Abstract
Delayed engraftment and graft failure represent major obstacles to successful umbilical cord blood (UCB) transplantation. Herein, we evaluated the use of hyperbaric oxygen (HBO) therapy as an intervention to improve human UCB stem/progenitor cell engraftment in an immune deficient mouse model. Six-to eight-week old NSG mice were sublethally irradiated 24 hours prior to CD34+ UCB cell transplant. Irradiated mice were separated into a non-HBO group (where mice remained under normoxic conditions) and the HBO group (where mice received two hours of HBO therapy; 100% oxygen at 2.5 atmospheres absolute). Four hours after completing HBO therapy, both groups intravenously received CD34+ UCB cells that were transduced with a lentivirus carrying luciferase gene and expanded for in vivo imaging. Mice were imaged and then sacrificed at one of 10 times up to 4.5 months post-transplant. HBO treated mice demonstrated significantly improved bone marrow, peripheral blood , and spleen (p=0.0293) retention and subsequent engraftment. In addition, HBO significantly improved peripheral, spleen and bone marrow engraftment of human myeloid and B-cell subsets. In vivo imaging demonstrated that HBO mice had significantly higher ventral and dorsal bioluminescence values. These studies suggest that HBO treatment of NSG mice prior to UCB CD34+ cell infusion significantly improves engraftment.
Keywords: Umbilical cord blood, CD34+, engraftment, hyperbaric oxygen
Introduction
Umbilical cord blood (UCB) may be the only allogeneic transplant option for patients who lack matched sibling or well matched unrelated donors. Unfortunately, UCB units have limited cell doses available for transplantation, which translates into delayed neutrophil and platelet engraftment and higher rates of engraftment failure [1; 2; 3]. This delay in engraftment is also associated with a delay in immune reconstitution post cord blood transplantation[1; 3; 4; 5; 6], which leads to higher incidence of infections early post UCB transplant[1; 5; 7; 8].
Hematopoietic stem cell (HSC) homing precedes engraftment, which corresponds to the proliferation and differentiation of hematopoietic stem cells to produce mature, functional hematopoietic cells within the bone marrow[9]. During HSC homing, HSCs actively cross the blood/bone marrow endothelium barrier and lodge at least transiently in the bone marrow compartment[10]. This process is fairly rapid and occurs within hours and no longer than two days after stem cell infusion[10]. HSC homing is mediated by the binding of CXCR4 receptor on the surface of HSCs to their ligand, stromal cell-derived factor-1 (SDF-1/CXCL12) expressed by bone marrow endothelium and endosteum[11; 12].
Several approaches have been investigated to improve UCB cell homing/engraftment[1; 3]. For example, after mouse bone marrow studies in lethally irradiated mouse recipients[13], inhibition of dipeptidylpeptidase 4 (DDP4)/CD26 activity by pretreating purified CD34+ human CB cells with Diprotin A significantly enhanced engraftment of HSCs from human UCB into non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice[14]. A DPP4 inhibitor, sitagliptin, after studies of DPP4 inhibitor in mouse recipients enhanced engraftment of mouse bone marrow cells[15], has been investigated in a clinical trial with encouraging results in engraftment of adults with hematological malignancies after single unit UCB transplantation[16], Another strategy involves direct intrabone administration of cord blood cells into the superior-posterior iliac crest under rapid general anesthesia. Though this strategy produced impressive results in some studies[17], another study did not show similar results[18]. In exploring defects in cord blood stem cell homing, it was found that cord blood CD34+ cells have reduced alpha1,3-fucosyltransferase (FucT) expression and activity, which results in absence of cord blood stem cell surface ligands needed to interact with adhesion molecules at time of stem cell homing[19]. Although the use of recombinant human FucTVI corrected the deficiency and helped generate ligands, these changes enhanced initial interaction with microvessels but not homing to bone marrow, according to one study[19]. Another study argued that ex vivo fucosylation improved engraftment in a murine model through enhanced homing[20]. Despite this progress in understanding the biology of UCB homing and engraftment, there is an urgent need to identify safe and practical interventions to enhance UCB homing and engraftment for transplant patients.
In this study, we evaluated the use of hyperbaric oxygen (HBO) therapy as an intervention to improve UCB stem/progenitor cell engraftment. HBO involves inhalation of 100% oxygen intermittently under a pressure greater than 1 atmosphere (ATM), which results in both mechanical effects related to increased pressure and physiologic effects related to hyperoxia[21]. We hypothesized that HBO therapy given prior to UCB CD34+ cell infusion will result in improved engraftment of intravenously infused UCB CD34+ cells. In these experiments, we treated sublethally irradiated NOD/SCID/IL-2Rgcnull (NSG) mice with HBO 6 hours prior to cord blood CD34+ cell infusion and assessed their bone marrow retention and their engraftment as a continuum at several time points. We have chosen NSG mice as they were reported to more efficiently support human cell engraftment [22]. In order to avoid any potential impact of HBO on the actual infused UCB CD34+ cells, we chose to infuse the UCB CD34+ cells 6 hours after the start of HBO therapy. In these studies, UCB CD34+ cells were transduced with luciferase lentivirus to allow for in vivo imaging of transplanted UCB CD34+ cells.
Materials and Methods
Isolation of CD 34+ cells from UCB units
UCB units freshly obtained from St. Louis Cord Blood Bank were processed for CD34+ selection according to manufacturer’s recommendation using StemSep® Human CD34 Positive Selection Cocktail purchased from (STEMCELL Technologies). Isolated CD34+ cells were then transduced and expanded as described in the next section.
UCB CD34 cell transduction
Lentivirus particles harboring the Luc2/mCherry gene and pseudotyped with VSV-G envelope was used to transduce CD 34+ cells at a multiplicity of Infection (MOI) of 10 – 20. The method of spin-infection was used as previously described [23]. Briefly, 1 – 5 × 106 CD34+ cells were centrifuged at 500 × g in 5 mL round bottom sterile tube and cells resuspended in 1 mL of serum-free StemSpan® media (Stemcell Technologies, Vancouver, BC). Lentiviral stock particles were added to 5 mL tube to the desired MOI and gently mixed. Polybrene (Sigma, St. Louis, MO) was added to a final concentration of 4 μg/ml. Cells were then centrifuged continuously at 1000 × g in a swinging bucket rotor for 120 minutes at ambient temperature. Finally, the supernatant was gently aspirated and the cells resuspended in StemSpan® media supplemented with 50 ng/ml each of Flt-3 ligand, Stem cell factor 1 (SCF-1) and thrombopoietin (TPO) (Stemcell Technologies). UCB CD34+ cells were initially expanded up to 10 days as described in the literature[24] and to increase the number of transplanted UCB CD34+ cells to allow better in vivo imaging. Expansion was carried out using StemSpan® media (Stemcell Technologies, Vancouver, BC) supplemented with growth factors as described previously.
Transplantation of NSG mice
Institutional Animal Care and Use Committee approval was obtained prior to the animal experiments. Six to eight week old female NSG mice, obtained from the Jackson Laboratory, Bar Harbor, Maine, were maintained in the animal facilities at the University of Kansas Medical Center. All animals were handled under sterile conditions as cages, water bottles, water, and food were sterilized and animals were maintained in cage microisolators. Mice were given a sublethal dose of 270 cGy using A J.L. Shepherd and Associates Mark I Model 68A Cesium-137 source irradiator 24 hours prior to UCB CD34+cell infusion. Animals were divided into two groups: HBO (n=37) and non-HBO (n=38) mice. (There were 4 mice for each group at each time point except for month 4 time point where only 3 non-HBO mice survived for analysis. Also, one mouse was available for the HBO group and three for the non-HBO group for the time point of 4 ½ months.) Twenty-four hours following irradiation, the HBO mice received HBO therapy in a locally designed hyperbaric chamber with 100% oxygen for 2 hours at 2.5 atmospheres absolute (ATA). Four hours after the end of HBO therapy, HBO and control mice were ready for UCB CD34+ cell infusion. UCB CD34+ cells (1 × 105 / 100 uL sterile PBS per mouse) were administered by tail vein injection using a mouse restraint. The injected UCB CD34 cell dose was calculated based on the total nucleated cell number and the percentage of CD34+ cells, determined by flow cytometry, in the prepared UCB CD34+ sample the day of cell injection. Additional irradiated mice (control) did not receive human UCB CD34+ cells and served as negative controls. Using isoflurane inhalation, mice from HBO and non-HBO groups were euthanized at the following time points: 24 hours, 48 hours, 72 hours, 1 week, 2 weeks, 4 weeks, 2 months, 3 months, 4 months, and 4.5 months. Four mice per group were euthanized at each time point; except for the month 4 time point when only 3 non-HBO mice survived and were available for analysis. Also, one mouse was available for the HBO group and three for the non-HBO group for the time point of 4 ½ months. Peripheral blood, bone marrow, and spleen were harvested and processed for engraftment as described later.
In vivo imaging
Unless otherwise indicated, mice (n=4 for each group for most time points) were imaged 1 day prior to their euthanization using IVIS Spectrum imaging unit. The following post-transplant time points were assessed: 3 hours (only ventral as no dorsal activity was detected in either group for this time point), 2 weeks, 4 weeks, 3 months and 4 months. For the 3 hour time point, 5 mice from the non-HBO pool and 5 mice from HBO pool were grouped. These mice were imaged also for one additional later time point. Imaging kinetics revealed peak signal was achieved at 12 minutes and remained stable up to 25 minutes post injection. Accordingly, we imaged the animals at 12 minute time-points. Briefly, animals were injected with potassium salt of D-Luciferin (15 mg/ml at 10 μl/gm bodyweight) followed by isoflurane induced anesthesia. Animals were imaged ventrally then dorsally, as ventral images correlate with engraftment in the sternum and rib cage while dorsal images correlate with engraftment in the vertebral column and cranium[20]. Images were quantified using living image software version 4.0. Region of interest (ROI) boxes were drawn around the entire body (excluding tail) of the animals. Measurements were expressed as flux, i.e. photons/second (p/s).
Staining of mouse peripheral blood and bone marrow for engraftment
Samples were stained using human CD45-FITC (Miltenyi Biotec, Bergisch Gladbach, Germany), human CD3-PE-Cy5 (BD Biosciences, San Jose, CA), human CD19-APC (Miltenyi Biotec) and human CD33-PE (Miltenyi Biotec) antibodies. Peripheral blood samples were collected into heparinized microcapillary tubes, incubated in FcR Blocking Reagent (Miltenyi Biotec), and stained with the appropriate antibody combination. Once stained, the red blood cells (RBCs) were lysed using EasySep® RBC Lysis Buffer (Stemcell Technologies, Vancouver, BC, Canada) per the manufacturer’s instructions, fixed in 2% paraformaldehyde in PBS and washed in PBS with 1% FBS. Both femurs and tibiae were collected for bone marrow harvest. Bone marrow samples were lysed for RBCs prior to being blocked with FcR Blocking Reagent and stained with the appropriate antibody combination. The cells were fixed with 2% paraformaldehyde in PBS and washed in PBS with 1% FBS. Ten-thousand events were acquired for each peripheral blood and bone marrow sample using the LSRII flow cytometer (BD Biosciences), and the data was analyzed using FACSDiva software.
Statistical analysis
Scatter plots and descriptive statistics were generated. The general model building plan used ordinary least squares (OLS) regression with backward elimination to derive the models. The relationships of study measures over time for each treatment group were determined. The exploratory flow cytometry models of this experiment allowed for separate curves by group. Initial assessment of the full model utilized all possible terms for this model. Model diagnostics included histograms of the residuals, quantile-quantile (q-q) plots, and plots of residuals against both the predicted values and against time. Results of these diagnostic evaluations led to the decision to utilize the natural log transformation of the response measures to stabilize model’s variance, in keeping with the OLS assumptions of constant variance. Factors were individually removed starting with the highest order interaction terms, with a criteria of p>0.05 for the corresponding F test to remain in the model. In the presence of higher ordered terms, all lower order effects were retained in the model.
Once the model was derived using the backwards elimination approach, contrast matrices were used to test the global hypotheses that the resulting curves (if quadratic terms for time remained in the model), lines (if linear terms were the highest order of time that remained in the model), or means (if no time terms remained in the model) were equal. This was done for each tissue-by-cell type combination. For the bioluminescence data, the backwards elimination model building approach of was again used.
Due to the multiple measures analyzed in these studies, the approach to control experiment wise type I error was as follows. CD45 measures in the bone marrow were considered the primary endpoint. The other measures (CD45 in other tissue; CD3, CD19, and bioluminescence) were treated as secondary measures. Hence, no additional type I error adjustments were included.
Results
Percentage of human UCB CD34+ cells pre- and post- transduction and expansion
Prior to transduction and expansion, human UCB units enriched for CD34+ cells contained an average of 91.4% (+/- 4%) CD34+ cells. Post- transduction and expansion, UCB units contained an average of 42% (+/- 37%) CD34+ cells. Expansion for less than 3 days resulted in 89% (+/-7%) CD34+ UCB cells, while expansion of three or more days resulted in 14% (+/-4%) CD34+ cells. For initial time points (24-72 hours) UCB CD34+ cells expanded up to 10 days were used for transplant. Because of the drop in CD34 percentage, for the remaining time points, UCB CD34+ cells were expanded for less than 3 days.
HBO significantly improved peripheral blood, bone marrow, and spleen engraftment of expanded and transduced human UCB CD34+ cells
In these experiments, we evaluated early peripheral blood, bone marrow, and spleen retention of intravenously infused human UCB CD34+ cell by determining the percentage of human CD45+ cells in the respective peripheral blood, bone marrow, and spleen tissue of transplanted mice within 72 hours of UCB CD34+ cell infusion. We also evaluated peripheral blood, bone marrow, and spleen engraftment by determining the percentage of human CD45+ cells in the respective peripheral blood, bone marrow and spleen tissue of transplanted mice starting at 1 week time point and up to 4 1/2 weeks following transplant. UCB CD34+ cell tissue retention within 72 hours of UCB CD34+ cell infusion and engraftment at later time points were assessed in the same tissue as a continuum. In our model, HBO treated mice demonstrated significantly improved bone marrow (p=0.0067), peripheral blood (p=0.0131), and spleen (p=0.0293) retention and subsequent engraftment (Fig.1). The impact on engraftment in these tissues was more pronounced toward later engraftment time points at 3 and 4 months. Collectively, these results suggest a potential role for HBO in improving engraftment of UCB CD34+ cells.
Figure-1. Impact of hyperbaric oxygen on umbilical cord blood CD34+ cell tissue retention/engraftment measured by flow cytometry.
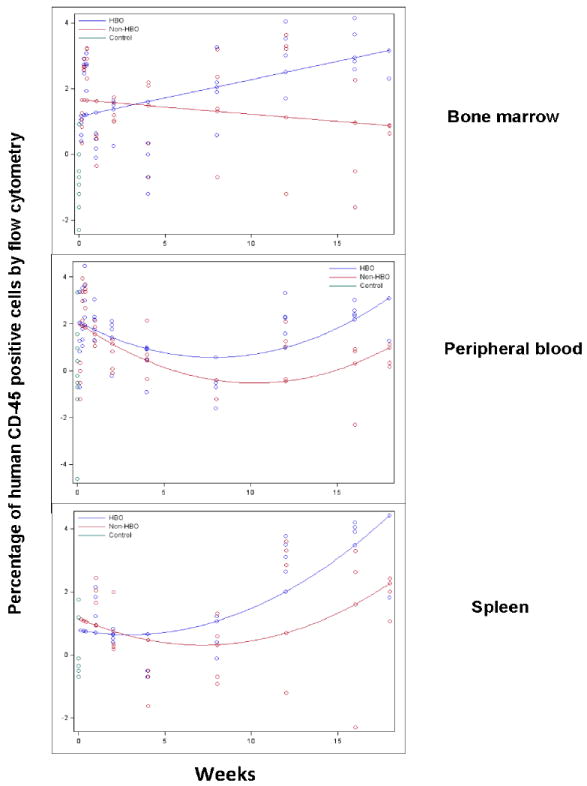
Peripheral blood, bone marrow, and spleen homogenates were processed for human CD45 staining and flow cytometry to determine percentage of human CD45+ cell retention (up to 72 hours) or engraftment (data after 72 hours). The percentage of human CD45+ cells was measured for each mouse and time point for each tissue. The data was log-transformed and plotted on the scatter plot graph. Mean human CD45+ cells were connected for each group at each time point (Blue: HBO, red: non-HBO, and green: control). In these experiments, HBO mice demonstrated a statistically significant increase in the percentage of human CD45+ cells in peripheral blood, bone marrow, and spleen over non-HBO mice. The difference in the percentage of human CD45+ cells between the two groups widened overtime.
HBO treated mice demonstrated significantly higher photon flux values
In these experiments, we used bioluminescent imaging to demonstrate repopulation of bone marrow by transduced human CD34-selected umbilical cord blood (UCB) cells as described previously[24]. We evaluated photon flux values obtained from in vivo images as a measure of engraftment, as others have shown correlation between bioluminescence and engraftment[25]. Higher dorsal and ventral photon flux values were observed in HBO treated mice compared to non-HBO treated mice (Fig.2A). Again, the impact of HBO on ventral photon flux values (p=0.0002) and dorsal photon flux values (p=0.0009) was more pronounced at later time points (Fig.2B). Accordingly, the impact of HBO on engraftment was universal without favoring certain bones for engraftment. On the other hand, HBO treated mice demonstrated on average higher ventral photon flux values at the very early time point of 3 hours (Fig.2C). In these images, bioluminescence activity was demonstrated in the thoracic area of HBO mice. Baseline images taken within 10 minutes of UCB CD34+ cell injection showed bioluminescence activity in mice thoraces consistent with trafficking of the infused cells to the lungs. On the other hand, the 3 hour images of HBO mice were more consistent with bioluminescence activity in the heart, given the central location of the bioluminescence, and less likely related to entrapment of UCB CD34+ cells in the lungs.
Figure-2. Impact of hyperbaric oxygen (HBO) on umbilical cord blood CD34+ cell engraftment determined by bioluminescence.
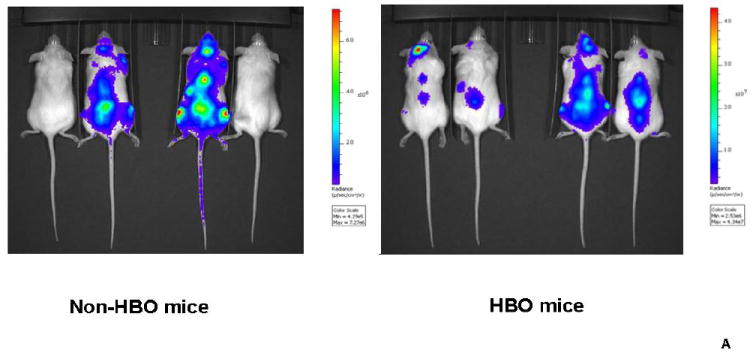
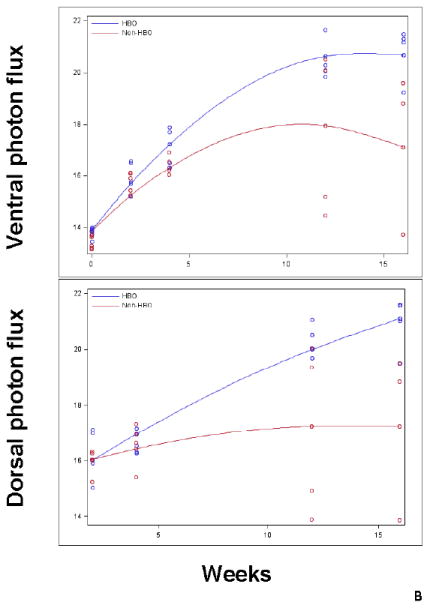
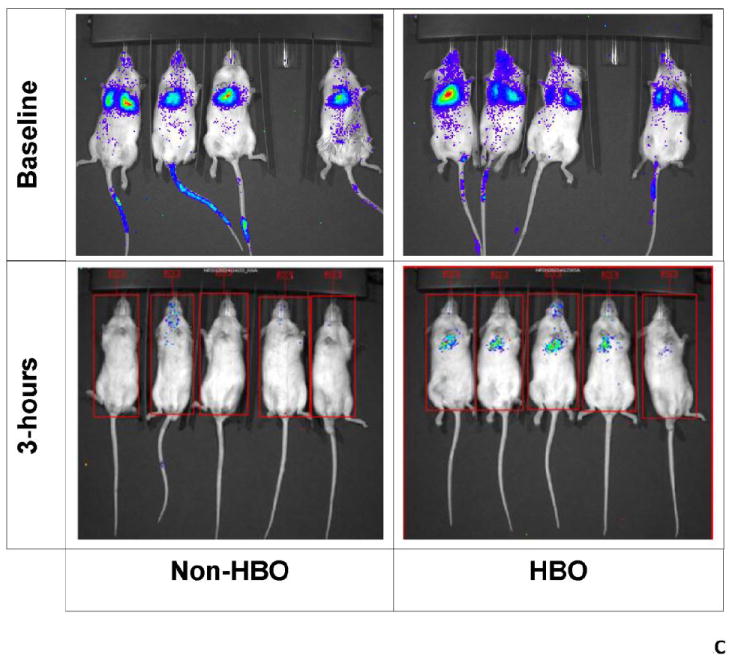
Transplanted mice were imaged in vivo using IVIS spectrum imager. Prior to imaging, mice were injected with D-Luciferin. Subsequently, mice were imaged ventrally then dorsally. Images were quantified using living image software version 4.0 and) measurements were expressed as flux i.e. photons/second (p/s). A: a representative in vivo image of the dorsal aspect of HBO and non-HBO mice obtained at month 3 time point. A measureable difference in bioluminescence signal between the 2 groups is demonstrated. Individual scales are shown to correspond to bioluminescence. B: photon flux log transformed data depicted in a scatter-plot. Mean photon flux values were connected for each group at each time point (Blue: HBO and red: non-HBO). In these experiments, HBO mice demonstrated a statistically significant increase in photon flux over non-HBO mice. The difference between the two groups of mice was more significant at the later time points of 3 and 4 months. C: In vivo imaging of the ventral aspect of HBO and non-HBO mice at baseline and 3 hours post-transplant. At baseline, (10 minutes after UCB CD34+ cell injection) both groups of mice demonstrated bioluminescence in the thoracic area. On the other hand, only HBO mice demonstrated central thoracic bioluminescence 3-hours post-transplant. For the 3 hour time point, 5 mice from the non-HBO pool and 5 mice from HBO pool were grouped. Abbreviation: HBO stands for hyperbaric oxygen.
These results suggest that HBO treatment might have altered early UCB CD34+ cell distribution or viability and resulted in improvement in photon flux values overtime mirroring engraftment data.
HBO significantly improved peripheral blood, spleen and bone marrow engraftment of human CD19+ and CD33+ cell subsets while only increased peripheral blood engraftment of CD3+ cells
We also determined if HBO impact on engraftment is related to improved engraftment of a certain subset of hematopoietic cells. Accordingly, we evaluated human CD33+ cells for myeloid engraftment, human CD19+ cells for B-cell engraftment, and human CD3+ cells for T-cell engraftment starting 4 weeks following UC B CD34+ cell infusion. In terms of CD33+ cell engraftment, it was significantly improved in peripheral blood (p=0.0091), bone marrow (p=0.0066), and spleen (0.0399) of HBO treated mice (Fig.3A). Similar to overall engraftment and B-cell engraftment patterns, the impact of HBO on CD33+ cell engraftment was more pronounced at later time points (Fig.3A).
Figure-3. Impact of hyperbaric oxygen on umbilical cord blood CD34+ cell subsets engraftment.
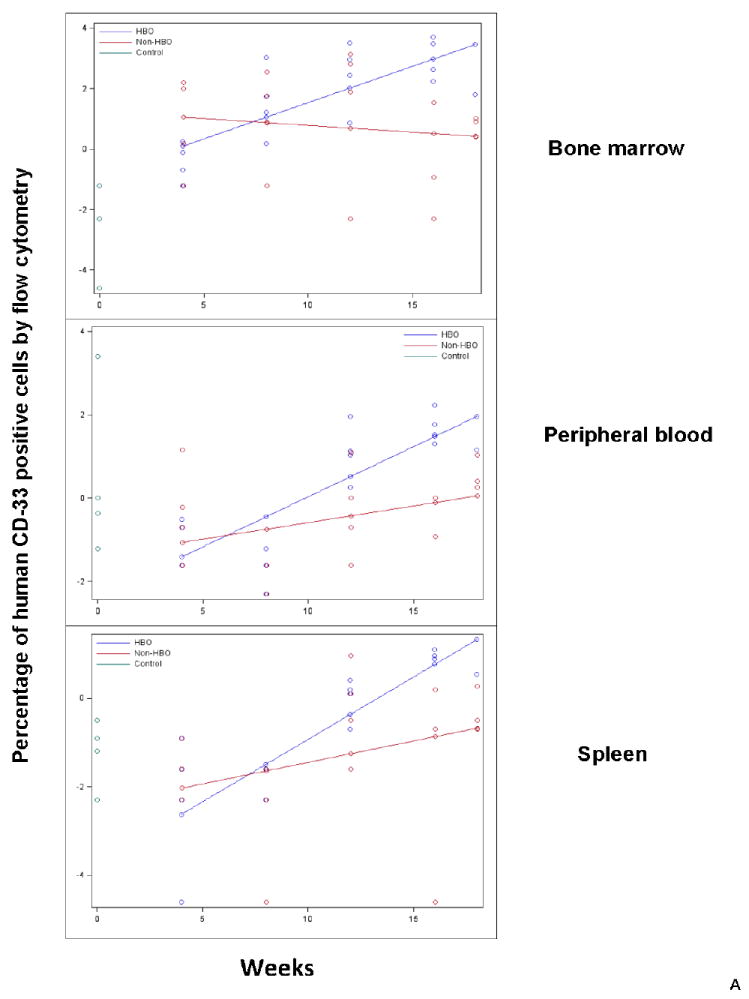
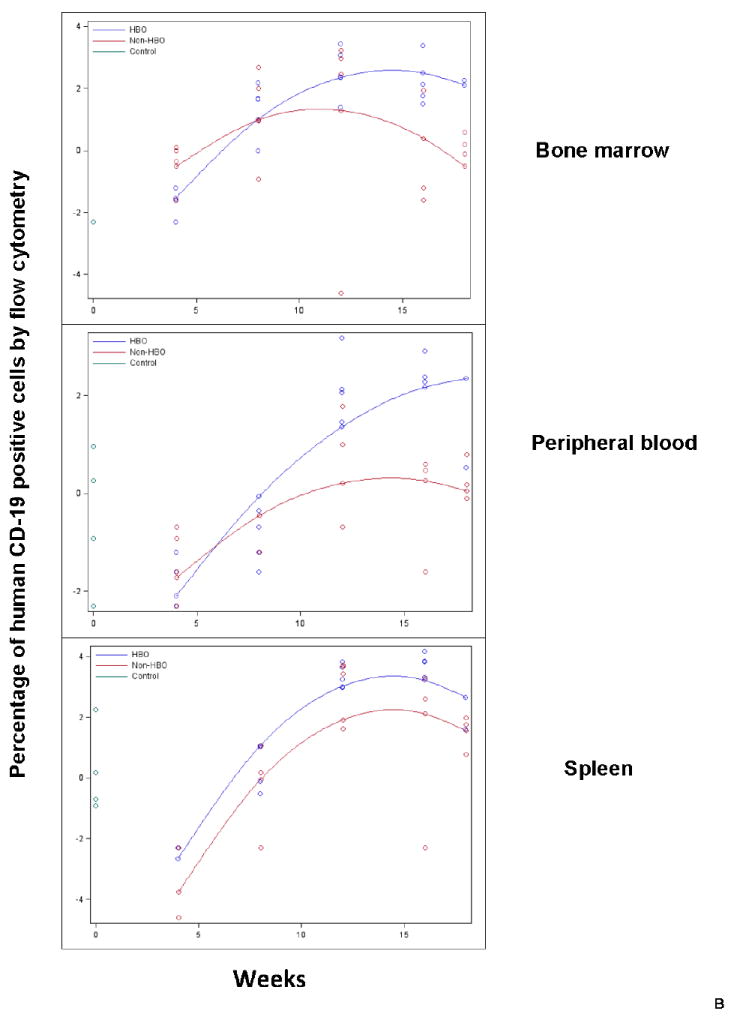
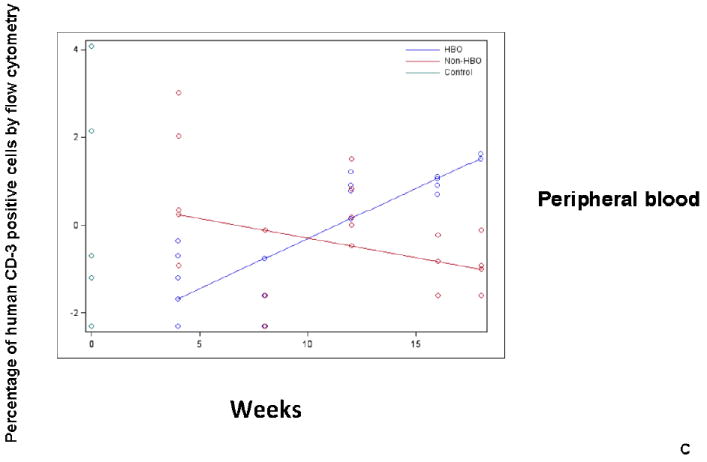
Peripheral blood, bone marrow, and spleen were harvested from HBO and non-HBO mice. Subset engraftment was assessed by flow cytometry staining for human CD33 (myeloid subset-Fig.3A), human CD 19 (B-cell subset-Fig.3B), and human CD3 (T cell subset-Fig.3C) antibodies. The percentage of human CD19, CD33, or CD3 positive cells was measured for each mouse and time point for each tissue. The data was log-transformed and plotted on the scatterplot graph. Mean human CD19, CD33, and CD3 positive cells were connected for each group for each time point (Blue: HBO, red: non-HBO, and green: control). A: HBO mice demonstrated a statistically significant increase in the percentage of human CD33+ cells in peripheral blood, bone marrow, and spleen over non-HBO mice. B:HBO mice demonstrated a statistically significant increase in the percentage of human CD19+ cells in peripheral blood, bone marrow, and spleen over non-HBO mice. The difference in the percentage of human CD33+ and CD19+ cells between the two groups widened overtime. C: HBO mice demonstrated a statistically significant increase in the percentage of human CD3+ cells across time only in peripheral blood compared to non-HBO mice.
In the case of CD19+, statistically significant improvement in CD19+ cell engraftment in peripheral blood (p=0.0016), bone marrow (p=0.0341), and spleen (p=0.0239) of HBO treated mice was demonstrated (Fig.3B). Similar to overall engraftment, the impact of HBO on engraftment was more pronounced at later time points for peripheral blood and bone marrow (Fig.3B). CD3+ cell engraftment, on the other hand, was significantly increased in peripheral blood (p=0.0032) of HBO treated mice (Fig.3C). Spleen and bone marrow CD3+ cell engraftment was comparable between HBO and non-HBO treated mice (p>0.05 for all model terms related to treatment; thus removed from the model via backwards elimination). Collectively, these data suggest that HBO improved overall engraftment mainly by improving B-cell and myeloid cell engraftment.
Discussion
In these experiments we investigated the use of HBO as a modality to improve human UCB CD34+ cell engraftment in sublethally irradiated NSG mice. We demonstrated that HBO treatment prior to UCB CD34+ cell infusion significantly improved engraftment of transduced and expanded UCB CD34+ cells, especially at later time points post-transplant. Improved long-term engraftment is a desirable outcome in UCB transplantation where non-engraftment remains a major complication of UCB transplantation. This procedure might be of advantage when using gene transduced CD34+ UCB cells, as expansion itself may decrease the homing characteristics of CD34+ cells.
In our experiments, we observed a significant impact of HBO on long-term engraftment of the transduced and expanded UCB CD34+ cells. Following transduction, UCB CD34+ cells were expanded using cytokine rich media to allow for their in vivo imaging and, as a result, we exposed these stem/progenitor cells to cytokines in the media. This exposure to cytokines may have altered homing mechanisms in this model, as has been shown previously[26]. Additionally, ex vivo cycling of hematopoietic stem cells results in changes in polarized membrane domain. This change in membrane polarization may impair certain adhesion molecules and, subsequently, the adhesion of hematopoietic stem cells to osteoblasts. These changes seem to disrupt early events like hematopoietic stem cell adhesion and homing and limit subsequent engraftment in NSG mice[27]. Due to these potential effects of cytokine exposure, expanded UCB CD34+ cells might have lost engraftment potential as has been described previously[28]. In our experiments, this loss was potentially restored by using HBO, while the loss of engraftment was evident in the case of non-HBO treated mice.
In our in vivo imaging experiments, we noticed that the difference in bioluminescence between HBO and non-HBO treated mice to have widened overtime and at the same time we noticed similar patterns for engraftment assessed by flow cytometry. These findings suggest that bioluminescence at later time points correlates with long-term engraftment. Consistent with this finding, others have found that bioluminescence in the first 3 weeks post-transplant reflects engraftment of short-term repopulating progenitors, while bioluminescence afterwards reflects engraftment of long-term repopulating progenitors[25].
Though HBO therapy was given prior to UCB CD34+ cell infusion and only for one treatment, the effects of such therapy on engraftment became more pronounced at later time points that correlate with long-term engraftment. Based on this observation, we hypothesize that HBO treatment must have changed engraftment kinetics in favor of improved long-term engraftment. Additionally, HBO supported peripheral blood engraftment of all lineages, especially myeloid and B-cell lineages, in HBO mice. A skewed engraftment of CD19+ cell population seems to occur upon repopulation of NOD/SCID mice by CD34+ UCB cells [29].
Outcomes of UCB transplantation reflect the interaction of the transplanted cells and the recipient bone marrow microenvironment. Most current approaches to improve UCB homing and engraftment involve manipulation of UCB unit, or graft, itself prior to transplantation. The use of HBO to improve UCB engraftment is a new approach as it does not involve graft manipulation, and instead may alter the host microenvironment conditions in favor of engraftment.
HBO is considered a safe procedure when standard protocols with oxygen pressures not exceeding 3 atmospheres and treatment sessions limited to a maximum of 120 minutes are used [30]. Therefore, we are investigating HBO therapy in a pilot clinical trial to primarily evaluate the safety and tolerability of this procedure in UCB transplantation. Secondarily, we will also assess the impact of HBO on engraftment. Applying methods that improve engraftment has the potential to greatly impact the application of this type of transplant to a larger population in need.
Acknowledgments
This work was mainly supported by a career grant award provided by Office of Scholarly, Academic & Research Mentoring (OSARM), Department of Internal Medicine, University of Kansas Medical Center. OSARM participated in the study design, collection, analysis, data interpretation, and the writing. O.S.A. is a recipient of a research career award OSARM at home institution. This work was partially funded by the Robert K. Dempski Cord Blood Research Fund, though had no involvement in study design, collection, analysis, data interpretation, writing or the decision to submit this article. We also thank St. Louis Cord blood Bank for providing umbilical cord blood units used in this research. We also acknowledge the Flow Cytometry Core Laboratory, which is sponsored, in part, by the NIH/NIGMS COBRE grant P30 GM103326.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yinghua Xiao, Email: xyhpotato@gmail.com.
Jeff D. Eskew, Email: jeskew@kumc.edu.
Nikhil K. Parelkar, Email: nparelkar@kumc.edu.
Megan Swink, Email: mswink@kumc.edu.
Jeff Radel, Email: JRADEL@kumc.edu.
Tara L. Lin, Email: tlin@kumc.edu.
Bruce F. Kimler, Email: bkimler@kumc.edu.
Jonathan D. Mahnken, Email: jmahnken@kumc.edu.
Joseph P. McGuirk, Email: jmcguirk@kumc.edu.
Hal E. Broxmeyer, Email: hbroxmey@iupui.edu.
George Vielhauer, Email: gvielhauer@kumc.edu.
References
- 1.Broxmeyer HE, Farag SS, Rocha V. Cord Blood Hematopoietic Cell Transplantation. In: Appelbaum FR, Forman SJ, Negrin RS, Antin JH, editors. Thomas’ Hematopoietic Cell Transplantation. 5. Wiley-Blackwell; Oxford, England: 2013. [Google Scholar]
- 2.Migliaccio AR, Adamson JW, Stevens CE, et al. Cell dose and speed of engraftment in placental/umbilical cord blood transplantation: graft progenitor cell content is a better predictor than nucleated cell quantity. Blood. 2000;96:2717–22. [PubMed] [Google Scholar]
- 3.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013 doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9:111–22. doi: 10.1016/j.bbmt.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JA, Boussiotis VA. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin Immunol. 2008;127:286–97. doi: 10.1016/j.clim.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–51. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabolcs P, Cairo MS. Unrelated umbilical cord blood transplantation and immune reconstitution. Semin Hematol. 2010;47:22–36. doi: 10.1053/j.seminhematol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandenbosch K, Ovetchkine P, Champagne MA, et al. Varicella-zoster virus disease is more frequent after cord blood than after bone marrow transplantation. Biol Blood Marrow Transplant. 2008;14:867–71. doi: 10.1016/j.bbmt.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson SK, Simmons PJ. Transplantable stem cells: home to specific niches. Curr Opin Hematol. 2004;11:102–6. doi: 10.1097/01.moh.0000133651.06863.9c. [DOI] [PubMed] [Google Scholar]
- 10.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 11.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 12.Lapidot T, Kollet O. The brain-bone-blood triad: traffic lights for stem-cell homing and mobilization. Hematology Am Soc Hematol Educ Program. 2010;2010:1–6. doi: 10.1182/asheducation-2010.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Christopherson KW, 2, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–3. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 14.Campbell TB, Hangoc G, Liu Y, Pollok K, Broxmeyer HE. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 2007;16:347–54. doi: 10.1089/scd.2007.9995. [DOI] [PubMed] [Google Scholar]
- 15.Broxmeyer HE, Hoggatt J, O’Leary HA, et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med. 2012;18:1786–96. doi: 10.1038/nm.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farag SS, Srivastava S, Messina-Graham S, et al. In Vivo DPP-4 Inhibition to Enhance Engraftment of Single-Unit Cord Blood Transplants in Adults with Hematological Malignancies. Stem Cells Dev. 2013;22:1007–15. doi: 10.1089/scd.2012.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frassoni F, Gualandi F, Podesta M, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008;9:831–9. doi: 10.1016/S1470-2045(08)70180-3. [DOI] [PubMed] [Google Scholar]
- 18.Brunstein CG, Barker JN, Weisdorf DJ, et al. Intra-BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA-matched units. Bone Marrow Transplant. 2009;43:935–40. doi: 10.1038/bmt.2008.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hidalgo A, Frenette PS. Enforced fucosylation of neonatal CD34+ cells generates selectin ligands that enhance the initial interactions with microvessels but not homing to bone marrow. Blood. 2005;105:567–75. doi: 10.1182/blood-2004-03-1026. [DOI] [PubMed] [Google Scholar]
- 20.Robinson SN, Simmons PJ, Thomas MW et al. Ex vivo fucosylation improves human cord blood engraftment in NOD-SCID IL-2Rgamma(null) mice. Exp Hematol. 2012;40:445–56. doi: 10.1016/j.exphem.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grim PS, Gottlieb LJ, Boddie A, Batson E. Hyperbaric oxygen therapy. JAMA. 1990;263:2216–20. [PubMed] [Google Scholar]
- 22.Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–82. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 23.Wrzesinski S, Seguin R, Liu Y, et al. HTLV type 1 Tax transduction in microglial cells and astrocytes by lentiviral vectors. AIDS Res Hum Retroviruses. 2000;16:1771–6. doi: 10.1089/08892220050193290. [DOI] [PubMed] [Google Scholar]
- 24.Steiner D, Gelovani J, Savoldo B, et al. Noninvasive bioluminescent imaging demonstrates long-term multilineage engraftment of ex vivo-expanded CD34-selected umbilical cord blood cells. Stem Cells. 2009;27:1932–40. doi: 10.1002/stem.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Rosol M, Ge S, et al. Dynamic tracking of human hematopoietic stem cell engraftment using in vivo bioluminescence imaging. Blood. 2003;102:3478–82. doi: 10.1182/blood-2003-05-1432. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed F, Ings SJ, Pizzey AR, et al. Impaired bone marrow homing of cytokine-activated CD34+ cells in the NOD/SCID model. Blood. 2004;103:2079–87. doi: 10.1182/blood-2003-06-1770. [DOI] [PubMed] [Google Scholar]
- 27.Larochelle A, Gillette JM, Desmond R, et al. Bone marrow homing and engraftment of human hematopoietic stem and progenitor cells is mediated by a polarized membrane domain. Blood. 2012;119:1848–55. doi: 10.1182/blood-2011-08-371583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters SO, Kittler EL, Ramshaw PJ. Quesenberry, Murine marrow cells expanded in culture with IL-3, IL-6, IL-11, and SCF acquire an engraftment defect in normal hosts. Exp Hematol. 1995;23:461–9. [PubMed] [Google Scholar]
- 29.Noort WA, Wilpshaar J, Hertogh CD, et al. Similar myeloid recovery despite superior overall engraftment in NOD/SCID mice after transplantation of human CD34(+) cells from umbilical cord blood as compared to adult sources. Bone Marrow Transplant. 2001;28:163–71. doi: 10.1038/sj.bmt.1703120. [DOI] [PubMed] [Google Scholar]
- 30.Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642–8. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]


