Abstract
Maxillary reconstruction is still an evolving art when compared to the reconstruction of the mandible. The defects of maxilla apart from affecting the functions of the speech, swallowing and mastication also cause cosmetic disfigurement. Rehabilitation of the form and function in patients with maxillary defects is either by using an obturator prosthesis or by a surgical reconstruction. Literature is abundant with a variety of reconstructive methods. The classification systems are also varied, with no universal acceptance of any one of them. The oncologic safety of these procedures is still debated, and conclusive evidence in this regard has not emerged yet. Management of the orbit is also not yet addressed properly. Tissue engineering, that has been hyped to be one of the possible solutions for this vexing reconstructive problem, has not come out with reliable and reproducible results so far. This review article discusses the rationale and oncological safety of the reconstructing the maxillary defects, critically analyzes the classification systems, offers the different reconstructive methods and touches upon the controversies in this subject. The management of the retained and exenterated orbit associated with maxillectomy is reviewed. The surgical morbidity, complications and the recent advances in this field are also looked into. An algorithm, based on our experience, is presented.
KEY WORDS: Free fibula, free flaps, head and neck cancer, head and neck reconstruction, maxillary, maxillectomy, reconstruction, orbital exenteration, orbital reconstruction
INTRODUCTION
The maxillary bone is important both for aesthesis of the face and function of the oral cavity. It separates the oral and orbital cavities and also provides skeletal support to the orbital contents. The defects of maxilla affect the speech, swallowing and mastication. Rehabilitation of the form and function in patients with maxillary defects is either by using an obturator prosthesis or by a surgical reconstruction. Traditionally, these defects were prosthetically rehabilitated, but, in the last two decades, several publications have demonstrated the advantages of surgically reconstructing these defects. A variety of regional and free flaps have been reported for this purpose. In order to facilitate logical thinking in maxillary reconstruction, various systems have been proposed for classifying these defects. These classifications, though have made the reporting of the results more comparable, still lack the uniformity and have differing levels of peer acceptance. In spite of the significant advances in the understanding of rehabilitating the maxillary defects, disagreement still exists in the methods suitable to address the associated orbital floor and exenteration defects. The oncologic safety of these procedures is still debated, and conclusive evidence in this regard has not emerged yet. Tissue engineering, that has been hyped to be one of the possible solutions for this vexing reconstructive problem, has not come out with reliable and reproducible results so far. Maxillary reconstruction is still an evolving art when compared to the reconstruction of the mandible. This review article will aim at looking at these various issues related to maxillary reconstruction.
RATIONALE AND ONCOLOGICAL SAFETY
Traditionally, post-maxillectomy defects were reconstructed using split-thickness skin grafts to line the post-resection cavity so as to facilitate a prosthetic obturator placement. Following healing of the cavity, an interim, and later on, a permanent obturator was provided. The retention was achieved by anchoring to the remaining teeth.[1] To facilitate obturator placement in edentulous patients as well as cases of bilateral maxillectomy, osseous integrated implants have been used locating them in the adjacent bony structures like the zygoma.[2] Obturation of the maxillary defects as a permanent definitive option is still practised widely due to its relative safety and least operative time. This method has an additional advantage of immediately restoring the dentition, whereas the flap reconstructions require further procedures or dentures. Above all, it has been believed that permanent sealing of the maxillary cavity would hinder its inspection during follow up for any recurrences. However, there are numerous disadvantages with the use of the obturator, which include inadequate sealing leading to poor oro-nasal separation and instability of the prosthesis due to the lack of buttressing areas. These become more evident when the resection defect is wider and posteriorly placed.[2,3] Few of the quality of life studies (QOL) showed low scores in patients using obturators when compared to the surgical reconstruction. Kornblith et al.[4] observed that patients with more than a third of the soft palate and a fourth of the hard palate resected had poor speech scores and overall obturator function. Rogers et al.[5] compared the QOL in a two similar small groups of patients having either prosthetic or surgical obturation of maxillectomy cavities. These patients completed a postoperative semi-structured interview with eight sets of questionnaires. No statistically significant differences were seen between the obturator and free flap groups. But, they reported borderline trends for obturator patients being more self-conscious, concerned about their appearance, to have more pain and soreness in their mouths, more and less satisfied with their upper dentures and function. The problems as noted by the authors not favourable to the use of obturator included larger soft or hard palatal resection defects, which included facial skin or orbital contents and presence of trismus. Okay et al. found that the stability of the prosthesis compromised as defect size increased, resulting in poor obturator function and QOL. They concluded that the defects that involved more than half the hard palate or included the premaxilla and both canines were poor candidates for prosthetic reconstruction.[6] The influence of the horizontal extent of the palatal defect in determining the success of the use of obturators was stressed by Moreno and Hanasano in their comparative study of 73 cases with obturation and 40 cases with free flap reconstruction. They found a statistically significant reduction in the speech and swallowing outcome with the use of obturators where the horizontal defect was large.[7] The superiority of free flaps in improving the quality of life even in small and medium defects of maxilla was reported by genden et al.[8] The influence of post-operative radiotherapy that most of these patients have to undergo can be a cause for significant problems with obturator rehabilitation. The radiation could lead to trismus and resultant difficulty in insertion of the prosthesis, as well as dryness and soreness of the mucosa as reported by Chigurupati et al.[9] Genden et al. noted that, in the group of patients who had undergone radiotherapy, there was pain and difficulty tolerating the prosthesis. They also needed frequent adjustment of the prosthesis.[10] The importance of surveillance of the cavity for recurrences by keeping it open has been supported or negated by only very few studies. Moreno et al.[7] compared the average time of presentation of recurrence in two groups of patients who had undergone maxillectomy; one group had reconstruction and the other had patients with obturator. Each group was matched for cancer stage and histology. No statistically significant differences between the two groups could be detected. Also, they found that the diagnosis of recurrence was more frequently made by physical examination in both groups than by imaging with CT or MRI scans. But, the number of cases included in this analysis was too small to reach into any reliable conclusion. But, the papers supporting reconstruction, without the support of any evidence, state that endoscopic examination and use of imaging modalities like CT and MRI scan can negate the necessity of keeping the cavity open.
CLASSIFICATION SYSTEMS
Various classification systems have tried to look at the defects either from their functional and/or aesthetic effects. Some of them have mainly looked at the reconstructive or rehabilitative considerations only. Brown et al.[11] initially classified maxillectomy defects into four, based on the vertical and horizontal defect components. The vertical divisions (Class I-IV) denote the extent of unilateral involvement. The sub-classifications into a-c qualify the defect horizontally, denoting the amount of palate and alveolus removed. The loss of the vertical component causes more of an aesthetic problem, while the horizontal component loss results in greater functional deficits. The dental, masticatory and the articulatory aspects are given by the horizontal component, and the aesthetic and orbital aspects are highlighted by the vertical components. Recently, the authors modified the classification system[12] to include a class V defect, the orbito-maxillary defect and class VI defect, the naso-maxillary defect. These two additions do not involve the ablation of the palate or the dental alveolus. They also included minimal changes in the horizontal classification into a-d.
Cordeirro et al. has suggested a four-part classification system.[13,14,15] They could use this classification system to assess the surface area to volume requirement, the need for the palatal closure and the need for orbital reconstruction. Triana et al.[16] classified the defects into (1) inferior partial maxillectomy, subdivided based on the extent of the palate lost and (2) Total maxillectomy, subdivided depending on whether the orbit was removed, and the amount of the malar bone and zygomatic arch lost. Okay et al.[6] has described a scheme based on the defect in the horizontal planes. This classification system mainly takes into account the obturator stability and the retention.
CRITICAL REVIEW OF THE CLASSIFICATION SYSTEMS
A classification system should be valid, reliable, rationale and should grade the defects according to the reconstructive difficulty. It should describe the defect with increasing loss of the reconstructive components, and it should be the guide to an ideal reconstructive method. It should also be a means of documentation for the comparison of results and should identify the ability and deficits in achieving the reconstructive goals. While other system gives much importance on the restoration of the soft issue and the bony components, the classification proposed by Okay et al. stressed on importance to the dental and alveolar restoration and rehabilitation. The extent of the palatal defect itself or the stability for successful obturator retention was given prime consideration in their classification. Moreno et al.[7] in their retrospective analysis pointed out that the success of denture obturator rehabilitation correlated very well when the defects were type 3 as per the Okay classification. They conclude that these defects are better covered with free flaps. Brown's classification[12] also incorporates the horizontal extent of the defect grading them a-c depending on the extent of resection of the hard palate and alveolus. But, while considering the options in choosing the reconstructive choice of the flap, they stress on the vertical extent of the resection than on the horizontal extent. Cordeiro et al.,[17] in their most recent modification, added two subgroups based on whether less or more than 50% of palate was resected, but failed substantiate this with any implications in the functional outcome.
The vertical extent of the defect as well as the components involved has been the mainstay of the well-publicized classification systems proposed by Cordeiro[3,17] and Brown. The four-part classification system proposed by Cordeiro and colleagues seems simple to follow and is based on the number of walls resected as well whether the orbital contents were included in the resection. They used this classification system to assess the surface area to volume requirement, the need for the palatal closure and the need for orbital reconstruction. They also tried to identify the best flap for a particular defect. But, since they relied mostly on soft tissue reconstruction only, especially with radial forearm, the guidance as to the ideal reconstructive choice based on their system of classification fails to serve in this purpose. Adding to the deficiencies of the system, this classification does not give much importance to the dental restoration. The Brown et al. classification is more sound in its principles both in terms of the description of the defects as well as the help rendered for planning the reconstruction and rehabilitation. This system does not quantify the amount of skin loss and skull base defect. Combining the Okay system palatal defect and the Browns classification as done in the series reported by Moreno et al.l seems most encompassing but may be difficult to follow in all future studies.
RECONSTRUCTIVE METHODS
The reconstructive options described for maxillectomy defects vary from regional soft tissue and bone containing flaps, free flaps with either soft tissue alone or with bone or combinations of soft tissue flaps and alloplastic implants. The regional soft tissue flaps used for reconstruction of these defects include temporalis myofacial flaps,[18] Facial artery myomucosal flaps (FAMM),[19,20] buccal pad of fat[21,22] and reverse submental flaps.[23,24] Of these, the buccal pad of fat flaps and the FAMM [Figure 1a, b] flaps are found to be useful in small and relatively lateral defects. The temporalis flap [Figure 2a–d] which served as the workhorse in several initial publications[25] on maxillary reconstructions is still useful and popular. But, the disadvantages of this flap include dehiscence in larger defects more than 4 cm and immediate or late trismus. The reverse submental artery flap based on the distal facial artery was reported to be successful in a series of 13 cases by Wang et al.[24] The flaps were de-epithelialised and used to cover defects of inferior maxillary defects and allowed to epithelise similarly to the temporalis myofacial flaps. These flaps undergo a phase of inflammation, granulation tissue re-formation followed by epithelisation.[26] But, all these flaps allowing epithelisation could lead to contraction and obliteration of the sulcus, making dental rehabilitation difficult.
Figure 1.
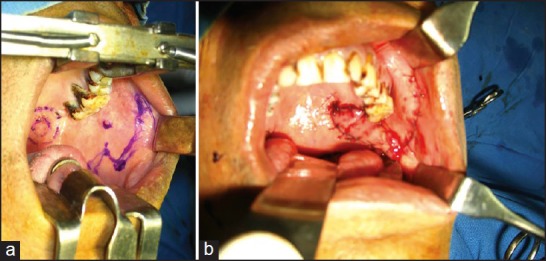
(a) Fascial Artery Myo-mucosal (FAMM) flap used for a lateral defect. The lesion and the flap marked (b) FAMM flap sutured to the defect
Figure 2.
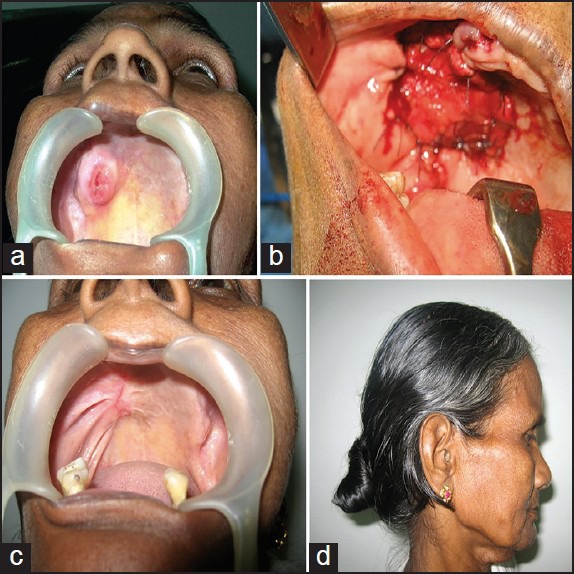
(a) Tumour involving the posterior superior alveolar area (b) Temporalis flap sutured to the defect (c) Reconstructive outcome two years after surgery (d) Temporal deformity
The free soft tissue flaps described are radial forearm flaps,[27] rectus abdominis,[28] Anterolateral thigh flap[29,30] and DIEP flaps.[31] The main objective of reconstruction using these flaps has been to seal the palatal defects. Hence, most of these flaps have been used in cases of maxillectomy, where orbital floor is preserved. Figure 3 shows an infrastructure maxillectomy defect reconstructed with free radial forearm flap. The advantage of these flaps includes their long pedicle that makes the vascular anastamosis in the neck easier.[32] The drawback of these flaps is their inability to address the need of orbital support, the inability to prevent hollowing of cheek and unsuitability to place dental implants. Dental rehabilitation becomes difficult in these cases due to the insufficiency of the gingivo-buccal sulcus.
Figure 3.
Infrastructure maxillectomy defect reconstructed with free radial forearm flap
The disadvantages of lack of bony support to the orbit and the absence of skeletal support to the cheek, while using soft tissue flap alone, have been addressed in several reports by using implants or bone grafts along with them. Bianchi et al. reported successful use of iliac crest bone grafts along with both ALT[33] and vertical rectus abdominis flaps.[34] The number of cases reported was small, but they claimed the bone grafts withstanding post-operative radiation in some cases. Hashikawa et al.[35,36] used titanium mesh for reconstruction of floor of the orbit only, radial forearm free flap for covering of titanium mesh and lining of the cheek flap and obturator prosthesis for palatal and dental rehabilitation. Sun et al.[37] also reported reconstruction of maxillary defects with titanium mesh and radial forearm free flap. Nakayama et al.[38] reported using various soft tissue flaps like rectus abdominis muscle and ALT in combination with titanium mesh for maxillectomy defect reconstruction. The soft tissue flaps were put both in front and behind the mesh to prevent its exposure. Emil Dediol et al.[39] in a recent report used a prefabricated titanium mesh, not only the orbital floor but also the infraorbital rim, the zygomatic prominence, the anterior wall of maxilla and the alveolus. The titanium mesh was bent intraoperatively on a 3D skeletal model. They used ALT flaps in majority of cases for palatal obturation. Part of the flap was de-epithelialised and used to cover the mesh from its anterior aspect, leaving it exposed in the sinus cavity.
Various bone flaps have been used in maxillary reconstructions. They provide a) support for the orbital contents, b) alveolar reconstruction and c) prominence to the cheek. Few regional bone containing flaps have been used for this purpose, but most of the reports are those using free bone flaps. The use of the coronoid process of the mandible based on the temporalis muscle for orbital support was reported by Curioni[40] and Pryor et al.[41] The coronoid process is harvested long enough into the ramus of the mandible, enabling it to reach the medial nasal wall, where it is attached with plate or wires. In the cases reported by Pryor et al., the maxillary cavity was covered with an obturator. Bilen et al.[42] described a superficial temporal artery (STA) and vein based calvarial bone flaps using its outer table. Without disrupting the integrity of fascia and periosteum, the bone was separated into two segments. The two bone segments were transferred as one single flap, and one segment of the flap was used to reconstruct the orbital floor and the other for reconstruction of the anterior maxillary wall. Out of the five cases, in two, large skin defects were also covered with lateral frontal skin supplied by the STA. Yang et al.[43] used the reverse submental de-epithelialised flap to carry the lower border of the mandible for reconstructing the upper alveolar defect. They even used immediate dental implants in these cases successfully. The mandibular donor site was filled with a MEDPOR Surgical Implant. The disadvantages of these pedicled flaps include difficulty in manoeuvring them as well as insufficient soft tissue cover. The behaviour of these to irradiation is also unpredictable.[44]
The literature is abundant on the use of free bone flaps for maxillary reconstruction. In fact, the spurt in the number of maxillary reconstruction owes a lot to the use of different types of free bone flaps. The flaps that has been used include fibula osteocutaneous,[45,46,47,48] scapula,[49,50,51] iliac crest,[52,53,54] radial forearm,[55,56] Tensor facial lata — iliac crest,[57] rectus abdominis with ribs[58] and TFL — iliac crest with internal oblique[59] medial femoral condyle flap.[60] The need of the bone in maxillary reconstructions is a) to restore midface contour, b) to provide orbital floor support, c) to replace the missing alveolar bone and d) to act as a base for dental implants. The various bone flaps and the methods described utilising these flaps achieve these goals to widely variable extent. The radial forearm osteocutaneous flaps have the advantage of a long pedicle, large skin paddle for cheek or palatal lining, but with a small thin bony segment. Andredes et al.[56] used these for zygomatic maxillary buttress with the skin paddle being used for covering the intraoral defect and the external skin defect. The orbital support was given by a mesh. Chepeha et al.[55] used the radial bone for orbital floor support and obturator for the palatal defect. In general, their use is limited in maxillary reconstruction.
The advantages of the fibular flap are the sufficient bone length that allows multiple osteotomies to be made if needed. This becomes important when separate bone segments are needed for alveolus and the orbital floor support. The intervening segment of bone can be removed for this purpose [Figure 4]. But, the greater advantage of fibula is the long pedicle length allowing a tension-free anastamosis in the neck. The skin paddle can be used for palatal obturation as well as skin cover if needed. The disadvantages of fibula include the lack of soft tissue to fill the maxillary cavity, especially if a mesh is used for orbital floor reconstruction and the inability to contour it to the needs of orbital floor support.
Figure 4.
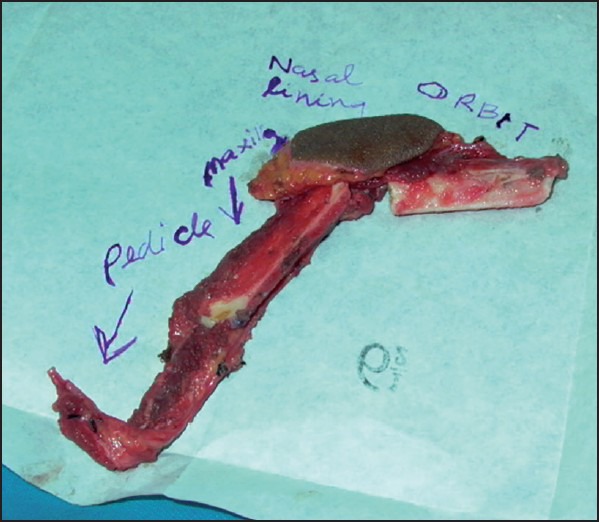
Free fibula osteocutaneous flap with osteotomies and an intervening segment of bone removed to facilitate orbital and alveolar bone support
The deep circumflex iliac artery-based iliac crest flap was suggested as a better option by Brown et al.[61] They oriented the iliac crest horizontally in their class 2 defects and vertically in the class 3 and 4 defects. The advantage of the iliac crest, when vertically placed for larger defects in restoration of the facial bone buttress, support to the nose and superior lip and reconstruction of the orbital rim were stressed by Futran[62] and Bianchi.[54] The drawbacks of the iliac crest flap have been the short pedicle length as well as the donor side morbidity. The solutions for the short pedicle length have been high dissection of the facial vessels in the cheek as the donor vessels or by extending the pedicle length by use of the ALT flap pedicle as an arterio-venous graft.[54]
The scapula and parascapular flaps have been increasingly used in maxillary reconstruction.[63,64] The advantages of the scapula include long pedicle length, the two pedicle systems that can be used to vascularise the bone namely the subscapular vessels and the angular artery from thoracodorsal system, the availability of large amount of soft tissues with minimal donor site morbidity. The disadvantage of this flap includes the difficulty in harvesting the flap simultaneously, but Clark et al.[50] refutes it by positioning the patient in such a way that they are able to harvest the flap without change of position, thereby reducing the time requires in the harvest. The thinness of the bone has been criticized as not to be suitable for implant placement.[65]
Among the less commonly used free bone flaps, the medial femoral condyle based on the descending genicular vessels was reported to be useful in a case of small anterior or anterolateral alveolar defects by Kadameni et al.[66] The periosteum provided the soft tissue surface to get mucosalised. In another single case report, Seikido et al.[58] harvested a DIEP flap, dissected the vessels through the rectus abdominis and used the cranial part of the rectus muscle to vascularise the 9th and 10th ribs. They used these ribs for zygomatico maxillary buttress reconstruction. We have described the use of the iliac crest-tensor facia lata muscle with or without the overlying skin for maxillary reconstruction, where support of the globe was needed. Seven successful cases were reported where the bone used was nourished by the attachment of the TFL muscle.[67] The authors reported further refinements on this technique, by combining it with the internal oblique muscle for orbital lining when orbital exenteration was carried out.[59]
MANAGEMENT OF ORBITAL CAVITY IN MAXILLARY DEFECTS
Two types of defects may be encountered during maxillary reconstruction depending on whether the orbital contents are preserved or not. When the orbital contents are preserved, the need for the support of the globe becomes important. This need will depend upon the amount of maxillary walls resected. Minimal loss of the orbital floor in a standard maxillectomy may not result in loss of orbital support since the periorbita will support it. But, when the loss is more, either in the medio-lateral direction, especially when three lateral orbital walls are also resected or in the antero-posterior direction when entire floor of orbit is removed, support for the globe may be necessary. This situation also occurs when large area of the periorbita is excised for tumour clearance.
The use of soft tissue flaps alone in these situations may not be ideal as reported by Rao et al. The number of cases with enopthalmos and hypophthalmos was higher when compared to bony reconstruction of the floor in the series reported by them. Figure shows a patient in whom rectus abdominis flap was used. [Figure 5a–d]. Provision for orbital floor support has been attempted by several methods[68] including use of prolene mesh, facial or musculofacial slings,[69] titanium mesh in combination with other flaps,[35,38] free bone grafts along with soft tissue flaps,[70] pedicled calvarial[71] and coronoid flaps[40,41] and free bone flaps. The free bone flaps suitable in these cases include fibula,[72] Scapula,[50] DCIA[52,54] and TFL-IC flap.[57] The simplest solution for orbital support is the use of a titanium mesh that can be contoured to the defect very well [Figure 6]. The problem with using the mesh is its high extrusion rate, especially with the post-operative radiation therapy. The importance of keeping the mesh posterior and not extending it to form the infraorbital rim in order to reduce the extrusion was stressed by Sarukawa et al.[73] They also observed that, if a mesh is to be used, it needs to be straddled on all sides with a robust and vascular soft tissue. The use of other non-vascularised tissues is also debatable in view of the radiation therapy, even though few case reports support them. In a recent article from MD Anderson group,[74] out of the 246 cases, 59 needed orbital floor reconstruction. The majority of the cases (30 patients) had non-vascularised tissue or alloplast-titanium mesh; bone grafts were used in 20 patients, porous polyethylene in four patients and a fascia lata graft in one patient. Four patients had bony free flaps. The implant exposure rate in their series of non-vascularised methods was very low. The implant got exposed in two cases of bone graft and in one case of titanium mesh. The authors’ preference[72] is for a bone flap, either fibula or TFL-Iliac crest. Figure shows a patient with maxillectomy defect with orbital floor loss, reconstructed with free fibula osteo-cutaneous flap [Figure 7a–f]. Titanium mesh was used in the cases where the bone flap failed to provide the support, either due to the architecture of the defect or poor pedicle length and in patients with risk for long surgery due to systemic problems. Figure shows a schematic representation of the three different options of reconstructing the orbital floor defects associated with maxillectomy with free flaps [Figure 8a–c].
Figure 5.
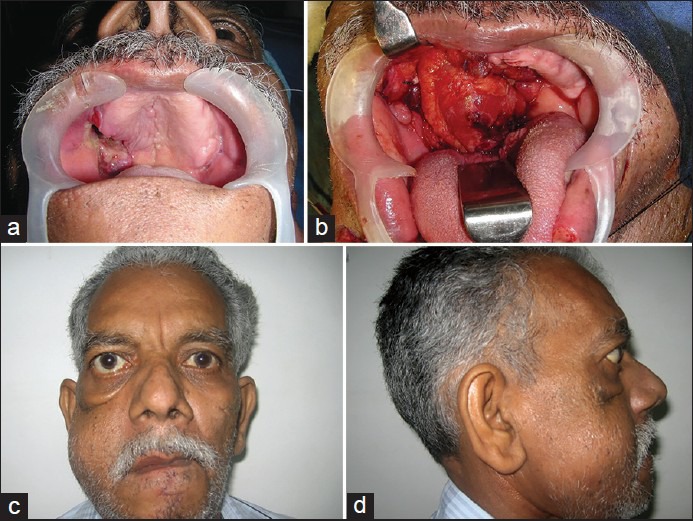
(a) Palatal involvement by the tumour (b) Rectus abdominis free flap used to fill the maxillectomy cavity and the palatal defect (c) Reconstructive outcome one year after surgery and radiotherapy, frontal view. Note the sagging of the eye on the reconstructed side (d) Reconstructive outcome one year after surgery and radiotherapy, lateral view
Figure 6.
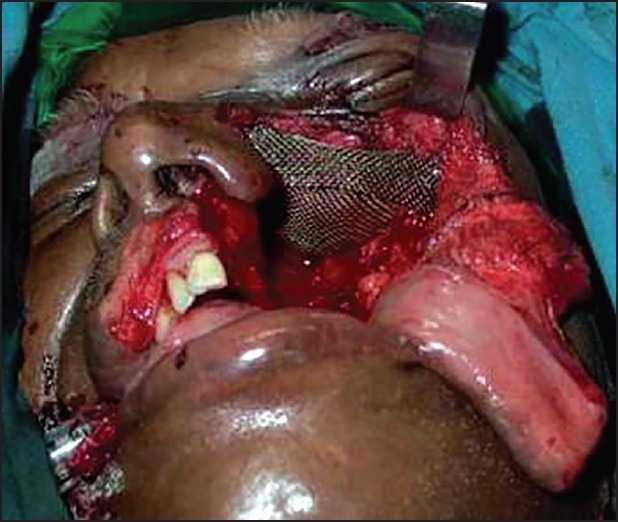
Titanium mesh for orbital support
Figure 7.
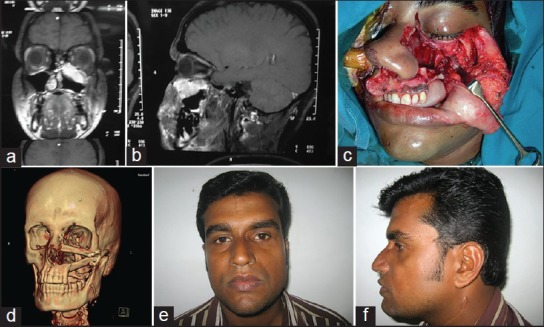
(a)Computed Tomogram of a patient with maxillary tumour with orbital floor involvement. Coronal view (b) Computed tomogram, Sagittal view (c) Total maxillectomy defect with orbital floor removed. Class III as per Brown's classification (d) Computed tomogram, three dimensional view showing free fibula flap used for reconstruction (e) Reconstructive outcome two years after surgery and radiotherapy. Frontal view (f) Reconstructive outcome, Lateral view
Figure 8.
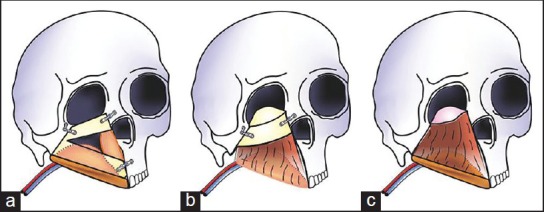
(a) Line diagram showing the reconstruction with free fibula flap (b) Line diagram showing the reconstruction with free tensor fascia lata with iliac crest flap (c) Line diagram showing the reconstruction with a soft tissue flap, free rectus abdominis. The fascia of the flap (shown in pink) was used as a sling for the orbital contents
MANAGEMENT OF THE ORBITAL EXENTERATION DEFECTS ASSOCIATED WITH MAXILLECTOMY
When the orbital contents are exenterated along with a maxillectomy, the defect becomes complex with communication of the oral and nasal cavities with exterior and occasionally with the cranial cavity. Immediate goal in the reconstruction is achieved by using a soft tissue flap like rectus abdominis as advised by Hanasono et al.[75] and Cordeiro et al.[27] Figure 9 shows a patient with carcinoma of maxillary sinus with orbital content involvement who underwent orbital exenteration along with maxillectomy. Reconstruction was with free rectus abdominis flap [Figure 9a–e]. The orbital rehabilitation becomes difficult in this method of reconstruction as only a spectacle borne external prosthesis could be used. Though attempts have been to fit an ocular prosthesis in the cavity formed by a muscle flap, the fibrosis that sets in pushes out the ocular prosthesis. Use of conventional bone flaps like The DCIA flap/fibula or scapula does not provide satisfactory reconstruction of the orbital cavity as the relative orientation of the bone, muscle and the skin paddle restricts this complex multi-axial reconstruction. A titanium mesh may be used for the orbital floor combined with a free soft tissue flap. But, technically, it is difficult to cover the mesh on the orbital cavity side, making this method not satisfactory. Using two flaps like DCIA or fibula for the suprastructure and a soft tissue flap like radial forearm flap for the palate may be considered. The authors described a new technique to address this issue by using a TFL- iliac crest- internal oblique flap (TFL-IC-IO) based on dual blood supply from the DCIA and TFL vessels[59] [Figure 10a–d].
Figure 9.
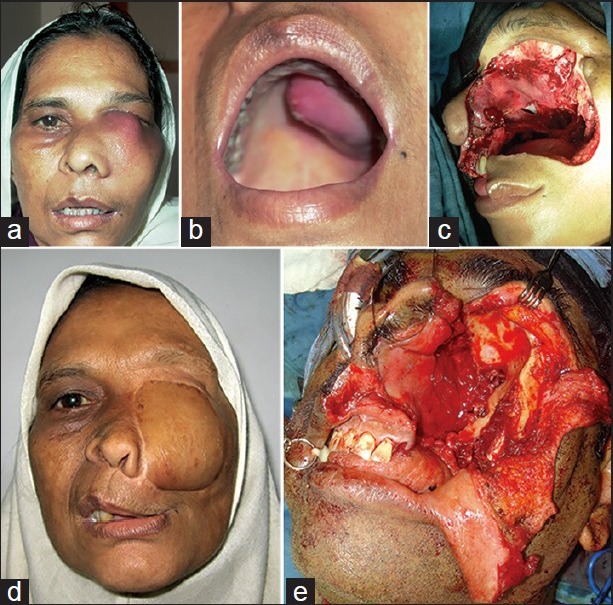
(a) Patient with squamous cell carcinoma of the maxilla, pre-operative frontal view (b) Intraoral view showing the palatal involvement (c) Maxillectomy defect with orbital exenteration, Brown's class IV (d) Reconstructed outcome with free rectus abdominis flap, two years after treatment (e) Maxillectomy with orbital exenteration defect
Figure 10.
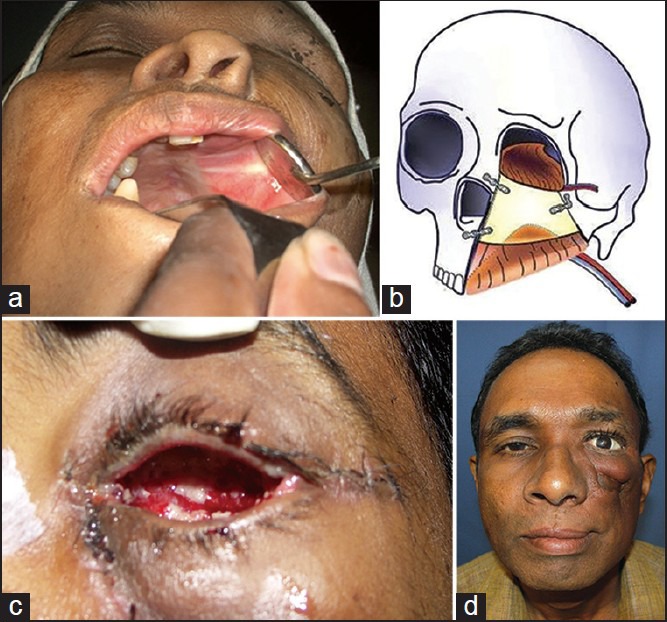
(a) Reconstructed outcome - intraoral view (b) Line drawing showing the reconstruction with Tensor fascia lata-Iliac crest-Internal Oblique flap (TFL-IC-IO) with dual anastamosis (c) Outcome showing the reconstructed eye socket (d) Reconstructed outcome one year after treatment, frontal view
ALGORITHMIC APPROACH FOR MAXILLARY AND ORBITAL RECONSTRUCTION
Based on different classification systems available and personal experience, different authors have put up algorithmic approaches for maxillary reconstruction. Brown[76] discusses the reconstructive options according to his earlier classification as well as its modification. He looked at the reconstructions reported till 2009, and after reclassifying them according to his method, found that, in class I, radial forearm flap dominated the method of reconstruction. In class II and III, more number of free bone flaps, especially fibula was used. Other bone flaps used included DCIA and scapular tip. In these defects, soft tissue flaps were also used; the radial forearm flap in class II and rectus abdominis in class III. In class IV, majority had either rectus abdominis or lattismus dorsi. When bone flaps were used, DCIA predominated. He suggested either obturation or regional flaps or radial forearm flap for class I defects. Bone flaps like for class II and III and soft tissue flaps for class IV defects. The bone flap preferred by him was DCIA or scapula or scapular tip based on thoracodorsal vessels. Cordeiro and associates,[27] based on their 15 year experience in maxillary reconstruction and based on their classification system, proposed an algorithm for maxillary reconstruction. Their preference was for the use of radial forearm flaps as single, double paddles or along with the radius bone as a sandwich flap in majority of the cases. The rectus abdominis flap was used for defects requiring larger amounts of soft tissue. Okay[6] and associates, after suggesting their classification guided by prosthodontic requirements, suggested an algorithm. They suggested options similar to the ones by Brown et al. They preferred obturation, regional flaps or soft tissue flaps for their type I defects, and all others were preferably reconstructed with bone flaps. Obturation could also be used in class II defects. They found bone flaps to be superior since they suggested implant placement for proper dental rehabilitation. They did not specify the type of bone flaps to be used. While reporting one of the largest series of free flap reconstructions in maxilla, Hanasono et al.[15] from MD Anderson group looked at the defects more exhaustively using both Brown and Corderio classification. The number of subclasses in their algorithm was more in number, but essentially, the palato-alveolar, orbital floor and orbital exenteration defects were the main considerations for choosing the method of reconstruction. In general, they suggested bone flaps (free fibula osteo-cutaneous flap) for palate alveolar defects rather than soft tissue flaps since they felt that dental rehabilitation was difficult with the latter, mainly due to the sagging of the flaps. They opted for alloplastic implants or bone grafts in combination for the orbital floor defects and obliteration with soft tissue flaps for orbital exenteration defects. Others also have tried to suggest algorithms like the one suggested by Sarukawa et al.[73] based on their treatment philosophies. They used the Cordeiro system and reconstructed only the class III and IV defects with soft tissue flaps. For large palatal defects, they adopted the Brown's classification[76] and suggested bony reconstruction using fibula. Based on our experience of over 150 cases, we have evolved an algorithm as given in Figure 11. We found the Brown's classification[11,12] to be more useful and reproducible. Our choice is regional or free soft tissue flaps or obturator for the type I defects. Free flaps, especially radial forearm flap, were preferred when the defect was larger transversely. In class II defects, our choice is fibula, mainly for its long pedicle length and good bone support. The fibular skin paddle gives a satisfactory oro-nasal separation. In class III defects requiring orbital support, especially when the periorbita is removed or when the bone defect is large, the choice is still debated. We still use fibula, but find it difficult to orient the orbital support segment properly in all cases. The other choice is TFL iliac crest flap, especially when the skin defect also needs to be covered. We have found the DCIA also to be useful. The only limiting factor is the short pedicle length. The use of mesh has been found to be unsatisfactory due to their extrusion in majority of the cases where we have used it with the post-operative radiation. In class IV defects, we would prefer to provide a bony orbital floor and hence prefer to use the TFL-iliac crest-Internal oblique flaps on the dual blood supply or double flaps with radial forearm providing the palatal cover and DCIA-internal oblique for the suprastructure defects.
Figure 11.
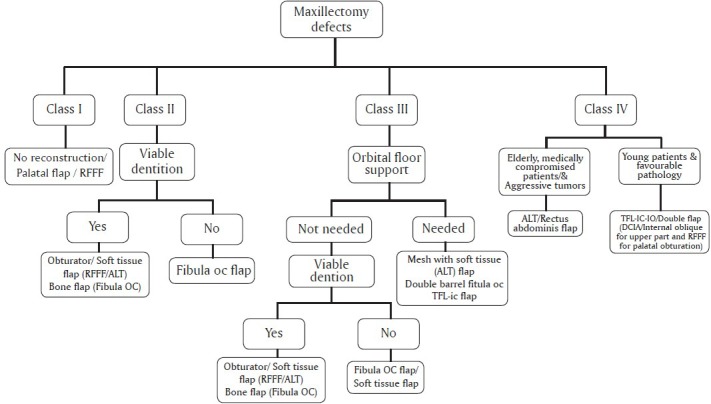
Amrita Algorithm for reconstructing the maxillectomy defects
SURGICAL MORBIDITY AND ASSOCIATED COMPLICATIONS
Maxillary reconstruction has been looked at with caution by many centres due to the perceived technical difficulties and the associated surgical morbidity. The pedicle length has been a worrying problem for the novice in selecting the flap and by experienced to limit their flap choices to soft tissue flaps with long pedicles in the majority of situations.[15] The other alternative was to use superficial temporal vessels as a donor.[77] The length of facial artery available in the neck can be increased by dissecting it into the cheek, with a vertical incision on the face, use of vein grafts and the use of composite ALT arteriovenous grafts.[78] The flap loss in larger series has been between 3% and 7%. Hanasono et al.[15] reported an overall complication rate of 37.8%, which included wound infection, dehiscence, fistulae and medical complications. Notable medical complication was pneumonia, which was reported in 6% cases.
OTHER METHODS AND DEVELOPMENTS IN TISSUE ENGINEERING IN MAXILLARY RECONSTRUCTION
Distraction osteogenesis has been used for maxilla and mandible in correcting bony deficiencies. This method was used by Xue-Gang Niu et al.[79] in a case of maxillary ameloblastoma treated by maxillectomy. During the initial surgery, an internal curvilinear distraction device was put in the residual zygoma. After about a month, the wound was re-opened, and this distractor was replaced with another to achieve curvilinear distraction osteogenesis of the maxillary anterior alveolar process and straight distraction of the palate. After couple of months, these distractors were removed and small area of the defect was bridged with a bone graft. Though the final outcome was satisfactory, the authors concede that the procedure was lengthy as well as needed multiple procedures.
Tissue engineering has been considered as a possible solution to replace complex reconstructive methods. But, it has been hampered by the lack of adequate vascularisation of the engineered constructs and the lack of clinically usable methods of engineering the constructs. Good manufacturing practices in cell culture and seeding have been available and have been reported[80] to be used successfully in reconstructing a segmental mandibular and maxillary defects. The autologous cells are handled and prepared without animal-derived material in good manufacturing practice (GMP) standard clean rooms; the cells can be considered safe for clinical cell therapy applications. Mesimäki et al.[81] for the first time described a novel method of maxillary reconstruction using tissue engineering methods. In a case of maxillectomy for a keratocyst, they harvested abdominal and adipose tissue stem cells. These cells were then isolated and expanded under GMP facilities without contamination. After 17 days, a titanium cage was inserted, filling it with mixture of auto ASCs, beta-tricalcium phosphate and bone morphogenetic protein-into the rectus abdominis flap area. After 8 months of follow-up, the flap had developed mature bone structures and vasculature. This was then transplanted into the defect. After the flap was settled, dental implants were successfully placed into the reconstruction. This method combined the use of the tissue engineering methods and utilized the microsurgical carrier for revascularising the construct. The Helsinki group has performed 10 cases, so far, for maxillary reconstructions using this method with 3 failures (personal communication). They use of the computer-aided design for prefabricating the tray, which at present has been changed to biodegradable materials. The anterolateral thigh flap with the vastus lateralis is the preferred carrier for the construct now.
SUMMARY
Maxillary reconstruction is still in an evolving stage; considerable understanding has been obtained in various aspects of maxillary reconstruction. Level of evidence is not very high by which the role of obturation versus reconstruction can be defined, but general trend seems to be more towards accepting the superiority of reconstruction, especially in larger defects. An ideal classification for the defects is still not agreed upon, but Brown's classification seems to be gaining widespread acceptance. The algorithm for reconstruction is again far from being universally agreed up on, and still reports claiming superiority of one flap over the other will be in vogue for some time. Strictly comparable studies will be difficult to carry out. Hence, individual surgeons or institutions practice may still persist as the standard for them. Tissue engineering methods may gain more acceptances and may throw up novel methods in the near future.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Parr GR, Tharp GE, Rahn AO. Prosthodontic principles in the framework design of maxillary obturator prostheses. J Prosthet Dent. 1989;62:205–12. doi: 10.1016/0022-3913(89)90315-6. [DOI] [PubMed] [Google Scholar]
- 2.Eckert SE, Carr AB. Implant-retained maxillary overdentures. Dent Clin North Am. 2004;48:585–601. doi: 10.1016/j.cden.2004.03.004. v. [DOI] [PubMed] [Google Scholar]
- 3.Cordeiro PG, Santamaria E, Kraus DH, Strong EW, Shah JP. Reconstruction of total maxillectomy defects with preservation of the orbital contents. Plast Reconstr Surg. 1998;102:1874–84. doi: 10.1097/00006534-199811000-00011. discussion 1885-7. [DOI] [PubMed] [Google Scholar]
- 4.Kornblith AB, Zlotolow IM, Gooen J, Huryn JM, Lerner T, Strong EW, et al. Quality of life of maxillectomy patients using an obturator prosthesis. Head Neck. 1996;18:323–34. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<323::AID-HED3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Rogers SN, Lowe D, McNally D, Brown JS, Vaughan ED. Health-related quality of life after maxillectomy: A comparison between prosthetic obturation and free flap. J Oral Maxillofac Surg. 2003;61:174–81. doi: 10.1053/joms.2003.50044. [DOI] [PubMed] [Google Scholar]
- 6.Okay DJ, Genden E, Buchbinder D, Urken M. Prosthodontic guidelines for surgical reconstruction of the maxilla: A classification system of defects. J Prosthet Dent. 2001;86:352–63. doi: 10.1067/mpr.2001.119524. [DOI] [PubMed] [Google Scholar]
- 7.Moreno MA, Skoracki RJ, Hanna EY, Hanasono MM. Microvascular free flap reconstruction versus palatal obturation for maxillectomy defects. Head Neck. 2010;32:860–8. doi: 10.1002/hed.21264. [DOI] [PubMed] [Google Scholar]
- 8.Genden EM, Wallace DI, Okay D, Urken ML. Reconstruction of the hard palate using the radial forearm free flap: Indications and outcomes. Head Neck. 2004;26:808–14. doi: 10.1002/hed.20026. [DOI] [PubMed] [Google Scholar]
- 9.Chigurupati R, Aloor N, Salas R, Schmidt BL. Quality of life after maxillectomy and prosthetic obturator rehabilitation. J Oral Maxillofac Surg. 2013;71:1471–8. doi: 10.1016/j.joms.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Genden EM, Okay D, Stepp MT, Rezaee RP, Mojica JS, Buchbinder D, et al. Comparison of functional and quality-of-life outcomes in patients with and without palatomaxillary reconstruction: A preliminary report. Arch Otolaryngol Head Neck Surg. 2003;129:775–80. doi: 10.1001/archotol.129.7.775. [DOI] [PubMed] [Google Scholar]
- 11.Brown JS, Rogers SN, McNally DN, Boyle M. A modified classification for the maxillectomy defect. Head Neck. 2000;22:17–26. doi: 10.1002/(sici)1097-0347(200001)22:1<17::aid-hed4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Brown JS, Shaw RJ. Reconstruction of the maxilla and midface: Introducing a new classification. Lancet Oncol. 2010;11:1001–8. doi: 10.1016/S1470-2045(10)70113-3. [DOI] [PubMed] [Google Scholar]
- 13.Cordeiro PG, Santamaria E. A classification system and algorithm for reconstruction of maxillectomy and midfacial defects. Plast Reconstr Surg. 2000;105:2331–46. doi: 10.1097/00006534-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Santamaria E, Cordeiro PG. Reconstruction of maxillectomy and midfacial defects with free tissue transfer. J Surg Oncol. 2006;94:522–31. doi: 10.1002/jso.20490. [DOI] [PubMed] [Google Scholar]
- 15.Cordeiro PG, Chen CM. A 15-year review of midface reconstruction after total and subtotal maxillectomy: Part I. Algorithm and outcomes. Plast Reconstr Surg. 2012;129:124–36. doi: 10.1097/PRS.0b013e318221dca4. [DOI] [PubMed] [Google Scholar]
- 16.Triana RJ, Uglesic V, Virag M, Varga SG, Knezevic P, Milenovic A, et al. Microvascular Free Flap Reconstructive Options in Patients With Partial and Total Maxillectomy Defects. Arch Facial Plast Surg. 2000;2:91–101. doi: 10.1001/archfaci.2.2.91. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy CM, Cordeiro PG. Microvascular reconstruction of oncologic defects of the midface. Plast Reconstr Surg. 2010;126:1947–59. doi: 10.1097/PRS.0b013e3181f446f1. [DOI] [PubMed] [Google Scholar]
- 18.Hanasono MM, Utley DS, Goode RL. The temporalis muscle flap for reconstruction after head and neck oncologic surgery. Laryngoscope. 2001;111:1719–25. doi: 10.1097/00005537-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Caubet Biayna J, Iriarte Ortabe JI, Pueyo J. Reconstruction of a palatal defect with pedicled myomucosal flap of buccinator muscle. An Otorrinolaringol Ibero Am. 1998;25:263–70. [PubMed] [Google Scholar]
- 20.Van Lierop AC, Fagan JJ. Buccinator myomucosal flap: Clinical results and review of anatomy, surgical technique and applications. J Laryngol Otol. 2008;122:181–7. doi: 10.1017/S0022215107008353. [DOI] [PubMed] [Google Scholar]
- 21.Amin MA, Bailey BM, Swinson B, Witherow H. Use of the buccal fat pad in the reconstruction and prosthetic rehabilitation of oncological maxillary defects. Br J Oral Maxillofac Surg. 2005;43:148–54. doi: 10.1016/j.bjoms.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Rapidis AD, Alexandridis CA, Eleftheriadis E, Angelopoulos AP. The use of the buccal fat pad for reconstruction of oral defects: Review of the literature and report of 15 cases. J Oral Maxillofac Surg. 2000;58:158–63. doi: 10.1016/s0278-2391(00)90330-6. [DOI] [PubMed] [Google Scholar]
- 23.You YH, Chen WL, Wang YP, Liang J, Zhang DM. Reverse facial-submental artery island flap for the reconstruction of maxillary defects after cancer ablation. J Craniofac Surg. 2009;20:2217–20. doi: 10.1097/SCS.0b013e3181bf84d7. [DOI] [PubMed] [Google Scholar]
- 24.Wang JG, Chen WL, Ye HS, Yang ZH, Chai Q. Reverse facial artery-submental artery deepithelialised submental island flap to reconstruct maxillary defects following cancer ablation. J Craniomaxillofac Surg. 2011;39:499–502. doi: 10.1016/j.jcms.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Clauser L, Curioni C, Spanio S. The use of the temporalis muscle flap in facial and craniofacial reconstructive surgery. A review of 182 cases. J Craniomaxillofac Surg. 1995;23:203–14. doi: 10.1016/s1010-5182(05)80209-4. [DOI] [PubMed] [Google Scholar]
- 26.Cheung LK, Samman N, Tideman H. Temporalis myofascial flap in maxillofacial reconstruction: Clinical and histological studies of the oral healing process. Br J Oral Maxillofac Surg. 1997;35:406–12. doi: 10.1016/s0266-4356(97)90717-8. [DOI] [PubMed] [Google Scholar]
- 27.Cordeiro PG, Chen CM. A 15-year review of midface reconstruction after total and subtotal maxillectomy: Part I. Algorithm and outcomes. Plast Reconstr Surg. 2012;129:124–36. doi: 10.1097/PRS.0b013e318221dca4. [DOI] [PubMed] [Google Scholar]
- 28.Butler CE, Lewin JS. Reconstruction of large composite oromandibulomaxillary defects with free vertical rectus abdominis myocutaneous flaps. Plast Reconstr Surg. 2004;113:499–507. doi: 10.1097/01.PRS.0000100810.21772.A1. [DOI] [PubMed] [Google Scholar]
- 29.Zaretski A, Wei FC, Lin CH, Cheng MH, Tsao CK, Wallace CG. Anterolateral Thigh Perforator Flaps in Head and Neck Reconstruction. Semin Plast Surg. 2006;20:64–72. [Google Scholar]
- 30.Rodríguez-Vegas JM, Angel PA, Manuela PR. Refining the anterolateral thigh free flap in complex orbitomaxillary reconstructions. Plast Reconstr Surg. 2008;121:481–6. doi: 10.1097/01.prs.0000299182.78690.f8. [DOI] [PubMed] [Google Scholar]
- 31.Zhang B, Li DZ, Xu ZG, Tang PZ. Deep inferior epigastric artery perforator free flaps in head and neck reconstruction. Oral Oncol. 2009;45:116–20. doi: 10.1016/j.oraloncology.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Chana JS, Wei FC. A review of the advantages of the anterolateral thigh flap in head and neck reconstruction. Br J Plast Surg. 2004;57:603–9. doi: 10.1016/j.bjps.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 33.Bianchi B, Ferri A, Ferrari S, Copelli C, Sesenna E. Maxillary reconstruction using anterolateral thigh flap and bone grafts. Microsurgery. 2009;29:430–6. doi: 10.1002/micr.20619. [DOI] [PubMed] [Google Scholar]
- 34.Bianchi B, Bertolini F, Ferrari S, Sesenna E. Maxillary reconstruction using rectus abdominis free flap and bone grafts. Br J Oral Maxillofac Surg. 2006;44:526–30. doi: 10.1016/j.bjoms.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Hashikawa K, Tahara S, Ishida H, Yokoo S, Sanno T, Terashi H, et al. Simple reconstruction with titanium mesh and radial forearm flap after globe-sparing total maxillectomy: A 5-year follow-up study. Plast Reconstr Surg. 2006;117:963–7. doi: 10.1097/01.prs.0000200623.91956.66. [DOI] [PubMed] [Google Scholar]
- 36.Schubert W, Gear AJL, Lee C, Hilger PA, Haus E, Migliori MR, et al. Incorporation of titanium mesh in orbital and midface reconstruction. Plast Reconstr Surg. 2002;110:1022–30. doi: 10.1097/01.PRS.0000021307.23118.E7. discussion 1031-2. [DOI] [PubMed] [Google Scholar]
- 37.Sun G, Yang X, Tang E, Wen J, Lu M, Hu Q. Palatomaxillary reconstruction with titanium mesh and radial forearm flap. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:514–9. doi: 10.1016/j.tripleo.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Nakayama B, Hasegawa Y, Hyodo I, Ogawa T, Fujimoto Y, Kitano H, et al. Reconstruction using a three-dimensional orbitozygomatic skeletal model of titanium mesh plate and soft-tissue free flap transfer following total maxillectomy. Plast Reconstr Surg. 2004;114:631–9. doi: 10.1097/01.prs.0000130940.46400.7f. [DOI] [PubMed] [Google Scholar]
- 39.Dediol E, Uglešić V, Zubčić V, Knežević P. Brown class III maxillectomy defects reconstruction with prefabricated titanium mesh and soft tissue free flap. Ann Plast Surg. 2013;71:63–7. doi: 10.1097/SAP.0b013e318246e895. [DOI] [PubMed] [Google Scholar]
- 40.Curioni C, Toscano P, Fioretti C, Salerno G. Reconstruction of the orbital floor with the muscle-bone flap (temporal muscle with coronoid process) J Maxillofac Surg. 1983;11:263–8. doi: 10.1016/s0301-0503(83)80063-0. [DOI] [PubMed] [Google Scholar]
- 41.Pryor SG, Moore EJ, Kasperbauer JL, Hayden RE, Strome SE. Coronoid-temporalis pedicled rotation flap for orbital floor reconstruction of the total maxillectomy defect. Laryngoscope. 2004;114:2051–5. doi: 10.1097/01.mlg.0000147948.51170.a7. [DOI] [PubMed] [Google Scholar]
- 42.Bilen BT, Kilinç H, Arslan A, Aslan S. Reconstruction of orbital floor and maxilla with divided vascularised calvarial bone flap in one session. J Plast Reconstr Aesthet Surg. 2006;59:1305–11. doi: 10.1016/j.bjps.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 43.Ye J, Yang Z, Zhang D, Chen W, Zhou M. Maxillary functional reconstruction using a reverse facial artery–submental artery mandibular osteomuscular flap with dental implants. J Oral Maxillofac Surg. 2011;69:2909–14. doi: 10.1016/j.joms.2011.02.043. [DOI] [PubMed] [Google Scholar]
- 44.Choung PH, Nam IW, Kim KS. Vascularized cranial bone grafts for mandibular and maxillary reconstruction. The parietal osteofascial flap. J Craniomaxillofac Surg. 1991;19:235–42. doi: 10.1016/s1010-5182(05)80063-0. [DOI] [PubMed] [Google Scholar]
- 45.Chang DW, Langstein HN. Use of the free fibula flap for restoration of orbital support and midfacial projection following maxillectomy. J Reconstr Microsurg. 2003;19:147–52. doi: 10.1055/s-2003-39826. [DOI] [PubMed] [Google Scholar]
- 46.Nakayama B, Matsuura H, Hasegawa Y, Ishihara O, Hasegawa H, Torii S. New reconstruction for total maxillectomy defect with a fibula osteocutaneous free flap. Br J Plast Surg. 1994;47:247–9. doi: 10.1016/0007-1226(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 47.Yim KK, Wei FC. Fibula osteoseptocutaneous free flap in maxillary reconstruction. Microsurgery. 1994;15:353–7. doi: 10.1002/micr.1920150513. [DOI] [PubMed] [Google Scholar]
- 48.Futran ND, Wadsworth JT, Villaret D, Farwell DG. Midface reconstruction with the fibula free flap. Arch Otolaryngol Head Neck Surg. 2002;128:161–6. doi: 10.1001/archotol.128.2.161. [DOI] [PubMed] [Google Scholar]
- 49.Gilbert RW, Miles BA. Maxillary reconstruction with the scapular angle osteomyogenous free flap. Arch Otolaryngol Head Neck Surg. 2011;137:1130–5. doi: 10.1001/archoto.2011.187. [DOI] [PubMed] [Google Scholar]
- 50.Clark JR, Vesely M, Gilbert R. Scapular angle osteomyogenous flap in postmaxillectomy reconstruction: Defect, reconstruction, shoulder function, and harvest technique. Head Neck. 2008;30:10–20. doi: 10.1002/hed.20649. [DOI] [PubMed] [Google Scholar]
- 51.Granick MS, Ramasastry SS, Newton ED, Solomon MP, Hanna DC, Kaltman S. Reconstruction of complex maxillectomy defects with the scapular-free flap. Head Neck. 1990;;12:377–85. doi: 10.1002/hed.2880120502. [DOI] [PubMed] [Google Scholar]
- 52.Brown JS, Jones DC, Summerwill A, Rogers SN, Howell RA, Cawood JI, et al. Vascularized iliac crest with internal oblique muscle for immediate reconstruction after maxillectomy. Br J Oral Maxillofac Surg. 2002;40:183–90. doi: 10.1054/bjom.2001.0774. [DOI] [PubMed] [Google Scholar]
- 53.Genden EM, Wallace D, Buchbinder D, Okay D, Urken ML. Iliac crest internal oblique osteomusculocutaneous free flap reconstruction of the postablative palatomaxillary defect. Arch Otolaryngol Head Neck Surg. 2001;127:854–61. [PubMed] [Google Scholar]
- 54.Bianchi B, Ferri A, Ferrari S, Copelli C, Boni P, Sesenna E. Iliac crest free flap for maxillary reconstruction. J Oral Maxillofac Surg. 2010;68:2706–13. doi: 10.1016/j.joms.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Chepeha DB, Moyer JS, Bradford CR, Prince ME, Marentette L, Teknos TN. Osseocutaneous radial forearm free tissue transfer for repair of complex midfacial defects. Arch Otolaryngol Head Neck Surg. 2005;131:513–7. doi: 10.1001/archotol.131.6.513. [DOI] [PubMed] [Google Scholar]
- 56.Andrades P, Rosenthal EL, Carroll WR, Baranano CF, Peters GE. Zygomatic-maxillary buttress reconstruction of midface defects with the osteocutaneous radial forearm free flap. Head Neck. 2008;30:1295–302. doi: 10.1002/hed.20874. [DOI] [PubMed] [Google Scholar]
- 57.Chatni S, Kuriakose MA, Iyer S. Free tensor fascia lata-iliac crest osteomusculocutaneous flap for reconstruction of combined maxillectomy and orbital floor defect. Ann Plast Surg. 2012;68:52–7. doi: 10.1097/SAP.0b013e31820ebc19. [DOI] [PubMed] [Google Scholar]
- 58.Sekido M, Yamamoto Y, Makino S. Maxillary reconstruction using a free deep inferior epigastric perforator (DIEP) flap combined with vascularised costal cartilages. J Plast Reconstr Aesthet Surg. 2006;59:1350–4. doi: 10.1016/j.bjps.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 59.Iyer S, Thankappan K, Kuriakose MA, Sampathirao LM, Mathew J, Sharma M. Tensor fascia lata-iliac crest-internal oblique free flap for composite orbito-maxillary defect with orbital exenteration. J Plast Reconstr Aesthet Surg. 2013;66:e116–8. doi: 10.1016/j.bjps.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 60.Kademani D, Mardini S, Moran SL. Reconstruction of head and neck defects: A systematic approach to treatment. Semin Plast Surg. 2008;22:141–55. doi: 10.1055/s-2008-1081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown JS. Deep circumflex iliac artery free flap with internal oblique muscle as a new method of immediate reconstruction of maxillectomy defect. Head Neck. 1996;18:412–21. doi: 10.1002/(SICI)1097-0347(199609/10)18:5<412::AID-HED4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 62.O’Connell DA, Futran ND. Reconstruction of the midface and maxilla. Curr Opin Otolaryngol Head Neck Surg. 2010;18:304–10. doi: 10.1097/MOO.0b013e32833b10b3. [DOI] [PubMed] [Google Scholar]
- 63.Ilankovan V, Ramchandani P, Walji S, Anand R. Reconstruction of maxillary defects with serratus anterior muscle and angle of the scapula. Br J Oral Maxillofac Surg. 2011;49:53–7. doi: 10.1016/j.bjoms.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 64.Mücke T, Hölzle F, Loeffelbein DJ, Ljubic A, Kesting M, Wolff KD, et al. Maxillary reconstruction using microvascular free flaps. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:51–7. doi: 10.1016/j.tripleo.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 65.Frodel JL, Funk GF, Capper DT, Fridrich KL, Blumer JR, Haller JR, et al. Osseointegrated implants: A comparative study of bone thickness in four vascularized bone flaps. Plast Reconstr Surg. 1993;92:449–55. discussion 456-8. [PubMed] [Google Scholar]
- 66.Kademani D, Salinas T, Moran SL. Medial femoral periosteal microvascular free flap: A new method for maxillary reconstruction. J Oral Maxillofac Surg. 2009;67:661–5. doi: 10.1016/j.joms.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 67.Iyer S, Chatni S, Kuriakose MA. Free tensor fascia lata-iliac crest osteomusculocutaneous flap for reconstruction of combined maxillectomy and orbital floor defect. Ann Plast Surg. 2012;68:52–7. doi: 10.1097/SAP.0b013e31820ebc19. [DOI] [PubMed] [Google Scholar]
- 68.Haug RH, Nuveen E, Bredbenner T. An evaluation of the support provided by common internal orbital reconstruction materials. J Oral Maxillofac Surg. 1999;57:564–70. doi: 10.1016/s0278-2391(99)90076-9. [DOI] [PubMed] [Google Scholar]
- 69.Cinar C, Arslan H, Ogur S, Kilic A, Bingol UA, Yucel A. Free rectus abdominis myocutaneous flap with anterior rectus sheath to provide the orbital support in globe-sparing total maxillectomy. J Craniofac Surg. 2006;17:986–91. doi: 10.1097/01.scs.0000234979.69368.79. [DOI] [PubMed] [Google Scholar]
- 70.Bianchi B, Bertolini F, Ferrari S, Sesenna E. Maxillary reconstruction using rectus abdominis free flap and bone grafts. Br J Oral Maxillofac Surg. 2006;44:526–30. doi: 10.1016/j.bjoms.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 71.Lee HB, Hong JP, Kim KT, Chung YK, Tark KC, Bong JP. Orbital floor and infraorbital rim reconstruction after total maxillectomy using a vascularized calvarial bone flap. Plast Reconstr Surg. 1999;104:646–53. doi: 10.1097/00006534-199909030-00005. [DOI] [PubMed] [Google Scholar]
- 72.Sampathirao L, Thankappan K, Duraisamy S, Hedne N, Sharma M, Mathew J, et al. Orbital Floor Reconstruction with Free Flaps after Maxillectomy. Craniomaxillofacial Trauma Reconstr. 2013;6:99–106. doi: 10.1055/s-0033-1343777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarukawa S, Sakuraba M, Asano T, Yano T, Kimata Y, Hayashi R, et al. Immediate maxillary reconstruction after malignant tumor extirpation. Eur J Surg Oncol. 2007;33:518–23. doi: 10.1016/j.ejso.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 74.Hanasono MM, Silva AK, Yu P, Skoracki RJ. A comprehensive algorithm for oncologic maxillary reconstruction. Plast Reconstr Surg. 2013;131:47–60. doi: 10.1097/PRS.0b013e3182729e73. [DOI] [PubMed] [Google Scholar]
- 75.Hanasono MM, Lee JC, Yang JS, Skoracki RJ, Reece GP, Esmaeli B. An algorithmic approach to reconstructive surgery and prosthetic rehabilitation after orbital exenteration. Plast Reconstr Surg. 2009;123:98–105. doi: 10.1097/PRS.0b013e3181904b95. [DOI] [PubMed] [Google Scholar]
- 76.Brown JS, Shaw RJ. Reconstruction of the maxilla and midface: Introducing a new classification. Lancet Oncol. 2010;11:1001–8. doi: 10.1016/S1470-2045(10)70113-3. [DOI] [PubMed] [Google Scholar]
- 77.Banks ND, Hui-Chou HG, Tripathi S, Collins BJ, Stanwix MG, Nam AJ, et al. An anatomical study of external carotid artery vascular territories in face and midface flaps for transplantation. Plast Reconstr Surg. 2009;123:1677–87. doi: 10.1097/PRS.0b013e3181a3f3ae. [DOI] [PubMed] [Google Scholar]
- 78.Bianchi B, Ferri A, Ferrari S, Copelli C, Boni P, Sesenna E. Iliac crest free flap for maxillary reconstruction. J Oral Maxillofac Surg. 2010;68:2706–13. doi: 10.1016/j.joms.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 79.Niu XG, Zhao YM, Han XX. Multiplanar and combined distraction osteogenesis for three-dimensional and functional reconstruction of unilateral large maxillary defects. Br J Oral Maxillofac Surg. 2009;47:106–10. doi: 10.1016/j.bjoms.2008.07.183. [DOI] [PubMed] [Google Scholar]
- 80.Sándor GK, Tuovinen VJ, Wolff J, Patrikoski M, Jokinen J, Nieminen E, et al. Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: A case report and review of the clinical application of good manufacturing practice-level adipose stem cells for bone regeneration. J Oral Maxillofac Surg. 2013;71:938–50. doi: 10.1016/j.joms.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 81.Mesimäki K, Lindroos B, Törnwall J, Mauno J, Lindqvist C, Kontio R, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–9. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]


