Abstract
Retinal neovascularization is a major cause of vision loss in diseases characterized by retinal ischemia and is characterized by the pathological growth of abnormal vessels. Vascular Endothelial Growth Factor (VEGF) is known to play an important role in this process. Oxidative stress has been strongly implicated in up regulation of VEGF associated with neovascularization in various tissues. Hence, compounds with anti-oxidant actions can prevent neovascularization. α-mangostin, a component of mangosteen (Garcinia mangostana Linn), has been shown to have an anti-oxidant property in pathological conditions involving angiogenesis such as cancer. However, the effect of α-mangostin on ROS formation and angiogenic function in microvascular endothelial cells has not been studied. Hence, this study demonstrated the anti-angiogenic effects of α-mangostin in relation to ROS formation in bovine retinal endothelial cells (REC). α-mangostin significantly and dose-dependently reduced formation of ROS in hypoxia-treated REC. α-mangostin also significantly and dose-dependently suppressed VEGF-induced increases in permeability, proliferation, migration and tube formation in REC and blocked angiogenic sprouting in the ex vivo aortic ring assay. In addition, α-mangostin inhibited VEGF-induced phosphorylation of VEGFR2 and ERK1/2-MAPK. According to our results, α-mangostin reduces oxidative stress and limits VEGF-induced angiogenesis through a process involving abrogation of VEGFR2 and ERK1/2-MAPK activation.
Keywords: angiogenesis, oxidative stress, vascular endothelial cells, vascular permeability, growth, migration, α-mangostin, VEGF, VEGFR2, signaling
INTRODUCTION
Angiogenesis is the process of vessel sprouting from pre-existing vessels that is involved in various pathological conditions such as cancer, rheumatoid arthritis and ocular diseases including diabetic retinopathy (DR), age-related macular degeneration (AMD) and retinopathy of prematurity (ROP). Retinal neovascularization is a major cause of vision loss and is characterized by disruption of endothelial cell–cell junctions, leading to increased permeability which is followed by endothelial cell migration, proliferation, and capillary tube formation. Vascular endothelial growth factor (VEGF), a potent angiogenic growth factor, is known to play an important role in these events.
Formation of reactive oxygen species (ROS) has been strongly implicated in up regulation of VEGF associated with neovascularization. ROS increase VEGF expression and activity by inhibition of the protein tyrosine phosphatase and activation of VEGFR and downstream signaling proteins (Colavitt et al., 2002; Ushio-Fukai and Nakamura, 2008). Hence, the use of anti-oxidants can suppress angiogenesis.
Mangosteen (Garcinia mangostana Linn) is a tropical fruit native to Southeast Asia including Indonesia, Malaysia, and Thailand. Various parts of mangosteen have been used as traditional medicine. For example, dried and powdered fruit hull is used as anti-microbial agent and anti-parasitic treatment for dysentery, whereas leaves and bark are used for eczema treatment (Obolskiy et al., 2009). At present, scientific reports demonstrate numerous actions of mangosteen including anti-tumor (Ho et al., 2002; Jung et al., 2006; Matsumoto et al., 2003, 2004; Moongkarndi et al., 2004a,b; Suksamrarn et al., 2006), anti-inflammation, anti-allergy (Chen et al., 2008; Nakatani et al., 2002a,b, 2004), and anti-oxidant activity (Chin et al., 2008; Jung et al., 2006; Yu et al., 2007). Mangosteen contains high amounts of xanthones which are secondary metabolites that demonstrate significant biological activities. α-mangostin is the one of the major biological components of mangosteen. Recently, it has been shown that α-mangostin inhibited growth of umbilical vein endothelial cells and suppressed ATP-induced phosphorylation of recombinant VEGFR2 at Y1175 (Shiozaki et al., 2012). However, the effect of α-mangostin on VEGF signaling and VEGF-induced hyperpermeability and angiogenesis has not been studied. Hence, this study determined the effects of α-mangostin on VEGF induced angiogenesis in retinal microvascular endothelial cells.
MATERIAL AND METHODS
1. Cell culture
Bovine retinal endothelial cells (REC) were used in these studies. Cells were cultured in M199 medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Gemini, Bio-product), penicillin and streptomycin (Gemini, Bio-product) at 37°C in 5% CO2. REC at passages 5–8 were used for all experiments. α-mangostin, at a purity of 96%, was kindly provided by Dr. Moongkarndi and dissolved in DMSO with a final DMSO concentration of less than 0.006 %.
2. Cytotoxicity assay
Cytotoxicity was studied using trypan blue staining. REC were seeded onto 6 well plates in complete medium. At 70% confluence, the cultures were switched to M199 with 0.1% bovine serum albumin (BSA). The next day, the cells were treated with or without α-mangostin in the same medium at doses 1, 4, 8, 12 and 16 μM for 24 hours. Subsequently, cells were trypsinized and stained with 0.25% trypan blue. Numbers of surviving cells were counted using a hemocytometer under an inverted microscope.
3. Dihydroethidium (DHE) and 2′, 7′ dichlorodihydrofluorescein diacetate (DCF-DA) assay
After overnight incubation in M199 with 0.1% BSA, confluent cultures were incubated in hypoxia (1% O2) or normoxia (20% O2) in α-mangostin or vehicle (DMSO) for 8 hours. Some cultures were also treated with SOD (superoxide dismutase, 400 units, Sigma-Aldrich) or catalase (400 units, Sigma-Aldrich). DHE (10 μM, Invitrogen, Carlsbad, CA) or DCF (25 μM, Sigma-Aldrich) in phenol red free M199 with 25 mM HEPES was added for 15 minutes. For DHE staining, images were taken at 200X magnification by using an inverted fluorescent microscope (Axiovert 135, Carl Zeiss, excitation, 480 nm; emission, 567 nm). The fluorescence intensity was quantified by MetaMorph Image System (Molecular Devices). For DCF staining, the fluorescence intensity was detected by microplate reader (Bio-Tek) with excitation 504 nm and emission 529 nm.
4. Electronic Cell-substrate Impedance Sensing (ECIS) assay
Cell barrier function was assessed by measuring the transendothelial electrical resistance using ECIS. REC (5×104) were seeded on the gelatin coated 8 well ECIS chambers (8W10E). At 70% confluence, the cultures were switched to M199 with 0.1% BSA. The next day, the ECIS chamber was connected to the ECIS machine and placed in a 5% CO2 incubator at 37°C. Current was applied and electrical resistance was recorded continuously until the cultures reached confluence and resistance became stable, indicating that the cells were fully confluent and well differentiated. Then the cultures were treated with VEGF (30 ng/ml) in the presence or absence of α-mangostin for 24 hours. Resistance was normalized to the baseline. Decreases in transendothelial electrical resistance across the cell monolayer indicates increases in paracellular permeability.
5. Cell proliferation assay
Cell proliferation was studied by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay. This assay is based on the conversion of MTT into formazan crystals by living cells, which determines mitochondrial activity, an indication of cell number. Cells (8×104/ml) were seeded on 96 well plates, incubated in complete medium for 24 hours and then switched to M199 containing 0.1% BSA. The next day the cells were treated with or without VEGF (20 ng/ml) in the presence or absence of α-mangostin or vehicle for 24 hours. MTT (Invitrogen, Carlsbad, CA) solution was added at a final concentration of 0.05% and the cultures were incubated for 4 hours at 37°C. Subsequently, 50 μl of DMSO was added to dissolve the formazen crystals, the cultures were placed on a shaker for 5 minutes and absorbance was measured at 540 nm by using a microplate reader.
6. Migration assay
REC were seeded onto 6 well plates, maintained in complete medium until they reached 100% confluence and then incubated in M199 containing 0.1% BSA overnight. The next day scrape wounds were made and cells were treated with VEGF (20 ng/ml) in the presence or absence of α-mangostin or vehicle for 24 hours. Pictures were taken at time 0 and 24 hours after scraping. The areas of cell migration into the scrape wound were analyzed by image J software.
7. 3D Matrigel tube formation assay
REC (2.5×104 cells) were suspended in matrigel (BD Biosciences) with or without VEGF (50 ng/ml) in the presence or absence of α-mangostin or vehicle. After 48 hours of treatment, images of the cultures were taken using an inverted confocal microscope (Zeiss 510; Carl Zeiss) and cell alignment into vessel-like networks was analyzed using image J software to measure the area of the aligned endothelial cells.
8. Ex- Vivo aortic ring assay
C57BL6 mice (8–12 weeks old) were euthanized by CO2 inhalation. Then the aorta was removed, isolated in Kreb’s solution and sectioned into 0.5–1 mm pieces. Subsequently, the aortic rings were embedded in fibrin matrigel and incubated in complete medium for 24 hours. Aortic rings were then pretreated with or without α-mangostin followed by incubation with or without VEGF (20 ng/ml) for 7 days. At the end of the experiment, aortic rings were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.3% triton X, in PBS with 3% normal goat serum and then stained with isolectin B4 (Invitrogen, Carlsbad, CA) overnight at 4°C. Images were taken by inverted fluorescent microscope at 50X magnification.
9. Western blot
Cellular protein was extracted in RIPA buffer containing protease inhibitor cocktail inhibitor and PMSF (phenyl methyl sulfonyl fluoride). Proteins (20 μg) were separated by 7.5–10% SDS-PAGE and transferred onto nitrocellulose membrane. The membrane was blocked with 5% non-fat dry milk in TBST for 60 minutes and then washed with TBST (5 minutes 3 times). Subsequently, the membrane was incubated in primary antibodies against phospho-ERK, total ERK, and phospho-VEGFR2 (Cell Signaling Technology, MA, USA) overnight at 4°C and then washed with TBST 3 times. Horseradish peroxidase-conjugated secondary antibody was added for 60 minutes followed by washing with TBST 3 times. Subsequently, the protein bands were detected using chemiluminescence (Amersham Pharmacia). The membranes were stripped and re-probed with an antibody against β-tubulin (Sigma-Aldrich, MO, USA) as a loading control.
10. Statistical analysis
Data were expressed as mean ± SE. Statistical analysis was performed using ANOVA. Tukey test was used for post-hoc analysis of individual group differences. A p-value is less than 0.05 was considered statistically significant.
RESULTS
1. Cytotoxicity
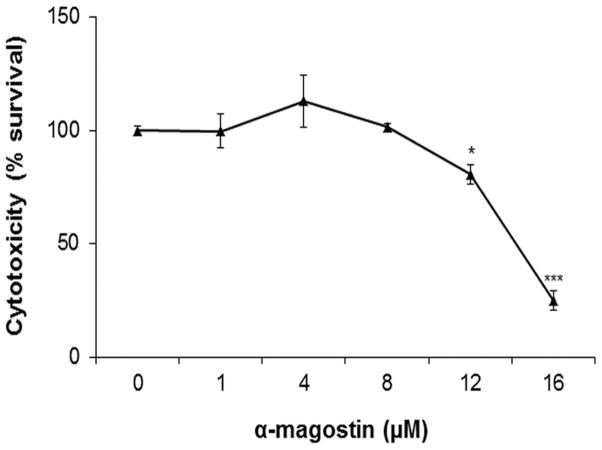
Because α-mangostin is a natural product that has potential cytotoxic effects, we first determined the non-toxic dose range of α-mangostin for treatment of REC by trypan blue staining. Cell viability was not significantly changed by 24 hours treatment with 1–8 μM of α-mangostin (Figure 1). However, concentrations of α-mangostin greater than 8 μM significantly reduced the number of surviving cells. Hence, α-mangostin concentrations at 1, 4 and 8 μM were used in further experiments.
Figure 1.
Effect of α-mangostin on REC viability. REC were treated with different concentrations of α-mangostin. Cytotoxicity was assessed by trypan blue staining. (n = 3–6 * p< 0.05, *** p< 0.001 vs control)
2. α-mangostin reduces intracellular oxidative stress
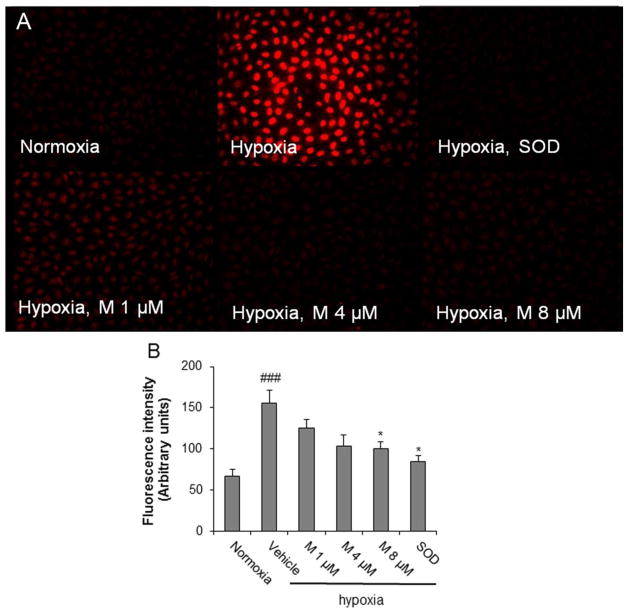
ROS formation plays a pivotal role on angiogenesis. Hence, we next determined the effects of α-mangostin on hypoxia-induced intracellular ROS formation by DHE assay. DHE is a cell-permeable probe that fluoresces red upon superoxide formation (O2.-). This analysis showed that α-mangostin treatment caused a dose-dependent inhibition of hypoxia-induced O2.- generation (Figure 2A and 2B). These results were confirmed by DCF-DA assay which showed a similar dose-dependent decrease in hypoxia induced ROS production following the α-mangostin treatment (data not shown).
Figure 2.
Impact of α-mangostin treatment on hypoxia-induced ROS formation as shown by DHE assay. REC were exposed to 1% or 20% O2 for 8 hours in the presence or absence of α-mangostin and reacted with DHE. A) Fluorescence microscope images of DHE staining. B) Quantification of DHE fluorescence intensity by MetaMorph Image System. (n = 3–6, ## p< 0.01, ### p< 0.001 vs control-normoxia, * p< 0.05, ** p< 0.01 vs vehicle-hypoxia)
3. α-mangostin inhibits VEGF-induced permeability increase
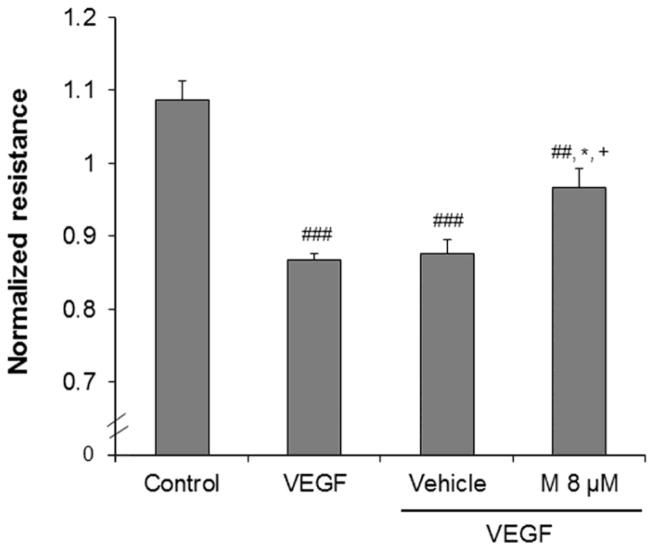
Hyperpermeability is one of the first steps in angiogenesis. In order to determine the effect of α-mangostin on VEGF-induced permeability we measured transendothelial electrical resistance (TER) of REC by using Electric Cell-substrate Impedance Sensing (ECIS). Decreases in TER are an indication of increases in paracellular permeability through the cell to cell junctions. This analysis showed that VEGF treatment caused a significant decrease in TER at 4 hour compared with the control group. On the other hand, this VEGF-induced decrease in TER was significantly inhibited by pretreatment with α-mangostin (8 μM) compared with VEGF + vehicle treated group (Figure 3).
Figure 3.
Effect of α-mangostin on VEGF induced decreases in TER. REC were seeded on ECIS chamber. At confluence, the cells were pretreated with α-mangostin (8 μM) for 30 minutes. Subsequently, VEGF (30 ng/ml) was added. VEGF caused a significant decrease in TER at 4 hr which was inhibited by α-mangostin. (n=3, ##p< 0.01, ###p< 0.001 vs control, *p< 0.05 vs VEGF, +p< 0.05 vs vehicle+VEGF)
4. α-mangostin inhibits VEGF-induced proliferation
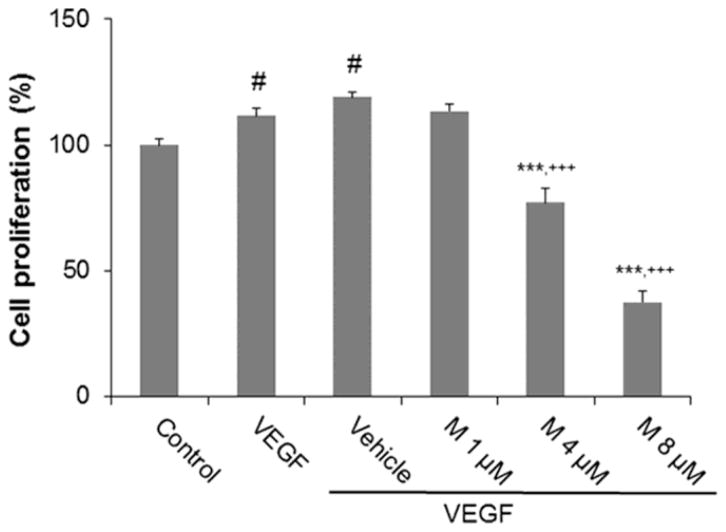
We investigated the effect of α-mangostin on REC proliferation by using the MTT assay. MTT (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazoliumbromide) is reduced by mitochondrial dehydrogenases in living cells to a formazan crystal. Thus, the amount of dissolved formazan is directly correlated with the number of living cells. α-mangostin at a dose 1, 4 and 8 μM inhibited VEGF-induced cell proliferation by 5, 42 and 81%, respectively, as compared to the vehicle control group. These results show that α-mangostin inhibits VEGF induced REC proliferation in a dose-dependent manner (Figure 4).
Figure 4.
Effect of α-mangostin on proliferation. REC were pretreated with different concentrations of α-mangostin and then treated with 20 ng/ml VEGF for 24 hours. Cell proliferation was determined by MTT assay. (n = 9, # p< 0.05 vs control, *** p< 0.001 vs VEGF, +++ p< 0.001 vs vehicle+VEGF)
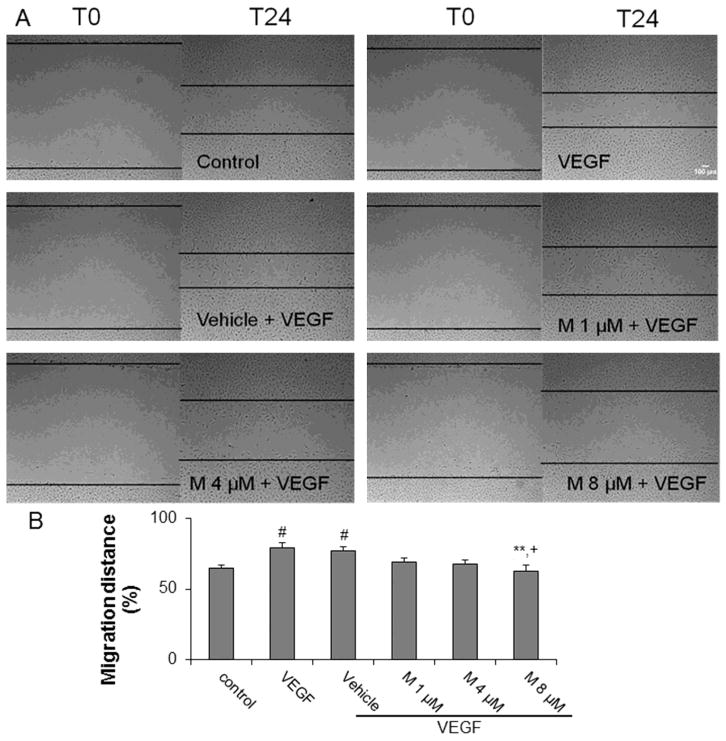
5. α-mangostin inhibits VEGF-induced migration
We used the scrape/wound assay to determine the effect of α-mangostin on VEGF-induced REC migration. At 24 hours after scraping, 20 ng/ml of VEGF enhanced REC migration and α-mangostin inhibited VEGF induced migration by 6%, 10% and 14% at concentrations of 1, 4 and 8 μM, respectively (Figure 5). This data shows that α-mangostin can suppress VEGF induced migration in a dose-dependent manner.
Figure 5.
Effect of α-mangostin on cell migration. A) REC were scraped and then treated with VEGF in the presence or absence of α-mangostin. Images were taken at 0 and 24 hrs after scraping. B) Cell migration across the scraped area was determined by image J software. (n = 12, # p< 0.05 vs control, ** p< 0.01 vs VEGF, + p< 0.05 vs vehicle+VEGF)
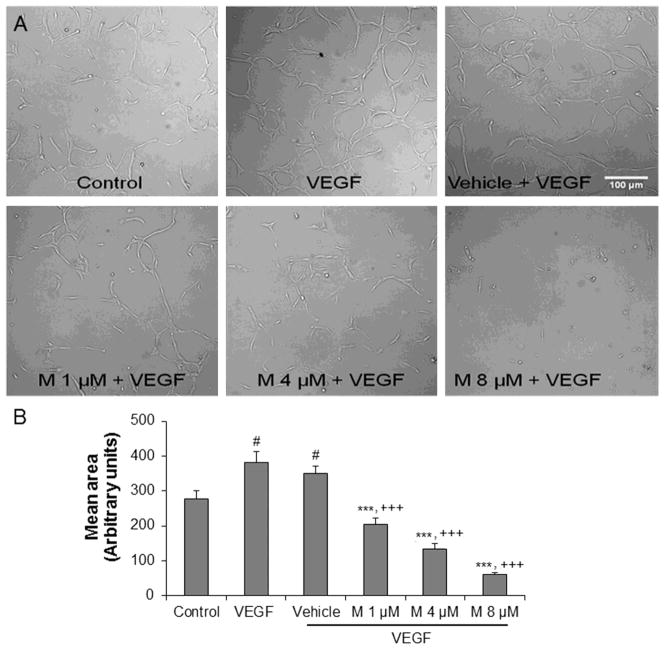
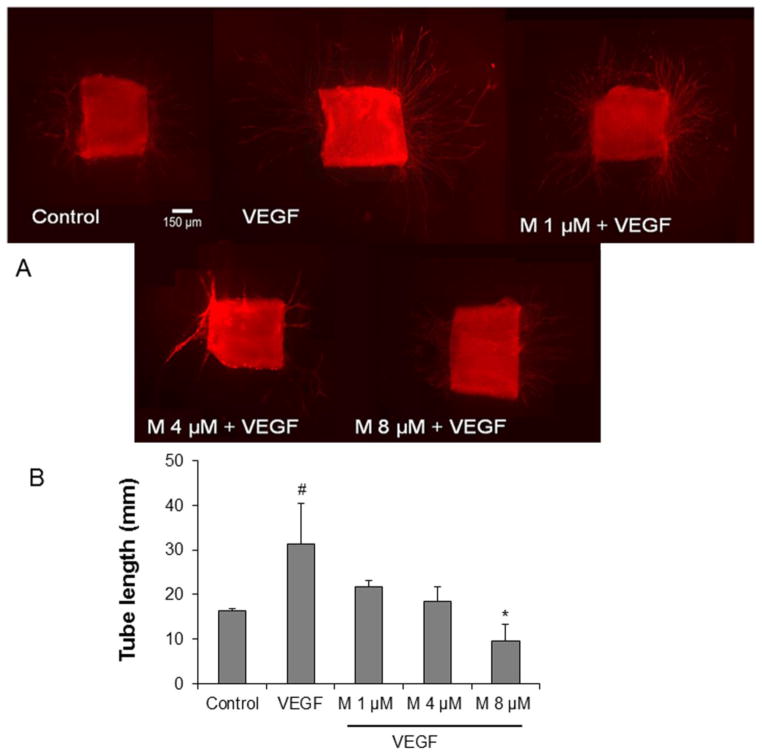
6. α-mangostin inhibits 3D tube formation and ex vivo aortic ring vascular sprouting
Tube formation and vascular sprouting are hallmarks of angiogenesis. Hence, we used the 3D Matrigel and aortic ring assays to determine the effects of α-mangostin on angiogenesis in vitro and ex vivo, respectively. VEGF (50 ng/ml) significantly increased cellular alignment by 37% compared with the control. On the other hand, α-mangostin pretreatment at doses 1, 4 and 8 μM significantly attenuated cellular alignment by 42%, 62% and 82% compared with vehicle pretreated group, respectively (Figure 6A and 6B). In the aortic ring assay, pretreatment with α-mangostin at doses 1, 4 and 8 μM also inhibited VEGF induced endothelial cell outgrowth significantly by 30%, 41% and 70%, respectively, compared with the VEGF group (Figure 7A and 7B). These results indicate that α-mangostin inhibits VEGF-induced endothelial cell alignment and vascular sprouting in a concentration dependent manner.
Figure 6.
Effect of α-mangostin on tube formation. A) REC were suspended in matrigel with or without VEGF (50 ng/ml) in the presence or absence of different concentrations of α-mangostin and maintained for 48 hours. B) Quantification of area of cell alignment was determined by image J software. (n=9, # p<0.05 vs control, *** p< 0.001 vs VEGF, +++ p<0.001 vs vehicle+VEGF)
Figure 7.
Effect of α-mangostin on aortic ring sprouting. A) Aortic rings were treated with VEGF (20 ng/ml) in the presence or absence of different concentrations of α-mangostin for 7 days. B) Quantification of vascular sprout length was determined by image J software. (n = 3–9, # p< 0.05 vs control, * p< 0.05 vs VEGF)
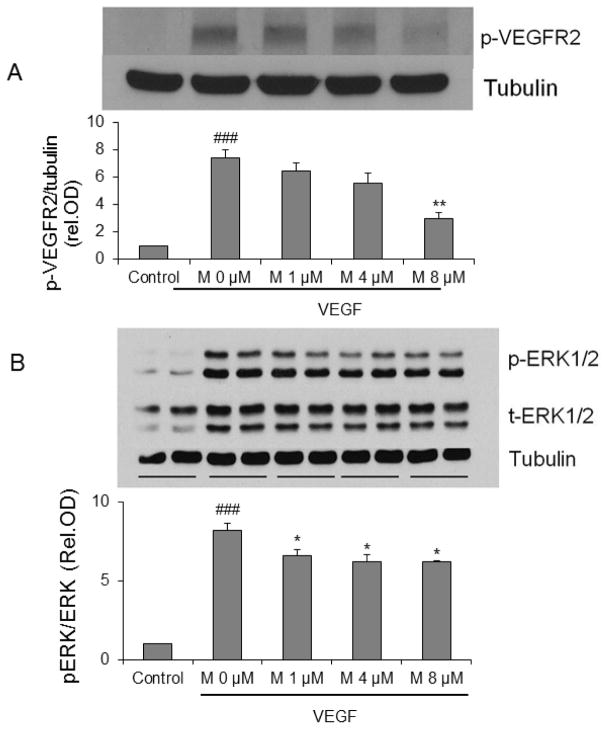
7. α-mangostin suppresses VEGFR2 and ERK1/2-MAPK signaling of angiogenesis
The biological action of VEGF in endothelial cells is mediated through VEGFR2. VEGF binding to VEGFR2 leads to dimerization and auto-phosphorylation. Subsequently, the downstream signaling proteins such as ERK1/2, p38 and Akt are activated. REC were pretreated with or without α-mangostin for 30 minutes and then treated with VEGF 30 ng/ml for 5 minutes. Immunoblotting using antibodies against phopho-VEGFR2 and ERK1/2 showed that α-mangostin suppresses VEGFR2 and ERK1/2-MAPK signaling significantly (Figure 8).
Figure 8.
Impact of α-mangostin on VEGF angiogenic signaling. REC were treated with VEGF (30 ng/ml) for 5 minutes in the presence or absence of different concentrations of α-mangostin. Cell lysates were subjected to SDS-PAGE for phosphorylation of VEGFR2 (A) and ERK1/2 (B). (n=3, ### p< 0.001 vs control, * p< 0.05, ** p< 0.01 vs VEGF)
DISCUSSION
In this study, we demonstrated that α-mangostin has anti-oxidant and anti-angiogenic effects on retinal endothelial cells. We have shown that (1) ROS formation induced by hypoxia treatment of REC is inhibited by α-mangostin, (2) VEGF induced angiogenic responses including increases in REC permeability, proliferation, migration and tube formation and vascular sprouting in the aortic ring assay are inhibited by α-mangostin, (3) VEGF induced phosphorylation of VEGFR2 and ERK1/2-MAPK in REC are reduced by α-mangostin.
The polyphenols have been shown to be effective in reducing ROS formation and preventing endothelial cell growth in other systems. In mangosteen extract, a polyphenolic xanthone derivative, has been claimed to have anti-oxidant activity. Mangosteen extract has been found to attenuate amyloid induced cytotoxicity and ROS production in human neuroblastoma cells (Moongkarndi et al., 2010). Another study showed that α-mangostin had cardioprotective effects due to suppression of ROS production in models of cardiac reperfusion injury (Buelna-Chontal et al., 2011) and in isoproterenol induced myocardial infarction in rats (Devi Sampath and Vijayaraghavan, 2007).
According to the chemical structure of α-mangostin, it is a polyphenolic xanthone. The anti-oxidant property of polyphenolic xanthones involves the donation of hydrogen atoms from hydroxyl groups to unpaired electrons of ROS (Rice-Evans et al., 1997; Soobrattee et al., 2005). It has been shown that various xanthones derived from mangosteen, 8-hydroxycudraxanthone G, gartanin, γ-mangostin, smeathxanthone A and α-mangostin have high anti-oxidant activity (Jung et al., 2006). ROS play a key role in angiogenesis. Numerous publications have been shown that hypoxia and growth factors induce ROS generation and up regulate VEGF and VEGFR expression. ROS also increase the DNA binding activity of the hypoxia-inducible factor 1α (HIF-1α) and promote VEGF expression (Chandel et al., 1998; Deudero et al., 2008; Wang et al., 2011). Moreover, VEGF signaling utilizes ROS as second messenger intermediates downstream of VEGFR2 during angiogenesis (Colavitt et al., 2002). Our previous study showed that VEGF induced peroxynitrite formation mediates angiogenesis in REC in vitro and in a mouse OIR model in vivo (El-Remessy et al., 2007). In the present study, we showed that α-mangostin inhibits hypoxia induced ROS production as detected by DHE and DCF-DA (data not shown). These data strongly suggest that the anti-angiogenic effects of α-mangostin could be associated with anti-oxidant actions.
Our present results show that α-mangostin significantly and dose-dependently suppresses VEGF induced angiogenic responses in retinal endothelial cells, including hyperpermeability, increased migration and proliferation and alignment into vessel-like structures. The α-mangostin treatment also limited vascular sprouting ex vivo in the aortic ring assay. Furthermore, α-mangostin inhibited VEGF induced phosphorylation of VEGFR2 and ERK1/2 but not Akt and p38 (data not shown). Increased permeability is one of first steps in neovascularization and supports the angiogenic process by allowing for the extravasation of growth factors. Hyperpermeability can also augment disease progression by inducing edema and tissue damage. We have shown previously that VEGF induced permeability in REC through activation of phosphorylation of VEGFR2, ERK1/2 and p38 (Yang et al., 2010). It has also been shown that hydrogen peroxide induced hyperpermeability involves the activation of ERK1/2 (Fischer et al., 2005).
Numerous studies have shown that α-mangostin has pro-apoptotic actions on cancer cells including human leukemia HL60 cells (Matsumoto et al., 2004), PC12 rat pheochromocytoma (Sato et al., 2004), human colorectal cancer DLD-1 cells (Nakagawa et al., 2007), human chondrosarcoma SW1353 cells (Krajarng et al., 2011) and human colon cancer cells (Watanapokasin et al., 2011). Thus, it is possible that the anti-angiogenic actions of α-mangostin involve apoptosis. However, stable cell-cell junctions are known to protect endothelial cells from apoptosis (Dejana, 2004) and our studies showed that treatment with 8 μM α-mangostin blunted VEGF-induced increases in TER, suggesting that the treatment preserved the cell-cell junctions. In order to further investigate whether the anti-angiogenic effect of α-mangostin may involve the apoptosis pathway, we performed preliminary studies in REC treated with VEGF in the presence or absence of 8 μM α-mangostin for 24 hours and assessed levels of cleaved PARP (a marker for apoptosis) by immunoblotting. This analysis showed low levels of cleaved PARP in the VEGF + vehicle-treated cultures. Consistent with our TER results showing preservation of the cell-cell junctions, cleaved PARP levels were even lower in the cultures treated with VEGF + α-mangostin as compared with the VEGF + vehicle cultures (data not shown). VEGF-induced cell death through activation of ERK1/2 has been reported in cerebral endothelial cells exposed to oxygen-glucose deprivation (Narasimhan et al., 2009). VEGF induces ROS formation which in turn triggers ERK1/2 signaling and cell death. Presumably, α-mangostin blocked this pathway by limiting VEGF-induced ROS formation. These data suggest that the anti-angiogenic action of α-mangostin does not involve endothelial cell death and indicate that α-mangostin has pleiotropic actions on apoptosis, depending on the cell type and experimental context.
Our previous investigations have also implicated low levels of oxidative stress in VEGF-induced angiogenesis in both cultured REC and in a mouse model of oxygen-induced retinopathy (OIR) (El-Remessy et al., 2007). It has also been shown that VEGF increases cell proliferation and tube formation through phosphorylation of ERK1/2 in retinal microvascular endothelial cells and in a rat OIR model (Bullard et al., 2003). A recent study reported that α-mangostin treatment of human umbilical vein endothelial cells (HUVEC) inhibited their proliferation, migration and tube formation (Shiozaki et al., 2012). These investigators also reported that α-mangostin treatment prevented ATP-induced phosphorylation of the tyrosine 1175 residue in recombinant VEGFR2. Our present results show for the first time that α-mangostin limits VEGF-induced activation of the VEGFR2 and ERK1/2-MAPK in microvascular endothelial cells. Further, the α-mangostin mediated inhibition of the VEGF signaling pathway is associated with blockade of angiogenic responses including increases in permeability, proliferation, migration and tube formation. Taken together, our results suggest that α-mangostin can suppress hypoxia induced ROS formation and VEGF induced angiogenic signaling in microvascular endothelial cells by inhibiting the activation of VEGFR2 and ERK1/2-MAPK.
Conclusion
α-mangostin has anti-oxidant and anti-angiogenic activities and may be useful as natural therapeutic agent to reduce oxidative stress and prevent retinal neovascularization.
Highlights.
α-mangostin inhibits hypoxia-induced ROS formation
α-mangostin attenuates VEGF-induced endothelial cell hyperpermeability
α-mangostin inhibits VEGF-induced endothelial cell proliferation and migration
α-mangostin inhibits VEGF-induced angiogenesis
α-mangostin limits VEGF-induced phosphorylation of VEGFR2 and ERK1/2-MAPK
Acknowledgments
This research was completed in partial fulfillment of requirements for Dr. Jittiporn’s PhD degree from Mahidol University in Bangkok, Thailand. The work was supported in part by The Ministry of Science and Technology, Thailand and grants from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (1I01BX001233), National Institute of Health (NIH grants R01-EY11766) and the Culver Vision Discovery Institute at Georgia Regents University. The contents do not represent the views of the Department of Veterans Affairs or the United States Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors are grateful to Dr. Priya Narayanan, Ms. Zhimin Xu and Ms. Tahira Lemtalsi for advice and assistance in completion of the studies.
Footnotes
Conflict of Interest Statement
None
Authors’ Contributions: Kanjana Jittiporn designed and performed all experiments, analyzed the data and wrote the manuscript; Jutamas Suwanpradid assisted with Western blot technique, Chintan Patel assisted with design and execution of Western blot and cell culture experiments; Modesto Rojas assisted with design and execution of aortic ring experiments and DHE assay, Primchanien Moongkarndi provided alpha-mangostin; Suwan Thirawarapan and Wisuda Suvitayavat provided guidance on research protocol and writing the results and discussion; Ruth B. Caldwell provided guidance on research design, data analysis and in writing and editing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buelna-Chontal M, Correa F, Hernandez-Resendiz S, Zazueta C, Pedraza-Chaverri J. Protective effect of α-mangostin on cardiac reperfusion damage by attenuation of oxidative stress. J Med Food. 2011;14:1370–1374. doi: 10.1089/jmf.2010.0238. [DOI] [PubMed] [Google Scholar]
- Bullard LE, Qi X, Penn JS. Role for extracellular signal-responsive kinase-1 and -2 in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2003;44:1722–1731. doi: 10.1167/iovs.01-1193. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LG, Yang LL, Wang CC. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol. 2008;46:688–693. doi: 10.1016/j.fct.2007.09.096. [DOI] [PubMed] [Google Scholar]
- Chin YW, Jung HA, Chai H, Keller WJ, Kinghorn AD. Xanthones with quinone reductase-inducing activity from the fruits of Garcinia mangostana (Mangosteen) Phytochemistry. 2008;69:754–758. doi: 10.1016/j.phytochem.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Colavitt R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- Deudero JJ, Caramelo C, Castellanos MC, Neria F, Fernandez-Sanchez R, Calabia O, Penate S, Gonzalez-Pacheco FR. Induction of hypoxia-inducible factor 1α gene expression by vascular endothelial growth factor. J Biol Chem. 2008;283:11435–11444. doi: 10.1074/jbc.M703875200. [DOI] [PubMed] [Google Scholar]
- Devi Sampath P, Vijayaraghavan K. Cardioprotective effect of α-mangostin, a xanthone derivative from mangosteen on tissue defense system against isoproterenol-induced myocardial infarction in rats. J Biochem Mol Toxicol. 2007;21:336–339. doi: 10.1002/jbt.20199. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Al-Shabrawey M, Platt DH, Bartoli M, Behzadian MA, Ghaly N, Tsai N, Motamed K, Caldwell RB. Peroxynitrite mediates VEGF’s angiogenic signal and function via a nitration-independent mechanism in endothelial cells. FASEB J. 2007;21:2528–2539. doi: 10.1096/fj.06-7854com. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wiesnet M, Renz D, Schaper W. H2O2 induces paracellular permeability of porcine brain-derived microvascular endothelial cells by activation of the p44/42 MAP kinase pathway. Eur J Cell Biol. 2005;84:687–697. doi: 10.1016/j.ejcb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Ho CK, Huang YL, Chen CC. Garcinone E, a xanthone derivative, has potent cytotoxic effect against hepatocellular carcinoma cell lines. Planta Med. 2002;68:975–979. doi: 10.1055/s-2002-35668. [DOI] [PubMed] [Google Scholar]
- Jung HA, Su BN, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen) J Agric Food Chem. 2006;54:2077–2082. doi: 10.1021/jf052649z. [DOI] [PubMed] [Google Scholar]
- Krajarng A, Nakamura Y, Suksamrarn S, Watanapokasin R. α-mangostin induces apoptosis in human chondrosarcoma cells through downregulation of ERK/JNK and Akt signaling pathway. J Agric Food Chem. 2011;59:5746–5754. doi: 10.1021/jf200620n. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Akao Y, Kobayashi E, Ohguchi K, Ito T, Tanaka T, Iinuma M, Nozawa Y. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J Nat Prod. 2003;66:1124–1127. doi: 10.1021/np020546u. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Akao Y, Yi H, Ohguchi K, Ito T, Tanaka T, Kobayashi E, Iinuma M, Nozawa Y. Preferential target is mitochondria in α-mangostin-induced apoptosis in human leukemia HL60 cells. Bioorg Med Chem. 2004;12:5799–5806. doi: 10.1016/j.bmc.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J Ethnopharmacol. 2004a;90:161–166. doi: 10.1016/j.jep.2003.09.048. [DOI] [PubMed] [Google Scholar]
- Moongkarndi P, Kosem N, Luanratana O, Jongsomboonkusol S, Pongpan N. Antiproliferative activity of Thai medicinal plant extracts on human breast adenocarcinoma cell line. Fitoterapia. 2004b;75:375–377. doi: 10.1016/j.fitote.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Moongkarndi P, Srisawat C, Saetun P, Jantaravinid J, Peerapittayamongkol C, Soi-ampornkul R, Junnu S, Sinchaikul S, Chen ST, Charoensilp P, Thongboonkerd V, Neungton N. Protective effect of mangosteen extract against β-amyloid-induced cytotoxicity, oxidative stress and altered proteome in SK-N-SH cells. J Proteome Res. 2010;9:2076–2086. doi: 10.1021/pr100049v. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Iinuma M, Naoe T, Nozawa Y, Akao Y. Characterized mechanism of α-mangostin-induced cell death: Caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg Med Chem. 2007;15:5620–5628. doi: 10.1016/j.bmc.2007.04.071. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Atsumi M, Arakawa T, Oosawa K, Shimura S, Nakahata N, Ohizumi Y. Inhibitions of histamine release and prostaglandin E2 synthesis by mangosteen, a Thai medicinal plant. Biol Pharm Bull. 2002a;25:1137–1141. doi: 10.1248/bpb.25.1137. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Nakahata N, Arakawa T, Yasuda H, Ohizumi Y. Inhibition of cyclooxygenase and prostaglandin E2 synthesis by γ-mangostin, a xanthone derivative in mangosteen, in C6 rat glioma cells. Biochem Pharmacol. 2002b;63:73–79. doi: 10.1016/s0006-2952(01)00810-3. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Yamakuni T, Kondo N, Arakawa T, Oosawa K, Shimura S, Inoue H, Ohizumi Y. γ-Mangostin inhibits inhibitor-kappaB kinase activity and decreases lipopolysaccharide-induced cyclooxygenase-2 gene expression in C6 rat glioma cells. Mol Pharmacol. 2004;66:667–674. doi: 10.1124/mol.104.002626. [DOI] [PubMed] [Google Scholar]
- Narasimhan P, Liu J, Song YS, Massengale JL, Chan PH. VEGF stimulates the ERK 1/2 signaling pathway and apoptosis in cerebral endothelial cells after ischemic conditions. Stroke. 2009;40:1467–1473. doi: 10.1161/STROKEAHA.108.534644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obolskiy D, Pischel I, Siriwatanametanon N, Heinrich M. Garcinia mangostana L.: a phytochemical and pharmacological review. Phytother Res. 2009;23:1047–1065. doi: 10.1002/ptr.2730. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- Sato A, Fujiwara H, Oku H, Ishiguro K, Ohizumi Y. α-Mangostin induces Ca2+-ATPase-dependent apoptosis via mitochondrial pathway in PC12 cells. J Pharmacol Sci. 2004;95:33–40. doi: 10.1254/jphs.95.33. [DOI] [PubMed] [Google Scholar]
- Shiozaki T, Fukai M, Hermawati E, Juliawaty L, Syah Y, Hakim E, Puthongking P, Suzuki T, Kinoshita K, Takahashi K, Koyama K. Anti-angiogenic effect of α-mangostin. J Nat Med. 2012;67:202–206. doi: 10.1007/s11418-012-0645-z. [DOI] [PubMed] [Google Scholar]
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat Res. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Suksamrarn S, Komutiban O, Ratananukul P, Chimnoi N, Lartpornmatulee N, Suksamrarn A. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem Pharm Bull. 2006;54:301–305. doi: 10.1248/cpb.54.301. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zang QS, Liu Z, Wu Q, Maass D, Dulan G, Shaul PW, Melito L, Frantz DE, Kilgore JA, Williams NS, Terada LS, Nwariaku FE. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am J Physiol Cell Physiol. 2011;301:C695–704. doi: 10.1152/ajpcell.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanapokasin R, Jarinthanan F, Nakamura Y, Sawasjirakij N, Jaratrungtawee A, Suksamrarn S. Effects of α-mangostin on apoptosis induction of human colon cancer. World J Gastroenterol. 2011;17:2086–2095. doi: 10.3748/wjg.v17.i16.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Duh EJ, Caldwell RB, Behzadian MA. Antipermeability function of PEDF involves blockade of the MAP kinase/GSK/β-catenin signaling pathway and uPAR expression. Invest Ophthalmol Vis Sci. 2010;51:3273–3280. doi: 10.1167/iovs.08-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Zhao M, Yang B, Zhao Q, Jiang Y. Phenolics from hull of Garcinia mangostana fruit and their antioxidant activities. Food Chem. 2007;104:176–181. [Google Scholar]