Abstract
Aim
Exosomes, the nano-units (<200nm), released from diverse cell types in the extracellular body fluid, possess non-immunogenic property and ability to cross blood-brain barrier (BBB). Since exosomes carry biological information from their cells of origin, we hypothesize that priming cells with potential therapeutic agents release improved cellular contents through exosomes. Curcumin possesses anti-oxidative, anti-inflammatory properties and provides a promising treatment for cerebral diseases and therefore, the aim of the study is to establish that mouse brain endothelial cells (MBEC) when primed with curcumin (7.5μM), release alleviated exosome population that can help recover endothelial cell (EC) layer permeability.
Methodology
Homocysteine is a well-known causative factor of BBB disruption; therefore, homocysteine-treated ECs were used as a model of BBB disruption and curcumin-primed exosomes were utilized to check their potential for mitigating EC disruption. MBEC were treated with curcumin and exosome were isolated by using ultracentrifugation and immunoprecipitation. Expression levels of junction proteins were detected by Western blot and Immunocytochemistry assays. Endothelial cell permeability was analyzed with FITC BSA leakage assay using transwell permeable supports.
Key Findings
Exosomes derived from curcumin-treated (primed) cells (CUR-EXO) alleviated oxidative stress, tight junctions (ZO-1, claudin-5, occludin), adherent junction (VE-cadherin) proteins and EC layer permeability induced during EC damage due to high homocysteine levels (hyperhomocysteinemia).
Significance
In conclusion, the study potentiates the use of CUR-EXO for cerebral diseases where drug delivery is still a challenge. The results also pave the way to novel translational therapies for cerebral diseases by maintaining and establishing therapeutic conservatories via primed exosomes.
Keywords: Blood-brain barrier, Curcumin, Endothelial cells, Exosomes, Homocysteine, Oxidative stress, Permeability, Tight Junctions
Introduction
Exosomes are nano-vesicles (<200nm) formed from the fusion of internal vesicles of multivesicular bodies to plasma membrane. These are found in extracellular fluids of the body (urine, breast milk, saliva, cerebrospinal fluid, brancheo-alveolar lavage) and culture conditioned media. These nano-units are believed as a molecular source of cellular factory and retain molecular conservatory of the cell type from where they are released (Kalani et al. 2013c). Apart from their use in biomarker exploration, mounting literature suggest their use in therapy for cerebral diseases, such as stroke (Xin et al. 2013). The major advantage of their use over other therapeutic systems in cerebral diseases is because of their ability to cross BBB, non-immunogenicity and targeted delivery (Kalani et al. 2013c;Zhuang et al. 2011); but the studies are largely lacking which provide sufficient information for their therapeutic aspects to combat and alleviate BBB disruption.
Blood-brain barrier (BBB) is exclusively maintained by cerebral blood vessels, which are lined by specialized endothelial cells (ECs). The integrity of cerebral ECs is retained by the cross talk of tight junctions (TJs, zona occludens, claudins) and adherent junctions (VE-cadherin). In pathological conditions, such as vascular complications and neurological disorders, cerebral junctions are disrupted that affect selective barrier function of BBB and eventually raise vascular permeability (de Vries et al. 1997;Kalani et al. 2013a). BBB has been reported to be affected significantly by homocysteine (Hcy) (Kalani et al. 2013a), which is a natural inhabitant molecule formed from methionine metabolism in the body. Being deficient in cystathionine-β synthetase (CBS) enzyme activity, which is responsible for Hcy clearance, the mammalian endothelium is the prime target of Hcy-induced toxicity (Shastry et al. 2006). The outcome of earlier studies reflect that subclinical elevation in Hcy levels (hyperhomocysteinemia; HHcy) (from baseline of ~3 μmoles/L to ~12 μmoles/L) induces toxicity resulting in considerable leakage of plasma proteins (Beard, Jr. et al. 2011;Kamath et al. 2006;Lominadze et al. 2006). The subsequent events that take place after the elevation of Hcy include: 1) activation of matrix metalloproteinases (MMPs), 2) disruption of membrane proteins including TJs and other junctional proteins and, 3) functional decline in endothelial cells forming BBB (Kumar et al. 2008;Lee et al. 2012).
Recently curcumin (diferuloylmethane; CUR) which is derived from the root of Curcumin longa (spice turmeric) has been used for neurological implications. Curcumin is also termed as yellow gold as it possesses anti-inflammtory, anti-lipidemic, and antioxidative properties, and has been recommended for the clinical trials to prevent / treat neurological diseases (Kulkarni and Dhir 2010). The findings of the earlier studies implicating in vivo myeloid cells suggest that curcumin delivered by exosomes is more stable, highly concentrated in the blood, and exhibits therapeutic effect, rather than toxic effects (Sun et al. 2010). The study by Liu et al. highlighted increase in exosomes/microvesicles secretion with curcumin that attenuates lysosomal cholesterol traffic impairment in HepG2 hepatocarcinoma cells and THP-1 differentiated macrophages (Canfran-Duque et al. 2013). Although these reports suggest beneficiary effect of curcumin on exosomes production and effect of curcumin-packed exosomes to surmount pathological conditions, the reports are largely lacking which establish the effect of curcumin-primed exosomes (CUR-EXO) in EC disruption and permeability. Curcumin-primed exosomes differ from curcumin packed exosomes in the respect that they are released by curcumin treated cells while curcumin packed exosomes are exosome entities encapsulated with curcumin. Therefore, we hypothesized that CUR-primed exosomes exhibit improved molecular constituents that help in regulation of ECs disruption. Mouse brain ECs were treated with Hcy to disrupt junction proteins, enhance permeability, and the therapeutic efficacy of CUR-EXO was observed for maintaining ECs integrity.
Meterials and methods
Cell culture
Mouse brain endothelial cell line (MBEC) was purchased from American Type Culture Collection (ATCC, Menassas, VA, USA) and grown in DMEM supplemented with 4.5 g/l glucose, 3.7 g/l sodium bicarbonate, 4 mM glutamine, exosome free 10% FBS (pH 7.4). The cells were maintained under an atmosphere of 5% CO2 and 95% air in 25 cm2 tissue culture flask.
Curcumin-primed exosomes collection
MBECs were treated with curcumin (7.5 μM) for 72 h. Culture media was collected and centrifuged at 3,000Xg (10 min) and supernatant was collected. Collected supernatant was further ultracentrifuged at 1,00,000 x g (1 h) to concentrate exosomes (CUR-EXO) in pellet (Thery et al. 2006). CUR-EXO were purified using exo-specific beads (Life technology, Grand Island, NY, USA) as per supplier’s protocol and either used for treatment or stored at −80°C till further use. Similarly, culture media from untreated cells was also used to concentrate exosomes (EXO).
Treatment groups
The cells were given following treatments: 1) Control, 2) Hcy (100μM), 3) Hcy-CUR-EXO (5μg/ml). In some experiments, cells were treated with EXO (5 μg) and compared with CUR-EXO treated cells for the protein expression analysis of junction proteins. The ECs proteins were extracted after 24 h treatment as described earlier (Thery et al. 2006). The protein concentration of CUR-EXO and EXO was determined through Bradford (Bio-Rad, Hercules, CA, USA) method as per manufacturer’s protocol.
Western blotting
Exosome preparations were run on SDS-PAGE, electro-transferred and immunoblotted with anti-TSG101 (Abcam, Cambridge, MA, USA), anti-TJs (claudin-5, ZO-1, occludin), and anti-VE cadherin specific antibodies (Santa Cruz, Dallas, Texas, USA). Images were recorded and data was analyzed with image lab software (Bio-Rad, Hercules, CA, USA).
Flow cytometry analysis
Flow cytometry was done as described earlier by (Bhatnagar et al. 2007)). Briefly, 5 μg CUR-EXO were incubated with 4 μm-diameter latex beads in PBS (50μl) at RT for 20 min. The volume was increased to 300 μl and the beads were incubated at 4°C overnight under gentle agitation. The reaction was stopped by incubation in 100 mM glycine (30 min). Coated beads were washed thrice and labelled with anti-CD63 PE and appropriate isotype control (Biolegend). After that, the beads were washed thrice and analyzed by flow cytometry (BD Accuri™ C6 Flow Cytometer, New Jersey, USA).
Gelatin Zymography
In-gel gelatin zymography was performed as per our previous reports (Tyagi et al. 2011). Briefly, the protein samples were run on SDS-PAGE. Gel was washed thrice with 2.5% Triton-X 100, incubated for 24 hrs (37°C) in activation buffer [5mmol/L Tris HCl (pH 7.4), 0.005% (v/v) Brij-35, and 1 mmol/L CaCl2], and stained with coomassie brilliant blue. Images were recorded and the data was analyzed with image-lab software (BioRad, USA).
AChE activity in exosome vesicles
AChE activity in exosome vesicles was determined as described earlier (Savina et al. 2002;Stoorvogel et al. 2002). Briefly, 15ml exosomal preparation was suspended in 100μl of PBS and incubated with acetylthiocholine (1.25mM) and dithio-bis-(2-nitrobenzoic acid) (0.1mM) in a final volume of 1ml. The incubation was carried out in cuvettes at 37°C and the change in absorbance (412nm) was monitored every 5 minutes for the total time 30 minutes.
Immunocytochemistry of ECs
Immunocytochemistry was performed to locate exosomes using PKH67 staining (Fitzner et al. 2011), determining ZO-1 cellular expression, and oxidative stress using DCFH-DA (Tyagi et al. 2009). For PKH67 staining, cells were grown in 8 well chamber slides and treated with tubulin red that binds to cytoskeleton proteins (16h), following PKH67 labelled exosome (2h). For ZO-1 expression, the cells were fixed with 4% paraformaldehyde (15 min), permeabilized (0.25% triton X-100 in PBS, 10 min) and blocked with 1% BSA solution (30 min). Cells were further incubated with primary antibody (overnight), and FITC labelled secondary antibody (1h) and washed thrice with PBS after antibody incubations. For DCFH-DA staining, ECs were treated with 5 μM DCFH-DA (20 min) and washed with PBS. In the above three staining, nuclei were stained with DAPI and cells were photographed using laser-scanning confocal microscope (FluoView 1000; Olympus, PA, USA). Total fluorescence intensity was measured with image analysis software (Image-Pro Plus; Media Cybernetics, Rockville, MD, USA).
ECs layer permeability
The transwell permeable supports with polycarbonate membranes (Nuclepore Track-Etch, 6.5 mm in diameter, 0.4μm pore size, and pore density of 108/cm2) coated with fibronectin were seeded with MBECs and grown in DMEM until they formed a complete monolayer (Muradashvili et al. 2012). Cells were washed with PBS and treated with 100μg/ml Hcy or with medium alone in the presence of FITC-BSA (0.2mg/ml). After 6, 12, and 24 h media samples (50 μl) were collected from lower chambers of the transwell system and the same volume of the sample was added to the each appropriate upper well. Fluorescent intensity in sample was measured by a microplate reader (SpectraMax M2e, Molecular Devices Corporation, Sunnyvale, CA, USA) with excitation at 488nm and emission at 520nm (cutoff 515nm). Results are expressed as a ratio of fluorescence intensity of each dye in the bottom chamber (of each experimental group) to fluorescence intensity of the respective dye in the original sample (of the respective group) at the end of the experiment.
Statistical analysis
The comparison between two groups was done by student’s-t test. The null hypothesis was rejected if p<0.05 and considered statistically significant.
Results
Characterization of CUR-EXO
To characterize CUR-EXO, Western blot, AChE activity, and flow cytometry were used. Western blot analysis revealed TSG101 band in purified 1, 00,000 x g CUR-EXO pellet but not in CUR-supernatant (Fig. 1A). Since exosome vesicles typically exhibit AChE activity; therefore, we determined the AChE activity for CUR-EXO preparations. We got high AChE activity levels in CUR-EXO and the activity increased over the observation period of 30 min while in CUR-supernatant, devoid of exosomes, very low activity was found throughout the observation period (Fig. 1B). We further characterized CUR-EXO by using exosome-specific CD63 antibody using flow cytometry. The data showed CD63-specific peak in the flow chart as given in Fig. 1C. These results suggested presence of CUR-EXO in purified 1, 00,000 x g fraction.
Fig. 1. Characterization of CUR-EXO.
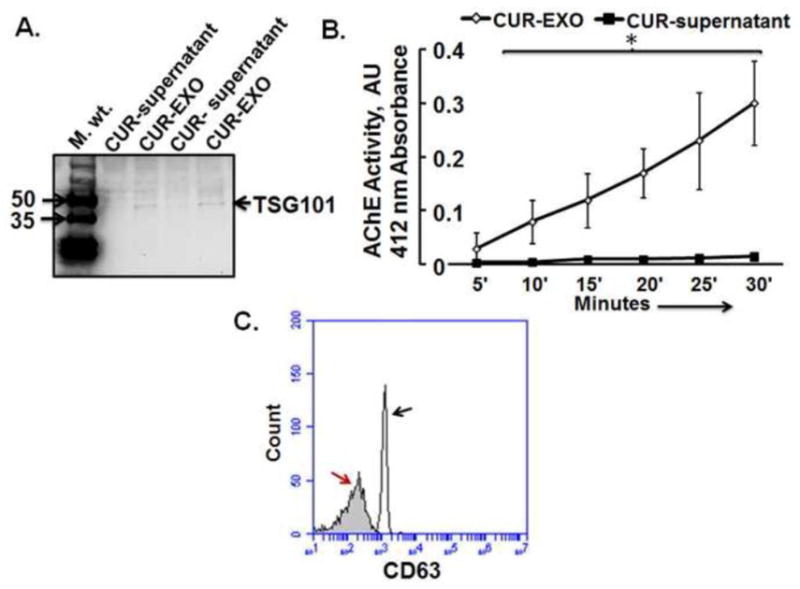
(A) Western blot analysis of CUR-EXO and CUR-supernatant fractions with anti-TSG101 antibody. (B) Line diagram for AChE activity in CUR-EXO pellet and CUR-supernatant. The OD was recorded at 419 nm at 5 min interval for the total time period of 30 min. Data represents mean ± SD, *p<0.05-versus control. (C) Flow cytometry detection of CUR-EXO captured on anti-CD63 coated latex beads. The exosome-bead complexes were immunostained against CD63 (open curve, black arrow) or their corresponding isotype control (filled curves, red arrow). Data is the depiction of the three independent experiments. Here, CUR-EXO, Curcumin-primed exosomes; TSG101, Tumor suppressive gene 101; CUR-supernatant, Supernatant collected after ultracentrifuging MBEC culture media at 1,00,000 X g (devoid of CUR-EXO).
CUR-EXO acquisition by ECs and its effect on oxidative stress
To address the effect of CUR-EXO, we first confirmed CUR-EXO acquisition by ECs, by labelling CUR-EXO with PKH67 fluorescent dye. As represented in fig. 2A, ECs incubated with labelled CUR-EXO showed green fluorescence dots as compared to control (Fig. 2A). These results suggest that CUR-EXOs are acquired by the ECs.
Fig. 2. CUR-EXO acquisition by the ECs and its effect on oxidative stress.
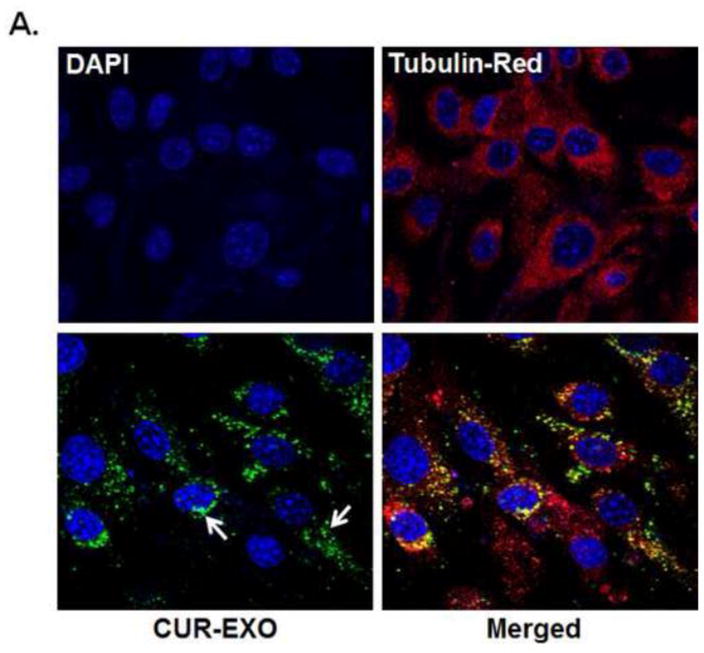
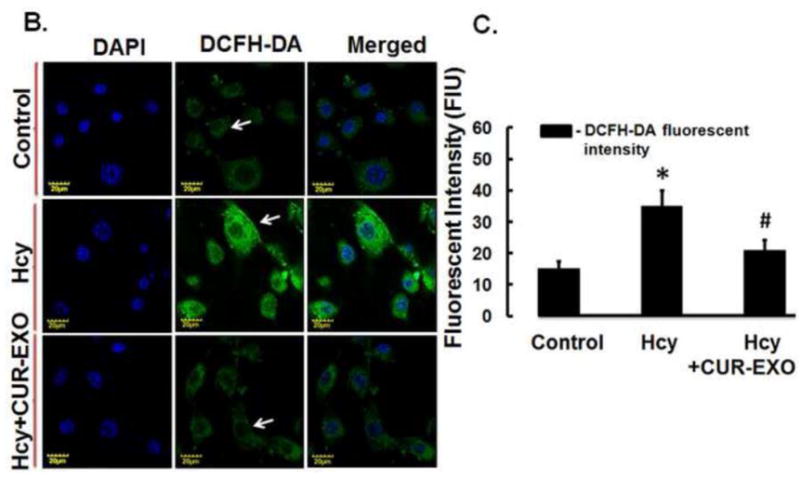
(A) Confocal images showing CUR-EXO acquisition by the ECs (bottom panel). CUR-EXOs were labelled with PKH67 (green dots; indicated with white arrow), ECs were treated with tubulin red (red) and nuclei were stained with DAPI (blue). However, the green fluorescent signal was not detected in ECs undelivered with CUR-EXO (top panel) (B) Confocal images depicting oxidative stress in control, Hcy, and Hcy-CUR-EXO treated cells using DCFH-DA fluorescent dye. Green color intensity is the representation of oxidative stress as indicated by white arrows. Nuclei are stained with DAPI (blue). (C) Bar graph representing intensity of DCFH-DA dye in ECs analysed with Image-Pro Plus 7.0 software. All Images were taken at X60 magnification, scale bar 20 μM. Data is expressed in arbitrary units. Data represents mean ± SD, *p<0.05-versus control, #p<0.05-versus Hcy-treated cells. The images and data are the representation of four independent experiments. Here, Hcy, homocysteine; CUR, curcumin; CUR-EXO-Curcumin-primed exosomes; DCFH-DA, Dichloro-dihydro-fluorescein diacetate (DCFH-DA); DAPI, 4′,6-diamidino-2-phenylindole; ECs, endothelial cells.
We next determined the effect of CUR-EXO on oxidative stress using DCFH-DA fluorescent dye. The confocal analysis showed increase in oxidative stress in Hcy-treated cells as high DCFH-DA fluorescence intensity was found in these cells as compared to control cells (Fig. 2B). On contrary, significant reduction in oxidative stress was observed in Hcy-CUR-EXO treated cells, when compared to Hcy-treated cells (Fig. 2B, 2C). The decrease in oxidative stress with CUR-EXO thereby indicates its anti-oxidative properties.
Effect of CUR-EXO on junction proteins and MMP-9 activity
To address the potential of CUR-EXO to overcome Hcy-induced ECs disruption, different concentrations (2, 5, 8, 10 μg) of CUR-EXO were introduced in Hcy-treated cells and protein expressions of junction proteins (occludin, claudin-5) were determined using Western blot. Significant low protein expressions of occludin and claudin-5 were observed in Hcy-treated cells as compared to control cells. On the other hand, CUR-EXO treatment alleviated the two protein levels in Hcy-treated cells (Fig. 3A, 3B).
Fig. 3. Effect of CUR-EXO on junction proteins and MMP-9 gelatin degradation activity.
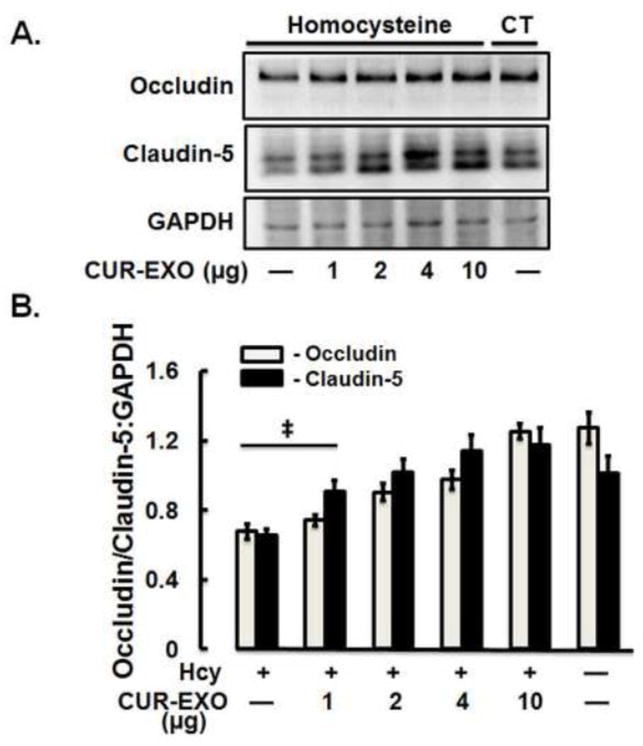
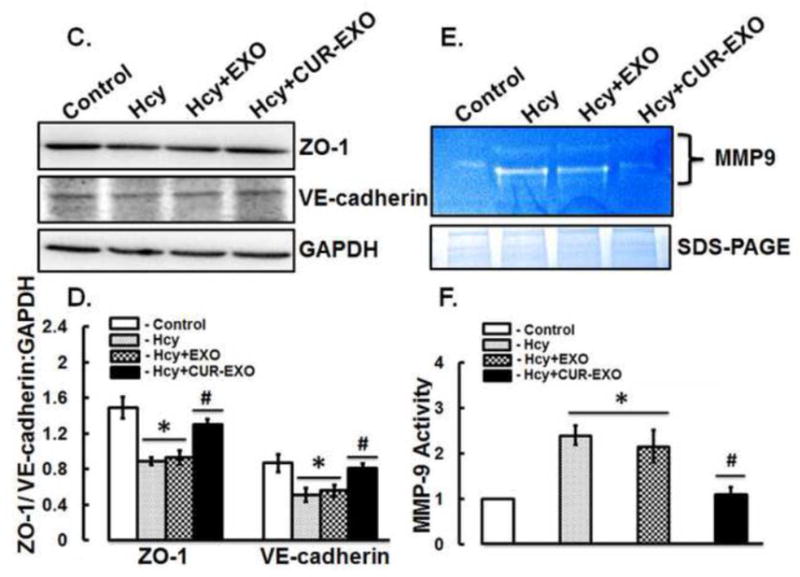
(A) Representative Western blot images showing expression levels of occludin, claudin -5 and GAPDH in control, Hcy, and Hcy-CUR-EXO treated cells. Different concentrations (μg) of CUR-EXO were used in Hcy-treated cells. (B) Bar diagram showing densitometry analysis of occludin and claudin-5 protein expressions normalized with GAPDH (C) Representative Western blot images showing protein expression levels of ZO-1, VE-cadherin and GAPDH in control, Hcy, Hcy-EXO, Hcy-CUR-EXO treated cells. 5μg concentration of EXO and CUR-EXO were used for the ECs treatment. (D) Bar graph showing densitometry analysis of ZO-1 and VE-cadherin protein expressions, normalized with GAPDH expression. (E) Representative zymograph image showing MMP-9 activity in control, Hcy, Hcy-EXO and Hcy-CUR-EXO treated cells. The EXO and CUR-EXO were used at 5μg concentration for treating ECs. Protein loading was shown by coomassie stained gel image given at the bottom of gelatin zymograph image. (F) Bar graph showing densitometry analysis of MMP-9 activity. All densitometry analysis was performed through image-lab software and values are expressed as mean ± SD. ‡p<0.05, *p<0.05-versus control, #p<0.05-versus Hcy-treated, and Hcy-EXO treated cells. Here, Hcy, homocysteine; CUR-EXO, curcumin-primed exosomes; EXO, exosomes MMP-9, matrix metalloproteinase-9.
In order to compare that the improvements of junction proteins are due to CUR-EXO, we compared the treatment effect of CUR-EXO with exosomes (EXO), derived from culture media of untreated cells, on the expression levels of TJs (ZO-1), and adherent junction (VE-cadherin) proteins. Western blot analysis revealed significant loss of ZO-1 and VE-cadherins in Hcy-treated cells when compared to control cells (Fig. 3C, 3D). Likewise, EXO treatment to Hcy-treated cells did not show any improvement in the protein expressions of ZO-1 and VE-cadherin. On contrary, Hcy-CUR-EXO cells showed significant improvement in the two protein expressions, as compared to Hcy-treated cells (Fig. 3C, 3D).
Furthermore, MMP-9 activity was determined using gelatin zymography assay. Gelatin zymography result depicted increase in MMP-9 activity in Hcy-treated and Hcy-EXO treated cells as compared to control cells. On the other side, Hcy-CUR-EXO treated cells represented less gelatin degradation activity as comparable to Hcy-treated cells (Fig. 3E, 3F). These results confirmed that CUR-EXO recovered the junction proteins and normalized the MMP-9 activity in Hcy-treated ECs to control cells.
Effect of CUR-EXO on ZO-1 expression and endothelial cell permeability
In the further sets of experiments, effect of CUR-EXO was observed on cellular expression of ZO-1 and EC layer permeability. The confocal analysis showed very low intensity of ZO-1 in Hcy-treated cells, as compared to control cells; however, intensity of ZO-1 was improved in Hcy-CUR-EXO treated cells (Fig. 4A).
Fig. 4. Effect of CUR-EXO in ZO-1 protein intensity and endothelial cell permeability.
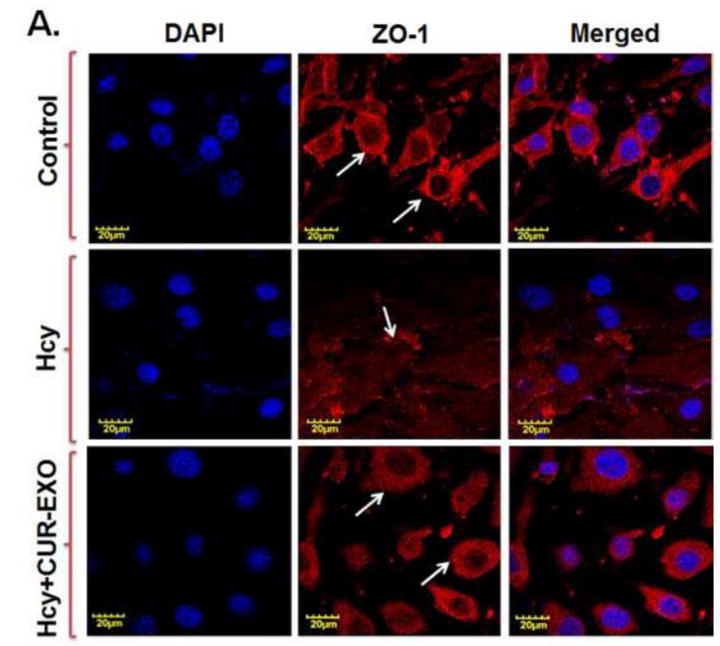
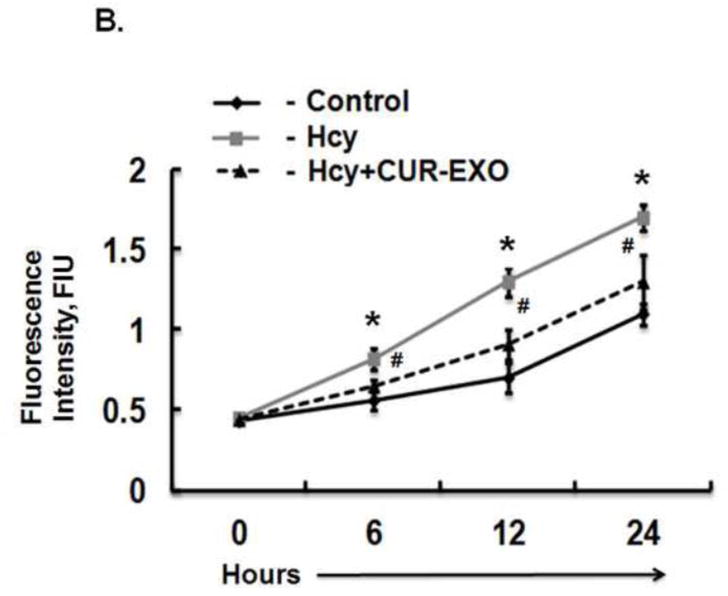
(A) Confocal images showing ZO-1 cellular expression levels (red) in control, Hcy, Hcy-CUR-EXO treated cells and indicated with white arrows. Nuclei are stained with DAPI (blue). All Images were taken at X60 magnification, scale bar 20 μM. (B) EC layer permeability was assessed with FITC-BSA leakage in control, Hcy, Hcy-CUR-EXO treated cells using transwell permeable supports thrice at 6h intervals for the total time period of 24 h. Data represents mean ± SD. *P<0.05 -versus control; #p<0.05-versus Hcy-treated cells. Here, Hcy, homocysteine; CUR-EXO, curcumin-primed exosomes; FITC-BSA, fluorescent isothiocyanate-bovine serum albumin; DAPI, 4′,6-diamidino-2-phenylindole; ECs, endothelial cells.
Permeability through ECs was determined with fluorescent probe (FITC-BSA) leakage assay at different time points. Hcy-treated cells showed increase in the leakage of FITC-BSA through ECs at all the observed time points (6, 12, 24 h) as compared to control cells. On the other side, Hcy-CUR-EXO treated cells showed significant decrease in leakage through ECs as compared to Hcy-treated cells (Fig. 4B). These results further confirmed the potential of CUR-EXO in amelioration of junction proteins and EC layer permeability.
Discussion
We studied the potential of curcumin-primed exosomes on ECs dysfunction by looking at their effect on junction proteins and ECs permeability. Exosomes carry specific biological information (protein, nucleic acid) from the tissue type they originate. Also, they possess potentials for cerebral disease therapy and potential to be used as biomarkers. Curcumin is an anti-oxidative, anti-inflammatory and neuroprotective agent. Accumulating evidences suggest curcumin not only induces exosome secretion but also increases its solubility, stability, and therapeutic potentials when encapsulated in exosomes (Canfran-Duque et al. 2013;Kulkarni and Dhir 2010;Sun et al. 2010;Zhuang et al. 2011). Hence in the present study, we prepared curcumin-primed exosomes and checked their potentials against oxidative stress, TJs, and ECs layer permeability.
Since the exosomes are nano-range vesicles, there have been different approaches to characterize its presence in biological fluids including culture conditioned media (Thery et al. 2006). We characterized CUR-EXO by Western blot, AChE activity and flow cytometry as described earlier (Kalani et al. 2013b;Stoorvogel et al. 2002;Thery et al. 2006). To confirm CUR-EXO acquisition by the ECs, we labelled exosomes with PKH67 fluorescent stain which specifically binds to exosomes. PKH labelling has been profoundly used to label exosomes in order to track their destinations (Fitzner et al. 2011;Zhuang et al. 2011). Increased levels of Hcy have been associated with increased permeability through ECs (Tyagi et al. 2007). Hence, we also considered Hcy-treated ECs as the model of BBB disruption. Previous studies have suggested the use of ECs as a convenient and useful model to study BBB function (Brown et al. 2007;Watanabe et al. 2013). We earlier established that Hcy causes redox stress which induces MMPs activation and increase in permeability (Tyagi et al. 2007). Therefore, in this study we checked CUR-EXO potentials to rescue these altered events.
Oxidative stress is the hallmark of pathological processes in brain ECs during HHcy (Abushik et al. 2013;Wu et al. 2013) which increases reactive oxygen species (ROS) (Kotamraju et al. 2000), and decreases endothelial nitric oxide (NO) bioavailability (Ryter and Choi 2002). On contrary, anti-oxidant therapy has been described to overcome the vascular remodeling and redox stress during endothelial damage (Tyagi et al. 2009). We found increase in oxidative stress in Hcy-treated cells which was significantly decreased with CUR-EXO treatment. This suggests anti-oxidative potential of CUR-EXO. Likewise the activity level of MMP-9, induced under HHcy, was also decreased with CUR-EXO treatment. It has also been well established that MMPs are induced in the brain ECs during Hcy-induced toxicity and lead to extracellular matrix remodeling (Kamat et al. 2013;Kumar et al. 2008;Shastry et al. 2006). The high activity of MMPs is known to disrupt BBB by eating up junction proteins that maintain BBB integrity (Kalani et al. 2013a;Valable et al. 2005).
Intercellular TJ protein complexes of the brain microvasculature limit the diffusion of the substances from the blood into the brain and provide cytoskeleton anchorage. ZO-1, ZO-2 and Occludin are proteins linked to actin cytoskeleton of ECs (Fanning et al. 1998;McCaffrey et al. 2009), while claudin-5 is a predominant claudin isoform, among other claudin isoforms, in TJs and its mRNA expression was found to be 600 times more as compared to other RNAs in brain ECs (Ohtsuki et al. 2008). Apart from TJs, adherent junctions are also important regulator of BBB permeability and attenuation of VE-cadherin, which is one of the important adherent junction proteins, showed increased monolayer permeability in culture cells, oedema and hemorrhage in in vivo model systems (Corada et al. 2001;Corada et al. 2002). These junction proteins are important to maintain selective barrier functions of ECs in brain vessels and therefore their loss affects BBB integrity. We observed that TJs are severely down-regulated under HHcy and treatment with CUR-EXO restored TJs expressions. Additionally, the EC layer permeability that increased with Hcy treatment was improved and became comparable to control using CUR-EXO. The mechanism that we believe for CUR-EXO to rescue ECs permeability is by decreasing oxidative stress which further decreased MMP-9 inpart by alleviating TJs-adherent junctions and ECs permeability.
In conclusion, our study determined the potential use of curcumin-primed exosomes in amelioration of junction proteins and ECs permeability. The beneficiary effect of CUR-EXO is believed to be exerted by maintaining redox homeostasis, lowering MMP-9 levels, and improving TJs that eventually improve ECs permeability. Since the exosomes carry biological information from the cells of their origin, we strongly believe that the alleviations of junction proteins maintaining ECs layer permeability is through the effective molecular contents in CUR-EXO imported to the cells by curcumin. Fig. 5 represents the overall hypothesis of the study.
Fig. 5. Hypothesis.
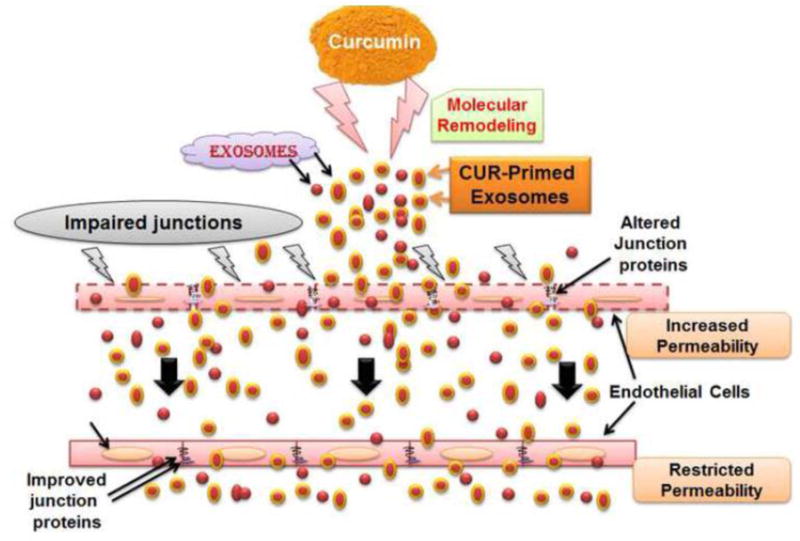
Overall hypothesis of the study showing treatment of curcumin-primed exosomes recovered junction proteins, adherent junction proteins and alleviated permeability of endothelial cells.
The results of the study also direct novel future therapies by preparing improved cellular and molecular conservatories via exosomes and use them to treat cerebral diseases, for which drug penetration and access is a challenge. However, the potential limitation of the study resided to resolve the molecular signalling via CUR-primed exosome.
Acknowledgments
This work was supported by National Institutes of Health grants HL107640-NT. The authors thank to Dr. Radha Munagala for her kind suggestions in this study.
Abbreviations
- Hcy
homocysteine
- HHcy
hyperhomocysteinemia
- CUR-EXO
curcumin-primed exosomes
- Exo
Exosomes
- BBB
blood-brain barrier
- TJs
tight junctions
- AChE
acetylcholinestrase
- ZO
Zona occludens-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declared no conflict of interest.
References
- Abushik PA, Niittykoski M, Giniatullina R, Shakirzyanova A, Bart G, Fayuk D, et al. The role of NMDA and mGluR5 receptors in calcium mobilization and neurotoxicity of homocysteine in trigeminal and cortical neurons and glial cells. J Neurochem. 2013 doi: 10.1111/jnc.12615. [DOI] [PubMed] [Google Scholar]
- Beard RS, Jr, Reynolds JJ, Bearden SE. Hyperhomocysteinemia increases permeability of the blood-brain barrier by NMDA receptor-dependent regulation of adherens and tight junctions. Blood. 2011;118:2007–14. doi: 10.1182/blood-2011-02-338269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–44. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Morris AP, O’Neil RG. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007;1130:17–30. doi: 10.1016/j.brainres.2006.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfran-Duque A, Pastor O, Quintana-Portillo R, Lerma M, de la Pena G, Martin-Hidalgo A, et al. Curcumin promotes exosomes/microvesicles secretion that attenuates lysosomal cholesterol traffic impairment. Mol Nutr Food Res. 2013 doi: 10.1002/mnfr.201300350. [DOI] [PubMed] [Google Scholar]
- Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, et al. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–84. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- Corada M, Zanetta L, Orsenigo F, Breviario F, Lampugnani MG, Bernasconi S, et al. A monoclonal antibody to vascular endothelial-cadherin inhibits tumor angiogenesis without side effects on endothelial permeability. Blood. 2002;100:905–11. doi: 10.1182/blood.v100.3.905. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD. The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49:143–55. [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–53. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Fitzner D, Schnaars M, van RD, Krishnamoorthy G, Dibaj P, Bakhti M, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–58. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- Kalani A, Kamat PK, Givvimani S, Brown K, Metreveli N, Tyagi SC, et al. Nutri-epigenetics Ameliorates Blood-Brain Barrier Damage and Neurodegeneration in Hyperhomocysteinemia: Role of Folic Acid. J Mol Neurosci. 2013a doi: 10.1007/s12031-013-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Mohan A, Godbole MM, Bhatia E, Gupta A, Sharma RK, et al. Wilm’s tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS One. 2013b;8:e60177. doi: 10.1371/journal.pone.0060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Tyagi A, Tyagi N. Exosomes: Mediators of Neurodegeneration, Neuroprotection and Therapeutics. Mol Neurobiol. 2013c doi: 10.1007/s12035-013-8544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat PK, Kalani A, Givvimani S, Sathnur PB, Tyagi SC, et al. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience. 2013;252:302–19. doi: 10.1016/j.neuroscience.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, et al. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107:591–93. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotamraju S, Konorev EA, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem. 2000;275:33585–92. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Dhir A. An overview of curcumin in neurological disorders. Indian J Pharm Sci. 2010;72:149–54. doi: 10.4103/0250-474X.65012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Tyagi N, Moshal KS, Sen U, Pushpakumar SB, Vacek T, et al. GABAA receptor agonist mitigates homocysteine-induced cerebrovascular remodeling in knockout mice. Brain Res. 2008;1221:147–53. doi: 10.1016/j.brainres.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Seo YH, Yang DJ, Kim KH, Park HW, Yuk HB, et al. Positive Vascular Remodeling in Culprit Coronary Lesion is Associated With Plaque Composition: An Intravascular Ultrasound-Virtual Histology Study. Korean Circ J. 2012;42:747–52. doi: 10.4070/kcj.2012.42.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC. Homocysteine causes cerebrovascular leakage in mice. Am J Physiol Heart Circ Physiol. 2006;290:H1206–H1213. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey G, Willis CL, Staatz WD, Nametz N, Quigley CA, Hom S, et al. Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem. 2009;110:58–71. doi: 10.1111/j.1471-4159.2009.06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradashvili N, Tyagi R, Lominadze D. A dual-tracer method for differentiating transendothelial transport from paracellular leakage in vivo and in vitro. Front Physiol. 2012;3:166. doi: 10.3389/fphys.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki S, Yamaguchi H, Katsukura Y, Asashima T, Terasaki T. mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J Neurochem. 2008;104:147–54. doi: 10.1111/j.1471-4159.2007.05008.x. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Choi AM. Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid Redox Signal. 2002;4:625–32. doi: 10.1089/15230860260220120. [DOI] [PubMed] [Google Scholar]
- Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002;115:2505–15. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- Shastry S, Tyagi N, Moshal KS, Lominadze D, Hayden MR, Tyagi SC. GABA receptors ameliorate Hcy-mediated integrin shedding and constrictive collagen remodeling in microvascular endothelial cells. Cell Biochem Biophys. 2006;45:157–65. doi: 10.1385/CBB:45:2:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–30. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–14. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3: Unit. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Moshal KS, Sen U, Vacek TP, Kumar M, Hughes WM, Jr, et al. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid Redox Signal. 2009;11:25–33. doi: 10.1089/ars.2008.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Moshal KS, Tyagi SC, Lominadze D. gamma-Aminbuturic acid A receptor mitigates homocysteine-induced endothelial cell permeability. Endothelium. 2007;14:315–23. doi: 10.1080/10623320701746164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Qipshidze N, Sen U, Rodriguez W, Ovechkin A, Tyagi SC. Cystathionine beta synthase gene dose dependent vascular remodeling in murine model of hyperhomocysteinemia. Int J Physiol Pathophysiol Pharmacol. 2011;3:210–22. [PMC free article] [PubMed] [Google Scholar]
- Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Dohgu S, Takata F, Nishioku T, Nakashima A, Futagami K, et al. Paracellular barrier and tight junction protein expression in the immortalized brain endothelial cell lines bEND.3, bEND.5 and mouse brain endothelial cell 4. Biol Pharm Bull. 2013;36:492–95. doi: 10.1248/bpb.b12-00915. [DOI] [PubMed] [Google Scholar]
- Wu S, Gao X, Yang S, Liu L, Ge B, Yang Q. Protective Effects of Cariporide on Endothelial Dysfunction Induced by Homocysteine. Pharmacology. 2013;92:303–09. doi: 10.1159/000356318. [DOI] [PubMed] [Google Scholar]
- Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–15. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–79. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]


