Introduction
Morphea, or localized scleroderma, can at times constitute a severely disabling and aggressive disease with no single therapy proven to be curative or the standard of care. Herein, we report the case of a patient with severe disabling generalized deep morphea who showed a marked clinical response to treatment with extracorporeal photochemotherapy (ECP).
Case Report
Ten years ago, a 49-year-old white patient presented with a reticulated erythema and induration of her abdominal skin. Over the next 5 years, the erythema and induration progressed to include the skin over her lower extremities, chest, back, and upper extremities. With the onset of new lesions, she frequently experienced pruritus and tenderness at the areas of involvement. She also noted decreased ranges of motion in her shoulders. After a 2-year period without progression, she again developed worsening of her symptoms with progression of existing lesions and new areas of involvement accompanied by arthralgias in her shoulders, ankles, knees, wrists, and elbows and myalgias in her thighs and calves. Her symptoms interfered with her mobility and her ability to work a full day owing to severe fatigue and pain. She described frequently awakening at night with pain and discomfort in her skin and joints. Her medical, surgical, and family histories were noncontributory. There was no history of exposure to toxins, including bleomycin, polyvinyl chloride, or tryptophan. A review of systems was negative for dysphagia and Raynaud phenomenon.
On physical examination, the bilateral extensor surfaces of the upper extremities from the deltoids to the triceps demonstrated a cobblestoned appearance. Hyperpigmentation was noted over the bilateral extensor forearms. Mild cobblestoning was present over the medial surface of the bilateral breasts and the bilateral buttocks. There were coalescing indurated, bound-down, reddish-purple, hypopigmented and hyperpigmented plaques over the abdomen (Figure 1). Thick, cobblestoned, erythematous, nearly circumferential plaques were found on the bilateral thighs with severe, deep erythematous bands over the sartorius muscles bilaterally.
Figure 1.
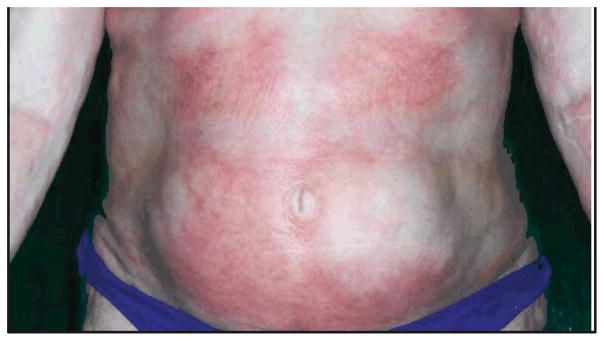
The patient’s abdominal skin prior to treatment. Indurated and bound-down plaques coalesced to involve most of the abdominal skin. These lesions were tender to palpation and pruritic.
Findings from a comprehensive metabolic profile and complete blood cell count were within reference range, including a normal eosinophil count. An antinuclear antibody test was positive with a titer of 1:320 and a speckled pattern. Findings from an upper endoscopy were unremarkable. Clinical presentation and findings from a punch biopsy specimen from the abdomen were consistent with generalized and deep morphea.
Previous treatments were numerous and included several topical corticosteroids, calcipotriene, and tacrolimus. Oral tetracycline antibiotics were prescribed for 6 years, including doxycycline, once daily, and minocycline, twice daily. Multiple prednisone tapers with a maintenance dose of 5 mg every other day were prescribed over a 2-year period. This was followed by several other unsuccessful therapeutic trials: psoralen–UV-A phototherapy 3 times a week for 7 months; azathioprine, 50 mg twice daily for 6 months; and mycophenolate mofetil for 1 year, up to a maximum oral dose of 6 g/d. Despite these therapeutic trials, the patient continued to experience progression of her disease.
Therapeutic Challenge
The treatment of generalized deep morphea is challenging, and no single therapy has proven to be very effective or significantly disease modifying.1,2 Numerous treatments, some with potentially hazardous adverse effects, are currently used with only limited success.3
Topical and intralesional corticosteroids may be useful for discrete lesions, and newer topical agents such as tacrolimus ointment and imiquimod cream have shown some benefit in open-label trials.2,4–6 Daily antimalarial agents, such as hydroxychloroquine, are prescribed, presumably for their immunomodulating properties.1,7 Systemic corticosteroids are used for more advanced disease and have been shown to be beneficial in an open-label study, as has methotrexate with and without pulsed methylprednisolone.1,2,4,6,7 Other agents that have been tried include vitamin E, topical and oral calcipotriol, aminobenzoate potassium, phenytoin sodium, penicillin and tetracycline antibiotics, retinoids, interferon gamma, cytotoxic agents, and bosentan (an endothelin receptor antagonist).1,4,6,7 Most of these therapies have shown mixed results.1 Partial responses have been reported with broadband UV-A treatment with and without 8-methoxypsoralen and with high- and low-dose long-wave UV-A1 therapy.1–4,6 Physical therapy is particularly important in deep morphea to prevent joint deformities and skin contractures.2,4,5,7
Solution
Extracorporeal photochemotherapy is an immunotherapy in which a patient’s peripheral blood leukocytes are isolated, bathed in the photosensitizing compound 8-methoxypsoralen, passed through a thin plastic exposure plate while irradiated with UV-A light, and returned intravenously.2,8–12 Based on our experience using ECP in the treatment of chronic graft-vs-host disease (GVHD), and because the patient had failed all previous attempted treatments, the decision was made to attempt a 4-month trial of ECP.
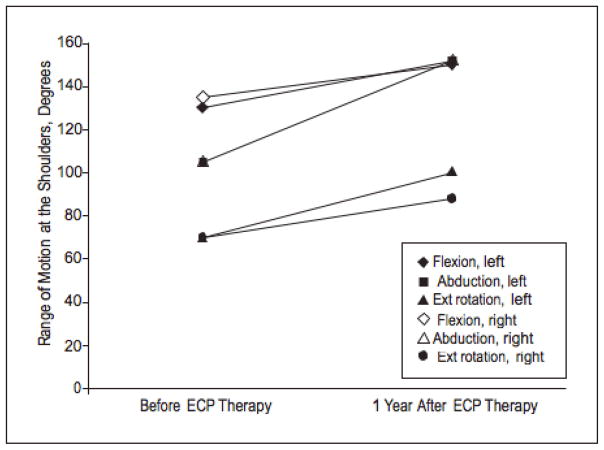
The patient initially began ECP with a regimen of 2 consecutive treatment days at 2-week intervals. She also began weekly physical therapy sessions. She reported improvement in her symptoms within 1 to 2 months of starting treatment. She first noticed an increased energy level, increased hair growth on her legs, and increased flexibility. She has had a dramatic improvement in her symptoms and has maintained a marked softening of hardened, sclerotic skin on her abdomen and upper and lower extremities, as well as increased ranges of motion in her shoulders. Of note, the plaques on her abdomen have resolved (Figure 2), and the near-circumferential plaques on her thighs have become mild and faded. There was a clear objective response to treatment as evidenced by her improved ranges of motion at the shoulders as measured by her physical therapist (Figure 3). By 4 months after starting ECP, her treatment interval was decreased to every 3 weeks, and then to every 4 weeks 3 months later.
Figure 2.
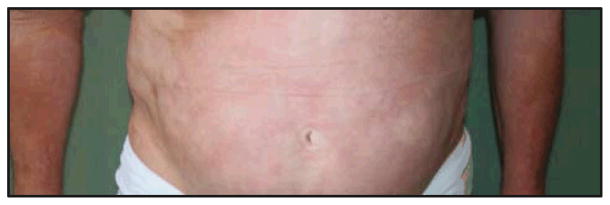
The patient’s abdominal skin after 15 months of treatment. The indurated plaques had completely resolved, with marked softening noted. A residual reticulated erythema remained.
Figure 3.
Improvement in the patient’s ranges of motion in the shoulders over 1 year during treatment with extracorporeal photochemotherapy (ECP). Ext indicates external.
Subjectively, the patient is, in her own words, able to “enjoy life again,” taking on projects like yard work and cooking, which were once not feasible owing to fatigue and decreased mobility. She is currently working her regularly scheduled hours and is no longer awakening at night with pain and discomfort in her skin and joints. We have been attempting to taper her treatments using a protocol based on our experience with chronic GVHD. She is currently being treated on 2 consecutive days every 5 weeks with a taper schedule as follows: 3 treatments at every 6 weeks, then 3 treatments at every 7 weeks, then 3 treatments at every 8 weeks, then, if stable, treatment will be discontinued.
Comment
Scleroderma encompasses a spectrum of disorders characterized by thickening of the skin and subcutaneous tissue.1,4 The 2 main clinical categories of scleroderma include systemic sclerosis, in which visceral changes are present, and localized scleroderma or morphea, in which lesions are limited to the skin and subcutaneous tissues.1,2 Peterson et al7 proposed a morphea classification that is used commonly today and includes the following types of morphea: plaque, generalized, bullous, linear and deep, with several types of morphea each having further subtypes.1,4 Deep morphea is present when inflammation and sclerosis are found in the deep dermis, panniculus, fascia, or superficial muscle.1,4,7 Generalized morphea occurs when individual plaques number 4 or more and are larger than 3 cm in diameter, become confluent lesions or multiply, and/or affect more than 2 anatomic sites.2,6,7 The distinction between deep and generalized morphea is not clear, and the patient presented in this case would most accurately be characterized as having both deep morphea (morphea profunda) and generalized morphea.1
Deep morphea (morphea profunda) is characterized by plaques that are diffuse, mildly inflamed, hyperpigmented, symmetrical, and somewhat ill defined.1 The skin feels thickened, taut, and bound down to the underlying fascia and muscle. 1,6,7 Plaques are smooth and shiny, but areas of both dermal and subcutaneous atrophy may be present, especially in chronic lesions.1 The onset of sclerosis is gradual and tends to occur over a period of several months.1 Flexion contractures of the joints are a relatively frequent finding, as is carpal tunnel syndrome.1,7 Arthralgias, arthritis, or myalgias are commonly present, as is the case with our patient.1
The onset of generalized morphea is usually insidious, and it can progress to involve widespread areas of the body.4 Lesions mainly involve the upper trunk, breast, abdomen, and upper thighs; however, the arms, face, neck, and scalp may also be involved.5 Despite extensive skin involvement, these patients rarely have internal organ involvement, but up to 40% may experience arthralgias.4,7
The cause of morphea is unknown.1,2,7 Fibroblast proliferation and excessive accumulation of extracellular matrix proteins, mainly collagen, is the final common pathway.1,4 Transforming growth factorβ, interleukin 1 (IL-1), IL-2, IL-4, IL-6, IL-8, IL-13, and connective tissue growth factor are thought to play important roles in pathogenesis, although the details have not yet been fully elucidated.1,2,4,7 An autoimmune cause is supported by the presence of autoantibodies.7 Diagnosis of morphea is based on clinicopathologic correlation.7 Homogenization of collagen bands is the basic histologic change.1,7 Histologic differentiation among the different types of morphea is determined by the depth of inflammation and sclerosis, although there is considerable histologic overlap.1,7
Hypergammaglobulinemia, peripheral eosinophilia, and an increased erythrocyte sedimentation rate can all be seen in patients with morphea.1,7 Various circulating autoantibodies can be found in all types of morphea, with most titers correlating with the burden of skin disease.4,7,13 Antinuclear antibodies are found in 46% to 80% of cases, anti–single-stranded DNA antibodies are found in 50% of cases, and antihistone antibodies are found in 47% to 87% of cases.1,4,5,7,13 Rheumatoid factor is seen in up to 60% of all cases of morphea and in up to 82% of cases of generalized morphea and may predict articular involvement.4,7,13 Antitopoisomerase IIa antibodies are found in up to 76% of patients with morphea and in 85% of those with generalized morphea.4,6,13
The natural history of disease progression is variable; however, the long-term prognosis of patients with morphea is generally good.4 Lesions of morphea tend to be self-limited with activity or progression lasting 3 to 5 years, followed by residual pigmentary and atrophic changes.2,4,7 Less commonly, reactivation can occur or cases will become generalized, progress for several years, and result in marked deformities.7
Extracorporeal photochemotherapy is one of the most successful and broadly applied forms of immunotherapy, used in the treatment of cutaneous T-cell lymphoma, transplant rejection, autoimmune diseases, and GVHD.9,11 Extracorporeal photochemotherapy showed beneficial results in a single study8 in 1 patient with linear morphea, with reduction in plaque number and decreased prominence of remaining plaques.4 It has also been shown to be effective to varying degrees in the treatment of systemic sclerosis and chronic GVHD, diseases that share many similarities with generalized deep morphea. 10–12 A multicenter, randomized, single blind controlled trial was performed to examine the benefit from ECP in the treatment of systemic sclerosis.8,10 After 6 months of treatment, a statistically significant reduction in skin severity scores was observed in 68% of patients receiving ECP compared with 32% of patients prescribed a regimen of D-penicillamine.8,10 The efficacy of ECP in systemic sclerosis was further evaluated in a more recent randomized, double-blind, placebo-controlled clinical trial conducted at 16 investigational sites world-wide.10 A statistically significant improvement in skin scores and joint involvement compared with baseline was observed at 6 months and 12 months among those who received active ECP.10
The mechanism by which ECP is of therapeutic benefit in morphea, systemic sclerosis, and chronic GVHD remains unproven.10 However, because systemic sclerosis, chronic GVHD, and generalized deep morphea share clinical and histologic skin findings, the proposed mechanisms of action of ECP in chronic GVHD and systemic sclerosis might apply to morphea as well. Experimental data from animal autoimmune and transplantation studies suggest that photopheresed cells induce an antigen-specific immune response against pathogenic T-cell populations without affecting general immunocompetence.10–12 It has also been shown that a considerable proportion of patients with systemic sclerosis and chronic GVHD have an expanded monoclonal T-cell population both in their peripheral blood and at the sites of tissue damage.10–12 These T-cell populations may play an autoreactive, disease-mediating role in morphea, systemic sclerosis, and chronic GVHD and may be the target of an immune response induced by ECP.10–12 It has been postulated that the immature dendritic cells shown to be produced by ECP may suppress such unwanted immune effector cells through induction of CD25+, CTLA-4+ regulatory T cells.9 Furthermore, at least with respect to patients with chronic GVHD, patients with a dominant peripheral T-cell clone seem to show the highest clinical response to ECP.10–12 This has yet to be studied in morphea.
In conclusion, the patient in the case presented herein experienced both a reactivation of disease and an aggressive, progressive clinical course prior to starting ECP. Fortunately, she has benefited profoundly from this treatment. Because ECP is one of the safest treatment options for morphea, with a very low adverse effect profile, it should be further investigated and incorporated in the treatment arsenal for this disease.
Footnotes
Financial Disclosure: None reported.
Author Contributions: All of the authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Neustadter and Girardi. Acquisition of data: Neustadter, Carlson, and Girardi. Analysis and interpretation of data: Neustadter, Samarin, and Girardi. Drafting of the manuscript: Neustadter and Samarin. Critical revision of the manuscript for important intellectual content: Neustadter, Carlson, and Girardi. Administrative, technical, and material support: Neustadter, Samarin, Carlson, and Girardi. Study supervision: Girardi.
References
- 1.Bielsa I, Ariza A. Deep Morphea. Semin Cutan Med Surg. 2007;26(2):90–95. doi: 10.1016/j.sder.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Rosenkranz ME, Agle LM, Efthimiou P, Lehman TJ. Systemic and localized scleroderma in children: current and future treatment options [published correction for error in dosage appears in Paedriatr Drugs. 2006;8(4):270] Paediatr Drugs. 2006;8(2):85–97. doi: 10.2165/00148581-200608020-00002. [DOI] [PubMed] [Google Scholar]
- 3.Brenner M, Herzinger T, Berking C, Plewig G, Degitz K. Phototherapy and photochemotherapy of sclerosing skin diseases. Photodermatol Photoimmunol Photomed. 2005;21(3):157–165. doi: 10.1111/j.1600-0781.2005.00154.x. [DOI] [PubMed] [Google Scholar]
- 4.Chung L, Lin J, Furst DE, Fiorentino D. Systemic and localized scleroderma. Clin Dermatol. 2006;24(5):374–392. doi: 10.1016/j.clindermatol.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Ghersetich I, Teofoli P, Benci M, Innocenti S, Lotti T. Localized scleroderma. Clin Dermatol. 1994;12(2):237–242. doi: 10.1016/s0738-081x(94)90327-1. [DOI] [PubMed] [Google Scholar]
- 6.Laxer RM, Zulian F. Localized scleroderma. Curr Opin Rheumatol. 2006;18(6):606–613. doi: 10.1097/01.bor.0000245727.40630.c3. [DOI] [PubMed] [Google Scholar]
- 7.Peterson LS, Nelson AM, Su DW. Classification of morphea (localized scleroderma) Mayo Clin Proc. 1995;70(11):1068–1076. doi: 10.4065/70.11.1068. [DOI] [PubMed] [Google Scholar]
- 8.Cribier B, Faradji T, Le Coz C, Oberling F, Grosshans E. Extracorporeal photochemotherapy in systemic sclerosis and severe morphea. Dermatology. 1995;191(1):25–31. doi: 10.1159/000246481. [DOI] [PubMed] [Google Scholar]
- 9.Berger CL, Hanlon D, Kanada D, Girardi M, Edelson RL. Transimmunization, a novel approach for tumor immunotherapy. Transfus Apher Sci. 2002;26(3):205–216. doi: 10.1016/s1473-0502(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 10.Knobler RM, French LE, Kim Y, et al. Systemic Sclerosis Study Group. A randomized, double-blind, placebo-controlled trial of photopheresis in systemic sclerosis. J Am Acad Dermatol. 2006;54(5):793–799. doi: 10.1016/j.jaad.2005.11.1091. [DOI] [PubMed] [Google Scholar]
- 11.French LE, Rook AH. T Cell clonality and the effect of photopheresis in systemic sclerosis and graft versus host disease. Transfus Apher Sci. 2002;26(3):191–196. doi: 10.1016/s1473-0502(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 12.French LE, Alcindor T, Shapiro M, et al. Identification of amplified clonal T cell populations in the blood of patients with chronic graft-versus-host disease: positive correlation with response to photopheresis. Bone Marrow Transplant. 2002;30(8):509–515. doi: 10.1038/sj.bmt.1703705. [DOI] [PubMed] [Google Scholar]
- 13.Takehara K, Sato S. Localized scleroderma is an autoimmune disorder. Rheumatology (Oxford) 2005;44(3):274–279. doi: 10.1093/rheumatology/keh487. [DOI] [PubMed] [Google Scholar]