the human condition. But a focus on dynamic mechanisms that support behavior and the use of electrophysiologic tools that complement human imaging methods may place us in an excellent position to understand the contribution of genetics and environmental variables to the biology of symptoms of psychiatric illnesses. This line of inquiry is consistent with the recently proposed Brain Activity Map Project10 supported by President Obama in the 2013 State of the Union Address (http://www.nytimes.com/2013/02/18/science/project-seeks-to-build-map-of-human-brain.html?_r=0).
Footnotes
Conflict of Interest Disclosures: None reported.
References
- 1.von der Malsburgh C, Phillips WA, Singer W, editors. Dynamic Coordination in the Brain. Cambridge, MA: The MIT Press; 2010. [Google Scholar]
- 2.Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62(4):2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 3.Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66(1):13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y, Wood J, Moghaddam B. Coordinated activity of ventral tegmental neurons adapts to appetitive and aversive learning. PLoS One. 2012;7(1):e29766. doi: 10.1371/journal.pone.0029766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood J, Kim Y, Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci. 2012;32(9):3022–3031. doi: 10.1523/JNEUROSCI.6377-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell KJ, Huang ZJ, Moghaddam B, Sawa A. Following the genes: a framework for animal modeling of psychiatric disorders. BMC Biol. 2011;9:76. doi: 10.1186/1741-7007-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzsáki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7(5):446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- 9.Totah NK, Jackson ME, Moghaddam B. Preparatory attention relies on dynamic interactions between prelimbic cortex and anterior cingulate cortex. Cereb Cortex. 2013;23(3):729–738. doi: 10.1093/cercor/bhs057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alivisatos AP, Chun M, Church GM, Greenspan RJ, Roukes ML, Yuste R. The Brain Activity Map Project and the challenge of functional connectomics. Neuron. 2012;74(6):970–974. doi: 10.1016/j.neuron.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]