Abstract
99mTc-mercaptoacetyltriglycine (99mTc-MAG3), 99mTc-dd- and ll-ethylene-di-cysteine (99mTc-EC) and 99mTc-mercaptoacetamide-ethylene-cysteine (99mTc-MAEC) contain N3S or N2S2 ligands designed to accommodate the four ligating sites of the {99mTcO}3+ core; they are all excellent renal imaging agents but have renal clearances less than that of 131I-orthoiodohippurate (131I-OIH). To explore the potential of the newly accessible but less polar {99mTc(CO)3}+ core having three ligating sites, we decided to build on the success of 99mTc-EC with its N2S2 ligand and two dangling carboxylates and have chosen an N2S ligand that also has two dangling carboxyls, lanthionine (LANH2), to form 99mTc(CO)3(LAN), a new renal radiopharmaceutical.
Methods
Biodistribution studies were performed on Sprague-Dawley rats by using 99mTc(CO)3(LAN) isomers, meso-LAN and dd,ll-LAN (an enantiomeric mixture), coinjected with 131I-OIH. Human studies were also performed by coinjecting each 99mTc product (~74 MBq [~2 mCi]) and 131I-OIH (~ 7.4 MBq [~ 0.2 mCi]) into 3 normal volunteers with dual-isotope imaging performed by using a camera system fitted with a high-energy collimator. Blood samples were obtained from 3 to 90 min after injection, and urine samples were obtained at 30, 90 and 180 min.
Results
Biodistribution studies in rats revealed a rapid blood clearance as well as rapid renal extraction for both preparations, with the dose in urine at 60 min averaging 88% that of 131I-OIH. In humans, both agents provided excellent renal images, with the plasma clearance averaging 228 mL/min for 99mTc(CO)3(meso-LAN) and 176 mL/min for 99mTc(CO)3(dd,ll-LAN), respectively. At 3 hours, both 99mTc(CO)3(meso-LAN) and 99mTc(CO)3(dd,ll-LAN), showed good renal excretion, averaging 85% and 77% that of 131I-OIH, respectively. Plasma protein binding was minimal (10% and 2%), and red cell uptake was similar (24% and 21%) for 99mTc(CO)3(meso-LAN) and 99mTc(CO)3(dd,ll-LAN), respectively.
Conclusion
Although the plasma clearance and the rate of renal excretion of the 99mTc(CO)3(LAN) complexes are still less than those of 131I-OIH, the results of this first application of a 99mTc tricarbonyl complex as a renal radiopharmaceutical in humans demonstrate that 99mTc(CO)3(LAN) complexes are excellent renal imaging agents and support continued renal radiopharmaceutical development based on the 99mTc tricarbonyl core.
Keywords: 99mTc tricarbonyl, lanthionine, 99mTc(CO)3(LAN), renal radiopharmaceuticals
INTRODUCTION
The development of technetium radiopharmaceuticals has relied heavily on the {TcO}3+ core with technetium in its +5 oxidation state, which is readily accessible by pertechnetate reduction in the presence of chelating ligands. Recently, the numerous synthetic advantages of the 99mTc water-stable organometallic precursor, [99mTc(CO)3(H2O)3]+ (with 99mTc in its low +1 oxidation state), have shifted the focus of 99mTc radiopharmaceutical development to agents with a fac-{99mTc(CO)3}+ core (1–11). Both cores are compact, form kinetically inert agents with suitable ligands, and are versatile for labeling many types of bioactive molecules. However, the fac-{99mTc(CO)3}+ moiety is non-polar, has an almost spherical shape, and offers only three sites on an octahedral face for ligand attachment. N2S2 and N3S ligands designed for the four co-planar sites of the polar {TcO}3+ core available for ligand attachment are generally unsuitable for the fac-{99mTc(CO)3}+ core; consequently new ligands are needed.
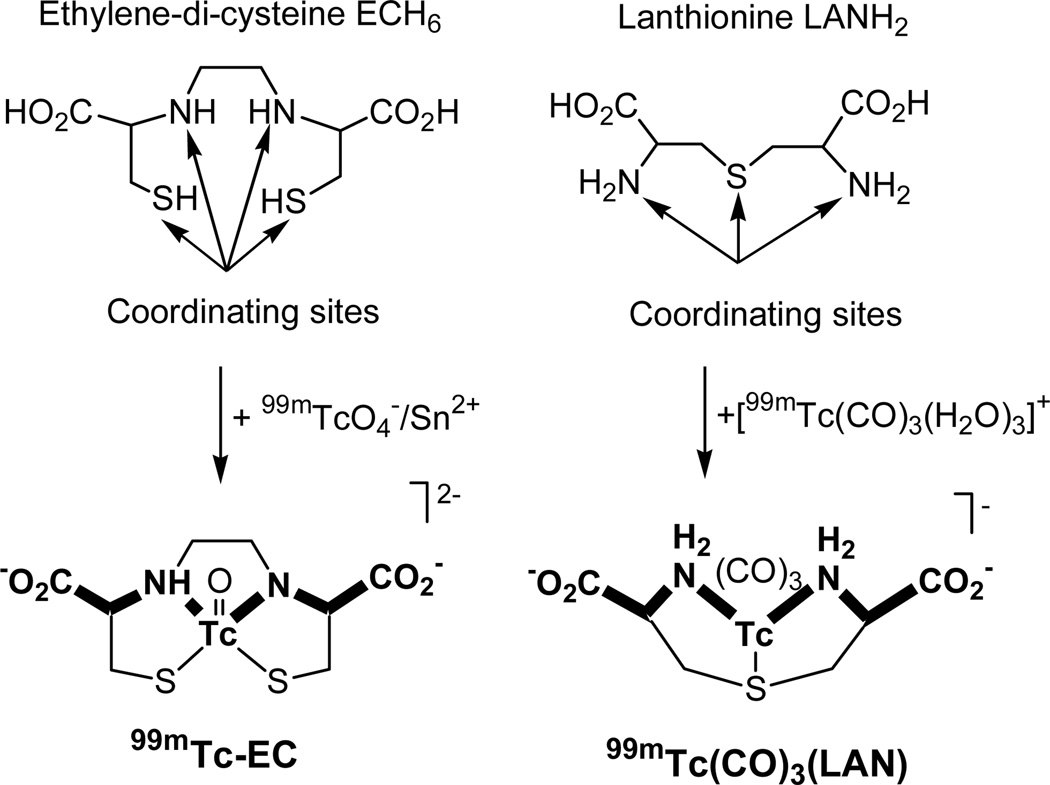
To date, renal radiopharmaceuticals designed around the {TcO}3+ core have not achieved a renal clearance in humans comparable to that of OIH. Thus, it seemed justified to redirect some of our effort to applying the tricarbonyl core approach to the goal of improving upon the performance of {TcO}3+ renal imaging agents. Our studies are focusing on relatively small ligands containing at least three N, O, or S ligating atoms (12, 13). An important focus of our work was to determine whether the effects of the less polar tricarbonyl core on the biodistribution and pharmacokinetics of a radiopharmaceutical designed around this core would preclude developing tracers with high renal clearance. One of the first tridentately coordinating ligands we selected to exploit for the fac-{99mTc(CO)3}+ core in the design of a novel renal radiopharmaceutical was lanthionine (Fig. 1, 3, 3'-thiodialanine, LANH2). We selected this design because it mirrors one of the best N2S2 renal agents, 99mTc-EC (99mTc-ethylene-di-cysteine), in that it contains a HO2C-CH2-NH-Tc-NH-CH2-CO2H sequence as well as two dangling carboxylate groups (Fig. 1). This similarity to 99mTc-EC and the promising initial results in rats led us to select this agent among those under study in our laboratories for our first assessment of a 99mTc-tricarbonyl core agent in humans. This report describing the biodistribution, excretion and imaging characteristics of the new renal imaging agent, 99mTc(CO)3(LAN), in fact presents one of the first human studies using any type of radiopharmaceutical containing the 99mTc-tricarbonyl core.
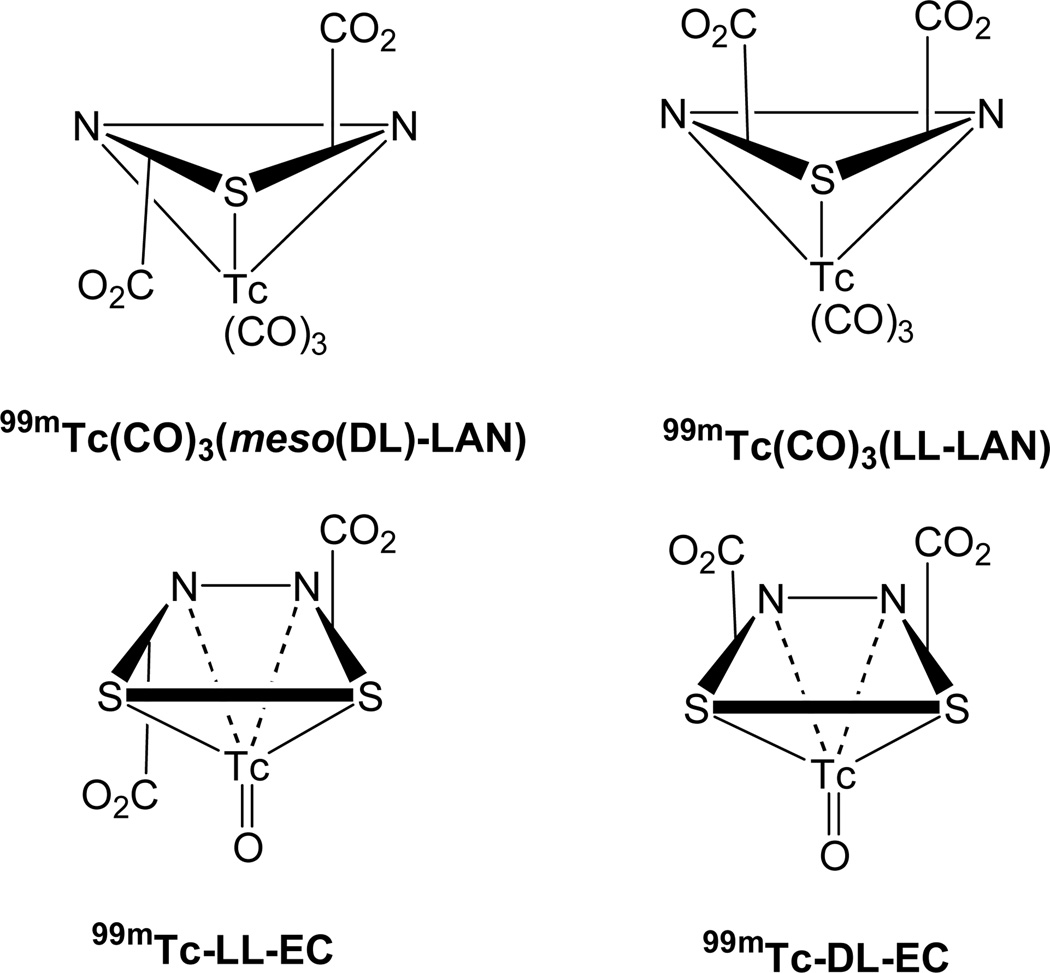
FIGURE 1.
Comparison of 99mTc-EC and 99mTc(CO)3(LAN), two agents with two-carboxylate structural features.
MATERIALS AND METHODS
All chemicals and solvents were of reagent grade and used without further purification. Lanthionine (LANH2, a mixture of dd-, ll-, and meso(dl)-LAN isomers) was purchased from TCI America (Portland, OR). 99mTc sodium pertechnetate (Na[99mTcO4]) was eluted from a 99Mo/99mTc generator (Amersham Health) using 0.9% saline. High-performance liquid chromatography (HPLC) analyses were performed on a Beckman System Gold Nouveau (for rat studies) and on a Beckman System Gold Bioessential (for human studies) equipped with model 170 radiometric detector, 166 UV/VIS detector, and 32 Karat chromatography software, using a C-18 RP Beckman Ultrasphere ODS 5 mm column (4.6 mm×250 mm); flow rate = 1 mL/min; mobile phase = 12% EtOH, 0.05 M tetraethylammonium phosphate buffer, pH 2.5. The [99mTc(CO)3(H2O)3]+ precursor was prepared directly from 99mTcO4− in saline solution under 1 atm of CO according to the published method (14).
99mTc Radiolabeling
LANH2 (1 mg) was dissolved in 1 N HCl (0.1 mL) and the pH of the solution was adjusted to ~ 9 with 1 N NaOH. An aliquot (0.1 mL) of this solution was added to 1 mL of the freshly prepared [99mTc(CO)3(H2O)3]+ solution and the mixture was heated at 70 °C for 30 min, cooled to room temperature, and analyzed by HPLC to show three resolved HPLC peaks with the following retention times: 6 min, 8 min, and 10 min (a minor peak), respectively. All three complexes were isolated by HPLC, and their radiochemical purities were > 98%. The first eluting peak was assigned as 99mTc(CO) 3(meso-LAN) and the second peak as 99mTc(CO) 3(dd,ll-LAN) (an enantiomeric mixture of dd- and ll-LAN isomers). Those two complexes were buffered to pH 7.4 and tested by HPLC for stability up to 6 hours; no measurable decomposition was observed and they were tested in rats and humans. The third peak represented the less stable isomer containing the meso-LAN ligand and was not used in our studies. We assigned those configurations to the 99mTc(CO)3(LAN) isomers because similar results were obtained for Re(CO)3(LAN) complexes, which we have fully characterized by analytical and spectroscopic methods (15).
Rat Studies
Biodistribution Studies
The animal experiments followed the principles of laboratory animal care and were approved by the Institutional Animal Care and Use Committee of Emory University. 99mTc(CO)3(meso-LAN) and 99mTc(CO)3(dd,ll-LAN) complexes were each evaluated in 5 Sprague-Dawley rats at 10 and 60 min, respectively. A solution of each 99mTc complex (3.7 MBq/mL [100 mCi/mL]) and 131I-OIH (925 kBq/mL [25 mCi/mL]) was prepared, and six 0.2-mL aliquots were drawn into insulin syringes. Five aliquots were used for doses; the sixth aliquot was diluted to 100 mL, and three 1-mL portions of the resulting solution were used as standards. Each rat was anesthetized with ketamine/xylazine (2 mg/kg body weight) injected intramuscularly with the additional supplemental anesthetic as needed. The bladder was catheterized by using heat-flared PE-50 tubing for urine collection.
The radiopharmaceutical solution was injected intravenously via a tail vein, and five animals were sacrificed at 10 min and five at 60 min post-injection. A blood sample was obtained, and the heart, lung, spleen, liver, intestine, stomach, and kidney were removed. The whole liver was weighed and random sections were obtained for counting. Blood, whole organs and tissue samples were placed in tubes, and each sample was weighed. Radioactivity of the sample and standards was measured in a dual-channel well counter with 20% windows centered on the photo peaks of 99mTc (140 keV) and 131I (360 keV). Counts were corrected for background radiation, physical decay and spillover of 131I counts into the 99mTc window. The percent dose in each tissue or organ was calculated by dividing the counts in each tissue or organ by the total injected counts. The value given for bowel represents combined stomach and intestine activity. The percent injected dose in whole blood was estimated assuming a blood volume of 6.5% of total body weight.
Metabolism Studies
Rats were prepared according to the procedure described above for the biodistribution studies. A bolus injection of each 99mTc(CO)3(LAN) complex (~ 7.4 MBq [0.2 mCi]) was intravenously administered to two rats and urine was collected for 30 min and analyzed by HPLC alone and with purified complex added to determine if the complex was metabolized or excreted unchanged in the urine.
Normal Volunteer Studies
All studies were performed with the approval of the Radioactive Drug Research Committee and the Emory University Institutional Review Board; signed consent was obtained from each volunteer. Six healthy volunteers (4 males, 2 females; mean age ± SD, 30.3 ± 5.5 y; range 25–38 y) participated in this study. Inclusion criteria required the absence of any history of kidney and bladder diseases and a normal review of systems. Pregnancy was excluded in females by means of a urine pregnancy test. Measurements of blood pressure, heart rate, and temperature were taken pre- and post-injection for each volunteer; in addition a CBC, standard chemistry panel and urinalysis were obtained pre- and 24 h post-injection. Volunteers were requested to drink approximately 500 mL of water prior to the study. 99mTc(CO)3(meso-LAN) and 99mTc(CO)3(dd,ll-LAN) complexes were each evaluated in three normal volunteers. HPLC-purified complexes and phosphate-buffered saline (pH 7.4) were passed through a Sep-Pak Plus C18 cartridge (Waters Co.) (primed with 4 mL of ethanol) and sterile Millex-GS 22 mm filter (Millipore Co.) (primed with 4 mL of saline) into a sterile, pyrogen-free empty vial. The final concentration was 37 MBq/mL (1 mCi/mL) and the final pH was 7.4. Test samples of each complex were sent for analysis and determined to be sterile and pyrogen free.
Approximately 74 MBq (~2 mCi) of each 99mTc(CO)3(LAN) complex was coinjected with 7.4–11.1 MBq (200–300 mCi) of 131I-OIH, and imaging was performed by using a General Electric Infinia (Milwaukee, WI) camera with a 3/8 inch crystal fitted with a high energy collimator; a 20% window was centered over the 365 KeV photopeak of 131I, and a second 20% window was centered over the 140 keV photopeak of 99mTc. Data were acquired in a 128 × 128 matrix by using a three-phase dynamic acquisition and processed on a General Electric Xeleris computer by using QuantEM™ renal software. Blood samples were obtained at 3, 5, 10, 20, 30, 45, 60, and 90 min after injection and plasma clearances for 131I-OIH and each 99mTc(CO)3(LAN) complex were determined by using the single injection, two-compartment model of Sapirstein et al. (16). The volunteers voided at 30, 90, and 180 min post-injection to determine the percent dose in the urine at each time period. Plasma protein binding (PPB) was determined by ultrafiltration (Centrifree® micropartition system, Amicon Inc.) of 1 mL of plasma: PPB = (1.0 – [ultrafiltrate concentration/plasma concentration])×100. A Beckman gamma counter system was used to determine the concentration of radioactivity in plasma, in red blood cells and in urine samples with correction for 131I scatter into the 99mTc window. To determine if the complex was metabolized or excreted unchanged in the urine, a 1 mL urine sample from the 30 min urine collection was obtained from each volunteer and analyzed by HPLC alone and with purified complex added.
RESULTS
99mTc Radiolabeling
Lanthionine was effectively radiolabeled with 99mTc under mild conditions (30 min at 70 °C, pH ~9) to form well-defined complexes with the 99mTc tricarbonyl core in high yield. In all complexes, the LANH2 ligand coordinated tridentately and facially to give a 99mTc(CO)3(N2S) coordination sphere, leaving both carboxyl groups uncoordinated. 99mTc(CO)3(meso-LAN) is a stable product of the meso-LAN ligand (there is also a less stable isomer containing the meso-LAN ligand that converts to more stable product), and 99mTc(CO)3(dd,ll-LAN) is an enantiomeric mixture of dd- and ll-LAN isomers.
Rat Biodistribution Studies
Both 99mTc(CO)3(meso-LAN) and 99mTc(CO)3(dd,ll-LAN) showed rapid blood clearance in rats with < 6% of the injected dose remaining in the blood at 10 min after injection (Table 1). Both complexes also demonstrated rapid renal extraction and high specificity for renal excretion, with the dose in the urine at 60 min (as a percent of 131I-OIH) to be 89 ± 6% for 99mTc(CO)3(meso-LAN) and 87 ± 4% for 99mTc(CO)3(dd,ll-LAN). Less than 1% of total activity was present in the spleen, heart, and lung; moreover, there was minimal gastrointestinal activity [4.6% for 99mTc(CO)3(dd,ll-LAN) and 1.7% for 99mTc(CO)3(meso-LAN)].
TABLE 1.
Percent injected dose in rats of the 99mTc(CO)3(meso-LAN) and 99mTc(CO)3(dd,ll-LAN) in the blood, urine and selected organs at 10 and 60 minutes compared with 131I OIH (n = 5).
| Blood |
Kidney |
Urine |
Urine |
Liver |
Bowel |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isomer | 99mTc | 131I-OIH | 99mTc | 131I-OIH | 99mTc | 131I-OIH | % 99mTc/ 131I-OIH |
99mTc | 131I-OIH | 99mTc | 131I-OIH | |
| meso-LAN | ||||||||||||
| 10 min | 4.1 ± 0.4 | 3.3 ± 0.5 | 6.5 ± 2.8 | 4.9 ± 3.5 | 41.0 ± 7.3 | 59.9 ± 11.5 | 69 ± 6 | 4.2 ± 0.6 | 2.0 ± 0.2 | 1.2 ± 0.3 | 1.0 ± 0.2 | |
| 60 min | 0.6 ± 0.1 | 0.5 ± 0.1 | 1.2 ± 0.3 | 0.7 ± 0.3 | 77.9 ± 3.2 | 87.5 ± 8.2 | 89 ± 6 | 1.2 ± 0.5 | 0.8 ± 0.5 | 1.7 ± 0.1 | 0.9 ± 0.2 | |
| dd,ll-LAN | ||||||||||||
| 10 min | 5.7 ± 0.7 | 4.0 ± 0.6 | 9.5 ± 0.9 | 4.6 ± 1.0 | 33.4 ± 3.9 | 59.2 ± 3.6 | 56 ± 4 | 3.9 ± 0.5 | 2.3 ± 0.2 | 1.7 ± 0.4 | 1.1 ± 0.2 | |
| 60 min | 0.7 ± 0.2 | 0.4 ± 0.1 | 1.2 ± 0.3 | 0.3 ± 0.1 | 76.4 ± 7.2 | 88.0 ± 7.8 | 87 ± 4 | 0.8 ± 0.3 | 0.5 ± 0.3 | 4.6 ± 0.7 | 0.9 ± 0.1 | |
Data are presented as mean ± SD.
Normal Volunteer Studies
There was no evidence of any toxicity based on measurements of blood pressure, heart rate, temperature, CBC, standard chemistry panel or urine analysis for any of the volunteers. The clearance of 99mTc(CO)3(meso-LAN) averaged 228 mL/min, as compared to 176 mL/min for 99mTc(CO) 3(dd,ll-LAN) (Table 2), both substantially lower than the 538 mL/min clearance of 131I-OIH. The plasma protein binding was minimal for both 99mTc(CO)3(LAN) isomers and averaged 10% for meso-LAN and 2% for dd,ll-LAN. Red cell uptake was similar for the two isomers: 24% for meso-LAN and 21% for dd,ll-LAN. Both complexes had a relatively rapid renal excretion, with the difference that the dd,ll-LAN isomer was excreted more slowly than the meso-LAN isomer (the activity in urine as a percent of 131I-OIH (99mTc(CO)3(LAN)/131I-OIH) at 30 min and 180 min was 57 ± 6 and 85 ± 6, respectively, for meso-LAN and 45 ± 3 and 77 ± 6, respectively, for dd,ll-LAN) (Table 2). Image quality was excellent with both agents (Fig. 2). The time to peak (TTP) appeared to be slightly more prolonged with 99mTc(CO3)(LAN) complexes than with 131I-OIH, and 20 min/max ratios for whole kidney and cortical regions of interest (ROIs) appeared to be higher (Table 3). Representative 99mTc(CO3)(LAN) images and renogram curves, as well as simultaneous 131I-OIH images and curves, are shown in Fig. 2.
TABLE 2.
Clearance, protein binding, red cell binding and urine excretion of the 99mTc(CO)3(LAN) complexes in humans compared to simultaneously injected 131I-OIH (n = 3).
| Isomer |
99mTc(CO)3(LAN) clearance mL/min |
131I-OIH clearance mL/min |
99mTc(CO)3(LAN) /131I-OIH clearance ratio (%) |
Protein binding (%) |
Red cell binding (%) |
99mTc(CO)3(LAN) /131I-OIH 30 min urine ratio (%) |
99mTc(CO)3(LAN) /131I-OIH 180 min urine ratio (%) |
|---|---|---|---|---|---|---|---|
| meso-LAN | 228 ± 33 | 548 ± 37 | 42 ± 5 | 10 ± 0.6 | 24 ± 3.6 | 57 ± 6 | 85 ± 6 |
| dd,ll-LAN | 176 ± 8 | 528 ± 13 | 33 ± 2 | 2 ± 0.0 | 21 ± 8.6 | 45 ± 3 | 77 ± 6 |
Data are presented as mean ± SD.
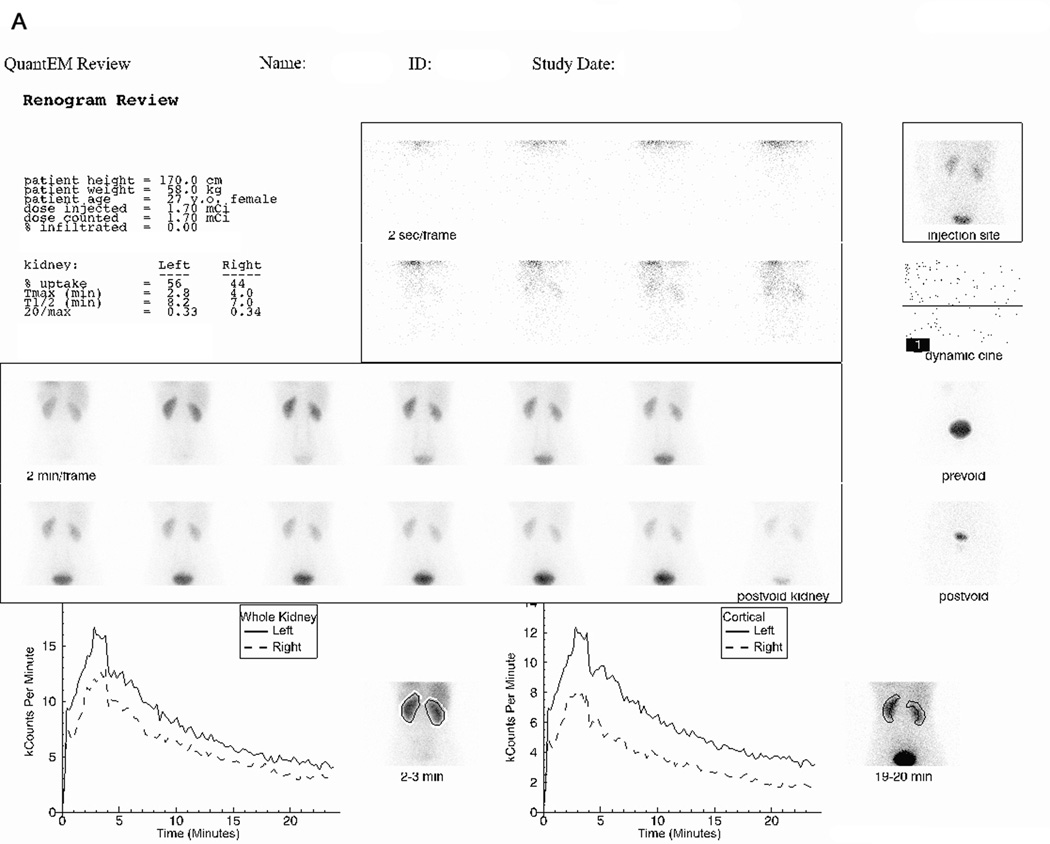
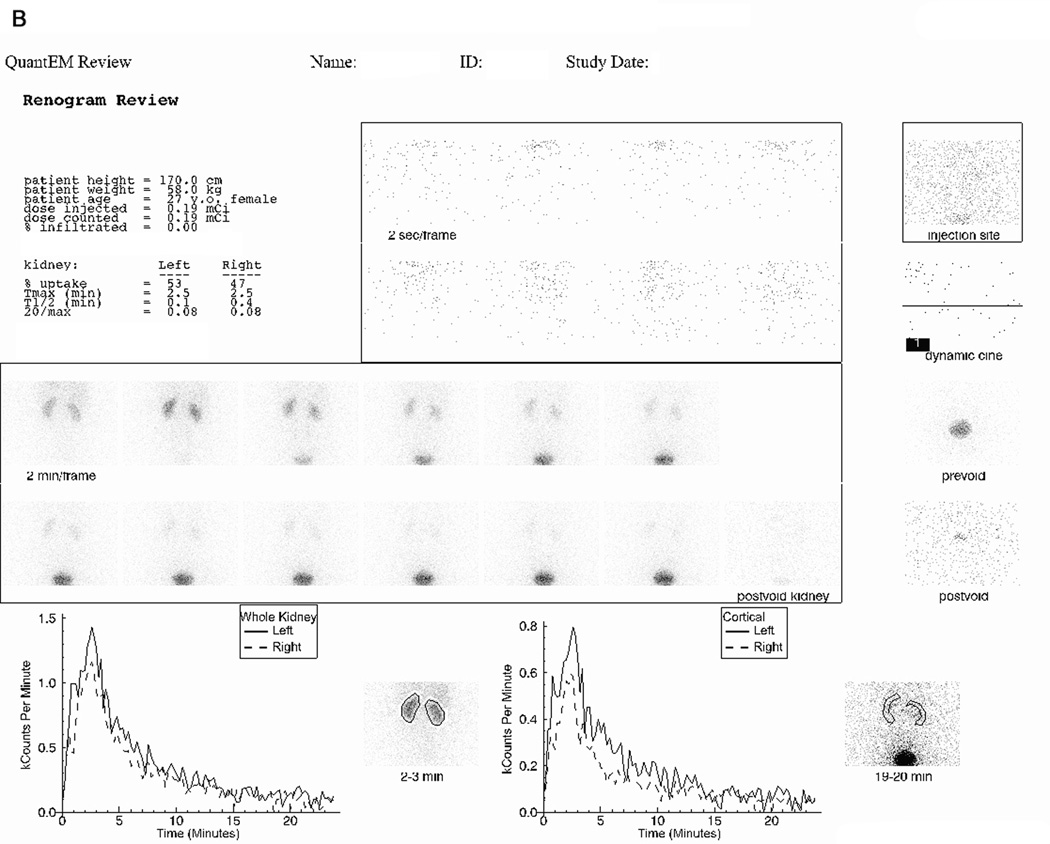
FIGURE 2.
(A) displays the 99mTc(CO)3(LAN) images and curves obtained from a 27-year-old female volunteer who received an intravenous injection containing 62.9 MBq (1.7 mCi) of 99mTc(CO)3(meso-LAN) and 7.03 MBq (0.19 mCi) of 131I-OIH followed by 24-minutes of data acquisition. Demographics and renogram data (relative uptake (% uptake), time to maximum counts (Tmax (min)), time to half maximum counts (T1/2 (min)), and the twenty minute to maximum count ratio (20/max) for the whole kidney region of interest) are displayed in the upper left panel. The middle upper panel shows flow images at 2-sec/frame. An image containing the injection site in her arm (right upper panel) showed no infiltration. The center panel shows good uptake by the kidneys bilaterally with prompt excretion into the bladder. Whole kidney renogram curves are shown in the left lower panel and cortical renogram curves in the right lower panel; and (B) displays the 131I-OIH images and renogram curves obtained from the volunteer described in (A) using identical regions of interest over the whole kidney and cortex. The 131I-OIH renogram curves are much noisier than the 99mTc(CO)3(LAN) renogram curves because of the lower dose of 131I-OIH and the fact that the camera is not optimized to image the high energy photon of 131I.
TABLE 3.
Renogram parameters of the 99mTc(CO)3(meso-LAN) and 99mTc(CO)3(dd,ll-LAN) complexes in humans compared to simultaneously injected 131I-OIH using whole kidney regions of interest (n = 3).
| Left Kidney |
Right Kidney |
Left Kidney |
Right Kidney |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Isomer | % 99mTc |
% 131I-OIH |
TTP* (min) 99mTc |
TTP* (min) 131I-OIH |
TTP* (min) 99mTc |
TTP* (min) 131I-OIH |
20 min/max† 99mTc |
20 min/max† 131I-OIH |
20 min/max† 99mTc |
20 min/max† 131I-OIH |
| meso-LAN(SD) | 47 (8.1) | 46 (7.0) | 4.66 (2.49) | 2.65 (0.53) | 3.58 (0.32) | 3.08 (0.59) | 0.35 (0.10) | 0.12 (0.09) | 0.29 (0.03) | 0.07 (0.02) |
| dd,ll-LAN (SD) | 60 (2.1) | 62 (4.2) | 3.17 (0.62) | 3.15 (0.75) | 3.01 (0.57) | 3.55 (0.19) | 0.39 (0.07) | 0.12 (0.06) | 0.33 (0.08) | 0.08 (0.04) |
TTP refers to time to peak height of the renogram curve.
The 20 min/max ratio refers to the counts in the kidney at 20 minutes post-injection divided by the maximum counts.
Metabolism Studies
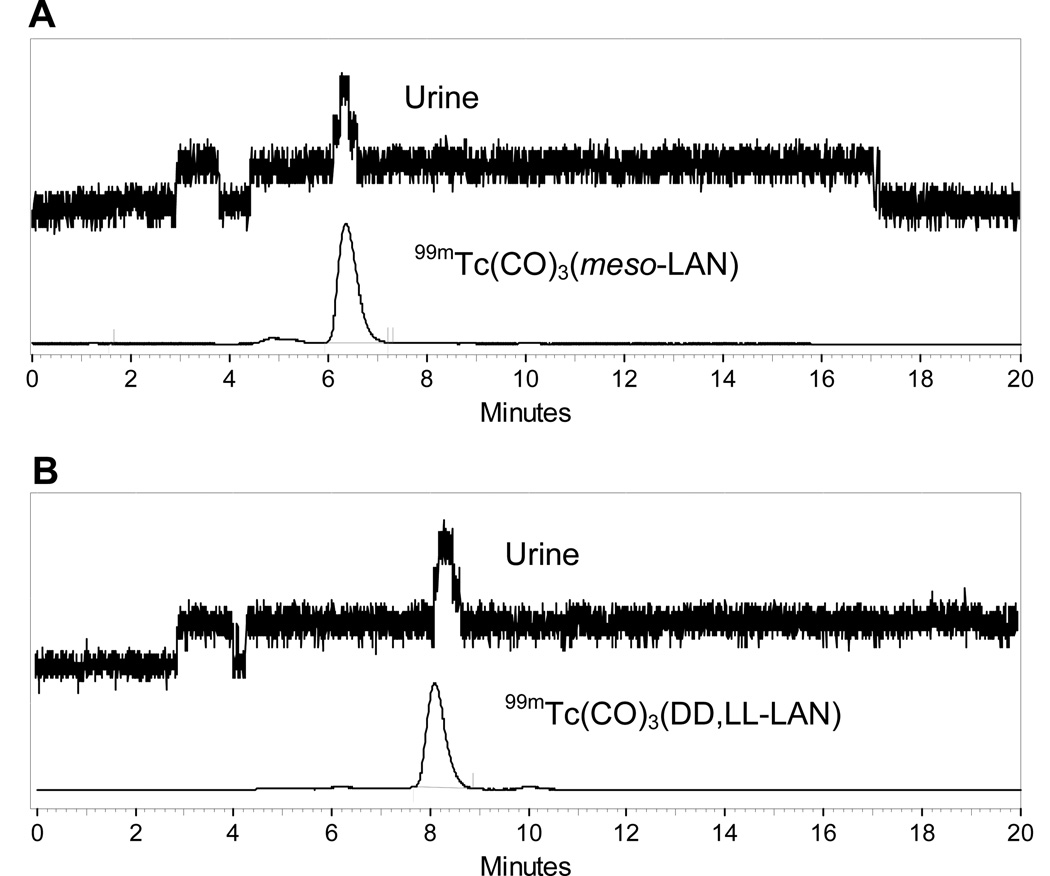
The urine was analyzed by HPLC to determine if the complexes were excreted intact. Greater than 95% of the activity recovered in the urine from both rats and humans coeluted with the respective HPLC-purified 99mTc(CO)3(meso-LAN) and 99mTc(CO)3(dd,ll-LAN) tracer, proving that each complex was excreted unchanged (Fig. 3).
FIGURE 3.
Urine samples from human volunteers injected with 99mTc(CO)3(meso-LAN) (panel A) and 99mTc(CO)3(dd,ll-LAN) (panel B) were subjected to γ-radioactive reversed-phase HPLC analysis. Corresponding reference HPLC traces show that both complexes were excreted unchanged in the urine.
DISCUSSION
A major focus of our research has been to develop radiopharmaceuticals possessing high renal clearance (13, 17–22). To obtain an agent with high renal clearance, 99mTc-labeled peptides and ligands are designed to target the organic anion tubular transporter of the proximal tubule (17, 22, 23). Small peptides are easy to synthesize and modify, are less likely than typical ligands to be immunogenic, and are more likely to exhibit rapid blood clearance. In most cases, the primary sites of interactions of the peptides are specific receptors on the outer surface of the cell membrane (extracellular). Thus, 99mTc-MAG3 (99mTc-mercaptoacetyltriglycine), 99mTc-EC, and 99mTc-MAEC (99mTc-mercaptoacetamide-ethylene-cysteine) (22)] are excreted primarily by tubular secretion, whereas the non-peptidic 99mTc-DTPA (99mTc-diethylenetriamine pentaacetic acid) is excreted by glomerular filtration and has a relatively low clearance compared to the other 99mTc renal agents.
All these factors make small peptides excellent candidates for the development of target-specific radiopharmaceuticals. However, as mentioned in the introduction, agents based on the newer peptidic ligands, while having clearances higher than that of 99mTc-MAG3, still have clearances less than those of 131I-OIH and PAH (p-aminohippurate). In an effort to define new cores for exploring ligands that could produce a superior 99mTc tubular agent, we decided to investigate the potential of the {99mTc(CO)3}+ core. This core has recently attracted growing interest, particularly after Alberto et al. reported an aqueous-based preparation of the [99mTc(CO)3(H2O)3]+ precursor (14, 24) and the introduction of the IsoLink boranocarbonate kit (Mallinckrodt, Petten, The Netherlands). As a relatively soft receptor, the {99mTc(CO)3}+ core prefers ligands with soft sp2 aromatic nitrogen and thioether donors (25–28). A bifunctional approach that incorporates ligating groups such as pyridyl or imidazole groups into amino acids or peptides has proved successful in labeling the {99mTc(CO)3}+ core (2, 29). However, we avoided incorporating pyridine rings into ligands to enhance labeling because pyridine rings tend to raise the overall lipophilicity of a complex, which usually leads to labeled agents with high hepatobiliary uptake, an undesirable property in a renal radiopharmaceutical (30).
Lanthionine (Fig. 1) is a small peptide (dipeptide) containing two free carboxyl groups that would be recognized by the anionic renal tubular transport system. Moreover, it is a simple NS ligand that efficiently produces uniform products when labeled with the 99mTc tricarbonyl core. In humans, only 10% of 99mTc(CO)3(meso-LAN) and 2 % of 99mTc(CO)3(dd,ll-LAN) are protein bound. These protein binding levels are much lower than those for 99mTc-MAG3 (PPB ~80%), 99mTc-dd-EC (PPB ~28%), or syn-99mTc-d-MAEC (PPB ~87%). Reduced protein binding is a desirable property in a renal radiopharmaceutical because it facilitates clearance by glomerular filtration as well as tubular extraction (31). The clearance of both 99mTc tricarbonyl agents exceeds the glomerular filtration rate; this fact indicates that these complexes must be transported by the renal tubules and, as anionic tracers, they likely share the same tubular transport process as for 131I-OIH, 99mTc-MAG3, 99mTc-EC, and 99mTc-MAEC.
All three renal tubular agents with the 99mTcO core and high renal clearance in humans, 99mTc-MAG3, 99mTc-dd-EC, and syn-99mTc-d-MAEC, contain an oxo-technetium-glycyl sequence with a CO2− group syn to the oxo ligand (syn-CO2−), and structure-distribution relationships suggest that the combination of the oxo and syn-CO2− groups is responsible for receptor recognition (32). Results in rodents generally show a similar dependence with syn isomers showing better clearance than anti isomers for agents with one carboxyl group. In rodents, however, the results for 99mTc-EC isomers do not show this dependence.
Labeling of a mixture of dd, ll, and dl-EC ligands resulted in a mixture of products that were resolved by HPLC into three peaks, one for the complexes with the chiral ligands (99mTc-dd-EC and 99mTc-ll-EC) and one for each of the two meso forms (syn- and anti-99mTc-dl-EC). In mice, biodistribution studies showed no significant differences in renal excretion, hepatobiliary excretion, or blood clearance between any of the three peaks (33–35). In rats, clearance, extraction efficiency, and biodistribution results were almost identical for all four separated 99mTc-EC isomers (17, 36). In humans, however, our results showed that 99mTc-dd-EC and 99mTc-ll-EC had similar clearance ratios (99mTc-EC/131I-OIH: 82% and 70%, respectively) which were significantly higher than the 40% clearance ratio for syn-99mTc-dl-EC (17). Similarly, the percent injected dose (99mTc-EC/131I-OIH) at 0–30 min in the urine was 90% and 92% for 99mTc-dd-EC and 99mTc-ll-EC, respectively, compared to 57% for syn-99mTc-dl-EC.
Our new 99mTc tricarbonyl agents are based on a completely different core with different physical properties and do not contain the oxo-technetium-glycyl sequence, but they still exhibit high specificity for renal excretion. In rats, there was no difference in the excretion of the 99mTc(CO)3(LAN) isomers at 60 min in spite of the relative absolute configuration of the asymmetric carbons; however, in humans, the meso-LAN isomer appears to be superior to the dd,ll-LAN isomer (Table 2). It should be noted that 99mTc(CO)3(dd,ll-LAN) should have been a superior tracer to 99mTc(CO)3(meso-LAN) by superficial analogy to 99mTc-EC biodistribution on the basis of the fact that both types of agents contain two dangling carboxylate groups. This similar lack of dependence on stereochemistry in rodent biodistribution, combined with a contrasting dependence on chiral vs meso stereochemistry, led us to analyze more deeply all these structures to understand better the relationship between a particular structure and its renal clearances.
The two CO2 groups project in opposite directions in the isomer with the better clearance and higher rate of excretion in urine, 99mTc(CO) 3(meso-LAN), and in the same direction in the isomer with the lower clearance and lower rate of excretion in urine, 99mTc(CO)3(dd,ll-LAN) (Fig. 4). In this regard, our new results parallel those with 99mTc-EC agents. For both dd- and ll-EC isomers the two CO2 groups are on opposite sides of the structure. The lower extraction efficiency of the dl-EC isomer and 99mTc(CO)3(dd,ll-LAN) may be due to the steric properties of two bulky carboxylate groups (CO2) on the same side of the molecule or to electrostatic effects due to the fact that the two CO2 groups are ionized and in close proximity to each other (Fig. 4). This feature appears to affect biodistribution in humans but not in rodents for agents with two carboxyl groups. This difference in the way the carboxyl groups project between the more classical 99mTc-EC agents and our new 99mTc(CO)3(LAN) agents is a direct consequence of the stereochemistry imposed by the cores: the Tc-tricarbonyl core imposes a triangular facial ligand coordination in an agent with a pseudo octahedral geometry, and the Tc-oxo core imposes a planar square-like ligand coordination in an agent with a pseudo square-pyramidal geometry. It is very interesting that one carboxyl group in the meso compound is situated very close to a carbonyl group, yet this agent has very good clearance. The result offers hope that the effects of the relatively non-polar carbonyl groups may not adversely impact the recognition of the tracer by the proximal tubular receptor.
FIGURE 4.
Schematic drawing of the spatial relationships of the carboxylate groups (CO2) to each other and to the plane defined by donor atoms in 99mTc-EC and 99mTc(CO)3(LAN) complexes.
Another approach toward understanding the effects of changes at the Tc-center on biological properties of 99mTc radiopharmaceuticals involves comparison of complexes containing the same chelating ligand but with different Tc-cores. In recent biodistribution experiments in mice, Rattat et al. (37) studied the characteristics of three different DTPA complexes: 99mTc-DTPA (with the Tc-oxo core), 99Tc(CO)3(DTPA) (with the Tc-tricarbonyl core), and 99mTc(CO)2(NO)(DTPA) (with a Tc-dicarbonyl-nitrosyl core). 99mTc-DTPA, a renal imaging radiopharmaceutical with the “classic” core, was excreted rapidly via the kidneys and had a low overall uptake in all other organs. Labeling DTPA with the 99mTc-tricarbonyl core led to an agent with a decreased excretion rate, a slightly higher liver uptake and a longer retention in blood. Introduction of the 99mTc-dicarbonyl-nitrosyl core resulted in a significant increase in the liver uptake, while excretion via the kidneys dropped to a negligible level as compared to those of 99mTc-DTPA. These three different DTPA agents showed different physical and biological characteristics, and these differences can be attributed to the consequences of the modifications at the Tc-center. However, because the exact chemical speciation of 99mTc(CO)3(DTPA) and 99mTc(CO)2(NO)(DTPA) has not been defined (38), the extent to which the spatial relationship of the carboxyl groups to each other and to the different cores influences biodistribution is unclear, and further studies are needed.
CONCLUSION
Results in rats show that both 99mTc(CO)3(LAN) isomers are rapidly excreted in the urine and have a high specificity for renal excretion. Moreover, we describe the first application of a 99mTc tricarbonyl renal radiopharmaceutical in humans and our results offer promise that a complex based on the {Tc(CO)3}+ core could be an excellent renal imaging agent with a high plasma clearance. Although the plasma clearance and the rate of renal excretion are still less than those of 131I-OIH, these data provide support for the continued development of renal and other radiopharmaceuticals based on the 99mTc tricarbonyl core. Additional ligand design and testing will be required to develop a 99mTc renal tracer that will provide a direct measurement of effective renal plasma flow.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health grant no. DK38842. We thank Dr. Patricia A. Marzilli for her invaluable comments during the preparation of the paper.
REFERENCES
- 1.Hoepping A, Reisgys M, Brust P, et al. TROTEC-1: A new high-affinity ligand for labeling of the dopamine transporter. J Med Chem. 1998;441:4429–4432. doi: 10.1021/jm981008a. [DOI] [PubMed] [Google Scholar]
- 2.Egli A, Alberto R, Tannahill L, et al. Organometallic 99mTc-aquaion labels peptide to an unprecedented high specific activity. J Nucl Med. 1999;40:1913–1917. [PubMed] [Google Scholar]
- 3.Schibli R, Katti KV, Higginbotham C, Volkert WA, Alberto R. In vitro and in vivo evaluation of bidentate, water-soluble phosphine ligands as anchor groups for the organometallic fac-[99mTc(CO)3]+-core. Nucl Med Biol. 1999;26:711–716. doi: 10.1016/s0969-8051(99)00028-1. [DOI] [PubMed] [Google Scholar]
- 4.Schibli R, La Bella R, Alberto R, et al. Influence of the denticity of ligand systems on the in vitro and in vivo behavior of99mTc(I)-tricarbonyl complexes: a hint for the future functionalization of biomolecules. Bioconjugate Chem. 2000;11:345–351. doi: 10.1021/bc990127h. [DOI] [PubMed] [Google Scholar]
- 5.Schibli R, Schubiger PA. Current use and future potential of organometallic radiopharmaceuticals. Eur J Nucl Med. 2002;29:1529–1542. doi: 10.1007/s00259-002-0900-8. [DOI] [PubMed] [Google Scholar]
- 6.Dyszlewski M, Blake HM, Dahlheimer JL, Pica CM, Piwnica-Worms D. Characterization of a novel99mTc-carbonyl complex as a functional probe of MDR1 P-glycoprotein transport activity. Molecular Imaging. 2002;1:24–35. doi: 10.1162/15353500200200002. [DOI] [PubMed] [Google Scholar]
- 7.Saidi A, Seifert S, Kretzschmar M, Bergmann R, Pietzsch H-J. Cyclopentadienyl tricarbonyl complexes of99mTc for the in vivo imaging of the serotonin 5-HT1Areceptor in the brain. J Organomet Chem. 2004;689:4739–4744. [Google Scholar]
- 8.Mundwiler S, Candreia L, Hafliger P, Ortner K, Alberto R. Preparation of no-carrier-added technetium-99m complexes via metal-assisted cleavage from a solid phase. Bioconjugate Chem. 2004;15:195–202. doi: 10.1021/bc034171f. [DOI] [PubMed] [Google Scholar]
- 9.Alberto R, Pak JK, van Staveren D, Mundwiler S, Benny P. Mono-, bi-, or tridentate ligands? The labeling of peptides with99mTc-carbonyls. Biopolymers. 2004;76:324–333. doi: 10.1002/bip.20129. [DOI] [PubMed] [Google Scholar]
- 10.Kothari KK, Satpati D, Joshi S, Venkatesh M, Ramamoorthy N, Pillai MRA. 99mTc carbonyl t-butyl isonitrile: a potential new agent for myocardial purfusion imaging. Nucl Med Commun. 2005;26:155–161. doi: 10.1097/00006231-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Buchegger F, Bonvin F, Kosinski M, et al. Radiolabeled neurotensin analog,99mTc-NT-XI, evaluated in ductal pancreatic adenocarcinoma patients. J Nucl Med. 2003;44:1649–1654. [PubMed] [Google Scholar]
- 12.Lipowska M, Cini R, Tamasi G, Xu X, Taylor TA, Marzilli LG. Complexes having the fac-{M(CO)3}+core (M = Tc, Re) useful in radiopharmaceuticals: X-ray and NMR structural characterization and density functional calculations of species containing two sp3N donors and one sp3O donor. Inorg Chem. 2004;43(24):7774–7783. doi: 10.1021/ic049544i. [DOI] [PubMed] [Google Scholar]
- 13.He H, Lipowska M, Xu X, Taylor A, Carlone M, Marzilli LG. Re(CO)3complexes synthesized via an improved preparation of aqueous fac-[Re(CO)3(H2O)3]+as an aid in assessing99mTc imaging agents. Structural characterization and solution behavior of complexes with thioether-bearing amino acids as tridentate ligands. Inorg Chem. 2005;44:5437–5446. doi: 10.1021/ic0501869. [DOI] [PubMed] [Google Scholar]
- 14.Alberto R, Schibli R, Egli A, Schubiger AP, Abram U, Kaden TA. A novel organometallic aqua complex of technetium for the labeling of biomolecules: synthesis of [99mTc(OH2)3(CO)3]+from [99mTcO4]-in aqueous solution and its reaction with a bifunctional ligand. J Am Chem Soc. 1998;120:7987–7988. [Google Scholar]
- 15.Lipowska M, He H, Malveaux E, Xu X, Marzilli LG, Taylor A. New Tc-99m tricarbonyl complexes with dipeptide (lanthionine): synthesis, characterization, and evaluation as potential renal imaging agents [abstract] J Nucl Med. 2005;46(suppl):363P. [Google Scholar]
- 16.Sapirstein L, Vidt DG, Mandel MJ, Hanusek G. Volumes of distribution and clearances of intravenously injected creatinine in the dog. Am J Physiol. 1955;181:330–336. doi: 10.1152/ajplegacy.1955.181.2.330. [DOI] [PubMed] [Google Scholar]
- 17.Taylor A, Hansen L, Eshima D, et al. Comparison of technetium-99m-LL-EC isomers in rats and humans. J Nucl Med. 1997;38(5):821–826. [PubMed] [Google Scholar]
- 18.Hansen L, Hirota S, Xu X, Taylor A, Marzilli LG. Nature of cysteine-based Re(V)=O(N2S2) radiopharmaceuticals at physiological pH ascertained by investigation of a new complex with a meso N2S2ligand having carboxyl group anti to the oxo group. Inorg Chem. 2000;39:5731–5740. doi: 10.1021/ic000183q. [DOI] [PubMed] [Google Scholar]
- 19.Lipowska M, Hansen L, Marzilli LG, Taylor A. The new renal imaging agent99mTc(CO)3(ENDAC) and the chemistry of the Re(CO)3(ENDAC) [abstract] J Nucl Med. 2001;42(suppl):259P. [Google Scholar]
- 20.Lipowska M, Hansen L, Cini R, et al. Synthesis of new N2S2ligands and Re(V)O(N2S2) analogues of99mTc renal imaging agents. Characterization by NMR spectroscopy, molecular mechanics calculations, and X-ray crystallography. Inorg Chim Acta. 2002;339:327–340. [Google Scholar]
- 21.Lipowska M, Malveaux E, He H, Marzilli LG, Taylor A. Synthesis, characterization, and evaluation of novel Tc-99m tricarbonyl complex as potential renal imaging agent [abstract] J Nucl Med. 2003;44(suppl):316P. [Google Scholar]
- 22.Taylor A, Lipowska M, Hansen L, Malveaux E, Marzilli LG. 99mTc-MAEC complexes: new renal radiopharmaceuticals combining characteristics of 99mTc-MAG3 and99mTc-EC. J Nucl Med. 2004;45:885–891. [PubMed] [Google Scholar]
- 23.Shikano N, Kanai Y, Kawai K, Ishikawa N, Endou H. Transport of99mTc-MAG3 via rat renal organic anion transporter 1. J Nucl Med. 2004;45:80–85. [PubMed] [Google Scholar]
- 24.Alberto R, Ortner K, Wheatley N, Schibli R, Schubiger AP. Synthesis and properties of boranocarbonate: a convenient in situ CO source for the aqueous preparation of [99mTc(OH2)3(CO)3]+ J Am Chem Soc. 2001;123:3135–3136. doi: 10.1021/ja003932b. [DOI] [PubMed] [Google Scholar]
- 25.Wust F, Skaddan MB, Leibnitz P, Spies H, Katzenellenbogen J, Johannsen B. Synthesis of novel progestin-rhenium conjugates as potential ligands for the progesterone receptor. Bioorg Med Chem. 1999;7:1827–1835. doi: 10.1016/s0968-0896(99)00119-4. [DOI] [PubMed] [Google Scholar]
- 26.Pietzsch H-J, Gupta A, Reisgys M, et al. Chemical and biological characterization of technetium(I) and rhenium(I) tricarbonyl complexes with dithioether ligands serving as linkers for coupling the Tc(CO)3and Re(CO)3moieties to biologically active molecules. Bioconjugate Chem. 2000;11:414–424. doi: 10.1021/bc990162o. [DOI] [PubMed] [Google Scholar]
- 27.Alves S, Paulo A, Correia JDG, Domingos A, Santos I. Coordination capabilities of pyrazolyl containing ligands towards the fac-[Re(CO)3]+moiety. J Chem Soc Dalton Trans. 2002:4714–4719. [Google Scholar]
- 28.Santos IG, Abram U, Alberto R, Lopez EV, Sanchez A. Tricarbonylrhenium(I) complexes with thiosemicarbazone derivatives of 2-acetylpyridine and 2-pyridine formamide showing two unusual coordination modes of tridentate thiosemicarbazone ligands. Inorg Chem. 2004;43(6):1834–1836. doi: 10.1021/ic035367u. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee SR, Levadala MK, Lazarova N, et al. Bifunctional single amino acid chelates for labeling of biomolecules with the {Tc(CO)3}+and {Re(CO)3}+cores. Crystal and molecular structures of [ReBr(CO)3(H2NCH2C5H4N)], [Re(CO)3{(C5H4NCH2)2NH}]Br, [Re(CO)3{(C5H4NCH2)2NCH2CO2H}]Br, [Re(CO)3{X(Y)NCH2CO2CH2CH3}]Br (X = Y = 2-pyridylmethyl X = 2-pyridylmethyl, Y = 2-(1-methylimidazolyl)methyl X = Y = 2-(1-methylimidazolyl)methyl), [ReBr(CO)3{(C5H4NCH2)NH(CH2C4H3S)}], and [Re(CO)3{(C5H4NCH2)N(CH2C4H3S)(CH2CO2)}] Inorg Chem. 2002;41:6417–6425. doi: 10.1021/ic020476e. [DOI] [PubMed] [Google Scholar]
- 30.Vanbilloen HP, Bormans GM, De Roo MJ, Verbruggen AM. Complexes of technetium-99m with tetrapeptides, a new class of 99mTc-labelled agents. Nucl Med Biol. 1995;22(3):325–338. doi: 10.1016/0969-8051(94)00110-6. [DOI] [PubMed] [Google Scholar]
- 31.Eshima D, Eshima L, Hansen L, Lipowska M, Marzilli LG, Taylor A. Effect of protein binding on renal extraction of 131I-OIH and 99mTc-labeled tubular agents. J Nucl Med. 2000;41:2077–2082. [PubMed] [Google Scholar]
- 32.Fritzberg AR, Kuni CC, Klingensmith III WC, Stevens J, Whitney WP. Synthesis and bioligical evaluation of Tc-99m N,N'-bis(mercaptoacetyl)-2,3-diaminopropanoate: a potential replacement for [131I] o-iodohippurate. J Nucl Med. 1982;23:592–598. [PubMed] [Google Scholar]
- 33.Verbruggen AM, Nosco DL, Van Nerom CG, Bormans GM, Adriaens PJ, De Roo MJ. Technetium 99m-L,L-ethylenedicysteine: a renal imaging agent. I. Labeling and evaluation in animals. J Nucl Med. 1992;33:551–557. [PubMed] [Google Scholar]
- 34.Van Nerom C, Bormans G, Cleynhens B, Nosco D, De Roo M, Verbruggen A. Comparison of renal excretion characteristics of isomers L,L and D,D of 99mTc ethylenedicysteine [abstract] J Nucl Med. 1990;31:806. [Google Scholar]
- 35.Verbruggen A, Bormans G, Van Nerom C, Cleynhens B, Osiadacz D, De Roo M. Is the syn and anti orientation of the oxotechnetium and carboxyl group in 99mTc renal function agents affecting the renal excretion rate? J Labl Comp Radiopharm. 1991;30:86–88. [Google Scholar]
- 36.Hansen L, Taylor A, Marzilli LG. Influence of stereoisomerism on structure and renal clearance. J Nucl Med. 1999;40(suppl):320P. [Google Scholar]
- 37.Rattat D, Terwinghe C, Verbruggen A. Comparison of 'classic'99mTc-DTPA,99mTc(CO)3-DTPA and 99mTc(CO)2(NO)-DTPA. Tetrahedron. 2005;61:9563–9568. [Google Scholar]
- 38.Rattat D, Eraets K, Cleynhens B, Knight H, Fonge H, Verbruggen A. Comparison of tridentate ligands in competition experiments for their ability to form a [99mTc(CO)3] complex. Tetrahedron Lett. 2004;45:2531–2534. [Google Scholar]