Abstract
Adolescence is a period of increased vulnerability to psychiatric illnesses such as addiction, mood disorders, and schizophrenia. Rats provide a useful animal model for investigating the differences in behavior and biology between adults and adolescents that stem from ongoing brain development. We developed the Cued Response Inhibition Task, or CRIT, to assess response inhibition and initiation processes by measuring the ability of rodents to withhold a response during an inhibitory cue and then to respond promptly after cue termination. We found no difference between adult and adolescent rats in the ability to appropriately inhibit a response during cue presentation. Adolescents, however, were unable to initiate a response as quickly as adults after cue termination. Further, we observed that this difference in responding was abolished after adolescent rats aged to adulthood with no additional training. In a separate experiment, adult and adolescent rats were trained in CRIT and then trained in another protocol in which the response inhibitory cue from CRIT was used as a Pavlovian cue predictive of reward. Adolescents demonstrated more reward-seeking behavior during the previously inhibitory Pavlovian cue than adults, indicative of greater behavioral flexibility. Taken together, these data suggest that, compared with adults, adolescent rats (a) are less able to initiate a response after response inhibition, (b) equally inhibit behavioral responses, and (c) are more adept at flexibly switching behavioral patterns. Furthermore, this study characterizes a task that is well suited for future pharmacological and electrophysiological investigations for assessing neuronal processing differences between adolescents and adults.
Keywords: adolescent, cognition, impulsivity, flexibility
Adolescence is associated with symptomatic onset of various psychiatric illnesses such as schizophrenia and drug addiction (Doremus-Fitzwater, Varlinskaya, & Spear, 2010; Kessler et al., 2005; Uhlhaas & Singer, 2011; Weinberger, 1987). Although this vulnerability may be due in part to life changes and social stressors, this period is characterized also by ongoing maturation of brain regions, including the prefrontal cortex and striatum (Casey, Jones, & Hare, 2008; Ernst, Pine, & Hardin, 2006; Sowell, Thompson, Holmes, Jernigan, & Toga, 1999; Sturman & Moghaddam, 2011a, 2012). Rodent models of adolescence can be utilized for mechanistic studies to delineate the impact of development on behavior while controlling social and environmental factors.
Several theories have proposed differences in cognitive processing between adolescents and adults. Adolescents are thought to attribute abnormally high salience to external cues and objects, which may promote enhanced novelty seeking (Spear, 2004; Stansfield & Kirstein, 2006). Furthermore, it has been demonstrated that reward processing differs between adolescents and adults, which may then affect subsequent expression of goal-directed behavior (Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Sturman & Moghaddam, 2011b). Both of these mechanisms offer explanations for some of the behavioral phenotypes that occur during adolescence, such as risk taking and impulsivity (Adriani & Laviola, 2003; Doremus-Fitzwater et al., 2010; Steinberg, 2005; Van Leijenhorst et al., 2010). However, it is technically challenging to uncover cognitive distinctions in support of these mechanisms, due to the brevity of the adolescent period in rodents (Spear, 2000), which precludes the use of intricate behavioral tasks. Although differences have been observed in a delayed alternation task (Koss, Franklin, & Juraska, 2011) and different aspects of impulsivity (Adriani & Laviola, 2003; Andrzejewski et al., 2011; Burton & Fletcher, 2012; Pinkston & Lamb, 2011), there remains a paucity of rodent tasks capturing complex aspects of cognition that reveal age-related differences. Uncovering differences between adult and adolescent rodents is critical for future mechanistic studies toward a better understanding of the neurobiological basis of adolescent vulnerabilities.
The aim of this study was to develop and characterize a rodent cognitive task that captured multiple aspects of cognition during adolescence. The Cued Response Inhibition Task, or CRIT, measured ability to withhold a previously reinforced response in the presence of a cue, then to respond quickly for reward following cue termination. CRIT also was modified to measure behavioral flexibility. Importantly, this task was designed not only to fit within the brief window of adolescence, but also to allow time for neurophysiological or pharmacological assessment following task acquisition in subsequent experiments. Our goal was to isolate elements of this task that reliably differ between adult and adolescent rats in order to provide behavioral correlates of the biological differences already observed between age groups. These data would allow for in-depth analysis of the specific aspects of adolescent brain development that influence behavior.
In Experiment 1, we trained adult and adolescent rats in CRIT and then compared behavior between groups. In Experiment 2, we tested adolescent rats in CRIT, and then allowed them to age to adulthood with no further training. After this aging period, we tested rats in CRIT a second time, allowing for a within-subjects comparison between the adolescent and adult period. In Experiment 3, we trained adult and adolescent rats in CRIT and then retrained rats in discriminative Pavlovian conditioning using the inhibitory cue from CRIT as the conditioned stimulus (CS) associated with reward. We then measured appetitive responding during this stimulus. This tested the ability to seek reward during a cue that had previously acted as a response inhibitor and, therefore, provided a novel assessment of behavioral flexibility.
Method
Subjects
Male adult (aged P60+, n = 36) and adolescent (aged prenatal day 28 to 48, n = 36) Sprague–Dawley rats were used for these experiments. All subjects were pair-housed with a similarly aged animal on a 12-hour reverse dark–light cycle (lights on at 7:00 p.m.). All rats were handled, habituated to operant boxes, and food deprived before behavioral training. We food deprived adolescents according to a schedule previously demonstrated to allow for weight gain but produce sufficient motivation in appetitive operant tasks without causing changes in learning rate (Sturman, Mandell, & Moghaddam, 2010). Adolescents were fed 5 g/cage (2 subjects per cage) on Day 1 and 8 g/cage on Day 2, followed by daily maintenance of 10 g/cage. Adults were given 15 g/cage daily.
Experiment 1: Comparing Response Inhibition and Cue-Based Reaction Time (RT) Between Adult and Adolescent Rats Using the CRIT
Shaping
Eighteen adult and 18 adolescent rats were utilized for this experiment, with age groups counterbalanced across two separate cohorts. Day 1 of shaping consisted of a 30-min magazine training session, with a single 45-mg food pellet reinforcer (Bioserv, Frenchtown, NJ) pseudorandomly delivered into the food trough at intervals of 75 ± 45 s. Day 2 consisted of training rats to perform a single nose poke when a port was illuminated, which was followed by immediate pellet delivery. The nose-poke port light was extinguished immediately after the nose poke was performed, and the food trough remained illuminated until rats collected the food. This training session culminated after rats completed 75 reinforced trials. On the final day of shaping, each nose poke into the illuminated port was again reinforced with a pellet and food trough illumination; however, this phase was divided into trials in which the nose-poke port only remained illuminated for a 10-s response period before progressing to an intertrial interval (intertribal interval [ITI]) during which reinforcement was not available. This phase of training was concluded also after 75 reinforced trials.
CRIT
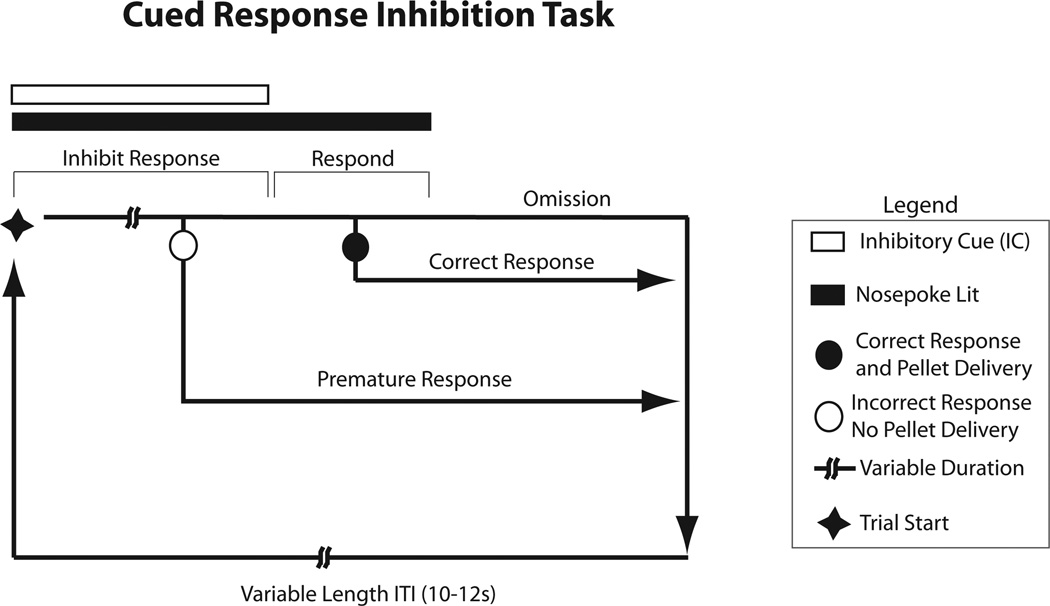
For a task schematic, see Figure 1. Each trial in the 60-min CRIT session began with simultaneous illumination of the nose-poke port (during which nose-poke responses had previously been reinforced) and an inhibitory cue (tone). During this “response inhibitory period,” a nose poke into the port resulted in termination of all cues and immediate nonreinforced entry into a 10- to 12-s ITI. The duration of this response inhibitory period varied pseudorandomly on a trial-by-trial basis (randomly selected length of 5, 10, 20, or 30 s). The variable length of the response inhibitory period required the rats to attend to the cue until offset rather than anticipating cue length and responding accordingly. Responses during this period were considered failure to appropriately withhold an action; these trials were classified as “incorrect trials.” After the inhibitory cue terminated, the nose-poke port cue remained illuminated for a 10-s period. If the rat responded with a nose poke during this “response period,” these trials were classified as “correct trials” and reinforced with pellet delivery, offset of the port light, and illumination of the food trough light. After the rat entered the food trough to collect the pellet, the trough light was extinguished and a 10- to 12-s ITI was initiated. A trial in which the subject failed to respond during the 10-s response period was classified as an “omission trial” and resulted in termination of the cue and entry into the 10- to 12-s ITI. Thus, three different types of trial responses were tabulated during the CRIT: incorrect, correct, and omission.
Figure 1.
Schematic detailing the Cued Response Inhibition Task (CRIT). Each trial begins with simultaneous presentation of the nose-poke light and an inhibitory cue (tone), which varies in duration from 5 to 30 s. During this response inhibitory period, a nose-poke response results in termination of all cues and immediate progression to the intertribal interval (ITI). After the response inhibitory cue, rats have a 5-s response period during which responses are reinforced with a pellet. The ITI begins after the rat enters the food trough to collect the pellet. There are three possible trial outcomes: correct responses, premature responses, and omissions. Both premature responses and omissions send the trial directly to the ITI.
After 7 days of training, the response period was shortened from 10 s to 5 s to increase task difficulty. Training continued until performance achieved stability, which was determined as a lack of repeated measures effect in inhibition ratio for each age group over the final 3 days of training. In order to control for total responses, a response inhibition ratio was calculated as correct trials/incorrect trials, with higher numbers indicative of greater ability to appropriately inhibit a response. Performance was calculated on the final 3 days of CRIT training and compared between adolescent and adult rats using a two-way ANOVA with age group as the between factor and day as the within factor.
Experiment 2: Within-Subjects Comparison of Response Inhibition and Cue-Based RT
Following completion of Experiment 1, the second cohort of 20 rats (10 adults, 10 adolescents) was taken off of food deprivation and kept in the animal colony with no behavioral testing for 21 days. This length was sufficient for the adolescent rats to reach adulthood prior to further testing (P70). After this, rats were given 4 days of food deprivation, then retested in CRIT. Subjects were given 1 day of baseline training to re-establish task performance, then 3 days of additional testing. CRIT performance was compared between groups both prior to and after the 21-day break to test if rats underwent behavioral changes after aging from adolescence to adulthood.
Experiment 3: Comparing Behavioral Flexibility in Adolescent and Adult Rats
A third group of 20 rats (10 adults, 10 adolescents) was trained to acquire CRIT using the same methodology as Experiment 1. After task acquisition, rats were trained in a discriminative Pavlovian conditioning protocol. Rats were given 100 trials per session using the (formerly) response inhibitory tone from CRIT as a 10-s CS (CS+), with a single pellet delivered at CS+ termination. A second stimulus (CS−)—illumination of light positioned on the wall opposite the food trough—terminated after 10 s without pellet delivery. The CS+ and CS− were presented pseudorandomly such that neither occurred more than twice consecutively, with an ITI of 30 ± 10 s between trials. Each session was 30 min long, and rats were tested for 10 consecutive days. This test determined whether adult and adolescent rats differed in behavioral flexibility, as defined by the propensity to perform an appetitive response to a cue that had previously acquired response inhibitory properties. Learning in the task was assessed using a measure of discriminative responding (Harmer & Phillips, 1998; Simon, Mendez, & Setlow, 2009; Simon & Setlow, 2006), which was the mean % time in food trough during the CS+ divided by mean % time in food trough during CS+ plus % time in food cup during the CS − (CS+/[CS+ +CS−]). A value of 0.5 represented equal time spent in the food trough during the CS+ and CS− periods (i.e., − no evidence of conditioned responding to the CS+). Data were compared between groups using a two-way ANOVA with each group and session serving as factors.
As a control experiment, a separate group of adolescent (n = 8) and adult (n = 8) rats were trained in an identical Pavlovian conditioning paradigm. These rats had no prior exposure to any task. These cues were not counterbalanced in order to replicate the parameters of the previous behavioral flexibility experiment as effectively as possible. After 8 days of conditioning, the predictive values of the cues was reversed, with the light acting as a CS+ (predicting pellet delivery) and the tone as a CS−. This control task allowed us to assess whether there were baseline differences in Pavlovian learning between adolescent and adult rats.
Results
Experiment 1: Comparing Response Inhibition and Response Initiation Between Adult and Adolescent Rats Using the CRIT
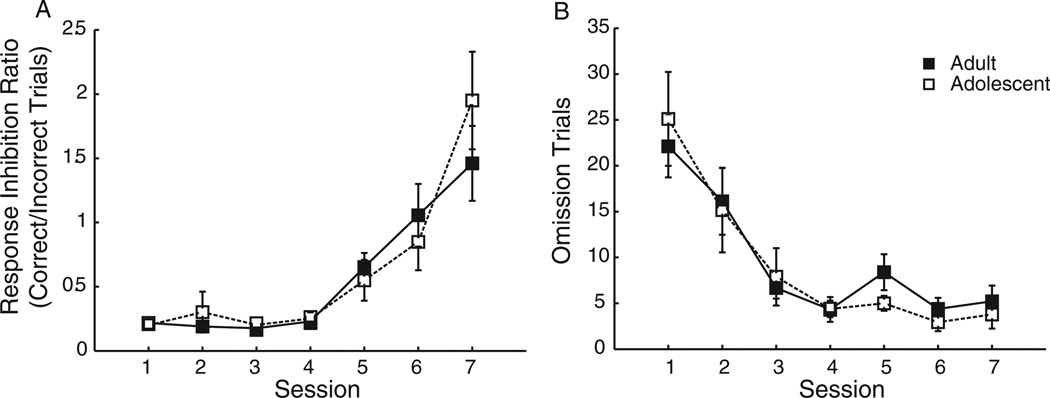
Because 4 of 36 rats (2 adolescent, 2 adult) experienced feeder jams that affected performance on at least one of these three sessions, these rats were removed from subsequent analyses. All rats demonstrated improved performance over the first 7 days of training in the simpler, 10-s response period CRIT in both response inhibition ratio, F(6, 204) = 22.98, p < .01 (Figure 2A) and omissions, F(6, 204) = 22.16, p = .01 (Figure 2B). There was no difference between adult and adolescent rats in acquisition of either response inhibition ratio, F(1, 31) =.20, p = .20, or omissions, F(1, 31) = .03, p = .86. Following the shift to the 5-s response period, CRIT performance was analyzed across the final 3 days of testing to probe for differences in behavior between adult and adolescent rats (aged P38 to P40). There was no difference between groups in the ability to withhold a response, as assessed by response inhibition ratio, F(1, 31) = .20, p = .66, and total incorrect trials, F(1, 31) = 1.27, p = .27 (Figure 3A). Omission trials occurred more frequently in adolescent than adult rats, F(1, 31) = 14.18, p < .01 (Figure 3B). Accordingly, there was a greater latency to respond during the 5-s response period in adolescent versus adult rats, F(1, 31) = 14.69, p < .01. Thus, during the response inhibition period when the cue was presented, adolescent rats were able to withhold a prepotent response as well as the adults. They, however, demonstrated reduced ability to initiate a response during the response period following termination of a cue.
Figure 2.
During initial acquisition of the Cued Response Inhibition Task (CRIT) (using a 10-s response window), there was no difference between adult and adolescent rats in acquisition of either (A) response inhibition, as assessed by response inhibition ratio (correct/incorrect trials), or (B) omission trials.
Figure 3.
(A) Following the shift to the more challenging, 5-s response period version of the Cued Response Inhibition Task (CRIT), there were still no age-related differences in response inhibition. (B) Adolescent rats were less able to respond quickly for reward following inhibitory cue termination, as evidenced by the increased number of omitted responses. Results are displayed from the final 3 days of CRIT training. Displayed: Means and ± SEM.
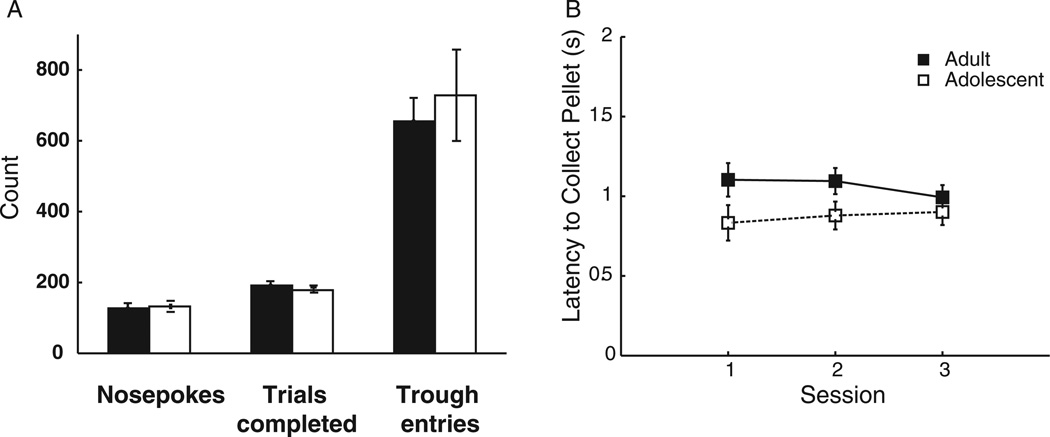
There were no differences between age groups in total completed trials (correct + incorrect trials) or total pokes into the nose-poke port (all ps > .72; Figure 4A), indicating that increased omission trials in adolescent rats compared with adults were not resulting from an inability to learn the task or the salience of the nose-poke response. There also was no difference between groups in terms of total pokes into the food trough, F(1, 31) = .13, p = .72 (Figure 4A) or latency to collect the pellet following delivery, F(1, 31) = .18, p = .67 (Figure 4B), suggesting that the difference in omissions was not a result of differences in motivation between groups. Further, during shaping, in which a less demanding version of CRIT was performed (with a 10-s rather than a 5-s rewarded response period), there was no difference between adults and adolescents in omission trials, F(1, 31) = 1.86, p = .18 (Figure 2B). This indicated that differences in response initiation only manifest when the cognitive demand of CRIT is increased, providing further evidence that the omissions were not related to motivational differences between groups. Therefore, it seems likely that the increase in omitted trials observed during adolescence is a result of either (a) inability to adequately sustain attention to the task during the cue, which would be required for a prompt instrumental response after cue termination, or (b) inability to quickly initiate a response following a shift from “no-go” to “go” response state.
Figure 4.
(A) There were no age-related differences in total nose pokes into the port, trials completed, or trough entries. (B) There was no difference in reaction time to food delivery.
Experiment 2: Within-Subjects Comparison of Response Inhibition and Cue-Based RT
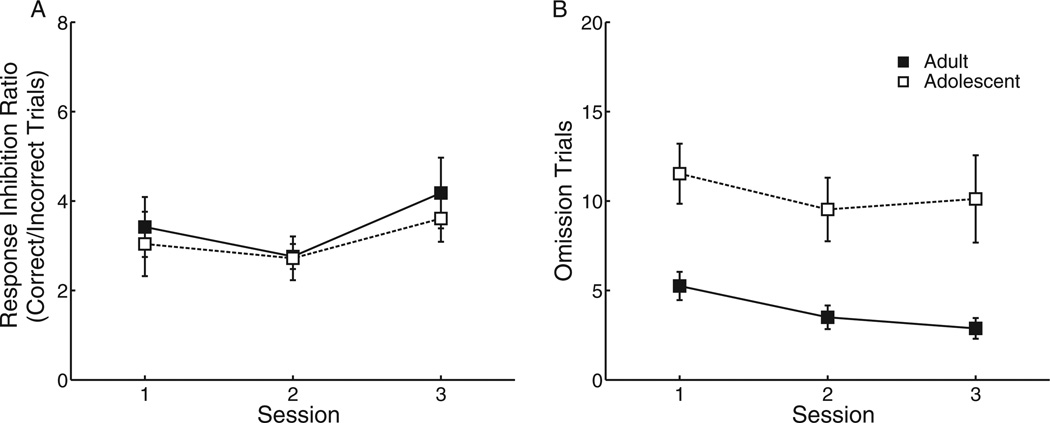
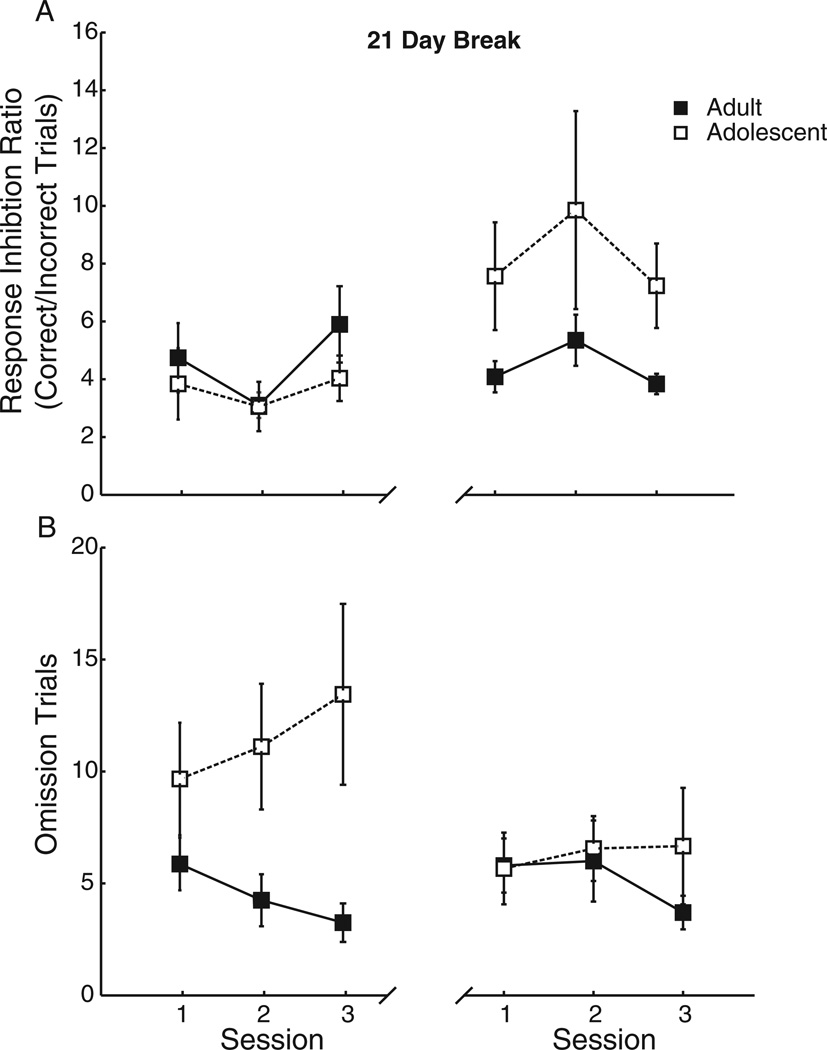
Rats were again trained in CRIT during either adolescence or adulthood. Two rats (1 adolescent, 1 adult) were removed from analyses due to feeder jams. Consistent with the results in Experiment 1, there was no difference in response inhibition ratio between adolescents and adults, F(1, 15) = .58, p = .46 (Figure 5A). Furthermore, adolescents had more omission trials than adults across the final 3 days of testing (adolescents, age 38 to 40, F[1, 15] = 5.67, p <.05; Figure 5B), and this was related to increased latency to respond during the rewarded response period, F(1, 15) = 9.15, p < .01. Both adolescent and adult rats were tested again in CRIT after 21 days with no additional behavioral training; this break was sufficient for adolescent rats to age to adulthood. Following this break period, the difference in omissions between groups was abolished, as the rats that began training during adolescence were equivalent in performance to the group of rats that began training in adulthood, F(1, 15) = .15, p = .70 (Figure 5B). There was no significant difference between groups in response inhibition ratio following the break, but there was a trend such that rats that began training during adolescence were better able to withhold responses than rats that began in adulthood, F(1, 15) = 3.70, p = .07 (Figure 5A). These data suggest (a) normal aging from adolescence to adulthood is characterized by an increased ability to initiate a response following inhibitory cue termination, and (b) rats trend toward performing a response inhibition task more effectively when training begins during adolescence versus in adulthood.
Figure 5.
(A) There was no difference between age groups in response inhibition. However, after a 21-day training-free period, allowing the adolescents to age to adulthood, the group that began training as adolescents showed a nonsignificant trend toward improved performance compared with the group that began in adulthood. (B) There were more omission trials during adolescence than during adulthood. After 21 days without training, this group difference was abolished. Data displayed are from the final three sessions of training (left), then the first three sessions after the break (right). Displayed: Means and ± SEM.
Experiment 3: Comparing Behavioral Flexibility in Adolescent and Adult Rats
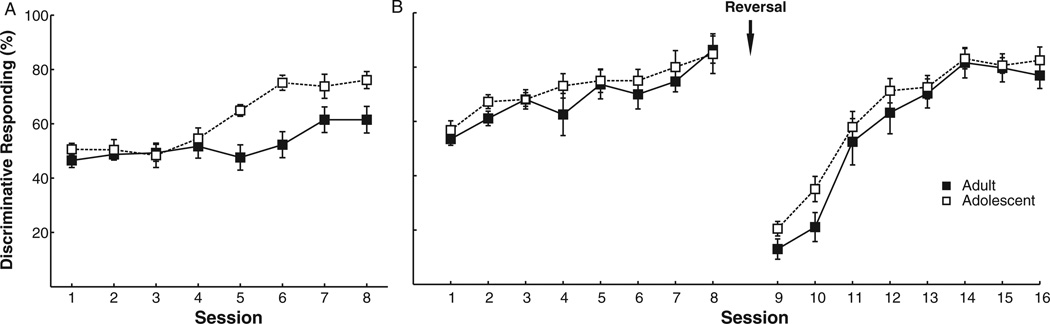
Following training in CRIT, a group of adolescent and adult rats were trained in discriminative Pavlovian conditioning using the same tone that served as the response inhibitory cue in the CRIT as a CS+. Across 8 days of conditioning, repeated measures ANOVA revealed that both adults and adolescents showed increased discriminative conditioning toward the CS+ (ps < .01); however, a Group × Day ANOVA revealed that adolescent rats demonstrated greater levels of discriminative conditioning than adults (between subjects, F[1, 18] = 8.31, p = .01; interaction, F[7, 126] = 3.62, p < .01; Figure 6A). Individual t tests revealed that this difference emerged after Session 5 (Sessions 1 through 4, all ps > .25; Sessions 5, 6, and 8, all ps < .01; Session 7, p = .07). Thus, adolescent rats demonstrated stronger appetitive conditioning to a previously inhibitory cue than adult rats, likely indicative of increased behavioral flexibility. It also is noteworthy that, during CRIT performance prior to discriminative conditioning, there was no difference between groups in ability to inhibit a response to the cue, F(1, 18) = .02, p = .88. This indicated that the difference between groups in ability to attribute salience to the cue during discriminative conditioning was not a result of group differences in the cue’s acquired inhibitory properties.
Figure 6.
(A) Following Cued Response Inhibition Task (CRIT) training, rats performed right sessions of discriminative Pavlovian conditioning using the previously response inhibitory cue as a CS+. Adolescents demonstrated more reward-seeking behavior during the cue than adults. (B) A separate group of rats were trained in discriminative Pavlovian conditioning with no previous cue exposure. There was no difference in reward seeking between groups and no difference in reward seeking following stimulus reversal. The y-axis indicates discriminative responding, which was calculated as time in food trough during the conditional stimulus (CS+) divided by both time in food trough during the CS+ and CS− (CS+/[CS+ + CS−). Displayed: Means and ± SEM.
It is possible that this increased approach behavior during the CS+ was a result of adolescent rats having a higher predisposition toward goal-tracking behavior (defined as approaching the site of reward delivery during periods of reward expectation). To control for this, a separate group of adolescent (n = 8) and adult (n = 8) rats were trained in Pavlovian conditioning with no previous training experience. After 8 days of training, no differences were found between groups in discriminative responding, F(1, 14) = 1.10, p = .31 (Figure 6B). Furthermore, when the predictive values of the CS+ and CS− were reversed for 8 days (with the light predicting a food pellet and the tone predicting no outcome), again there was no difference between groups in discriminative conditioning (between subjects, F[1, 14] = 3.02, p = .10; Group × Day interaction, F[7, 98] = .45, p = .87; Figure 6B). Thus, the difference in approach observed between adolescent and adult rats in the first portion of Experiment 3 was not a result of increased propensity to goal track by adolescent rats or a general impairment in reversal learning. Rather, adolescent rats only demonstrated greater behavioral flexibility than adults when the CS+ previously had response inhibitory properties due to negative consequences (i.e., withholding reinforcement).
Discussion
We designed a task to isolate behavioral differences between adult and adolescent rats. The CRIT measured response inhibition and the ability to initiate a response following inhibition, and could be modified to assess behavioral flexibility. We found that adolescents, compared with adults, demonstrate a reduced ability to initiate a response after termination of an inhibitory cue. This effect was abolished when the adolescent rats aged to adulthood. We found no difference between age groups in ability to inhibit a previously reinforced response during presentation of a response inhibitory cue. Finally, we found that adolescent rats had a greater ability to acquire an appetitive response to a cue that had previously acted as a signal for inhibiting an instrumental response, suggesting that adolescent rats are more effective at flexibly encoding these opposing relationships.
Increased Omission Trials During Adolescence
Adolescent rats performed more omission trials than adult rats in CRIT. We also found that aging from adolescence to adulthood reduced the number of response omissions. This finding is important because simply allowing normal development processes to proceed, without additional training or exposure to the task environment, improved this behavioral pattern. As there was no manipulation of the subjects during this aging period, we assert that the pattern of increased omissions seen in adolescent rats is the product of a neural circuitry that is not yet fully matured.
We hypothesize that the difference in omission trials likely is the result of either or both of two possibilities: adolescents being less able to shift from a response inhibition to a response state (i.e., selecting and initiating a behavioral response) or adolescents being less able to attend to the offset of response inhibitory cues than adults. The first possibility, response selection and initiation, depends upon the integrity of striatal circuits. “Go” response latency in the rat stop signal RT task (SSRT) is increased by lesions of dorsal striatum or D2 receptor blockade in dorsal striatum (Eagle & Robbins, 2003; Eagle et al., 2011). Previous work has shown that neural activity in dorsal striatum during an instrumental task differs between adults and adolescents even when behavior is consistent between groups (Sturman & Moghaddam, 2012). Additionally, reduced dopamine receptor availability has been observed in the dorsal striatum but not the nucleus accumbens of adolescents (Teicher, Andersen, & Hostetter, 1995). Therefore, the increase in omission trials and increase in latency to respond during the rewarded response period in adolescent rats may be related to immaturity of dorsal striatum activity in adolescents, possibly resulting from reduced dopamine transmission. In agreement with this mechanism, our preliminary microdialysis and protein analyses suggest that presynaptic indices of dopamine activity are lower in the dorsal striatum of adolescents compared with adults (Matthews & Moghaddam, 2011). However, it should be noted that response initiation during CRIT differs from that observed during “go” trials during SSRT. During CRIT, response initiation occurs following a period of response inhibition, whereas response initiation in SSRT precedes response inhibition. Therefore, more research is necessary to sufficiently demonstrate that response initiation in CRIT is modulated by dopaminergic transmission in dorsal striatum.
A second explanation for the difference in omissions may be attenuated ability to sustain attention to the duration of the inhibitory cue in adolescent rats. The adolescent prefrontal cortex undergoes development throughout adolescence (Casey, Jones, & Hare, 2008; Ernst, Pine, & Hardin, 2006; Geier et al., 2010; Sowell, Trauner, Gamst, & Jernigan, 2002), and previous work has demonstrated abnormal reward-related activity in the adolescent prefrontal cortex during learning and performance of an instrumental task (Sturman & Moghaddam, 2011b). The prefrontal cortex has been strongly implicated in sustained attention (Cohen & Maunsell, 2010; Miller & Cohen, 2001; Posner & Petersen, 1990; Totah, Jackson, & Moghaddam, 2012; Totah, Kim, Homayoun, & Moghaddam, 2009). Additionally, the presence of reward has been associated with greater activation of brain regions associated with top-down attention in adult compared with adolescent humans, which suggests greater integration between motivational and executive function networks in adulthood (Smith, Halari, Giampetro, Brammer, & Rubia 2011). As attention is required for efficient response initiation following cue termination in CRIT, it is possible that different patterns of activity in the prefrontal cortex and other structures implicated in attention during adolescence are related to the response initiation during CRIT. It also is important to note that adolescent rats perform more exploratory behaviors than adults (Spear, 2000), and are more likely to perform task-irrelevant behaviors during an instrumental task (Sturman et al., 2010), which also could be indicative of inability to sustain attention during an instrumental task.
The difference in omission trials did not appear to be a result of reduced learning of task contingencies in adolescents, as adults and adolescents both demonstrated an equal number of total trials completed and total nose-poke responses. The difference in omissions also was likely not a result of reduced motivation in adolescent rats compared with adults. First, there was no significant difference in total time spent in the food trough or food-trough entries between groups. Second, during task acquisition, in which rats performed a less demanding version of the task (with a 10-s rewarded response period), there was no difference in omissions between groups. As age-related differences in omissions only occurred with a shortened response period, these differences were likely a result of increased task difficulty rather than general motivational factors. Finally, there was no age-related difference in latency to collect pellets upon delivery, a measure shown to be reflective of reward value (Holland & Straub, 1979; Sage & Knowlton, 2000). Similar latencies to collect the reward also indicated that the increased omission trials during adolescence were not a result of general impairments in RT to salient stimuli.
Cued Response Inhibition Was Similar in Adolescents and Adults
We found no difference between adolescent and adult rats in response inhibition. The inability to withhold a previously reinforced response is termed impulsive action (Evenden, 1999; Winstanley, Eagle, & Robbins, 2006), and has been proposed to be greater in adolescents than adults (Romer, 2010; Spear, 2000; Whelan et al., 2012). Indeed, rat studies have shown increased impulsive action in adolescents (Andrzejewski et al., 2011; Burton & Fletcher, 2012). However, the nature of the impulsive behavior assessed in these studies differed from the current study. Andrzejewski et al. (2011) used the differential reinforcement of low rates of behavior (DRL) schedule to measure response inhibition in adolescent rats, and adolescents were less able to withhold a response than adults. However, because the DRL required the rats to withhold a response for a fixed interval, it is difficult to distinguish whether this age-related difference was a result of increased impulsive action or inaccurate assessment of the passage of time (Cheng, MacDonald, & Meck, 2006). Indeed, functional brain activity associated with temporal processing undergoes maturation throughout adolescence (Smith, Giampietro, et al., 2011). The CRIT used a response inhibition period of variable duration, rendering it impossible for subjects to determine when to respond, based on a mental representation of timing. A second study by Burton and Fletcher (2012) utilized a simplified version of the five-choice serial RT task (limiting it to two choices). This study also revealed increased impulsive action/reduced response inhibition in adolescent rats. However, this study differed from the current study because during CRIT, response inhibition is governed by the presence of a cue, whereas in Burton and Fletcher (2012) response inhibition was required during cue anticipation. In other words, CRIT measured whether adolescents were able to follow an environmental cue that signaled response suppression rather than an internal rule during the absence of a cue. Collectively, these data suggest adolescent rats are able to inhibit impulsive responses when an explicit environmental cue guides behavior (the current study), but demonstrate increased impulsivity when behavior is guided only by internal representations (Andrzejewski et al., 2011; Burton & Fletcher, 2012). To the best of our knowledge, our data provide the first evidence of this important distinction in the literature.
We also observed that, when rats acquired CRIT during adolescence, they showed improved ability to inhibit a response after a 21-day break period, whereas rats that began training as adults maintained the same level of performance after this break. These data, however, are somewhat noisy, as the effect does not achieve statistical significance (p = .07). Thus, this may provide preliminary evidence that learning during adolescence may lead to more effective performance in comparison with learning starting later in life, but further research is necessary to confirm this phenomenon.
Improved Behavioral Flexibility During Adolescence
We found that adolescent rats demonstrated enhanced behavioral flexibility compared with adults. This was determined by shifting the response inhibitory cue in CRIT to a Pavlovian CS, then measuring conditioned responding during presentation of that CS. Indeed, adolescent rats demonstrated greater acquisition of the cue as a CS, as assessed by increased reward approach behavior (time spent in food trough). The effect was not a result of adolescents learning Pavlovian associations more quickly or being more likely to goal track than adults. When trained in Pavlovian conditioning with no previous behavioral history, adults and adolescents acquired conditioned approach similarly. These data are consistent with previous data showing that, despite differences in propensity to approach and contact the CS (sign-tracking), adult and adolescent rats acquire goal tracking at a similar rate (Doremus-Fitzwater & Spear, 2011). Interestingly, when adult and adolescent rats were tested in a traditional reversal learning task, there was no significant difference in acquisition between groups. Thus, the age-related difference in behavioral flexibility did not reflect a general difference in the ability to shift responding from one discriminatory cue to another. Rather, this difference in response flexibility applied to situations in which the CS previously was associated with negative outcomes in an instrumental context. It should be noted that previous data have revealed improved performance on a reversal task in juvenile mice compared with adults (Johnson & Wilbrecht, 2011). However, these mice were only 3 days beyond the weaning period, whereas the rats in the current study were tested during late adolescence. Additionally, Johnson and Wilbrecht measured instrumental rather than Pavlovian reversal, and these learning processes are mechanistically distinct (Balleine & O’Doherty, 2010; Grau & Joynes, 2005; Wassum, Ostlund, Balleine, & Maidment, 2011). The current study highlights a potentially important difference between adolescent and adult rats: Adolescent rats may less readily generalize the predictive nature of environmental stimuli that have previously been experienced during instrumental tasks, and may encode these contextual relationships in a more flexible fashion.
This finding is of particular interest because adolescent research has often focused on seemingly maladaptive behavioral tendencies (Adriani & Laviola, 2003; Burton & Fletcher, 2012; Spear, 2011). However, enhanced behavioral flexibility is suggested to be an advantageous characteristic (Clarke, Dalley, Crofts, Robbins, & Roberts, 2004; Floresco & Magyar, 2006; Schoenbaum, Nugent, Saddoris, & Gallagher, 2002). Therefore, it should be noted that, much like during aging later in life (Simon et al., 2010), not all the cognitive consequences of development are negative. Rather, the adolescent brain may be better suited to certain forms of learning (Johnson & Wilbrecht, 2011; Qin et al., 2004), particularly learning that occurs in uncertain or changing environments.
Because differences between adult and adolescent rats have been observed in the dorsal striatum (Matthews, Torres, & Moghaddam, 2011; Sturman & Moghaddam, 2012; Teicher, Andersen, & Hostetter, 1995), it seems likely that differences in habit learning as defined by responding in which the value of the outcome exerts less of a bias on response selection (Dickinson, 1985; Poldrack & Packard, 2003) exists between age groups. Indeed, a stronger propensity to form habits has been associated with vulnerability to drug addiction (Everitt & Robbins, 2005; Gerdeman, Partridge, Lupica, & Lovinger, 2003; Porrino, Lyons, Smith, Daunais, & Nader, 2004). However, the data here suggest that, at least under certain conditions, adolescents demonstrate greater behavioral flexibility than adults. It is possible that agerelated differences in the dorsal striatum are manifested as reduced ability to shift from goal-directed to habitual responding, leading to less rigid behavior in adolescents.
Conclusions
We found that adolescents, compared with adults, demonstrate reduced ability to initiate a response quickly after termination of a response inhibitory cue. This appeared to be a function of age-related differences in attention or action selection or initiation rather than motivation. Importantly, this difference disappeared after rats aged into adulthood. We also found that adolescents and adults perform comparably on a measure of response inhibition. Finally, adolescents showed an increased ability to acquire a formerly response inhibitory cue as a CS compared with adults, likely a result of enhanced behavioral flexibility. We propose that this difference in behavioral flexibility may be related to previously observed differences between adolescent and adult dorsal striatum activity (Matthews, Torres, & Moghaddam, 2011; Sturman & Moghaddam, 2012). These results demonstrate differences in adult and adolescent rat behavior using a task ideally suited for pharmacological and electrophysiological investigation. This task can be utilized for assessment of how brain function and morphology contribute to the complex behavioral patterns observed during adolescence, a period associated with high vulnerability to the development or onset of psychiatric disorders.
Acknowledgments
This work was supported by R37 MH48404 (Bita Moghaddam) and T32 DA031111 (Nicholas W. Simon, Jesse Wood).
References
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: Two behavioral features of adolescence in mice. Behavioral Neuroscience. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Schochet TL, Feit EC, Harris R, Mc Kee BL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behavioral Neuroscience. 2011;125:93–105. doi: 10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: The role of dopamine and glutamate. Behavioural Brain Research. 2012;230:21–33. doi: 10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RK, MacDonald CJ, Meck WH. Differential effects of cocaine and ketamine on time estimation: Implications for neurobiological models of interval timing. Pharmacology, Biochemistry and Behavior. 2006;85:114–122. doi: 10.1016/j.pbb.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004 May 7;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR. A neuronal population measure of attention predicts behavioral performance on individual trials. The Journal of Neuroscience. 2010;30:15241–15253. doi: 10.1523/JNEUROSCI.2171-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A. Philosophical Transactions of the Royal Society of London. Vol. 308. Series B: Biological Sciences; 1985. Actions and habits: The development of behavioral autonomy; pp. 67–78. [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behavioral Neuroscience. 2011;125:661–667. doi: 10.1037/a0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: Effects of lesions of the medial striatum and d-amphetamine. Behavioral Neuroscience. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Wong JCK, Allan ME, Mar AC, Theobald DE, Robbins TW. Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the Stop-Signal Task in rats. The Journal of Neuroscience. 2011;31:7349–7356. doi: 10.1523/JNEUROSCI.6182-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: Beyond working memory. Psychopharmacology. 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: Drugs of abuse and striatal synaptic plasticity. Trends in Neurosciences. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Grau JW, Joynes RL. A neural-functionalist approach to learning. International Journal of Comparative Psychology. 2005;18:1–22. [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behavioural Pharmacology. 1998;9:299–308. [PubMed] [Google Scholar]
- Holland PC, Straub JJ. Differential effects of two ways of devaluing the unconditioned stimulus after Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:65–78. doi: 10.1037//0097-7403.5.1.65. [DOI] [PubMed] [Google Scholar]
- Johnson C, Wilbrecht L. Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Developmental Cognitive Neuroscience. 2011;1:540–551. doi: 10.1016/j.dcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas K, Walters E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Koss WA, Franklin AD, Juraska JM. Delayed alternation in adolescent and adult male and female rats. Developmental Psychobiology. 2011;53:724–731. doi: 10.1002/dev.20543. [DOI] [PubMed] [Google Scholar]
- Matthews M, Torres G, Moghaddam B. Neuroscience meeting planner. Washington, DC: Society for Neuroscience; 2011. Comparing striatal dopamine function in adolescent and adult rats. Program number 732.19/ZZ78, Online. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Pinkston JW, Lamb RJ. Delay discounting in C57BL/6J and DBA/2J mice: Adolescent-limited and life-persistent patterns of impulsivity. Behavioral Neuroscience. 2011;125:194–201. doi: 10.1037/a0022919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. The Journal of Neuroscience. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Qin Y, Carter CS, Silk EM, Stenger VA, Fissell K, Goode A, Anderson JR. The change of the brain activation patterns as children learn algebra equation solving. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5686–5691. doi: 10.1073/pnas.0401227101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D. Adolescent risk taking, impulsivity, and brain development: Implications for prevention. Developmental Psychobiology. 2010;52:263–276. doi: 10.1002/dev.20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage JR, Knowlton BJ. Effects of US devaluation on win-stay and win-shift radial maze performance in rats. Behavioral Neuroscience. 2000;114:295–306. doi: 10.1037//0735-7044.114.2.295. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Gallagher M. Teaching old rats new tricks: Age-related impairments in olfactory reversal learning. Neurobiology of Aging. 2002;23:555–564. doi: 10.1016/s0197-4580(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, Bizon JL. Good things come to those who wait: Attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiology of Aging. 2010;31:853–862. doi: 10.1016/j.neurobiolaging.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology. 2009;202:699–709. doi: 10.1007/s00213-008-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Setlow B. Post-training amphetamine administration enhances memory consolidation in appetitive Pavlovian conditioning: Implications for drug addiction. Neurobiology of Learning and Memory. 2006;86:305–310. doi: 10.1016/j.nlm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Smith A, Giampietro V, Brammer M, Halari R, Simmons A, Rubia K. Functional development of fronto-striato-parietal networks associated with time perception. Frontiers in Human Neuroscience. 2011;5:136. doi: 10.3389/fnhum.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Halari R, Giampetro V, Brammer M, Rubia K. Developmental effects of reward on sustained attention networks. NeuroImage. 2011;56:1693–1704. doi: 10.1016/j.neuroimage.2011.01.072. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In Vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Developmental Medicine & Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Annals of the New York Academy of Sciences. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1:392–400. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Developmental Psychobiology. 2006;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Sturman DA, Mandell DR, Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behavioral Neuroscience. 2010;124:16–25. doi: 10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B. The neurobiology of adolescence: Changes in brain architecture, functional dynamics, and behavioral tendencies. Neuroscience and Biobehavioral Reviews. 2011a;35:1704–1712. doi: 10.1016/j.neubiorev.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B. Reduced neuronal inhibition and coordination of adolescent prefrontal cortex during motivated behavior. The Journal of Neuroscience. 2011b;31:1471–1478. doi: 10.1523/JNEUROSCI.4210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B. Striatum processes reward differently in adolescents versus adults. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1719–1724. doi: 10.1073/pnas.1114137109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Totah NK, Jackson ME, Moghaddam B. Preparatory attention relies on dynamic interactions between prelimbic cortex and anterior cingulate cortex. Cerebral Cortex (New York, NY: 1991) 2012 doi: 10.1093/cercor/bhs057. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totah NK, Kim YB, Homayoun H, Moghaddam B. Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. The Journal of Neuroscience. 2009;29:6418–6426. doi: 10.1523/JNEUROSCI.1142-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: Relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophrenia Bulletin. 2011;37:514–523. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: Neurocognitive development of reward and control regions. NeuroImage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Balleine B, Maidment NT. Differential dependence of Pavlovian incentive motivation and instrumental learning processes on dopamine signaling. Learning & Memory. 2011;18:475–483. doi: 10.1101/lm.2229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of General Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline J-B, Lourdusamy A, Banaschewski T, Barker GJ, Garavan H. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clinical Psychology Review. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]