Abstract
Stressors that are controllable not only protect an individual from the acute consequences of the stressor, but also the consequences of stressors that occur later. This phenomenon, termed “behavioral immunization”, is studied in the rat by first administering tailshocks each of which can be terminated (escapable tailshock) by an instrumental wheel-turn response prior to exposure to a second stressor. Previous research has shown that exposure to escapable tailshock blocks the neurochemical and behavioral consequences of later inescapable tailshock or social defeat stress. Here we explored the generality of behavioral immunization by examining the impact of prior escapable tailshock on the behavioral consequences of cold swim stress. Exposure to a 5 min cold-water (19 °C) swim caused an anxiety-like reduction in social interaction that was dependent upon 5-HT2C receptor activation. Rats with prior exposure to escapable tailshock did not develop the swim-induced anxiety. Plasticity in the medial prefrontal cortex, a hypothetical neural mechanism underlying behavioral immunization, is discussed.
Keywords: 5-HT2C, Anxiety, Learned helplessness, Rat, Social interaction
1. Introduction
There is enormous variability in how individuals respond to stressors and whether the stressor episode will interact with the development of psychiatric disorders (Alleva and Francia, 2009; Dudley et al., 2011; Franklin et al., 2012). Although it is clear that stress history is an important modulating factor, the features of prior experience that are critical remain largely unknown. Often, prior exposure to a stressor or adverse circumstance increases the behavioral or neurochemical impact of a subsequent stressor, particularly if the prior stressor is intense and different from the later target stressor (Girotti et al., 2011; Johnson et al., 2002; Pelton et al., 1997). Until recently, the emphasis has been on attempting to determine the mechanisms by which prior experiences produce such potentiation. However, there are circumstances under which prior stress experience blunts the impact of subsequent stressors (Lyons and Macri, 2011), and there has been an increasing interest in understanding the processes that underlie “resilience” (Dudley et al., 2011; Russo et al., 2012; Southwick and Charney, 2012).
The degree of behavioral control that the organism is able to exert over the stressor is arguably the most potent experiential aspect of the stressor episode that modulates its influence on behavioral and neurochemical reactions to subsequent stressors (Cabib et al., 2012; Southwick and Charney, 2012; Southwick et al., 2005). The presence of behavioral control (the ability to alter the duration, intensity, temporal pattern, etc. of an adverse event by means of behavioral responses) not only blunts the impact of the stressor being experienced, but also potently reduces the behavioral impact of subsequent uncontrollable stressors. Exposure to intense stressors, such as social defeat or inescapable tailshocks, produces a constellation of behavioral changes that include both anxiety-like (e.g., reduced social investigation) and depressive-like (e.g., anhedonia) elements (Maier and Watkins, 2005; Overstreet, 2012). These behavioral effects are mediated, at least in part, by changes in dorsal raphe nucleus (DRN) 5-HT neurons that are consequent to the intense activation of these neurons produced by the stressor (for review see Maier and Watkins, 2005). Experience with a series of escapable tailshocks (ES) during which each tailshock can be terminated by turning a small wheel located in the front of the chamber, blocks both the anxiety and depression-like behavioral changes, as well as the 5-HT DRN activation produced by later inescapable tailshock (Amat et al., 2006), a phenomenon that has been called “behavioral immunization” (Williams and Maier, 1977). An initial experience with exactly matched inescapable tailshocks (IS) does not produce immunization, so the immunization depends on the controllability of the initial tailshocks. Strikingly, the immunizing effects of ES persist for at least 7 days, and may persist for at least a month, depending on the outcome measure (Kubala et al., 2012; Rozeske et al., 2012).
Because mechanisms of stress resistance and resilience are critically important to understanding the development of psychiatric disease (Russo et al., 2012), our laboratory has sought to identify the procedural limits of the behavioral immunization phenomenon. Although the work cited above involved administration of tailshock in different environments in the two phases of the procedure, the stressors were fundamentally the same. A critical issue with regard to the immunization phenomenon concerns whether control over tailshock would afford protection against qualitatively different stressors. At present, only one experiment has been directed at this issue. Amat et al. (2010) exposed rats to social defeat 7 days after ES or IS. As above, prior ES prevented the activation of the DRN and the anxiety-like behavioral changes associated with social defeat. This finding suggests a fundamental organismic change produced by an experience with control over tailshock and here we explore the generality of this trans-stressor effect by determining whether ES would blunt the changes produced by acute swim exposure. Because our interests focus on the consequences of stressor exposure on anxiety-like behavior, we first determined whether forced swim would induce social avoidance. In the first experiment the rats were exposed to a 5 minute swim in cold 19 °C water and tested for social exploration 1 h after swim. In the second experiment the rats have received either ES, IS or no stress 7 days before the forced swim and social exploration tests. Finally, to determine if forced swim-induced anxiety depends on 5-HT2C receptors, a critical mediator of anxiety in this test (Christianson et al., 2010), the rats were given the 5-HT2C receptor antagonist SB242084 after the swim.
2. Materials and methods
2.1. Animals
Adult male Sprague–Dawley (Harlan, IN) rats weighing 250–300 g at the time of experimentation were used in all experiments. The rats were housed in groups of 4 in plastic cages with shaved-wood bedding and free access to food and water at all times. Lighting in the vivarium was maintained on a 12 h light/dark cycle. All behavioral experiments were conducted in the first 4 h of the light cycle and were approved by the University of Colorado Institutional Animal Care and Use Committee.
2.2. Acute swim stress
The rats were placed in a clear glass beaker (Pyrex) 45.7 cm height×30.5 cm diameter with water filled to a depth of 30 cm. Behavior was recorded by a digital video camera for later continuous analysis of immobility, swimming and climbing according to the definitions of Detke et al. (1995). Water temperature was 19 °C or 35 °C (see experimental procedures in Section 2.6).
2.3. Social exploration test
The juvenile social exploration test was conducted exactly as described previously (Christianson et al., 2010). Briefly, the rats were transferred to a testing room and placed into individual plastic tub cages with bedding and a wire lid. After 60 min a 28(±2) day-old juvenile conspecific was introduced into the cage and exploratory behaviors (sniffing, grooming, and pinning) initiated by the adult test subject were quantified by a trained observer who was naïve to the treatment over a 3 min period.
2.4. Escapable/yoked inescapable stress
In Experiment 2, the rats were exposed to 100 trials of either controllable, escapable tailshock (ES), exactly equal uncontrollable, inescapable tailshock (IS) or no shock. This procedure was identical to that described previously (Christianson et al., 2010). In brief, the rats were placed in a plastic chamber and restrained by the tail with cloth tape. Electrodes were attached to the tail and augmented with electrolyte paste. 100 tailshocks (33 at 1 mA, 33 at 1.3 mA and 34 at 1.6 mA) were administered by a computer with Graphic State hardware and software (Coulbourn Instruments, PA) on a variable 60 s interval (range 30–90 s). Shock intensity was increased over the session to maintain response. The rats in the ES condition were able to turn a wheel in the chamber to terminate the shock. IS rats were physically yoked to an ES subject so that when the wheel turn requirement was reached, the shock was terminated for both subjects. The wheel turn requirement began with ¼ turn and increased to 4 full turns as previously described (Amat et al., 2006; Christianson et al., 2010). If an escape was not made within 30 s of shock onset the trial was automatically terminated by the computer. Then the stress rats were returned to the home cages thereafter.
2.5. Drugs
The brain penetrant and highly selective 5-HT2C receptor antagonist SB242084 (Tocris) was dissolved in saline by sonication and administered i.p. at a dose of 0.25 mg/kg in a volume of 1 ml/kg. 0.25 mg/kg was chosen based on a pilot study to minimize animal usage and was slightly smaller than previously used to reverse stressor-induced anxiety (Christianson et al., 2010; Strong et al., 2009).
2.6. Experimental procedures
2.6.1. Experiment 1
The purpose of Experiment 1 was to determine the effects of a brief cold water swim on anxiety-like behavior in the social exploration test. A pilot study indicated that a 15 min swim, the duration of a typical forced swim test (Detke et al., 1995) in 19 °C water, produced a robust decrease in social exploration but was confounded by severe hypothermia. Thus, the rats have received cold swims of 0, 5, 10 or 15 min at 19 °C. In order to determine whether the cold temperature of the water is critical, a final group received a 15 min swim in 35 °C water. After 60 min a juvenile was added and social exploration tests were conducted as described above.
2.6.2. Experiment 2
The goal of Experiment 2 was to test whether pretreatment with controllable or uncontrollable stress would alter the behavioral response to swim stress and the anxiety observed 60 min later. We first conducted a time course experiment in which separate groups of rats were exposed to ES, IS or HC treatments and subsequently were given social exploration tests 1, 3 or 7 days later. IS, but not ES, reduced social exploration time 1 and 3 days after stress and all groups appeared equal by day 7. In the critical experiment, the rats were assigned to 1 of 4 groups: no stress home cage control (HC), Swim only (Swim), escapable stress and swim (ES-Swim) or inescapable stress and swim (IS-Swim). Escapable or inescapable stress occurred on experimental day 1. HC and Swim rats remained undisturbed in the vivarium. On day 7, Swim, ES-Swim and IS-Swim rats received a 5 minute swim in 19 °C and social exploration tests were conducted 60 min later as described in Experiment 1. HC rats were given social exploration tests at the same time without any prior treatment.
2.6.3. Experiment 3
The purpose of Experiment 3 was to test whether the reduction in social exploration observed 60 min after swim stress was mediated by 5-HT2C receptors. This was done because reduction in social exploration produced by inescapable tailshocks and other stressors is mediated by these receptors (Christianson et al., 2010; Harvey et al., 2012; Overstreet et al., 2003, 2006). The rats were assigned to 1 of 4 treatment groups in a 2 (swim or no stress)×2 (0 or 0.25 mg/kg SB242084) design. The rats in the swim group were exposed to a 5 min swim in 19 °C water, the minimum required to produce anxiety like behavior followed immediately by 0 or 0.25 mg/kg SB242084 i.p. Rats in the no stress group were given injections before placement into social exploration test cages. The rats were transferred to social exploration test cages and tested for anxiety like behavior 60 min after stress as in Experiment 1.
2.7. Data analysis
In all experiments n=7–10 rats per group. Social exploration was analyzed as total exploration time in seconds and the swim behaviors, im-mobility, swimming and climbing, were analyzed as time in seconds per 30 s epochs of the test. Effects of stress and drug treatment were tested with analysis of variance (ANOVA) with stressor and drug groups as between subject factors and swim behaviors over time treated as a repeated measure, using Prism 6.0a for Mac OS X. Results were deemed significant if p<0.05. Significant main effects and interactions were followed by Fisher's LSD post hoc tests.
3. Results
3.1. Experiment 1
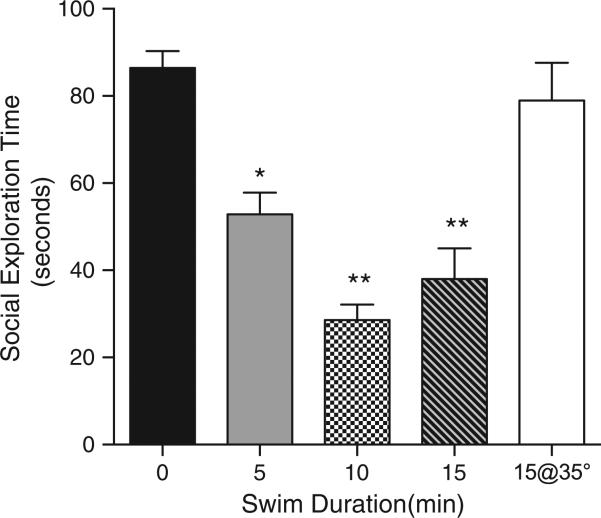
Rats exposed to either 5, 10 or 15 min swims in 19 °C water spent significantly less time interacting with a juvenile in the social exploration tests 60 min after the swim (Fig. 1). A one-way analysis of variance (ANOVA) revealed a significant main effect for swim time (0, 5, 10 or 15 min) on social exploration, F(3, 29)=26.80, p<0.0001. Post hoc comparisons between all groups revealed significant differences between no stress and each stress group, and a difference between 5 min and the 10 and 15 min groups, which did not differ from each other (ps<0.05, Fisher LSD). The mean did not differ from the unstressed 0 min group as compared with the rats exposed to a 15 min swim in 35 °C water.
Fig. 1.
Mean(+SEM) social exploration during a 3 minute test conducted 60 min after the swim in 19 °C water. Exposure to swims of increasing duration reduced subsequent exploratory behavior in a social interaction test. *5 min is significantly different from 0, 10 and 15 min groups. **10 and 15 min are significantly different than 0 and 5 min groups but not each other, ps<0.05.
3.2. Experiment 2
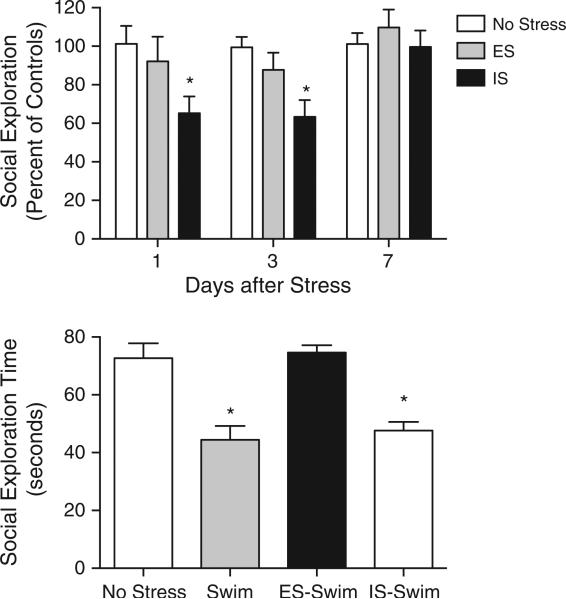
The rats exposed to escapable stress (ES) rapidly learned the wheel-turn escape response such that escape latencies were less than 5 s per trial, similar to the results we have published previously (data not shown for simplicity, see Amat et al., 2005). In the social exploration time course experiment, IS reduced social exploration in tests that occurred 1 or 3 days after stress (Fig. 2, upper panel). A two-way ANOVA with stress treatment and day as between-subject factors revealed significant main effects for stress, F(2, 66)=6.025, p=0.004 and day, F(2, 66)=4.075, p=0.024; the interaction did not reach significance (p=0.33). Post hoc comparisons between ES, IS and HC groups on each day revealed a significant reduction in social exploration in the IS group as compared to HC on days 1 and 3 (psb0.05, Fisher LSD). ES never differed from HC. In the critical behavioral immunization experiment the rats received ES, IS or no stress on day 1. On day 7 rats received a 5 min swim followed by social exploration tests 60 min later. Prior ES prevented the reduction in social exploration caused by acute swim, while prior IS had no effect (Fig. 2). A one-way ANOVA revealed a significant main effect of group, F(2, 28)= 15.91, p<0.0001. Post hoc analysis revealed that the HC-Swim and IS-Swim groups to be significantly different from the HC-No swim and ES-Swim groups (psb0.001, Fisher LSD), which did not differ (ps>0.5). Immediately after the social exploration test rectal temperatures were taken as a relatively non-invasive index of core body temperature. Consistent with the reports of Linthorst et al. (2008) and Kelly et al. (2011), no differences in rectal temperature were observed at this time between groups that received swim as compared to un-stressed controls (data not shown for simplicity).
Fig. 2.
TOP: Mean(+SEM) social exploration during a 3-minute test conducted 1, 3 or 7 days after ES, IS or no stress treatment. Data are expressed as the % of the mean HC value per test cohort (see the results in Section 3.2). *IS significantly decreased social exploration time 1- and 3-day post stress, ps<0.05 compared to no stress and ES groups). BOTTOM: Mean(+SEM) social exploration during a 3-minute test conducted 60 min after a 5 min swim in 19 °C water, or no stress. Rats were treated with escapable stress (ES) or inescapable stress (IS) 7 days prior to the swim. Prior ES completely blocked the reduction in swim typically observed after a 5 min swim. *Swim and IS-Swim groups are significantly different from no stress and ES-Swim groups, ps<0.05.
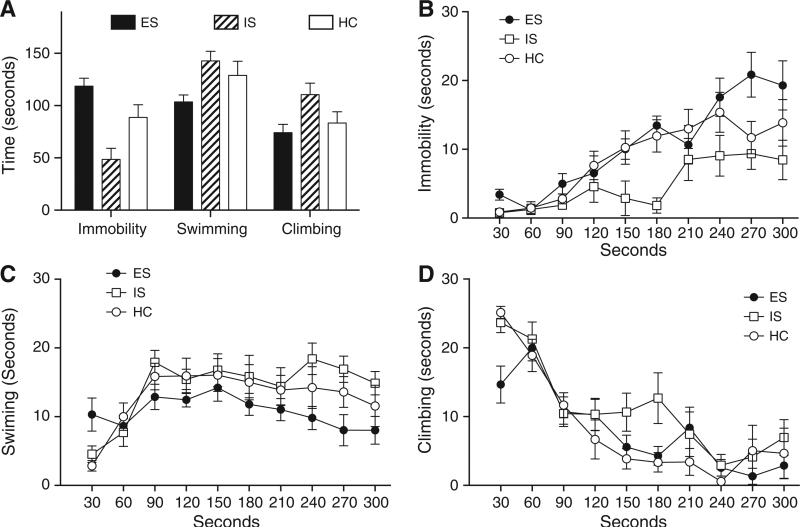
The controllability of prior stress appeared to bi-directionally modulate behavioral responses to the 5 min swim itself (Fig. 3). Immobility, swimming and climbing behaviors were continuously quantified by an observer blind to prior stress treatment. Three separate ANOVAs with stress treatment (ES, IS or HC) as a between subject factor and time spent in immobility, swimming or climbing per 30 s epoch as a repeated measure revealed numerous significant effects. For immobility, there is a significant main effect of stress on immobility F(2,20)=6.285, p= 0.007, a main effect of time, F(9, 180)=18.78, p<0.001, and a group by time interaction F(18, 180)=1.758, p=0.036. Post hoc comparisons based on the significant interaction between each stress group at each 30 s time point revealed significantly less time spent immobile in the IS group as compared to both HC and ES groups at 150, 180, and 240 s (ps<0.05, Fisher LSD), while the ES group spent more time in immobility than IS at 270 and 300 s (ps <0.05). For swimming, the main effect of stress approached significance, F(2, 20) 2.805, p=0.073 and a significant effect of time was found, F(8, 180)=7.718, p<0.001. Significant differences in time swimming were found between ES and IS groups at 240, 270 and 300 s and ES differed from HC at 30 s. For climbing, the main effect of stress was not significant (p=0.116) but a significant effect of time, F(9, 180) =28.39, p<0.001, and significant stress by time interaction, F(18, 180)=1.647, p=0.053 were found. Significant differences in time spent climbing were found between HC and IS groups at 150 and 180 s, between HC and ES at 30 s, and between ES and IS at 30 and 180 s timepoints. These statistical results support what is apparent from visual inspection of the data: prior ES increased time spent in immobility with a corresponding reduction in time spent swimming and climbing, while prior IS increased active swimming and climbing behaviors.
Fig. 3.
Immobility, swimming and climbing behaviors quantified during a 5 min swim in 19 °C water. A) Prior escapable or inescapable stress treatment resulted in very different behavioral topography in 5 min swim. Compared to stress-naïve (HC) rats, ES led to relatively more immobile postures, while IS reduced immobility. This trend is reflected as increases in active swimming and climbing behaviors in the IS group. Immobility, swimming and climbing were subsequently analyzed across the 5 min test. B) IS has reduced im-mobility as compared to HC and ES rats in the 3rd minute of the test while ES subjects spent more time in immobility in the last minute. C) ES rats spent less time swimming throughout the test and less time (D) climbing upon initial exposure. These data are shown for illustration; statistical comparisons are described in the text.
3.3. Experiment 3
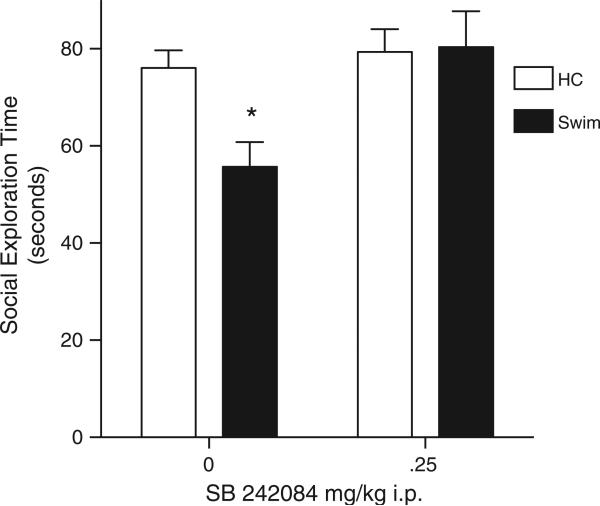
Systemic administration of the 5-HT2C receptor antagonist SB282084 immediately after stress prevented the development of the swim stress-induced anxiety typically observed 60 min later (Fig. 4). A significant main effect of the drug was found in a 2 (dose: 0 or 0.25 mg/kg SB242084) by 2 (stress: HC or 5 min swim) between subject ANOVA, F(1, 28)=6.774, p=0.015. The main effect of stress and the interaction did not reach significance (ps<0.0815). Post hoc comparisons found a significant difference between the Swim 0 mg/kg SB242084 group and all other groups (ps<0.05, Fisher LSD).
Fig. 4.
Mean(+SEM) social exploration during a 3 minute test conducted 60 min after a 5 min swim in 19 °C water, or no swim. The 5-HT2C receptor antagonist SB242084 completely blocked the acute reduction in social exploration observed after a 5 min swim. *Swim-0 mg/kg group is significantly different from all other groups, ps<0.05.
4. Discussion
Here we present a set of experiments designed to determine whether exposure to a controllable tailshock stressor would protect against the acute consequences of a cold water swim. An acute swim in relatively cold 19 °C water produced a reliable reduction in social exploration 60 min after stress. Exposure to escapable tailshock (ES) 7 days before prevented the acute swim-induced change in social exploration. In addition, prior stress altered the behavioral response to the cold-water swim itself, with controllable stress leading to passive, immobile behaviors and uncontrollable stress leading to active, swimming and climbing behaviors. Lastly, 5-HT2C receptors proved to be a critical mediator of anxiety expression, and here the 5-HT2C receptor antagonist SB242084 prevented the expression of swim stress induced anxiety.
The cold swim-induced anxiety-like behavior occurred at a time point is known to correlate with elevated extracellular 5-HT levels in the forebrain after swim stress (Linthorst et al., 2008). Although administered systemically, SB242084 which prevented the anxiety suggests that the acute rise in 5-HT likely mediates the acute anxiety by action at post synaptic 5-HT2C receptors, possibly by specific action in the basolateral amygdala (Christianson et al., 2010). Consistent with this possibility, prior ES prevents the 5-HT activation that results from inescapable tailshock and social defeat, as well as the resulting anxiety-like behaviors (Amat et al., 2006, 2010). Like swim, social defeat stress also activates the DRN in a manner comparable to inescapable tailshock (Amat et al., 2010). Thus, 5-HT activation is common to the three stressors, shock, social defeat and cold-water swim, so far studied with the behavioral immunization paradigm.
The majority of forebrain 5-HT input stems from the dorsal raphe nucleus (DRN). We have demonstrated that prior ES prevents subsequent DRN activation by tailshock via a top-down circuit involving the prelimbic medial prefrontal cortex (PL). Glutamatergic efferents from the PL to the DRN (Gabbott et al., 2005) synapse preferentially on GABAergic DRN interneurons (Jankowski and Sesack, 2004). Accordingly, activation of the PL input to the DRN results in inhibition of DRN 5-HT neuronal activity (Celada et al., 2001). Amat et al. (2006) tested whether behavioral immunization depends upon PL activation and, indeed, intra-PL muscimol prevented the effect of prior ES on both DRN reactivity and anxiety like behaviors. Conversely, pharmacological activation of the PL during exposure to an uncontrollable stressor itself produced immunization against later IS effects (Amat et al., 2008), suggesting that PL activation during stress is sufficient to cause behavioral immunization.
One possibility is that the immunization occurs because experience with control induces neuroplasticity in the PL. As a consequence, subsequent stressors that would not normally activate the PL-to-DRN projections, such as uncontrollable stressors, now do so. Indeed, Baratta et al. (2009) reported that prior ES caused PL neurons with known projections to the DRN to be selectively activated by IS occurring 7 days later. Consistent with the PL plasticity argument, the protein synthesis inhibitor anisomycin administered to the PL prevented behavioral immunization (Amat et al., 2006). Finally, it can be noted that the experience of ES increases the excitability of PL pyramidal neurons (Varela et al., 2012).
The existence of controllability-induced PL plasticity indicates that subsequent stimuli that would not normally activate PL top-down inhibitory control now do so after experiencing ES. Recently, Warden et al. (2012) demonstrated that the PL-to-DRN pathway modulates behavioral responses to swim and this is consistent. Plasticity within the PL-DRN neural circuit may account for the two effects of prior ES observed here. First, prior ES may have altered PL regulation of the behavioral responses to swim by favoring the strategy that conserves the most energy. And second, prior ES may have prevented the rise in 5-HT that likely altered social exploration, possibilities that require further investigation. In support of this speculation, Weinberg et al. (2010) observed that prior ES caused the rats exposed to subsequent restraint to engage in passive behaviors more rapidly than do stressor naïve rats or rats with prior IS. The meaning of climbing versus immobility in a swim test is at best arguable, however, adopting a passive response to cold swim would likely increase the likelihood of survival in the wild. Indeed, immobility has been argued to be a relatively “good” coping response to forced swim (West, 1990) and the result of behaviorally adaptive learning and retrieval processes (Mitchell and Meaney, 1991). Nevertheless, the evidence suggests that the behavioral immunization phenomenon is quite general, with prior ES blocking the effects of later stressors that are quite dissimilar from the inoculating stimulus. It may be that experiencing behavioral control over an intense stressor confers wide-ranging protection that depends upon plasticity in the PL, and we suggest that behavioral immunization will occur with regard to stimuli that evoke behavioral responses that are under the inhibitory control of the PL.
5. Conclusion
Learning to inhibit negative emotions is a feature of cognitive therapies and when successfully applied cognitive therapy provides a psychological tool to manage responses to multiple stressors (Clark and Beck, 2010). Turning a wheel to terminate a tailshock may capture a fundamental element of emotional control akin to the goal of cognitive therapy and so discerning the mechanisms underlying the behavioral immunization effect could improve cognitive therapies. The proactive effects of controllable tailshocks endure for a significant period (up to 35 days in rat, Kubala et al., 2012) and require synaptic plasticity within the medial prefrontal cortex. However, several important questions remain open: 1) what physiological and molecular mechanisms mediate plasticity underlying behavioral immunization and 2) what is the fundamental feature of a stressful event that activates the prefrontal cortex after controllable stress exposure? Clearly, the boundaries of this phenomenon are worthy of further investigation and these questions are the focus of ongoing research.
Abbreviations
- DRN
dorsal raphe nucleus
- ES
escapable tailshock
- IS
Inescapable tailshock
- 5-HT
5-hydroxytryptamine
- ANOVA
analysis of variance
- LSD
least signifi-cant difference
- SEM
standard error of the mean
- HC
home cage
References
- Alleva E, Francia N. Psychiatric vulnerability: suggestions from animal models and role of neurotrophins. Neurosci Biobehav Rev. 2009;33:525–36. doi: 10.1016/j.neubiorev.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–72. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–86. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Aleksejev RM, Paul E, Watkins LR, Maier SF. Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience. 2010;165:1031–8. doi: 10.1016/j.neuroscience.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur J Neurosci. 2009;30:1111–6. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Campus P, Colelli V. Learning to cope with stress: psychobiological mechanisms of stress resilience. Rev Neurosci. 2012;23:659–72. doi: 10.1515/revneuro-2012-0080. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–29. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, et al. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry. 2010;67:339–45. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Beck AT. Cognitive theory and therapy of anxiety and depression: convergence with neurobiological findings. Trends Cogn Sci. 2010;14:418–24. doi: 10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Dudley KJ, Li X, Kobor MS, Kippin TE, Bredy TW. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neurosci Biobehav Rev. 2011;35:1544–51. doi: 10.1016/j.neubiorev.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–61. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–77. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Girotti M, Donegan JJ, Morilak DA. Chronic intermittent cold stress sensitizes neuro-immune reactivity in the rat brain. Psychoneuroendocrinology. 2011;36:1164–74. doi: 10.1016/j.psyneuen.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey ML, Swallows CL, Cooper MA. A double dissociation in the effects of 5-HT2A and 5-HT2C receptors on the acquisition and expression of conditioned defeat in Syrian hamsters. Behav Neurosci. 2012;126:530–7. doi: 10.1037/a0029047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol. 2004;468:518–29. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Spencer RL, Watkins LR, Maier SF. Prior stressor exposure primes the HPA axis. Psychoneuroendocrinology. 2002;27:353–65. doi: 10.1016/s0306-4530(01)00057-9. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Donner NC, Hale MW, Lowry CA. Swim stress activates serotonergic and nonserotonergic neurons in specific subdivisions of the rat dorsal raphe nucleus in a temperature-dependent manner. Neuroscience. 2011;197:251–68. doi: 10.1016/j.neuroscience.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubala KH, Christianson JP, Kaufman RD, Watkins LR, Maier SF. Short- and long-term consequences of stressor controllability in adolescent rats. Behav Brain Res. 2012;234:278–84. doi: 10.1016/j.bbr.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst AC, Flachskamm C, Reul JM. Water temperature determines neurochemical and behavioural responses to forced swim stress: an in vivo microdialysis and biotelemetry study in rats. Stress. 2008;11:88–100. doi: 10.1080/10253890701533231. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Macri S. Resilience and adaptive aspects of stress in neurobehavioral development. Neurosci Biobehav Rev. 2011;35:1451. doi: 10.1016/j.neubiorev.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–41. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Meaney MJ. Effects of corticosterone on response consolidation and retrieval in the forced swim test. Behav Neurosci. 1991;105:798–803. doi: 10.1037//0735-7044.105.6.798. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. Modeling depression in animal models. Methods Mol Biol. 2012;829:125–44. doi: 10.1007/978-1-61779-458-2_7. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2c antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology (Berl) 2003;167:344–52. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Angel RA, Navarro M, Breese GR. Reduction in repeated ethanol-withdrawal-induced anxiety-like behavior by site-selective injections of 5-HT1A and 5-HT2C ligands. Psychopharmacology (Berl. 2006;187:1–12. doi: 10.1007/s00213-006-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton GH, Lee Y, Davis M. Repeated stress, like vasopressin, sensitizes the excitatory effects of corticotropin releasing factor on the acoustic startle reflex. Brain Res. 1997;778:381–7. doi: 10.1016/s0006-8993(97)00669-0. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Der-Avakian A, Watkins LR, Maier SF. Activation of the medial prefrontal cortex by escapable stress is necessary for protection against subsequent inescapable stress-induced potentiation of morphine conditioned place preference. Eur J Neurosci. 2012;35:160–5. doi: 10.1111/j.1460-9568.2011.07929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–84. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338:79–82. doi: 10.1126/science.1222942. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–91. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Strong PV, Greenwood BN, Fleshner M. The effects of the selective 5-HT(2C) receptor antagonist SB 242084 on learned helplessness in male Fischer 344 rats. Psychopharmacology (Berl) 2009;203:665–75. doi: 10.1007/s00213-008-1413-3. [DOI] [PubMed] [Google Scholar]
- Varela JA, Wang J, Christianson JP, Maier SF, Cooper DC. Control over stress, but not stress per se increases prefrontal cortical pyramidal neuron excitability. J Neurosci. 2012;32:12848–53. doi: 10.1523/JNEUROSCI.2669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492(7429):428–32. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Grissom N, Paul E, Bhatnagar S, Maier SF, Spencer RL. Inescapable but not escapable stress leads to increased struggling behavior and basolateral amygdala c-fos gene expression in response to subsequent novel stress challenge. Neuroscience. 2010;170:138–48. doi: 10.1016/j.neuroscience.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP. Neurobehavioral studies of forced swimming: the role of learning and memory in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:863–77. doi: 10.1016/0278-5846(90)90073-p. [DOI] [PubMed] [Google Scholar]
- Williams JL, Maier SF. Transituational immunization and therapy of learned helplessness in the rat. J Exp Psychol Anim Behav Process. 1977;3:240. [Google Scholar]