Abstract
Background:
The management of advanced cutaneous malignancies has been controversial. Thirteen patients with nonmelanoma skin neoplasias that had invaded the bone of the calvarium and scalp were treated in our centre.
Objective:
The purpose of this study was to evaluate our experience in treating these malignancies with scalp resection and full or partial thickness cranium reconstruction.
Patients and Methods:
From June 2008 to March 2012, thirteen patients with locally advanced tumours of the scalp invading the calvarium were treated with wide local excision of the scalp combined with an underlying craniectomy and dural resection if needed.
Results:
Using histopathological diagnosis eleven patients were diagnosed with basal cell carcinoma and two patients with squamous cell carcinoma. A full thickness cranium resection was performed in seven patients and partial in six patients.
Conclusion:
These large cancers occasionally invade adjacent structures, as well as bone, presenting a challenging surgical problem. In general, giant rotational or island scalp flaps and free tissue transfers are needed to close the area. Finding clean margins are an important part of treating patients with bone involvement and can usually be attained using outer tabula curettage thus preventing unnecessary morbidity.
KEY WORDS: Bone invasion, calvarium, non-melanocytic, reconstruction, scalp, skin malignancies
INTRODUCTION
Basal cell carcinomas (BCC) are the most common neoplasm in the craniofacial region and squamous cell carcinomas (SCC) are the second most frequently occurring. BCCs tend to have an aggressive growth pattern and quickly reach a large size.[1,2] These invasive tumours usually reach into deep tissue, affecting cartilage, bone, or muscle.[3] These lesions are generally caused by unprotected exposure to ultraviolet light, and common predisposing conditions including; lightly pigmented skin, family history of skin cancer and immunodeficiency states.-[4] BCC also occurs as part of familial neoplastic syndromes, like the Gorlin-Gotz syndrome.[5] SCC is the second most common malignant tumour of the craniofacial region. Its main difference from BCC is that they can often spread to the regional lymph nodes of the neck and head region. Cutaneous SCC risk factors include; long-term exposure to ultraviolet or ionising radiation, arsenic or repeated contact with polycyclic hydrocarbons. These cancers can also occur in chronic lesions caused by burns, ulcers, or infection of traumatic wounds.[6]
Reconstruction is usually accomplished by primary closure or local flaps after complete traditional or Mohs micrographic excision. Most patients have a benign clinical course with excellent cosmetic and functional outcomes. A small percentage of these patients will present with craniofacial skin cancers that involve large surface areas and to variable degrees, the subcutaneous structures, adjacent organ systems, or facial skeleton. These large locally advanced skin cancers frequently invade adjacent structures, requiring an extensive resection of soft-tissue and bone, creating a challenging reconstructive problem. In addition, the face and scalp over time tend to ulcerate and necrosis, creating significant hygiene and social problems.
The purpose of this study is to evaluate our experience with surgical resection of locally advanced scalp non-melanocytic skin malignancies (NMSMs), reconstruction of composite scalp defects using pedicle or free tissue transfer and to evaluate polymethyl-methacrylate cranioplasty in repairing large defects in the craniofacial region. At the same time outer tabula skull bone curettage is discussed as a treatment for patients with invasive partial skull defects. We retrospectively review our institutional experience with surgical excision of bone invaded NMSMs and immediate reconstruction. Patient characteristics, tumour size and location, recurrence and metastasis, type of reconstruction and complications are presented for 13 patients.
PATIENTS AND METHODS
Between March 2008 and January 2013, 292 patients were treated in our clinic for non-melanocytic skin cancer in the craniofacial region. Out of these, 13 patients with NMSMs that had invaded the bone of the craniofacial region were found and included in this study. A retrospective chart review of hospital and office records of all patients was performed. Patient demographics, tumour characteristics, reconstructive treatment and outcomes are summarized in Table 1.
Table 1.
Patient demographics, tumour characteristics, reconstructive treatment and outcomes were summarized
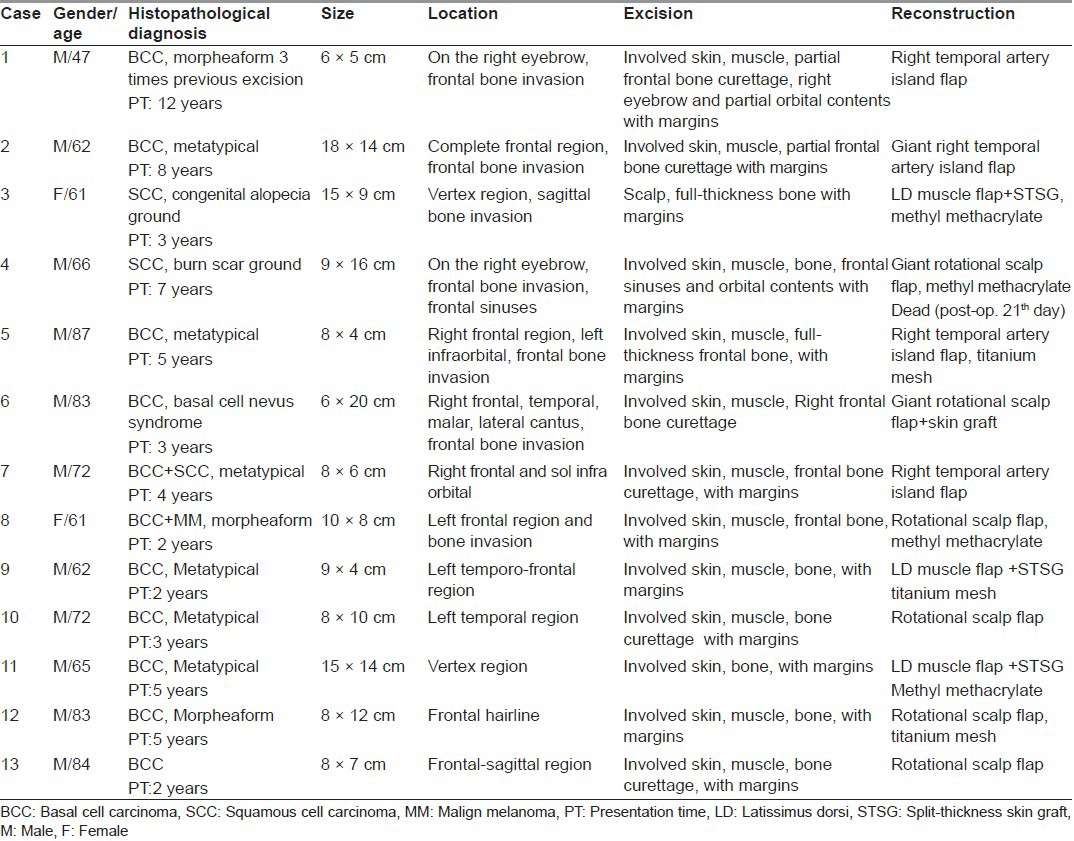
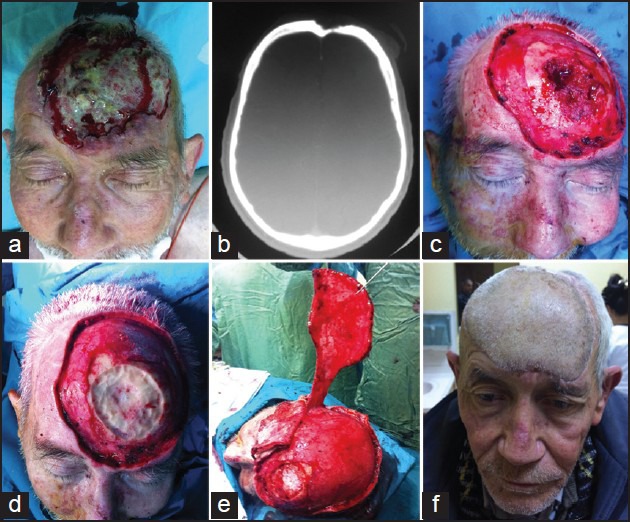
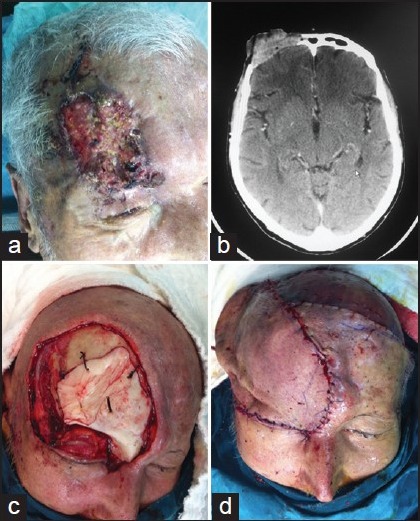
Patients with locally advanced tumours of the scalp invading the calvarium were treated with wide local excision of the scalp combined with underlying craniectomy and dural resection if needed. Curettage of the outer tabula of the skull bone was performed for the partial thickness cranium invasion and reconstruction of the skeletal structure was not needed [Figure 1d]. We visually confirmed clean margins on the bone structure. In the case of full thickness cranium defects reconstruction was completed using a fascial graft for the dura, methyl methacrylate and titanium mesh for the skull [Figure 2c]. External soft-tissue closure was performed with giant rotational flap [Figure 2], temporal artery island flap [Figure 1 and 3] and free tissue transfer.
Figure 1.
(a) Appearance of the nodular ulcerative lesion on the frontal and anterior sagittal region, (b) Axial plane, computerised tomography imaging scan. View of the frontal bone erosion, (c) Intraoperative appearance of the frontal bone tumoural erosion and (d) construction of bone curettage, (e) Intra-operative appearance of giant temporal artery island flap elevation, (f) Late post-operative view of the patient
Figure 2.
(a) Appearance of the nodular ulcerative lesion on the right eyebrow and frontal region, (b) Axial plane, computerised tomography imaging scan. View of the frontal bone erosion, (c) Appearance of the methyl methacrylate and (d) large rotational scalp flap reconstruction after wide full thickness scalp and skull excision
Figure 3.
(a) Appearance of the nodular ulcerative lesion on the right eyebrow and frontal region, (b) Three-dimensional: computerised tomography imaging scan. View of the right supraorbital frontal bone erosion, (c) Intra-operative appearance of the supraorbital bone tumoural erosion, (d) Intra-operative planning of the right temporal artery island flap, (e) Late post-operative view of the patient
RESULTS
Bone invasion was detected in 4.4% of our cases of NMSMs in the craniofacial region. Of these patients a histopathological diagnosis of BCC was confirmed in 11 cases and SCC in the remaining 2 cases. In our study 6 patients presented with metatypical type pathologies and 3 patients had morpheaform type pathologies. The mean diameter of the lesions was 12 cm (8-20 cm). The age range at presentation was from 43 to 87 years old, with a mean age of 70 years. 10 patients were male and 3 patients female. The lesions were located in the frontal (8), temporal (3) and vertex (2) regions. All patients had a history of lesion presentation longer than 2 years and 2 patients presented with recurrence after previous excisions. Significant medical history included 1 patient with burn scar ground, 1 patient with a history of basal cell nevus syndrome and 1 patient with congenital alopecia ground. None of our patients had evidence of metastasis at the time of presentation.
All patients underwent wide surgical excision to obtain free soft-tissue margins as confirmed by intraoperative frozen sections. One patient required 2 operative procedures to confirm a microsurgically free margin before reconstruction was performed. One patient had gross extension of tumour into the frontal sinuses, requiring a complete sinuses exenteration. Outer tabula resection was enough to get clear margins in 6 of the patients. Full thickness cranium resection and dura repair was needed for 7 of the patients. Large bony defects were reconstructed with polymethyl methacrylate (bone cement). Small defects were reconstructed with titanium mesh. Fascial grafts were used for dura repair. Soft tissue reconstruction required 3 free tissue transfers (Latissimus dorsi (LD) muscle flap and skin grafting were preferred), 4 temporal artery island flaps and 6 large rotational, tranpositional scalp flaps. Two patients developed flap complications. These were a partial distal loss of a scalp tranpositional flap on the patient with burn scar ground and a partial necrosis of an LD muscle flap. The patient's distal flap healed seamlessly after a minor revision while the partial necrosis healed after a major revision with a rotational scalp flap, all other reconstructions healed without complications.
Patients follow-up ranged from 10 to 52 months, with a mean follow-up of 26 months. Late complications include a strabismus due to flap contraction after radiotherapy. Peri-operative complications not associated with the operative site include a pneumonia requiring ventilatory support. This patient died on the 21st post-operative day for reasons not relating to the surgery. With the exception of the strabismus, no patient has undergone any revisions or additional reconstructions. No tumour recurrences or metastases have been identified in any of our patients thus far. Patient and family satisfaction has been good. A decrease in pain and improved appearance and hygiene has been reported.
DISCUSSION
The usual presentation of basal cell cancer, the most common cutaneous malignancy, is a lesion that is readily excised and closed, with negligible concern for metastasis. However, cutaneous SCCs have a risk of spreading to the lymph nodes. NMSM Invaded the bone are extremely rare tumours as most patients with non-healing lesions will typically seek medical attention before the ulceration reaches a large size.[7] There aren’t any studies specifically designed to study these tumours. Risk factors associated with giant skin malignancies that have invaded the bone include length of time to presentation, patient neglect, previous treatment, an aggressive histologic type (morpheaform, micronodular, and metatypical) and previous radiation therapy.[8] In addition, traumatic ground (congenital alopecia, burn scar) can also be seen as an additional risk as seen in two of our cases. In our study group more than half of the cases presented in the frontal region. The entire frontal area can be considered a risk factor. In this study only 2 of 13 patients were pathologically diagnosed as SCC and these had developed on the burn scars and congenital alopecia ground. We conclude that in order for these tumours to invade bone tissue before distant metastasis has occurred there must be other risk factors present. The size of a lesion results from a combination of rate of growth, as well as length of time to diagnosis, since there is no definitive evidence that malignancies that have invaded the bone grow at an increased rate, delayed diagnosis and treatment is presumed to be the primary cause of increased size.[9] Both our study and other studies report duration of greater than 2 years before diagnosis and treatment which also supports the idea that neglect is a contributing factor to the large tumour size.[8,9,10,11,12,13] In our group of patients, medical attention was sought at the insistence of family or friends, or the lesion was noted when the patient presented for evaluation of other medical conditions.
The large size and invasion of the bone have implications for metastatic potential[6,13] as well as reconstructive requirements. Metastatic potential, while increased in some studies of giant skin cancers[8,12,13] is not present at all in our series. Metastasis in basal cancer, overall, is a rare finding, often occurring after a long interval following the initial presentation.[14,15] None of the patients in our series exhibited distant metastasis. The development of metastases may be related to an immune deficient state.[14] Once metastases are present, survival is markedly diminished.[16]
Surgery is the primary treatment in NMSMs. Radiation therapy is an alternative to surgical excision and reconstruction in patients who are not candidates for prolonged anaesthesia or surgery or in patients who do not elect to undergo surgery. A study of T4 lesions of the head and neck with involvement of cartilage, muscle, or bone showed only 67% rate of success when using radiation therapy.[17] Interestingly, tumour size did not correlate with outcomes in this series. Short-term complications related to head and neck function and long-term complications such as carcinogenesis are considerations in choosing the appropriate course of treatment.
Reconstruction options are determined according to the size of the resulting defect and the location of vital structures. The removal of extensive face and scalp lesions can result in large complex soft-tissue and bone defects, with exenteration of the orbit, brain and frontal sinuses exposure. Exposure of the frontal sinus will require stripping the sinus mucosa and obliterating the frontonasal duct. Reconstruction options are planned in three stages, the layer of soft tissue, cranium and duramater. For external soft-tissue repair free flaps are considered today to be the first choice in most cases. Microsurgical reconstruction has the advantage of a single- stage closure of complex defects with well-vascularised tissue, particularly in the case of radiated or scarred tissue.[18] In the oncologic setting, large defects and composite defects are reliably treated with a free flap to provide stable immediate coverage.[19,20,21] If postoperative radiotherapy is necessary, fasciocutaneous coverage is preferable to muscle coverage with skin grafting because of the reliability and durability of cutaneous healing.[22,23] In thin patients, an anterolateral thigh flap is ideal because of its minimal donor-site morbidity and long pedicle length. For total scalp coverage, or in patients with a thick subcutaneous layer where an anterolateral thigh flap is inadequate, a LD muscle free flap with skin grafting may be preferable.[22] In our series we have used the free LD muscle flap in three patients. Alternatives such as the scapular flap, rectus abdominis flap and radial forearm flaps have been described but are limited to treating smaller sized defects.[19,23,24,25,26]
Our group was surgically treated; however, reconstruction with microsurgery is not always possible for reasons including advanced age of the patient, additional problems that increase the risk of anesthesia, extension of the duration of surgery. In these cases we chose to use scalp flaps if adequate for closure. The advantages of scalp flaps are that they provide repair with similar textures, short length of time in surgery and they have a tissue resistant to radiotherapy. The disadvantages of the use of skin grafts for closure of the donor area are development of dog-ear deformities and sometimes insufficient volume. Pinwheel flaps or a series of three rhomboid flaps as described by Raposio are useful in preserving the spiral hair growth pattern at the vertex.[27,28] However, we do not prefer this technique because suture lines end are in an area at the bottom of the devascularised cranioplasty. Larger defects have been reconstructed with rotation, advancement, or transposition scalping flaps based on a single axial blood supply as described by Converse, Juri and Juri and Orticochea.[29,30,31,32] Larger flaps may require skin grafting at the donor site that can be removed with subsequent tissue expansion. Regional flaps are limited largely to the trapezius myocutaneous flap for occipital defects. Tissue expansion provides a superior cosmetic result, allowing for like-with-like tissue replacement. However, tissue expansion typically requires multiple stages and a healed wound edge, precluding immediate coverage in the oncologic setting.[22] In our clinic, we use scalp flaps if adequate for closure and in conditions inappropriate for microsurgical reconstruction. In some patients using a temporal artery island flap is seen as a good option especially in the inferior frontal region.[33]
Composite defects of the cranial vault requiring both scalp and calvarial replacement can be challenging. In an otherwise healthy wound bed where radiotherapy is not anticipated, there are a variety of viable options, including skull reconstruction with rib grafts or titanium, and coverage with a free flap.[34] If adjuvant radiotherapy is planned, titanium mesh covered with a fasciocutaneous free flap is an appropriate option. If the wound bed has been previously infected or irradiated, non-vascularised grafts and alloplastic materials may not be sufficient. A vascularized rib based on a LD free flap has been used successfully in these cases.[35] In cases where the scalp is intact and there is an isolated defect of the calvaria, polymethyl-methacrylate has been used successfully for recontouring. Kumar et al. reported a 95 % success rate using customized methylmethacrylate implants to reconstruct craniectomy defects.[36] Disadvantages of the autogenous bone grafts are developing of donor site morbidity and they may sometimes prove inadequate. Animal (xenograft) or cadaver (allogeneic) source bone grafts are a viable option but the disadvantage is the cost. In our series, immediate skeletal reconstruction is performed using methyl metacrylate and metallic mesh (small defects) to restore integrity and contour to the cranial vault and facial skeleton.
We usually prefer to use fascial grafts in the inner layer duramater reconstruction. Although it seems against to oncological principles, we think that, curettage of the external tabula can be performed until the tumour free tissue is gained in cases such as superficial bone invasion and low grade bcc cases. In the 6 (6/13) patients in whom we reached clean margins with only the outer tabula curettage with no skeletal reconstruction required morbidity was reduced as was patient mortality and there was no recurrence during the 3 year follow-up period. Clean margins can be reached in appropriate patients with just the external tabula curettage. It has a high proportion of patients in our series.
Recurrence rates after treatment of giant basal cell cancers has been low in most studies, but patient numbers are also low. Overall, the 5-year recurrence rates for all types of basal cell cancers treated by non-Mohs surgery are 8.7 %.[37] The length of time of follow-up influences published recurrence rates. Risk of recurrence is also related to increasing size.[14,38] Prior to excision, patients with craniofacial lesions complained of pain and difficulty with hygiene due to drainage and odour from the NMSM. Relief of pain and improvements in hygiene were repeatedly cited as areas of satisfaction by patients and families postoperatively. Successful treatment of NMSMs can be accomplished in a one-stage procedure even in the presence of extensive involvement beyond the skin. The improved quality of life results in high patient and family satisfaction. Unfortunately, the very reasons why these patients did not seek earlier initial treatment may result in presentation of other illnesses in the postoperative period and may also limit their long-term follow-up.
CONCLUSION
The primary goal of surgical treatment of skin tumours with invasion of the craniofacial bone structure is three-dimensional tumour resection with histologically clear margins. This goal has to be balanced, however, with an acceptable functional and aesthetic result. These large cancers frequently invade adjacent structures, as well as bone, presenting a challenging surgical problem. Free tissue transfer has become a frequent first choice in reconstruction of major defects, but local flaps still have an important role. Clean margins in most patients with bony invasion can be obtained by curettage of the outer tabula. In order to reduce the risk of morbidity full-thickness resections should only be considered as the last alternative. Since these lesions have metastatic potential, an aggressive surgical resection to obtain clean margins offers the best chance for cure. Neglect is a major contribution to the development of NMSMs, with undiagnosed medical problems predisposing patients to postoperative complications. NMSMs which have invaded the bone can be successfully treated, with low complications, good oncologic outcomes and acceptable cosmetic results.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ko CB, Walton S, Keczkes K. Extensive and fatal basal cell carcinoma: A report of three cases. Br J Dermatol. 1992;127:164–7. doi: 10.1111/j.1365-2133.1992.tb08050.x. [DOI] [PubMed] [Google Scholar]
- 2.Gussack GS, Schlitt M, Lushington A, Woods KE. Invasive basal cell carcinoma of the temporal bone. (609-11).Ear Nose Throat J. 1989;68:605–6. [PubMed] [Google Scholar]
- 3.Papadopoulos O, Konofaos P, Chrisostomidis C, Champsas G, Frangoulis M, Karakitsos P, et al. Nonmelanoma skin tumors involving the craniofacial region: Our 22 years of experience. J Craniofac Surg. 2007;18:1021–33. doi: 10.1097/scs.0b013e3180f6112f. [DOI] [PubMed] [Google Scholar]
- 4.Wong CS, Strange RC, Lear JT. Basal cell carcinoma. BMJ. 2003;327:794–8. doi: 10.1136/bmj.327.7418.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasso JM, García-Tutor E, Bazán A. Aggressive basal cell carcinoma of the temporal region in a patient with Gorlin-Goltz syndrome. Ann Plast Surg. 2000;44:429–34. doi: 10.1097/00000637-200044040-00014. [DOI] [PubMed] [Google Scholar]
- 6.Fleming MD, Hunt JL, Purdue GF, Sandstad J. Marjolin's ulcer: A review and reevaluation of a difficult problem. J Burn Care Rehabil. 1990;11:460–9. [PubMed] [Google Scholar]
- 7.Lackey PL, Sargent LA, Wong L, Brzezienski M, Kennedy JW. Giant basal cell carcinoma surgical management and reconstructive challenges. Ann Plast Surg. 2007;58:250–4. doi: 10.1097/01.sap.0000250842.96272.37. [DOI] [PubMed] [Google Scholar]
- 8.Randle HW, Roenigk RK, Brodland DG. Giant basal cell carcinoma (T3). Who is at risk? Cancer. 1993;72:1624–30. doi: 10.1002/1097-0142(19930901)72:5<1624::aid-cncr2820720522>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Betti R, Inselvini E, Moneghini L, Crosti C. Giant basal cell carcinomas: Report of four cases and considerations. J Dermatol. 1997;24:317–21. doi: 10.1111/j.1346-8138.1997.tb02797.x. [DOI] [PubMed] [Google Scholar]
- 10.Manstein CH, Gottlieb N, Manstein ME, Manstein G. Giant basal cell carcinoma: A series of seven T3 tumors without metastasis. Plast Reconstr Surg. 2000;106:653–6. doi: 10.1097/00006534-200009030-00021. [DOI] [PubMed] [Google Scholar]
- 11.Kokavec R, Fedeles J. Giant basal cell carcinomas: A result of neglect? Acta Chir Plast. 2004;46:67–9. [PubMed] [Google Scholar]
- 12.Sahl WJ, Jr, Snow SN, Levine NS. Giant basal cell carcinoma. Report of two cases and review of the literature. J Am Acad Dermatol. 1994;30:856–9. [PubMed] [Google Scholar]
- 13.Sahl WJ. Basal cell carcinoma: Influence of tumor size on mortality and morbidity. Int J Dermatol. 1995;34:319–21. doi: 10.1111/j.1365-4362.1995.tb03610.x. [DOI] [PubMed] [Google Scholar]
- 14.Lo JS, Snow SN, Reizner GT, Mohs FE, Larson PO, Hruza GJ. Metastatic basal cell carcinoma: Report of twelve cases with a review of the literature. J Am Acad Dermatol. 1991;24:715–9. doi: 10.1016/0190-9622(91)70108-e. [DOI] [PubMed] [Google Scholar]
- 15.Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262–9. doi: 10.1056/NEJMra044151. [DOI] [PubMed] [Google Scholar]
- 16.Raszewski RL, Guyuron B. Long-term survival following nodal metastases from basal cell carcinoma. Ann Plast Surg. 1990;24:170–5. doi: 10.1097/00000637-199002000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Lee WR, Mendenhall WM, Parsons JT, Million RR. Radical radiotherapy for T4 carcinoma of the skin of the head and neck: A multivariate analysis. Head Neck. 1993;15:320–4. doi: 10.1002/hed.2880150409. [DOI] [PubMed] [Google Scholar]
- 18.McCombe D, Donato R, Hofer SO, Morrison W. Free flaps in the treatment of locally advanced malignancy of the scalp and forehead. Ann Plast Surg. 2002;48:600–6. doi: 10.1097/00000637-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Lutz BS, Wei FC, Chen HC, Lin CH, Wei CY. Reconstruction of scalp defects with free flaps in 30 cases. Br J Plast Surg. 1998;51:186–90. doi: 10.1054/bjps.1997.0182. [DOI] [PubMed] [Google Scholar]
- 20.Leedy JE, Janis JE, Rohrich RJ. Reconstruction of acquired scalp defects: An algorithmic approach. Plast Reconstr Surg. 2005;116:54e–72. doi: 10.1097/01.prs.0000179188.25019.6c. [DOI] [PubMed] [Google Scholar]
- 21.Mehrara BJ, Disa JJ, Pusic A. Scalp reconstruction. J Surg Oncol. 2006;94:504–8. doi: 10.1002/jso.20487. [DOI] [PubMed] [Google Scholar]
- 22.Wei FC, Dayan JH. Scalp, skull, orbit, and maxilla reconstruction and hair transplantation. Plast Reconstr Surg. 2013;131:411e–24. doi: 10.1097/PRS.0b013e31827c7167. [DOI] [PubMed] [Google Scholar]
- 23.Lin PY, Miguel R, Chew KY, Kuo YR, Yang JC. The role of the anterolateral thigh flap in complex defects of the scalp and cranium. Microsurgery. 2014;34:14–9. doi: 10.1002/micr.22103. [DOI] [PubMed] [Google Scholar]
- 24.Miller MJ, Schusterman MA, Reece GP, Kroll SS. Microvascular craniofacial reconstruction in cancer patients. Ann Surg Oncol. 1995;2:145–50. doi: 10.1007/BF02303630. [DOI] [PubMed] [Google Scholar]
- 25.Wang HT, Erdmann D, Olbrich KC, Friedman AH, Levin LS, Zenn MR. Free flap reconstruction of the scalp and calvaria of major neurosurgical resections in cancer patients: Lessons learned closing large, difficult wounds of the dura and skull. Plast Reconstr Surg. 2007;119:865–72. doi: 10.1097/01.prs.0000240830.19716.c2. [DOI] [PubMed] [Google Scholar]
- 26.van Driel AA, Mureau MA, Goldstein DP, Gilbert RW, Irish JC, Gullane PJ, et al. Aesthetic and oncologic outcome after microsurgical reconstruction of complex scalp and forehead defects after malignant tumor resection: An algorithm for treatment. Plast Reconstr Surg. 2010;126:460–70. doi: 10.1097/PRS.0b013e3181de2260. [DOI] [PubMed] [Google Scholar]
- 27.Raposio E, Nordström RE, Santi P. Aesthetic reconstruction of the vertex area of the scalp. Case report. Scand J Plast Reconstr Surg Hand Surg. 1998;32:339–41. doi: 10.1080/02844319850158705. [DOI] [PubMed] [Google Scholar]
- 28.Dowbak G. V-Y-S plasty for scalp defects. Plast Reconstr Surg. 2004;113:1889–90. doi: 10.1097/01.prs.0000119862.61832.87. [DOI] [PubMed] [Google Scholar]
- 29.Orticochea M. Four flap scalp reconstruction technique. Br J Plast Surg. 1967;20:159–71. doi: 10.1016/s0007-1226(67)80032-8. [DOI] [PubMed] [Google Scholar]
- 30.Orticochea M. New three-flap reconstruction technique. Br J Plast Surg. 1971;24:184–8. doi: 10.1016/s0007-1226(71)80038-3. [DOI] [PubMed] [Google Scholar]
- 31.Converse JM. The technique of closure of scalp defects. Clin Neurosurg. 1964;11:21–31. doi: 10.1093/neurosurgery/11.cn_suppl_1.21. [DOI] [PubMed] [Google Scholar]
- 32.Juri J, Juri C. Aesthetic aspects of reconstructive scalp surgery. Clin Plast Surg. 1981;8:243–54. [PubMed] [Google Scholar]
- 33.Cöloğlu H, Koçer U, Oruç M, Sahin B, Ozdemir R. Axial bilobed superficial temporal artery island flap (tulip flap): Reconstruction of combined defects of the lateral canthus including the lower and upper eyelids. Plast Reconstr Surg. 2007;119:2080–7. doi: 10.1097/01.prs.0000260602.32664.ba. [DOI] [PubMed] [Google Scholar]
- 34.Wei FC, Tsao SB, Chang CN, Noordhoff MS. Scalp, skull, and dura reconstruction on an emergency basis. Ann Plast Surg. 1987;18:252–6. doi: 10.1097/00000637-198703000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Seitz IA, Adler N, Odessey E, Reid RR, Gottlieb LJ. Latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap reconstruction in composite defects of the scalp: Case series and review of literature. J Reconstr Microsurg. 2009;25:559–67. doi: 10.1055/s-0029-1236834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar AR, Bradley JP, Harshbarger R, Stevens F, Bell R, Moores L, et al. Warfare-related craniectomy defect reconstruction: Early success using custom alloplast implants. Plast Reconstr Surg. 2011;127:1279–87. doi: 10.1097/PRS.0b013e318205f47c. [DOI] [PubMed] [Google Scholar]
- 37.Rowe DE, Carroll RJ, Day CL., Jr Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: Implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315–28. doi: 10.1111/j.1524-4725.1989.tb03166.x. [DOI] [PubMed] [Google Scholar]
- 38.Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373–7. [PubMed] [Google Scholar]


