Abstract
AngII (angiotensin II) is a potent neurohormone responsible for cardiac hypertrophy, in which TGF (transforming growth factor)-β serves as a principal downstream mediator. We recently found that ablation of fibulin-2 in mice attenuated TGF-β signalling, protected mice against progressive ventricular dysfunction, and significantly reduced the mortality after experimental MI (myocardial infarction). In the present study, we investigated the role of fibulin-2 in AngII-induced TGF-β signalling and subsequent cardiac hypertrophy. We performed chronic subcutaneous infusion of AngII in fibulin-2 null (Fbln2−/− ), heterozygous (Fbln2+/− ) and WT (wild-type) mice by a mini-osmotic pump. After 4 weeks of subpressor dosage of AngII infusion (0.2 μg/kg of body weight per min), WT mice developed significant hypertrophy, whereas the Fbln2−/− showed no response. In WT, AngII treatment significantly up-regulated mRNAs for fibulin-2, ANP (atrial natriuretic peptide), TGF-β1, Col I (collagen type I), Col III (collagen type III), MMP (matrix metalloproteinase)-2 and MMP-9, and increased the phosphorylation of TGF-β-downstream signalling markers, Smad2, TAK1 (TGF-β-activated kinase 1) and p38 MAPK (mitogen-activated protein kinase), which were all unchanged in AngII-treated Fbln2−/− mice. The Fbln2+/− mice consistently displayed AngII-induced effects intermediate between WT and Fbln2−/− . Pressor dosage of AngII (2 mg/kg of body weight per min) induced significant fibrosis in WT but not in Fbln2−/− mice with comparable hypertension and hypertrophy in both groups. Isolated CFs (cardiac fibroblasts) were treated with AngII, in which direct AngII effects and TGF-β-mediated autocrine effects was observed in WT. The latter effects were totally abolished in Fbln2−/− cells, suggesting that fibulin-2 is essential for AngII-induced TGF-β activation. In conclusion our data indicate that fibulin-2 is essential for AngII-induced TGF-β-mediated cardiac hypertrophy via enhanced TGF-β activation and suggest that fibulin-2 is a potential therapeutic target to inhibit AngII-induced cardiac remodelling.
Keywords: angiotensin II (AngII), autoinduction, cardiac hypertrophy, extracellular matrix, signalling, transforming growth factor-β (TGF-β)
INTRODUCTION
Cardiac remodelling refers to a dynamic pathological process that induces progressive geometric changes and functional deterioration of the heart in response to biomechanical stress, direct myocardial insults or genetic predisposition [1,2]. Multiple neurohormonal pathways are responsible for the progression of cardiac remodelling, including roles for norad renaline (norepinephrine), AngII (angiotensin II), endothelin-1 and natriuretic peptides [3]. AngII, a major bioactive peptide of the rennin–angiotensin–aldosterone system, plays a critical role in maintaining cardiovascular homoeostasis [4], and its dysregulation results in cardiac remodelling [5,6]. The beneficial effects of AngII inhibition in preventing heart failure after MI (myocardial infarction) have been shown in clinical settings [7,8] and in experimental animal studies [9,10]. The underlying network interactions involving growth factors, receptors and intracellular signalling pathways responsible for the failing heart have been studied extensively [11,12], but the critical factors that mediate maladaptive signalling to induce pathological changes in myocytes and cardiac ECM (extracellular matrix) are not fully elucidated [13–15].
TGF (transforming growth factor)-β is a multifunctional growth factor that has a vital role in the regulation of cell growth, differentiation and repair in a variety of tissues, and dysregulation of TGF-β function is associated with a number of pathological states including tumour cell growth, fibrosis and autoimmune disease [15]. The significance of TGF-β in the progression of myocardial fibrosis and heart failure has been increasingly emphasized in recent years [16,17]. AngII stimulates autocrine production and release of TGF-β1 in rat CFs (cardiac fibroblasts) [18,19], and AngII-induced ECM synthesis occurs through increased expression of TGF-β1 in CFs [20,21]. TGF-β is a central mediator of AngII-induced cardiac hypertrophy because TGF-β1-deficient mice fail to develop myocardial hypertrophy by chronic subpressor AngII infusion [22]. TGF-β plays a key role in hypertrophic and fibrotic remodelling of the heart by mediating cardiomyocyte growth, fibroblast activation and ECM deposition [23]. The deleterious effect of TGF-β in the progression of ventricular remodelling has been supported by the study in which inhibition of TGF-β by gene therapy after MI attenuated progression of ventricular remodelling and heart failure [24].
Fibulin-2, a 180 kDa protein belonging to the fibulin protein family, is predominantly expressed at sites of epithelial– mesenchymal transformation during cardiovascular development, including formation of endocardial cushion, coronary arteries and aortic arch vessels [25]. It is down-regulated in most tissues at postnatal stages but remains expressed in the perivascular space of large- and medium-sized arteries and in cardiac valves. The expression of fibulin-2 is up-regulated during tissue remodelling, such as in skin wounds and vascular lesions [26,27]. Mice lacking fibulin-2 do not show any obvious phenotypic anomalies [28], but our recent studies revealed that loss of fibulin-2 in mice significantly improved the survival rate after experimental MI through attenuating progressive ventricular dysfunction accompanied by reduced TGF-β activation compared with WT (wild-type) mice [29]. Thus we hypothesized that fibulin-2 positively modulates TGF-β activation during cardiac remodelling.
In the present study, we have performed experiments with chronic infusion of subpressor and pressor dosage of AngII in fibulin-2 null (Fbln2−/− ), heterozygous (Fbln2+/− ) and WT (Fbln2+/+ ) mice and investigated the role of fibulin-2 in AngII-induced hypertrophic growth response of myocytes and ECM changes. We have found that fibulin-2 is required for AngII-induced cardiac hypertrophy, a process predominantly mediated by TGF-β signalling. We also performed cell culture experiments with isolated CFs from WT and Fbln2−/− mice and demonstrated that fibulin-2 is essential in AngII-induced TGF-β signalling. We propose that a positive-feedback loop involving fibulin-2 synthesis and subsequent TGF-β activation is a key process that promotes cardiac hypertrophy induced by continuous AngII infusion. This is the first study to demonstrate that fibulin-2 is essential for TGF-β-mediated cardiac hypertrophy induced by AngII in vivo.
MATERIALS AND METHODS
Animals
Adult male mice, 12–20 weeks of age, BW (body weight) 25– 35 g, were used in the present study. The Fbln2−/− mice were maintained on the C57BL/6 genetic background after backcrossing for 10 generations as described elsewhere [28]. Age-matched WT C57BL/6 mice were purchased from the Jackson Laboratory. Fbln2+/− mice were generated by mating Fbln2−/− and WT mice. All animal procedures in this study were performed in adherence to the National Institutes of Health Guidelines on the Use of Laboratory Animals and were approved by the IACUC (Institutional Animal Care and Use Committee) of Alfred I. duPont Hospital for Children.
Chronic subcutaneous AngII infusion
Under isoflurane anaesthesia (1.5%), a mini-osmotic pump (ALZET, model 2004; ALZACorp) filled with either subpressor or pressor dose of AngII or normal saline was inserted underneath the skin via mid-scapular incision. This mini-osmotic pump allows consistent release of solution at a rate of 0.25 μl/h for 4 weeks to deliver a subpressor or pressor dose of AngII (0.2 or 2.0 μg/kg of body weight per min). SBP (systolic blood pressure) and HR (heart rate) were measured by a tail cuff method (SC1000; Hatteras Instruments). During the measurement, each mouse was kept in restrainers heated to 37°C under unanaesthetized condition. After 4 weeks of AngII infusion, all mice were killed with excessive CO2 inhalation. Hearts were quickly dissected out and rinsed with sterile PBS. The weight of the left ventricle was measured, and the LVW [LV (left ventricular) weight]/BW ratio was calculated as an index of LV hypertrophy.
Echocardiography
Under isoflurane anaesthesia (1.5%), M-mode echocardiography was performed before and biweekly after the surgery with a 30 MHz probe (Vevo770 System; VisualSonics). Body temperature was maintained at 37°C using a thermally controlled surgical table and monitored with a rectal probe. Diastolic measurements of interventricular septum (IVSd), LV internal dimension (LVIDd), LV posterior wall thickness (LVPWd) and systolic measurement of LV internal dimension (LVIDs) were made from original M-mode tracings. The LV FS (fractional shortening) was calculated as:
| (1) |
Histopathology and immunohistochemistry
The LV myocardium was stored in either 10% formalin/PBS or frozen medium (OCT) for routine histology or immunohisto-chemistry, respectively. Paraffin-embedded specimens were sectioned at 6 μm thickness for H&E (haematoxylin and eosin) and Masson's Trichrome staining. Serial 8-μm-thick cryosections of frozen specimens were prepared for immunohistochemistry. The detailed protocol for immunostaining was described elsewhere [25]. Primary antibodies used were fibulin-2 (1:1000 dilution; a gift from Dr Takako Sasaki, University of Erlangen-Nuremberg, Erlangen, Germany), Col I (collagen type I) (1:100 dilution), Col III (collagen type III) (1:100 dilution) (Rockland Immunochemicals) and TGF-β1 (1:50 dilution; Santa Cruz Biotechnology). Alexa Fluor® 546 goat anti-rabbit IgG antibody (Invitrogen) was used as a secondary antibody. The specimens were observed under epifluorescent microscope (Olympus). Microscopic images of LV myocardium were captured digitally, and myocyte cross-sectional area was analysed using Image-Pro plus 5.1 (Media Cybernetics).
qRT–PCR (real-time reverse transcription–PCR)
Total RNA was extracted from the fresh LV myocardium using TRI Reagent (Applied Biosystems) according to the manufacturer’s instruction. The cDNA was synthesized from 2 μg of total RNA by oligo(dT)-primed RT (iScript cDNA synthesis kit; Bio-Rad Laboratories). qRT–PCR was performed with MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad Laboratories). A 20 μl reaction mixture was used that contained 10 μl of iQ SYBR Green supermix (Bio-Rad Laboratories), 100 ng of cDNA template and primers shown in Supplementary Table S1 (at http://www.clinsci.org/cs/126/cs1260275add.htm). The expression for each gene was determined as the relative expression or CT (threshold cycle value) of the gene of interest to the expression of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) by the formula:
| (2) |
Western blot analysis
Myocardial tissue was homogenized with RIPA lysis buffer (Santa Cruz Biotechnology) and protein concentration of the supernatants was determined by BCA Assays (Pierce Biotechnology). Equal amounts of protein (50 μg) were electrophoresed on a 14% SDS-polyacrylamide gel and then electrophoretic-ally transferred on to a PVDF membrane (Millipore). After blocking with 5% non-fat dried skimmed milk in TBS (Tris-buffered saline) containing 0.05% Tween 20 at room temperature (25°C) for 1 h, the membranes were incubated at 4°C with primary antibodies at 1:1000 dilutions on a rocking platform overnight. Antibodies used were phospho-Smad2, Smad2, phospho-TAK1 (TGF-β-activated kinase 1), TAK1, phospho-p38 MAPK (mitogen-activated protein kinase), p38 MAPK, phospho-ERK (extracellular-signal-regulated kinase) 1/2, ERK1/2, phospho-JNK (c-Jun N-terminal kinase), JNK (all from Cell Signaling Technology), TGF-β1 and β-actin (Santa Cruz Biotechnology). Blots were incubated with HRP (horseradish peroxidase)-labelled secondary antibodies (anti-mouse IgG or anti-rabbit IgG) for 1 h at room temperature, and signals were detected using Pierce ECL (enhanced chemiluminescence) Western Blotting Substrate. Membrane was exposed to Fuji radiograph film, and signal intensities were analysed with the NIH ImageJ software (version 1.38×).
Cell culture
Mouse CFs were isolated from ventricular myocardium of WT and Fbln2−/− mice, as reported previously [29]. Briefly, after repeated digestion with collagenase IV (100 units/ml)/trypsin (0.6 mg/ml) (Invitrogen), CFs were suspended in DMEM (Dulbecco's modified Eagle's medium) with 20% FBS (fetal bovine serum; Mediatech) and 1% penicillin and streptomycin (Invitrogen). CFs were seeded at 1×106 cells/cm3 at the third passage on laminin-coated dishes (BD Biosciences) for study. After serum starvation for 24 h, CFs were incubated with AngII (10− 7 M), AngII (10− 7 M) and TGF-β nAb (neutralizing antibody: 10 ng/ml; R&D Systems), and recombinant TGF-β1 (10 ng/ml; Roche) for 24 h.
Statistical analysis
All values in the text and Figures are presented as means ± S.E.M. All data (except Western blot density) were subjected to one-way ANOVA followed by Bonferoni correction for a post-hoc Student's t test. Western blot densities were analysed with the Kruskal–Wallis test followed by Dunn's post-hoc test. P values <0.05 were considered statistically significant.
RESULTS
Subpressor dosage of AngII infusion had no systemic effects
BW, HR and SBP were measured before, 2 weeks and 4 weeks after AngII infusion (Table 1). There was no noticeable change in BW, HR and SBP during the 4-week observation period, suggesting that a subpressor dose of AngII did not induce noticeable systemic effects. These results are consistent with the previously published results by other investigators [22]. No mice died during the 4 weeks of AngII infusion.
Table 1.
Physiological parameters during subpressor dosage of Angll infusion over 4 weeks
| Group | 0 day |
14 day |
28 day |
||||||
|---|---|---|---|---|---|---|---|---|---|
| BW(g) | HR (beats/min) | SBP (mmHg) | BW(g) | HR (beats/min) | SBP (mmHg) | BW (g) | HR (beats/min) | SBP (mmHg) | |
| WT +saline (n = 10) | 24.7 ± 2.2 | 666 ± 46 | 112.6 ± 6.3 | 27.6 ± 2.4 | 687 ± 58 | 115.2 ±5.5 | 29.4 ± 2.7 | 702 ± 61 | 117.0 ± 2.7 |
| WT +Angll (n = 15) | 23.9 ± 1.8 | 645±71 | 116.0 ± 10.1 | 28.3±1.6 | 701 ± 46 | 116.2 ± 3.7 | 29.6 ± 1.5 | 13 ± 59 | 115.4 ± 9.4 |
| Fbln2+/- +saline (n = 7) | 24.2 ± 0.9 | 645 ± 31 | 110.4 ± 3.7 | 27.1 ± 0.5 | 672 ± 52 | 114.2 ± 3.8 | 28.6 ± 1.0 | 695 ± 58 | 114.7 ± 9.3 |
| Fbln+/- + Angll (n = 7) | 24.1 ± 0.5 | 669 ± 64 | 112.3 ± 5.9 | 27.2 ± 0.5 | 688 ± 39 | 116.2 ± 6.1 | 28.7 ± 0.5 | 703 ± 42 | 117.9 ± 4.0 |
| Fbln2-/- +sallne (n = 10) | 24.2 ±2.3 | 660 ±53 | 113.4±8.9 | 27.4±3.0 | 697 ±44 | 120.2 ±3.6 | 28.0±1.2 | 697 ±57 | 119.6±5.7 |
| Fbln2-/- + Angll (n = 15) | 25.4±2.4 | 658 ±63 | 114.2±8.6 | 28.2± 1.8 | 664 ±67 | 123.2 ±6.4 | 29.6±1.9 | 684 ± 44 | 120.4±3.2 |
Fibulin-2 deficiency attenuated AngII-induced LV hypertrophy
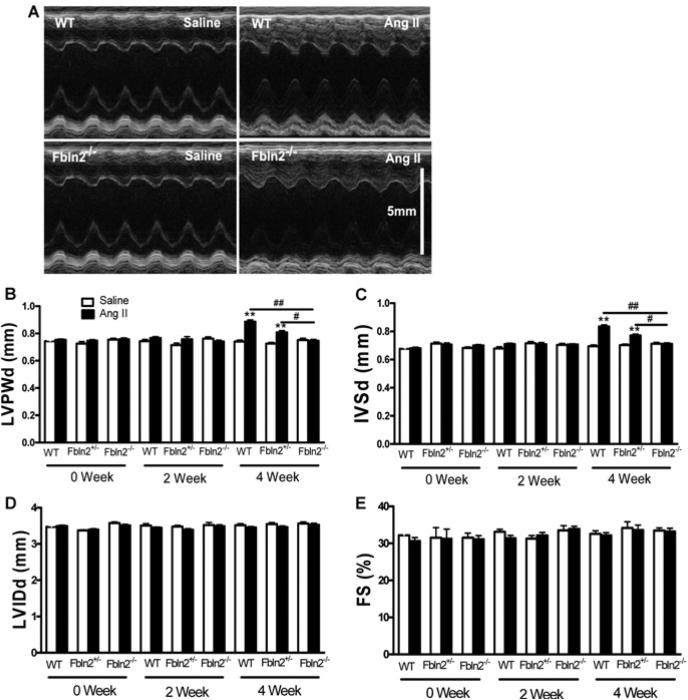
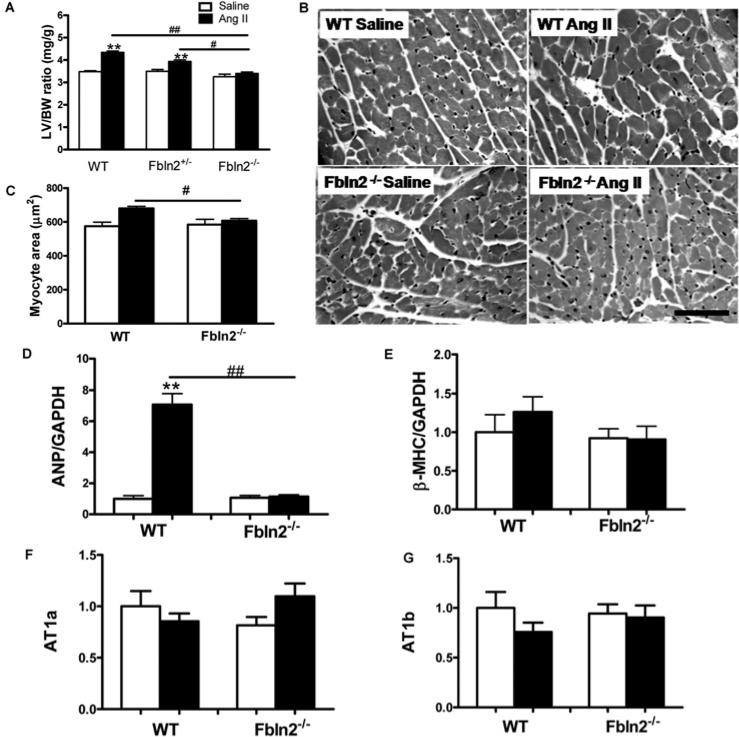
M-mode echocardiography was performed before, 2 weeks and 4 weeks after AngII infusion (Figure 1). Left ventricle wall thickness increased significantly only at 4 weeks in WT (Figures 1B and 1C), whereas no increase was seen in Fbln2−/− ; Fbln2+/− showed an intermediate increase in LV wall thickness between WT and Fbln2−/− by AngII. There was no change in LV chamber size (LVIDd) or LV systolic function (%FS) in all three groups throughout AngII infusion (Figures 1D and 1E). The degree of LV hypertrophy was also assessed by LVW/BW. By AngII treatment, LVW/BW was significantly increased (21% increase) in WT, whereas intermediate and no increase were seen in Fbln2+/− and Fbln2−/− mice, respectively (Figure 2A), consistent with echocardiographic findings. The degree of individual myocyte hypertrophy by AngII was assessed by cross-sectional area measurement of each myocyte in H&E staining. With AngII treatment, the measured cross-sectional area was significantly increased in WT but not in Fbln2−/− (Figures 2B and 2C). To gain further insight into the nature of the hypertrophy, we studied mRNA level of ANP (atrial natriuretic peptide), a myocardial hypertrophic marker. The ANP was markedly increased after AngII infusion only in WT mice, whereas no increase was observed in Fbln2−/− mice (Figure 2D). There was no statistically significant increase in β-MHC (β myosin heavy chain) expression by AngII infusion in either group, suggesting that the nature of hypertrophy induced by subpressor dosage of AngII does not involve the MHC isoform switch frequently seen in uncompensated pathological hypertrophy [30]. AT1 receptors (AngII type I receptors, AT1a and AT1b) were not up-regulated by AngII treatment in either group (Figures 2F and 2G).
Figure 1. In vivo assessment of LV hypertrophy.
M-mode echocardiographic measurements were performed to assess LV wall thickness (LVPWd), ventricular dimension (LVIDd) and systolic performance (%FS) at 0, 2 and 4 weeks after treatment in WT (saline, n = 10; AngII, n = 15), Fbln2+/− (saline, n = 7; AngII, n = 7), and Fbln2−/− (saline: n = 10, AngII: n = 15) mice. (A) Representative photographs of M-mode echocardiography WT and Fbln2−/− mice under saline and AngII treatment after 4 weeks. LVPWd (B), IVSd (C), LVIDd (D) and %FS (E). #P < 0.05 and ##P < 0.01. **P < 0.01 compared with corresponding saline-treated group.
Figure 2. Myocardial hypertrophy induced by continuous subpressor AngII infusion.
(A) LVW/BW ratio of saline and AngII-treated WT, Fbln2+/− and Fbln2−/− mice. #P < 0.05 and ##P < 0.01. **P < 0.01 compared with corresponding saline group (n = 10). (B) Microscopic images (H&E staining) of LV myocardium after 4 weeks of treatment with saline and AngII in WT and Fbln2−/− mice. Note the increased myocyte cross-sectional area in AngII-treated WT myocardium. (C) Cardiomyocyte cross-sectional area of 4-week saline and AngII-infused WT and Fbln-2−/− hearts. Measured cross-sectional area of myocytes was significantly increased in AngII-treated WT, whereas there was no increase in Fbln2−/− by AngII treatment (n = 3). (D) and (E) ANP and β-MHC mRNA levels by qRT–PCR. WT (saline, n = 10; AngII, n = 15) and Fbln2−/− (saline, n = 10; AngII, n = 15). (F) and (G) AT1a and AT1b. WT (saline, n 5; AngII: n = 5) and Fbln2−/− (saline: n 5, AngII: n = 5). The mRNA levels of WT-saline were arbitrarily set at 1. #P < 0.05 ## and P < 0.01. **P < 0.01 compared with corresponding saline-treated group.
Deficiency of fibulin-2 attenuated ECM changes in response to subpressor dose of AngII infusion
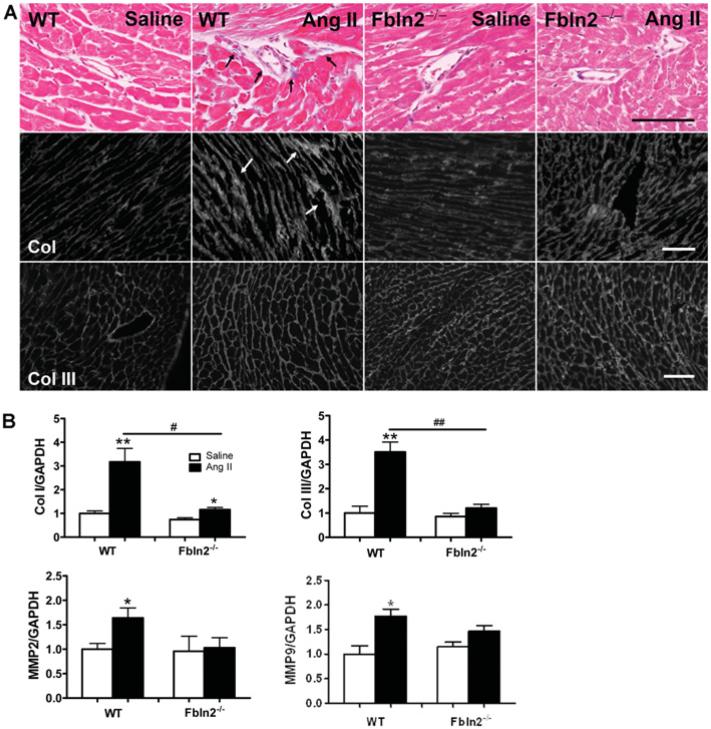
Histological assessment by Masson's Trichrome staining revealed that there was no noticeable induction of myocardial fibrosis by chronic subpressor dosage of AngII in either WT or Fbln2−/− mice (Figure 3A). However, Col I protein deposition in tissue shown by immunohistochemistry was markedly increased by AngII infusion in both WT and Fbln2−/− mice, and the increase was more prominent in WT than in Fbln2−/− , as shown by the width of Col I-positive interstitial space (Figure 3A). There was no significant increase in the Col III immunolocalization pattern in either group by AngII. The mRNA levels of Col I, Col III, MMP (matrix metalloproteinase)-2 and MMP-9 were significantly up-regulated by AngII in WT mice, whereas no notable increase in Col III, MMP-2 or MMP-9 was observed in Fbln2−/− mice by AngII (Figure 3B).
Figure 3. Assessment of ECM remodelling and tissue fibrosis in LV myocardium.
(A) Masson's Trichrome (Ma-Tri) staining and immunohistological staining (Col I and Col III). In Masson's Trichrome, blue colour indicates the area of collagen fibres (see arrows), which is exclusively seen in the perivascular area. There was no noticeable increase in tissue collagens by AngII in either WT or Fbln2−/− . However, by immunostaining, interstitial deposition of Col I was markedly increased in AngII-treated WT (see arrows) compared with AngII-treated Fbln2−/− . There was no significant difference in Col III protein deposition in all four groups. Also note that the size of each myocyte was markedly enlarged in AngII-treated WT compared with other three groups, consistent with Figure 2(B). Scale bar, 100 μm. (B) Col I, Col III, MMP-2 and MMP-9 mRNA levels by qRT–PCR. The mRNA levels of saline-treated WT were arbitrarily set at 1. WT (saline, n = 10; AngII, n = 15) and Fbln2−/− (saline, n = 10; AngII: n = 15). #P < 0.05 and ##P < 0.01. *P < 0.05 and **P < 0.01 compared with corresponding saline-treated group.
Fibulin-2 deficiency attenuated AngII-induced TGF-β signalling
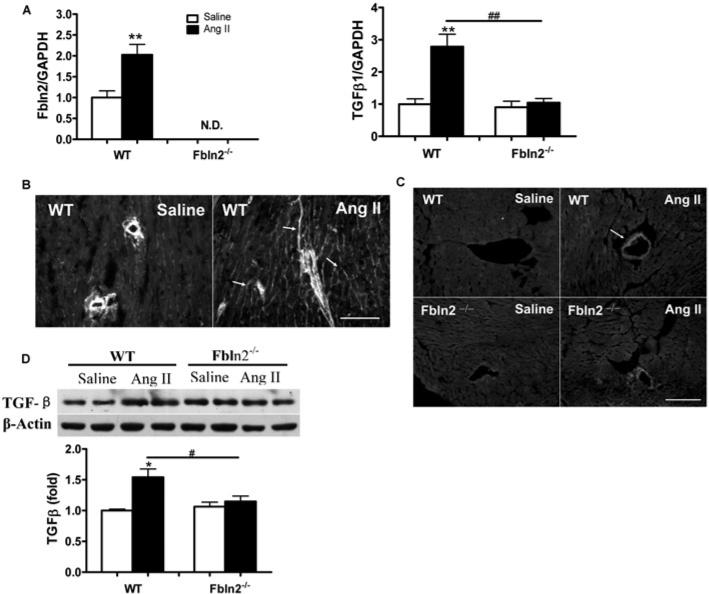
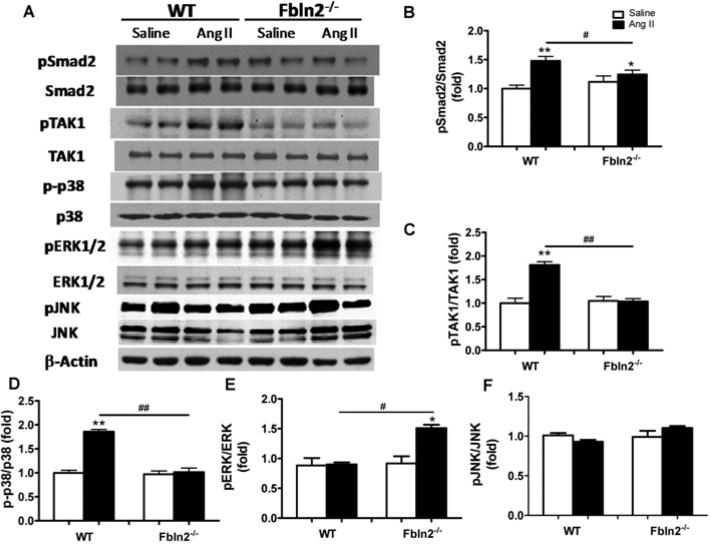
Having demonstrated that fibulin-2 is required for AngII-induced cardiac hypertrophy, we tested whether fibulin-2 is required for AngII-induced TGF-β activation. As shown in Figure 4(A), fibulin-2 mRNA levels were significantly up-regulated in AngII-treated WT mice compared with the control mice. In WT mice, AngII induced interstitial localization of fibulin-2 protein in addition to pre-existing perivascular localization (Figure 4B). TGF-β1 levels of both mRNA and protein were significantly up-regulated in WT mice by AngII, but unchanged in Fbln2−/− mice (Figures 4A and 4D). TGF-β1 protein expression was predominantly up-regulated around the blood vessels with AngII in both WT and Fbln2−/− , more in WT than in Fbln2−/− (Figure 4C). The localization of TGF-β protein in WT overlaps the perivascular localization of fibulin-2. TGF-β protein expression was significantly increased in WT by AngII, but not increased in Fbln2−/− mice (Figure 4D). TGF-β induces downstream signalling pathways that promote cardiac remodelling: Smad-dependent pathway and Smad-independent pathways, such as TAK1 and p38 MAPK. In parallel with the increase of TGF-β1 secretion in the tissue by AngII, phosphorylation of Smad2, TAK1 and p38 MAPK was significantly increased in WT mice, whereas no discernible increase was seen in Fbln2−/− mice (Figure 5). On the contrary, phosphorylation of ERK1/2 was significantly increased only in Fbln2−/− mice, whereas no change was detected in WT mice by AngII. Phosphorylation of JNK was not induced by AngII in either WT or Fbln2−/− mice. Our data suggest that fibulin-2 is essential for AngII-induced TGF-β synthesis, secretion, and downstream signalling, Smad2, TAK1 and p38 MAPK, and that fibulin-2 plays a critical role in AngII-induced cardiac hypertrophy by mediating TGF-β signalling pathways.
Figure 4. Fibulin-2 and TGF-β expression induced by AngII.
(A) Fibulin-2 and TGF-β1 mRNA levels in WT and Fbln2−/− . ##P < 0.01. **P < 0.01 compared with corresponding saline-treated group. (B) Immunostaining of fibulin-2 in WT mice. Fibulin-2 protein localization is restricted to perivascular area in the saline-treated group, but is markedly increased and also diffusely seen in the interstitial areas upon AngII treatment (see arrows). Scale bar, 100 μm. (C) Immunostaining of TGF-β1 in WT and Fbln2−/− mice. Protein localization of TGF-β1 is not clear in either WT or Fbln2−/− mice with saline treatment. When treated with AngII, perivascular expression of TGF-β1 is increased (arrow) in WT. Scale bar, 100 μm. (D) Quantitative assessment of TGF-β1 protein in LV myocardium in WT and Fbln2−/− by Western blots. Lower panel, densitometric analysis of TGF-β1 expression (n = 5 from each group). #P < 0.05. *P < 0.05 compared with corresponding saline-treated group.
Figure 5. Assessment of TGF-β downstream signalling pathways by Western blot analyses.
Activity levels of each signalling protein are indicated by the ratio of phosphorylated form/total form. (A) Representative Western blots of Smad2, TAK1, p38 MAPK, ERK1/2 and JNK with each corresponding phosphorylated form. Smad2 (B), TAK1 (C) and p38 MAPK (D) were all significantly up-regulated in AngII-treated WT mice, whereas no significant AngII-induced increase was seen in Fbln2−/− . On the contrary, ERK1/2 (E) showed significant increase only in Fbln2−/− but not in WT. JNK (F) did not change with AngII either in WT or Fbln2−/− . The ratio in saline-treated WT is arbitrarily set at 1. Sample size: n = 6 from each group for Smad2, TAK1 and p38 MAPK, and n = 4 for ERK1/2 and JNK. #P < 0.05 and ##P < 0.01. *P < 0.05 and **P < 0.01 compared with the corresponding saline-treated groups.
Cardiac hypertrophy and TGF-β activation parallel the fibulin-2 expression levels
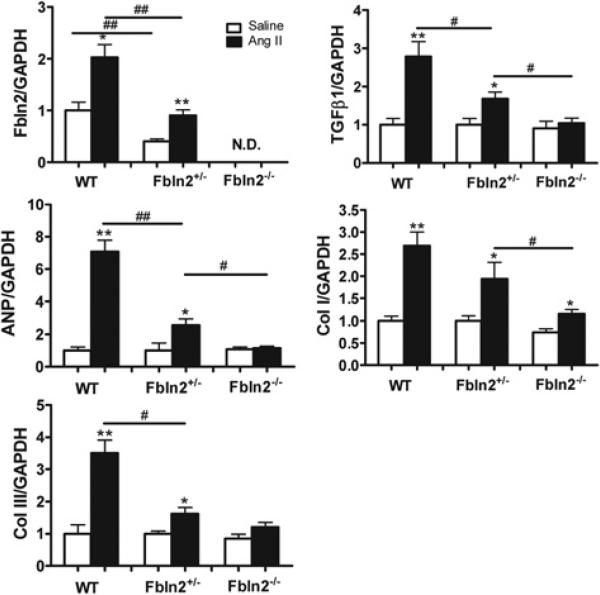
To obtain more evidence to support our hypothesis that fibulin-2 is required for TGF-β-induced cardiac hypertrophy, we included Fbln2+/− mice in the study to examine whether fibulin-2 has dose-dependent effects in AngII-induced TGF-β activation. First, baseline fibulin-2 mRNA level in Fbln2+/− was shown to be approximately half that of WT (Figure 6A). With AngII treatment, fibulin-2 mRNA in Fbln2+/− increased 2-fold, which is still approximately one half of that in AngII-treated WT. In the previous section, we have shown that Fbln2+/− developed intermediate hypertrophy between WT and Fbln2−/− by AngII treatment (Figures 1B, 1C and 2A). The ANP, Col I, Col III and TGF-β1 in WT, Fbln2+/− and Fbln2−/− were in parallel with the fibulin-2 mRNA levels (Figure 6A). Our data suggest that fibulin-2 mRNA expression levels were proportional to the degree of AngII-induced mRNA levels of TGF-β1, ANP, Col I and Col III and the degree of AngII-induced hypertrophy.
Figure 6. Intrinsic fibulin-2 mRNA expression levels paralleled those of remodelling genes in subpressor infusion of AngII.
Quantification of mRNA levels of fibulin-2, TGF-β1, ANP, Col I and Col III. The data of each mRNA were normalized by GAPDH mRNA and expressed as a fold increase compared with WT-saline mice (n 6 from each group). These are separate samples from those shown in Figures 2–4. #P < 0.05 and ##P < 0.01. *P < 0.05 and **P < 0.01 compared with saline infusion group.
Fibulin-2 is essential for AngII-induced TGF-β signalling in isolated CFs
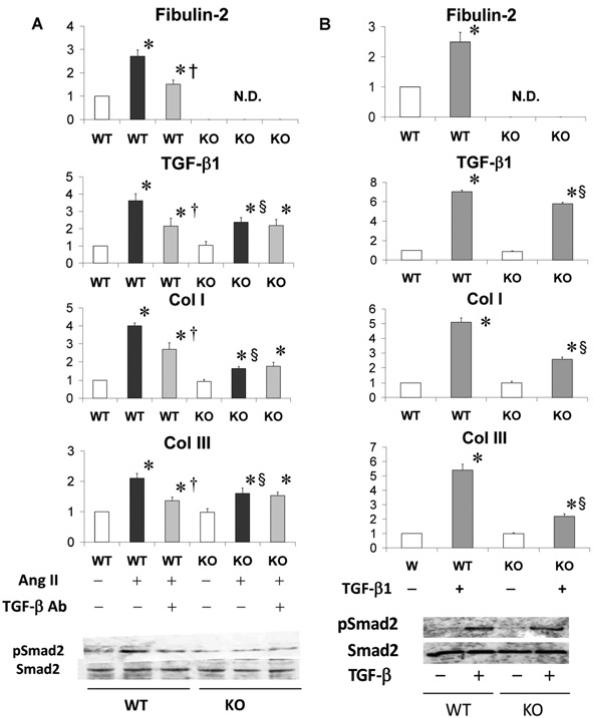
We treated isolated CFs from WT and Fbln2−/− (KO) ventricular myocardium with (a) AngII, (b) AngII and TGF-β nAb (both in Figure 7A) and (c) recombinant TGF-β1 (Figure 7B). With AngII treatment, up-regulation of ECM markers including fibulin-2, TGF-β1, Col I and Col III were all significantly higher in WT than in Fbln2−/− . Co-treatment with TGF-β nAb partially inhibited AngII-induced up-regulation of these markers in WT CFs, whereas there was no change in AngII-treated Fbln2−/− CFs. TGF-β1 and Col III mRNA levels after co-treatment with AngII and TGF-β nAb were relatively comparable between WT and Fbln2−/− . These data suggest that AngII induced (1) direct AngII effects and (2) indirect TGF-β-mediated autocrine effects in WT CFs, and that there were no TGF-β-mediated effects in Fblin2−/− CFs. In other words, the AngII-induced TGF-β-mediated auto-crine pathway is fibulin-2-dependent.
Figure 7. Isolated CFs from WT and KO (Fbln2−/−) mice treated with AngII + TGF-β nAb (A) and recombinant TGF-β (B).
Upper histograms indicate mRNA levels of fibulin-2, TGF-β1, Col I and Col III. The mRNA levels of WT controls were arbitrarily set at 1. Note that there were no effects by TGF-β nAb in AngII-treated KO cells, suggesting that KO cells did not show TGF-β-mediated effects when stimulated with AngII (A). Shown below are pSmad2 and Smad2 expression by Western blots. In KO cells, pSmad2 showed no change with either AngII or AngII + TGF-β nAb. *P < 0.05 compared with sham (white bar), P§ < 0.05 compared with WT, and †P < 0.05 compared with AngII-treated group (black bar).
Exogenous TGF-β1 up-regulated mRNA of ECM markers including TGF-β1 in both WT and Fbln2−/− CFs, but significantly more in WT than in Fbln2−/− mice. The difference in response between the two may be explained by the lack of additional TGF-β-mediated autocrine effects in Fbln2−/− CFs, although AngII-induced pSmad2 level was not significantly higher in WT than in Fbln2−/− (Figure 7B). This is probably because the amount of exogenous TGF-β significantly outweighs the produced secondary endogenous TGF-β. These findings were consistent with the results in an in vivo animal model.
Absence of fibulin-2 attenuated myocardial fibrosis in pressor dosage of AngII infusion
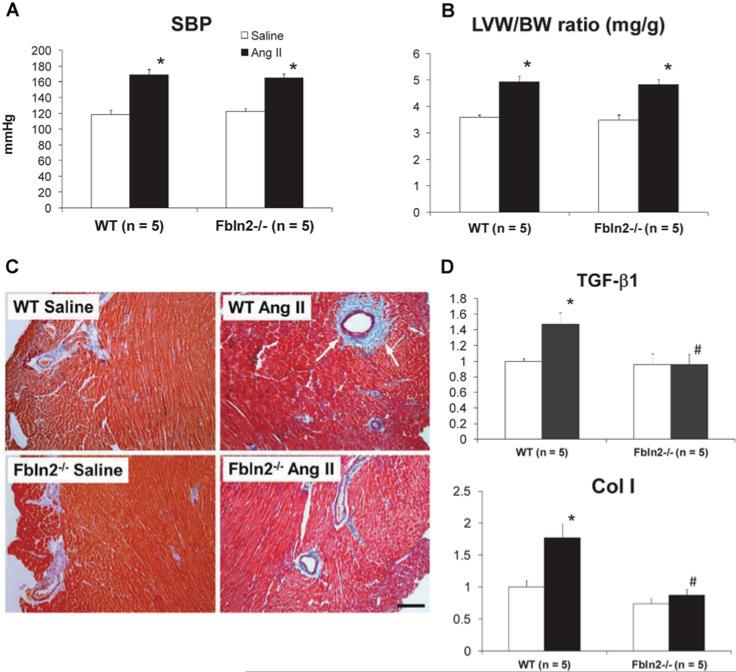
We also treated WT and Fbln2−/− mice with pressor dosage of AngII (2.0 μg/kg of body weight per min; 10 times higher than the subpressor dosage) (Figure 8). With this treatment, both mice developed comparable hypertension (SBP was 169 ± 7.5 in WT and 165 ± 5.5 mmHg in Fbln2−/− ; not significant).−The degree of myocardial hypertrophy was also comparable (LVW/BW ratio was 4.93 ± 0.22 in WT and 4.83 ± 0.19 in Fbln2−/− : not significant). However, myocardial fibrosis was markedly attenuated in Fbln2−/− compared with WT. The mRNA levels of TGF-β1 and Col I were significantly increased by AngII in WT, whereas there was no increase by AngII in Fbln2−/− mice. Although fibulin-2 protein is predominantly expressed in the perivascular space and vascular basement membrane [25], increase in BP by AngII was not different between WT and Fbln2−/− . The absence of fibulin-2 protected against AngII-induced myocardial fibrosis in vivo.
Figure 8. Pressor dosage of AngII infusion (2.0 μg/kg of BW per min) induced a comparable degree of hypertension in both WT and Fbln2−/−.
After 4 weeks of AngII treatment, both groups developed equal degrees of hypertension (A) and hypertrophy shown by LVW/BW (B). (C) Masson's Trichrome staining of LV myocardium. The development of fibrosis was significantly attenuated in Fbln2−/− compared with WT. See marked perivascular fibrosis in WT AngII (see arrows). Scale bar, 100 μm. (D) The mRNA levels of TGF-β1 and Col I were significantly increased in WT by pressor dose of AngII infusion, whereas no noticeable increase was seen in Fbln2−/− . *P < 0.05 compared with saline group, #P < 0.05 compared with WT. Results are means± S.E.M.
DISCUSSION
A biological role of fibulin-2 in TGF-β signalling
Because AngII plays a principal role in post-MI ventricular remodelling and progressive heart failure [9,10] and because AngII induces TGF-β activation in the myocardial tissue [31], we introduced an in vivo chronic infusion of subpressor dosage of AngII in Fbln2−/− , Fbln2+/− and WT mice to determine whether fibulin-2 is involved in AngII-induced TGF-β-mediated cardiac remodelling. TGF-β is known as a critical mediator in AngII-induced cardiac remodelling [22]. Our present data indicated that fibulin-2 is essential in TGF-β up-regulation induced by AngII and that fibulin-2 is likely to participate in the pathogenesis of the AngII-induced hypertrophic and fibrotic process. TGF-β is a multi-functional growth factor that has a vital role in the regulation of cell growth, differentiation and repair in a variety of tissues [15]. Dysregulated TGF-β amplification is considered a pathological process that leads to multiple disease conditions via tissue fibrosis and cardiac remodelling [17,32] and cancer progression [33]. TGF-β activation is regulated at multiple different sites including within intracellular signalling pathways and through extracellular interactions. Because a substantial amount of latent forms of TGF-β protein are stored in the ECM as a sequest-rated form, up-regulation of TGF-β activity depends upon the release of free soluble TGF-β from the latent complex. Several ECM proteins are known to participate in modulating TGF-β activation in ECM. Fibrillin-1 is a microfibrillar protein that associates with elastic fibres and regulates bioactivity of TGF-β1 [34]. Tsp (thrombospondin)-1 plays a critical role in TGF-β activation in vivo [35], and absence of Tsp-1 resulted in worsening of ventricular remodelling after experimental MI [36], suggesting Tsp-1 is a major activator of TGF-β, although molecular roles of Tsp-1 are considered multifold, including inhibition of angiogenesis and de-adhesion from a variety of cells in addition to TGF-β activation [37].
Our data indicate that fibulin-2 is a critical mediator of TGF-β under AngII-stimulated conditions. The ECM serves not only as a silent structural scaffold to support physical integrity of tissue but also as a complex functional modulator to generate growth phenomena by activating latent growth factors directly or indirectly in response to various external stresses. Exaggerated growth phenomena or failure to regulate this positive growth response is known to be induced, in part, by TGF-β dysregulation. Thus it is likely that fibulin-2 plays a critical role in various pathological conditions mediated by excessive TGF-β activation.
Fibulin-2 not only enhanced AngII-induced TGF-β synthesis and secretion, but also modulated activation of TGF-β downstream signalling pathways. All pro-remodelling signalling pathways including Smad2, TAK1 and p38 MAPK were activated only in WT by AngII, whereas EKR1/2 activation was induced only in Fbln2−/− mice. ERK1/2 becomes activated in cardiac myocytes in response to any type of stress stimulation and is required for cardiac growth response [38], but there is no stretch effect in this model (no change in BP with AngII treatment). Despite the wealth of studies on the subject, however, the precise role of ERK1/2 as a necessary mediator of cardiac hypertrophy is not fully understood [38]. Inhibition or genetic targeting of ERK1/2 predisposes the heart to decompensation or failure in response to haemodynamic stress, suggesting that ERK1/2 signalling has some cardioprotective effects rather than being a hypertrophic mediator [39]. In our model, Fbln2−/− mice did not develop AngII-induced hypertrophy, and this anti-hypertrophic effect may be associated with ERK1/2 activation. The mechanism of AngII-induced ERK1/2 activation in Fbln2−/− mice is currently under investigation by the authors.
Fibulin-2 as an essential enhancer of TGF-β autoinduction
Fbln2−/− mice failed to show AngII-induced TGF-β synthesis and activation, and, thus, were protected from hypertrophy by AngII treatment (Figure 4). How can the ECM protein fibulin-2 modulate AngII-induced up-regulation of TGF-β and activate its downstream signalling pathways? First, AngII directly up-regulated fibulin-2 mRNA expression in myocardium, as we recently showed that fibulin-2 mRNA was up-regulated by AngII in isolated CFs [29]. It is plausible that the increase in secreted fibulin-2 enhanced free TGF-β release from the latent complex in the ECM by competing TGF-β binding sites with other ECM proteins [40]. Fibulin-2 is predominantly synthesized by activated CFs or myofibroblasts, not by myocytes, and is secreted into ECM in post-MI ventricular remodelling in vivo as well as in isolated CFs in vitro [29]. Biologically active free TGF-β then further induced mRNA expression of fibulin-2 [41] and TGF-β in fibroblasts [42,43] as well as mRNAs of other ECM proteins. This activation of endogenous TGF-β is triggered and enhanced by fibulin-2 which is important in growth phenomena including wound healing, in which fibulin-2 is markedly up-regulated [27,29]. Thus fibulin-2 functions both upstream and downstream of TGF-β. This unique role of fibulin-2 in TGF-β autoinduction is probably performed via a positive-feedback loop. Because AngII cannot induce cardiac hypertrophy without up-regulation of endogenous TGF-β [22,44] and because fibulin-2 is necessary in AngII-induced TGF-β activation, fibulin-2 serves as a critical mediator in this positive autocrine and paracrine feedback loop induced by AngII. Although in vivo study has a limitation in addressing specific signalling pathways in each cell type, we propose possible signalling pathways in Figure 9 based upon our present findings and some published studies. Smad2 activation was induced by AngII in isolated CFs, as we demonstrated in Figure 7(A) and in our previous study [29]. AngII-induced myocyte hypertrophy is not only exclusively dependent upon endogenous TGF-β but also is TAK1-dependent and Smad2/3-independent in vitro [45]. Matsumoto-Ida et al. [46] showed that TAK1 protein is exclusively localized in myocytes in the non-infarct zone after experimental MI and thus postulated that the activation of the TGF-β1/TAK1/p38 MAPK pathway in myocyte is involved in ventricular remodelling. Unregulated growth response involving the TGF-β-mediated positive-feedback loop may be one possible underlying cause of various progressive pathological disorders.
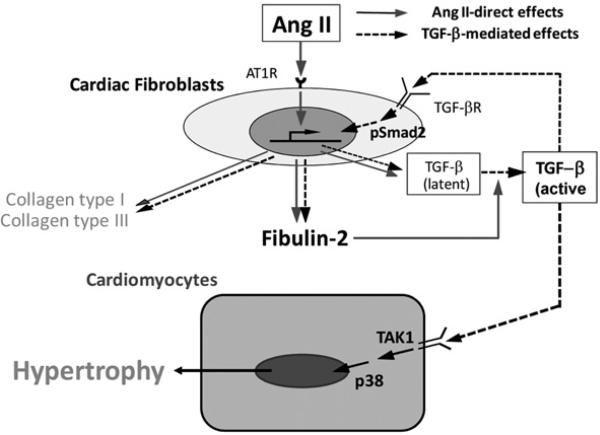
Figure 9. Positive autocrine (cardiac fibroblasts) and paracrine (cardiomyocytes) pathway during AngII-induced cardiac hypertrophy.
AngII directly induces multiple ECM protein synthesis including fibulin-2 and TGF-β. We postulate that the increased fibulin-2 in the ECM stimulates TGF-β activation, by which TGF-β is further up-regulated in an autocrine fashion. The additional TGF-β-mediated effects, cardiac hypertrophy and increased ECM protein synthesis are only seen in WT mice. Thus fibulin-2 is necessary in activating endogenous TGF-β in the ECM.
A positive-feedback loop involving TGF-β has been documented in other pathological processes. TGF-β and endogenous AngII mutually enhance their activity to induce renal injury via a positive-feedback loop [47,48]. TGF-β and PAI-1 (plasminogen-activator inhibitor-1) together constitute a positive-feedback loop in the development of renal fibrosis in diabetics [49]. In our study, fibulin-2 was found to be a novel and unique extracellular enhancer of AngII-induced autocrine and paracrine TGF-β synthesis in the mouse model.
Fibulin-2 as a novel therapeutic target in heart failure
Uncontrolled activation of TGF-β is thought to be a central process in the progression of ventricular remodelling after MI [23], and its inhibition has been proved to be an effective way to prevent post-MI ventricular remodelling and heart failure [24,50]. On the other hand, a couple of studies indicated an opposite effect by TGF-β inhibition [51,52]. This is because biological effects of TGF-β are complicated and sometimes even paradoxical in physiological growth phenomena as well as pathological processes in multiple different organ systems [34]. Simply inhibiting TGF-β may create a wide spectrum of complex combined effects depending upon timing, duration and the degree of inhibition. Thus a selective inhibition of TGF-β autoinduction by disrupting fibulin-2 would have more therapeutic efficiency. Together with our recent study showing that absence of fibulin-2 protects against progressive ventricular dysfunction and death after experimental MI [29], fibulin-2 can be considered as a novel therapeutic target in progressive cardiac remodelling. Fibulin-2 ablation prevents excessive TGF-β activation but still allows necessary TGF-β activity for baseline homoeostasis of all organs. Moreover, a recent study by Karakikes et al. [53] identified fibulin-2 as a direct target in miRNA (microRNA) treatment that prevented pressure overload-induced pathological remodelling in conjunction with attenuated TGF-β signalling. Together with our current data and previous work [29], it strongly suggested that fibulin-2 modulates common downstream pathways of cardiac remodelling that can be targeted for prevention of heart failure. In conclusion, we have demonstrated for the first time that fibulin-2 is essential for TGF-β activation in AngII-induced cardiac hypertrophy in vivo. In combination with our recent report asserting that absence of fibulin-2 protects against progressive ventricular dys-function after MI, in part, by attenuating TGF-β activation [29], our present study strengthened the evidence of fibulin-2 participation in TGF-β activation during various pathological conditions. This unique feature of fibulin-2 addresses a significant possibility for its use in the treatment of multiple progressive disorders involving dysregulation of TGF-β signalling.
Limitations
Because myocardial tissue consists of multiple cell types including cardiomyocytes, CFs, endothelial cells, VSMCs (vascular smooth muscle cells) and circulating macrophages, our proposed model of signalling pathways only between CFs and myocytes may be too simplified. We proposed this model because CFs and myocytes are two predominant cell types in the myocardium that determine ventricular function and morphology. Although isolated CFs synthesize TGF-β in response to AngII, VSMCs are another source of TGF-β synthesis in response to AngII [54], as shown in Figure 4(C). Spatial expression of TGF-β protein overlaps with that of fibulin-2. In this study, however, we did not specifically address the role of endothelial cells or VSMCs in fibulin-2 synthesis and TGF-β activation. In addition, the AngII effects were not exactly the same between in vivo and in vitro models. Although Fbln2−/− myocardium did not respond to AngII with regard to TGF-β1, Col I, Col III and MMP-2 mRNA expression in vivo, there were slight increases of these markers in isolated CFs when treated with AngII. These differences may be due to involvement of multiple other cell types in in vivo experiments.
CLINICAL PERSPECTIVES.
Excessive activation of the AngII/TGF-β axis is thought to play a central role in the transition from compensatory responses to maladaptive remodelling, but the underlying mechanism of this pathological transition is poorly understood. Recently, we have demonstrated that the loss of fibulin-2 protects against progressive remodelling after MI by attenuating TGF-β activation. In the present study, we investigated the role of fibulin-2 in the activation of TGF-β during AngII-induced myocardial remodelling in the mouse.
We have demonstrated that fibulin-2 is necessary for AngII-induced TGF-β activation and subsequent myocardial hypertrophy and fibrosis. Excessive TGF-β activation can be targeted to prevent progressive remodelling, but its baseline activity is essential for cardiovascular homoeostasis. The absence of fibulin-2 inhibited AngII-induced myocardial hyper-trophy and fibrosis by attenuating excessive TGF-β activation, but still maintaining its baseline activity.
Our findings may generate a new treatment strategy of targeting fibulin-2 in preventing progressive heart failure in humans.
Acknowledgments
FUNDING
This work was supported by the National Institutes of Health (NIH) [grant number 1P20RR020173-01] and an American Health Association Beginning Grant-in-Aid [grant number 0665433U] (both to T.T).
Abbreviations
- AngII
angiotensin II
- ANP
atrial natriuretic peptide
- AT1 receptor
AngII type I receptor
- BW
body weight
- CF
cardiac fibroblast
- Col I
collagen type I
- Col III
collagen type III
- CT
threshold cycle value
- ECM
extracellular matrix
- ERK
extracellular-signal-regulated kinase
- FS
fractional shortening
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- H&E
haematoxylin and eosin
- HR
heart rate
- IVSd
inter-ventricular septum dimension
- JNK
c-Jun N-terminal kinase
- LV
left ventricular
- LVIDd
LV internal dimension
- LVPWd
LV posterior wall thickness
- LVW
LV weight
- MAPK
mitogen-activated protein kinase
- MHC
myosin heavy chain
- MI
myocardial infarction
- MMP
matrix metalloproteinase
- nAb
neutralizing antibody
- qRT-PCR
quantitative real-time reverse transcription PCR
- SBP
systolic blood pressure
- TGF
transforming growth factor
- TAK1
TGF-β-activated kinase 1
- Tsp
thrombospondin
- VSMC
vascular smooth muscle cell
- WT
wild-type
Footnotes
AUTHOR CONTRIBUTION
Hangxiang Zhang carried out most of the in vivo experiments, obtained the data and contributed to the preparation of the paper. Jing Wu participated in animal experiments, obtained the data and independently performed cell culture experiments. Both authors contributed equally in this study. Hailong Dong initially designed the study protocol and set-up for animal experiments. Shaukat Khan performed all the additional new experiments, qRT–PCR of MMP-9 and Western blots of ERK1/2 and JNK, requested for the revision. Mon-Li Chu provided Fbln2−/− mice, critically read the paper and provided a number of important comments and suggestions. Take-shi Tsuda supervised the entire experiments, took part in the daily discussions with the data obtained, and critically read and revised the entire paper as a corresponding author.
REFERENCES
- 1.Olson EN. A decade of discoveries in cardiac biology. Nat. Med. 2004;10:467–474. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- 2.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 3.Mann DL. Heart Failure. W.B. Saunders; Philadelphia: 2004. Heart failure as a progressive disease. pp. 123–128. [Google Scholar]
- 4.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin. Sci. 2007;112:417–428. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 5.Weber KT. Extracellular matrix remodeling in heart failure: a role for de novo angiotensin II generation. Circulation. 1997;96:4065–4082. doi: 10.1161/01.cir.96.11.4065. [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer JM, Fischer TA, Pfeffer MA. Angiotensin-converting enzyme inhibition and ventricular remodeling after myocardial infarction. Ann. Rev. Physiol. 1995;57:805–826. doi: 10.1146/annurev.ph.57.030195.004105. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N. Engl. J. Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 8.Schieffer B, Wirger A, Meybrunn M, Seitz S, Holtz J, Riede UN, Drexler H. Comparative effects of chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation. 1994;89:2273–2282. doi: 10.1161/01.cir.89.5.2273. [DOI] [PubMed] [Google Scholar]
- 9.Harada K, Sugaya T, Murakami K, Yazaki Y, Komuro I. Angiotensin II type 1A receptor knockout mice display less left ventricular remodeling and improved survival after myocardial infarction. Circulation. 1999;100:2093–2099. doi: 10.1161/01.cir.100.20.2093. [DOI] [PubMed] [Google Scholar]
- 10.Jain M, Liao R, Ngoy S, Whittaker P, Apstein CS, Eberli FR. Angiotensin II receptor blockade attenuates the deleterious effects of exercise training on post-MI ventricular remodelling in rats. Cardiovasc. Res. 2000;46:66–72. doi: 10.1016/s0008-6363(99)00429-0. [DOI] [PubMed] [Google Scholar]
- 11.Selvetella G, Hirsch E, Notte A, Tarone G, Lembo G. Adaptive and maladaptive hypertrophic pathways: points of convergence and divergence. Cardiovasc. Res. 2004;63:373–380. doi: 10.1016/j.cardiores.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodeling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 13.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ. Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- 14.Cleutjens JP, Creemers EE. Integration of concepts: cardiac extracellular matrix remodeling after myocardial infarction. J. Card. Failure. 2002;8:S344–S348. doi: 10.1054/jcaf.2002.129261. [DOI] [PubMed] [Google Scholar]
- 15.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N. Engl. J. Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 16.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-β1. Mol. Genet. Metab. 2000;71:418–435. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 17.Hao J, Ju H, Zhao S, Junaid A, Scammell-La Fleur T, Dixon IM. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-β in the chronic phase of myocardial infarct scar healing. J. Mol. Cell. Cardiol. 1999;31:667–678. doi: 10.1006/jmcc.1998.0902. [DOI] [PubMed] [Google Scholar]
- 18.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-β1 in cardiac fibroblasts and myofibroblasts. J. Mol. Cell. Cardiol. 1997;29:1947–1958. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- 19.Lee AA, Dillmann WH, McCulloch AD, Villarreal FJ. Angiotensin II stimulates the autocrine production of transforming growth factor-β1 in adult rat cardiac fibroblasts. J. Mol. Cell. Cardiol. 1995;27:2347–2357. doi: 10.1016/s0022-2828(95)91983-x. [DOI] [PubMed] [Google Scholar]
- 20.Sadoshima J, Izumo S. Molecular characterization of angiotensin II–induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ. Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 21.Schorb W, Booz GW, Dostal DE, Conrad KM, Chang KC, Baker KM. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ. Res. 1993;72:1245–1254. doi: 10.1161/01.res.72.6.1245. [DOI] [PubMed] [Google Scholar]
- 22.Schultz, Jel J, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T. TGF-β1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J. Clin. Invest. 2002;109:787–796. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bujak M, Frangogiannis NG. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc. Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada H, Takemura G, Kosai K, Li Y, Takahashi T, Esaki M, Yuge K, Miyata S, Maruyama R, Mikami A, et al. Postinfarction gene therapy against transforming growth factor-β signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation. 2005;111:2430–2437. doi: 10.1161/01.CIR.0000165066.71481.8E. [DOI] [PubMed] [Google Scholar]
- 25.Tsuda T, Wang H, Timpl R, Chu ML. Fibulin-2 expression marks transformed mesenchymal cells in developing cardiac valves, aortic arch vessels, and coronary vessels. Dev. Dyn. 2001;222:89–100. doi: 10.1002/dvdy.1172. [DOI] [PubMed] [Google Scholar]
- 26.Strom A, Olin AI, Aspberg A, Hultgardh-Nilsson A. Fibulin-2 is present in murine vascular lesions and is important for smooth muscle cell migration. Cardiovasc. Res. 2006;69:755–763. doi: 10.1016/j.cardiores.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Fassler R, Sasaki T, Timpl R, Chu ML, Werner S. Differential regulation of fibulin, tenascin-C, and nidogen expression during wound healing of normal and glucocorticoid-treated mice. Exp. Cell Res. 1996;222:111–116. doi: 10.1006/excr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 28.Sicot FX, Tsuda T, Markova D, Klement JF, Arita M, Zhang RZ, Pan TC, Mecham RP, Birk DE, Chu ML. Fibulin-2 is dispensable for mouse development and elastic fiber formation. Mol. Cell. Biol. 2008;28:1061–1067. doi: 10.1128/MCB.01876-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuda T, Wu J, Gao E, Joyce J, Markova D, Dong H, Liu Y, Zhang H, Zou Y, Gao F, et al. Loss of fibulin-2 protects against progressive ventricular dysfunction after myocardial infarction. J. Mol. Cell. Cardiol. 2012;52:273–282. doi: 10.1016/j.yjmcc.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Susic D, Nunez E, Frohlich ED, Prakash O. Angiotensin II increases left ventricular mass without affecting myosin isoform mRNAs. Hypertension. 1996;28:265–268. doi: 10.1161/01.hyp.28.2.265. [DOI] [PubMed] [Google Scholar]
- 31.Rosenkranz S. TGF-β1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 32.Deten A, Holzl A, Leicht M, Barth W, Zimmer HG. Changes in extracellular matrix and in transforming growth factor β isoforms after coronary artery ligation in rats. J. Mol. Cell. Cardiol. 2001;33:1191–1207. doi: 10.1006/jmcc.2001.1383. [DOI] [PubMed] [Google Scholar]
- 33.Meulmeester E, Ten Dijke P. The dynamic roles of TGF-β in cancer. J. Pathol. 2011;223:205–218. doi: 10.1002/path.2785. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM. Fibrillin-1 regulates the bioavailability of TGFβ1. J. Cell. Biol. 2007;176:355–367. doi: 10.1083/jcb.200608167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 36.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 37.Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc. Res. 2004;64:24–31. doi: 10.1016/j.cardiores.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Kehat I, Molkentin JD. Extracellular signal-regulated kinase 1/2 (ERK1/2) signaling in cardiac hypertrophy. Ann. N. Y. Acad. Sci. 2010;1188:96–102. doi: 10.1111/j.1749-6632.2009.05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc. Natl. Acad. Sci. U.S.A. 2007;104:14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ono RN, Sengle G, Charbonneau NL, Carlberg V, Bachinger HP, Sasaki T, Lee-Arteaga S, Zilberberg L, Rifkin DB, Ramirez F, et al. Latent transforming growth factor β-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. J. Biol. Chem. 2009;284:16872–16881. doi: 10.1074/jbc.M809348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji Y, Prasad NB, Novotny EA, Kaur S, Elkahloun A, Chen Y, Zhang RZ, Chu ML, Agarwal SK, Marx SJ, et al. Mouse embryo fibroblasts lacking the tumor suppressor menin show altered expression of extracellular matrix protein genes. Mol. Cancer Res. 2007;5:1041–1051. doi: 10.1158/1541-7786.MCR-06-0379. [DOI] [PubMed] [Google Scholar]
- 42.Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB, Roberts AB. Transforming growth factor β 1 positively regulates its own expression in normal and transformed cells. J. Biol. Chem. 1988;263:7741–7746. [PubMed] [Google Scholar]
- 43.Bascom CC, Wolfshohl JR, Coffey RJ, Jr, Madisen L, Webb NR, Purchio AR, Derynck R, Moses HL. Complex regulation of transforming growth factor β1, β2, and β3 mRNA expression in mouse fibroblasts and keratinocytes by transforming growth factors β1 and β2. Mol. Cell. Biol. 1989;9:5508–5515. doi: 10.1128/mcb.9.12.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang XR, Chung AC, Yang F, Yue W, Deng C, Lau CP, Tse HF, Lan HY. Smad3 mediates cardiac inflammation and fibrosis in angiotensin II-induced hypertensive cardiac remodeling. Hypertension. 2010;55:1165–1171. doi: 10.1161/HYPERTENSIONAHA.109.147611. [DOI] [PubMed] [Google Scholar]
- 45.Watkins SJ, Borthwick GK, Oakenfull R, Robson A, Authur HM. Angiotensin II-induced cardiomyocyte hypertrophy in vitro is TAK1-dependent and Smad2/3-independent. Hypertens. Res. 2012;35:393–398. doi: 10.1038/hr.2011.196. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto-Ida M, Takimoto Y, Aoyama T, Akao M, Takeda T, Kita T. Activation of TGF-β1-TAK1-p38 MAPK pathway in spared cardiomyocytes is involved in left ventricular remodeling after myocardial infarction in rats. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H709–H715. doi: 10.1152/ajpheart.00186.2005. [DOI] [PubMed] [Google Scholar]
- 47.Brezniceanu ML, Wei CC, Zhang SL, Hsieh TJ, Guo DF, Hebert MJ, Ingelfinger JR, Filep JG, Chan JS. Transforming growth factor-β 1 stimulates angiotensinogen gene expression in kidney proximal tubular cells. Kidney Int. 2006;69:1977–1985. doi: 10.1038/sj.ki.5000396. [DOI] [PubMed] [Google Scholar]
- 48.Zhang M, Fraser D, Phillips A. ERK, p38, and Smad signaling pathways differentially regulate transforming growth factor-β1 autoinduction in proximal tubular epithelial cells. Am. J. Pathol. 2006;169:1282–1293. doi: 10.2353/ajpath.2006.050921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seo JY, Park J, Yu MR, Kim YS, Ha H, Lee H,B. Positive feedback loop between plasminogen activator inhibitor-1 and transforming growth factor-β1 during renal fibrosis in diabetes. Am. J. Nephrol. 2009;30:481–490. doi: 10.1159/000242477. [DOI] [PubMed] [Google Scholar]
- 50.Ellmers LJ, Scott NJ, Medicherla S, Pilbrow AP, Bridgman PG, Yandle TG, Richards AM, Protter AA, Cameron VA. Transforming growth factor-β blockade down-regulates the renin-angiotensin system and modifies cardiac remodeling after myocardial infarction. Endocrinology. 2008;149:5828–5834. doi: 10.1210/en.2008-0165. [DOI] [PubMed] [Google Scholar]
- 51.Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, Lonning S, Ling H, Ertl G, Bauersachs J. Transforming growth factor β inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res. Cardiol. 2008;103:485–492. doi: 10.1007/s00395-008-0739-7. [DOI] [PubMed] [Google Scholar]
- 52.Lucas JA, Zhang Y, Li P, Gong K, Miller AP, Hassan E, Hage F, Xing D, Wells B, Oparil S, Chen YF. Inhibition of transforming growth factor-β signaling induces left ventricular dilation and dysfunction in the pressure-overloaded heart. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H424–H432. doi: 10.1152/ajpheart.00529.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karakikes I, Chaanine AH, Kang S, Mukete BN, Jeong D, Hajjar RJ, Lebeche D. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J. Am. Heart Assoc. 2013;2:e000078. doi: 10.1161/JAHA.113.000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-β signaling in vascular fibrosis. Cardiovasc. Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]