Abstract
Background
Anaphylaxis is the most severe manifestation of a mast cell–dependent immediate reaction and may be fatal. According to data from the Berlin region, its incidence is 2–3 cases per 100 000 persons per year.
Methods
We evaluated data from the anaphylaxis registry of the German-speaking countries for 2006–2013 and data from the protocols of the ADAC air rescue service for 2010–2011 to study the triggers, clinical manifestations, and treatment of anaphylaxis.
Results
The registry contained data on 4141 patients, and the ADAC air rescue protocols concerned 1123 patients. In the registry, the most common triggers for anaphylaxis were insect venom (n = 2074; 50.1%), foods (n = 1039; 25.1%), and drugs (n = 627; 15.1%). Within these groups, the most common triggers were wasp (n = 1460) and bee stings (n = 412), legumes (n = 241), animal proteins (n = 225), and analgesic drugs (n = 277). Food anaphylaxis was most frequently induced by peanuts, cow milk, and hen’s egg in children and by wheat and shellfish in adults. An analysis of the medical emergency cases revealed that epinephrine was given for grade 3 or 4 anaphylaxis to 14.5% and 43.9% (respectively) of the patients in the anaphylaxis registry and to 19% and 78% of the patients in the air rescue protocols.
Conclusion
Wasp and bee venom, legumes, animal proteins, and analgesic drugs were the commonest triggers of anaphylaxis. Their relative frequency was age-dependent. Epinephrine was given too rarely, as it is recommended in the guidelines for all cases of grade 2 and above.
Anaphylaxis is defined as an acute systemic reaction with the symptoms of an immediate allergic reaction that can affect the entire organism and is potentially life-threatening (1– 3). Anaphylaxis can be fatal or lead to permanent damage (3). Pathophysiologically, anaphylactic reactions—especially those triggered by foods or insect venom—are predominantly IgE-mediated, but in rare cases direct activation of the mast cells can be the cause.
At present no uniform worldwide definition of anaphylaxis exists. A mechanistic approach uses the term “anaphylaxis” for systemic generalized reactions of all degrees of severity (4). The definition most often used today describes anaphylaxis as a severe generalized reaction with symptoms in various organ systems (skin and mucosa, respiratory tract, cardiovascular system, gastrointestinal tract) (5). The differential diagnosis of anaphylaxis includes a large number of possible conditions (Box).
Box. Differential diagnoses of anaphylaxis.
-
Cardiovascular events
Vasovagal syncope
-
Other forms of shock
(e.g., hemorrhagic, cardiogenic)
Cardiac arrhythmias
Hypertensive crisis
Pulmonary embolism
Capillary leak syndrome
-
Endocrinological/metabolic events
Carcinoid syndrome
Pheochromocytoma
Thyrotoxic crisis
Hypoglycemia
-
Psychogenic events
Hyperventilation, especially with globus hystericus attacks
Multiple chemical sensitivity (MCS)
Münchhausen syndrome (anaphylaxis as artifact)
-
Cerebral events
Epilepsy
Stroke
Coma (without anaphylaxis), e.g., metabolic, traumatic
-
Airway events
Vocal cord dysfunction, psychogenic respiratory distress
Tracheal/bronchial obstruction (e.g., foreign body, tumor)
Asthma (without anaphylaxis)
-
Pharmacological/toxic events
Due to drugs (e.g., local anesthetic i.v.)
Due to alcohol and administration of substances that cause a disulfiram-like reaction (e.g., griseofulvin, sulfonyl ureas, certain fungal toxins)
(Modified from Ring et al. [12])
Epidemiology
Although data exist worldwide on the epidemiology of anaphylaxis, the triggers and their prevalences vary from region to region and depending on the patient group(s) studied (6, 7). One central problem is that the ICD-10 coding system does not make provision for coding the various forms of anaphylactic reaction or for anaphylactic shock with a single code, so studies working on the basis of ICD-10 diagnoses have to take account of several code numbers in order to collect data on anaphylaxis in an appropriate way.
Data from the US on the epidemiology of anaphylaxis suggest an incidence of up to 40 to 50 people per 100 000 population per year (8), whereas the figures for England are somewhat lower at 6 to 8 cases per 100 000 population (9).
A recently published study by our working group in collaboration with Berlin emergency physicians showed an anaphylaxis incidence of 2 to 3 people per 100 000 population (10). Although the data vary according to where they were collected, who the target group was, and the selection criteria for the group, nevertheless, overall they show that anaphylaxis, the most severe manifestation of an allergic reaction, is a rare event.
Studies from the USA and Australia suggest that the number of patients experiencing anaphylaxis has increased during the past few decades (8, 11). This relates most of all to severe allergic reactions triggered by food or drugs (8).
Treatment of anaphylaxis
According to the current guidelines for the acute treatment of anaphylaxis, the first-line treatment for patients with severe allergic reactions who show respiratory and/or cardiovascular symptoms is intramuscular adminstration of epinephrine (12)—although the evidence for this is poor because of a lack of controlled studies (13).
Measures such as appropriate positioning of the patient and the giving of fluids and oxygen are further basics of treatment. Additional drugs that should be given in the acute situation are H1-receptor–blocking antihistamines and corticosteroids, although here too controlled studies on the effectiveness of these drugs in treating anaphylaxis are lacking (9). In patients with symptoms of asthma, additional treatment with inhalational β2-sympathomimetics is recommended. A summary of currently recommended therapeutic measures for anaphylaxis is given in Table 1.
Table 1. Recommendations for emergency drug treatment in patients with anaphylaxis (adapted from Ring et al. [12]).
| Drug substance | Application route and dosage |
|---|---|
| Epinephrine | Autoinjector for intramuscular administration, weight-adjusted: >10 kg 150 µg epinephrine >30 kg 300 µg epinephrine |
| Antihistamine | Depending on patient age and preference, oral as a fluid or as (soluble) tablets; the permitted daily dose of any antihistamine can be recommended as a single dose. For dimetindene drops, similarly, a weight-adjusted dosage of the i.v. formulation can be recommended as dose to be taken orally |
| Corticosteroid | Depending on patient age and preference, rectal or oral (as fluid or tablets) 50–100 mg prednisolone-equivalent dose |
| Optional β-mimetic, e.g., salbutamol | In patients with known bronchial asthma: β2-sympathomimetic; in patients with expected obstruction of the airways: inhalational epinephrine preparation with spray head for drug vial (ask the pharmacist) |
Note: An emergency kit for immediate treatment should include written instructions for using the medication (e.g., anaphylaxis card and/or anaphylaxis emergency plan)
Aim of the study
The aim of the present study was to identify the triggers of anaphylaxis from data in the anaphylaxis registry of the German speaking countries, and to present them and their relative frequencies in relation to patient age. To gain a picture of the care situation for anaphylaxis patients in the German-speaking countries, data from the anaphylaxis registry relating to care are presented in comparison with primary data obtained from emergency physicians (from the Berlin area as a sample, and from the ADAC air rescue service for the whole of Germany).
Methods
The study was carried out as a retrospective evaluation of data from the anaphylaxis registry (this records data about patients with severe allergic symptoms; that is, skin or mucosal symptoms and respiratory and/or cardiovascular symptoms in relation to triggers, symptoms, diagnostic procedures, concomitant diseases, and care) from 2006 to 2013, and from the documented callouts of the ADAC air rescue service from 2010 to 2011.
The data from the survey of Berlin area emergency physicians have been published previously; in the present analysis they are reused only for purposes of comparison for the 2010 to 2011 time period (10). A detailed description of the methods and statistical evaluation is provided in eBox 1.
eBox 1. Methods.
Data collection by the anaphylaxis registry in the German-speaking countries (Germany, Austria, Switzerland) began in 2006. Participating centers enter the data on their anaphylaxis patients in a password-protected questionnaire in a pseudonymized and standardized manner. The data collected include details of trigger and circumstances accompanying the anaphylactic reactions, together with information about the emergency treatment provided. Patients who have cardiovascular and/or respiratory symptoms in the course of an anaphylactic reaction are included. Data from patients who have only skin reactions, and reported data from patients whose anaphylactic episode occurred more than a year before they first attended the reporting center, are not included in the analysis.
The ethical commission and data protection officer of the Berlin Charité Hospital approved the study (EA1/079/06).
For the data collection from the Berlin emergency physicians, a shortened, one-page questionnaire generated by the anaphylaxis registry was used. The data collected include:
Patient sex
Patient age
Symptoms
Location where the reaction occurred
Outcome of the reaction
Triggering factors
Emergency measures carried out.
The detailed demographic and clinical data from this project have already been published, and for the purposes of the present study were referred to for comparative purposes exclusively for the period from 2010 to 2011 (10). This relates to 120 out of a total of 375 reported cases. At the beginning of the project, the emergency physicians were informed of the aim and data collection parameters of the project. During the project, the data recorded were communicated to the emergency physicians in a newsletter at quarterly intervals.
In addition to the survey of emergency physicians in the Berlin area, we also carried out a retrospective analysis of the digitally documented emergency physician calls of the ADAC air rescue service with the key word “Anaphylaxie” (anaphylaxis) (2010 to 2011, n = 994) in the whole of Germany. This analysis included the documentation of every callout of the ADAC air rescue service in the indicated area archived under the emergency service diagnosis „anaphylaxis.“ Patient demographic data, the standard information on signs of alertness, data on the patient’s respiratory and cardiovascular status, and details of treatment given were analyzed.
Statistical analysis
Data management and descriptive analysis were carried out using SPSS for Windows version 19.0 and multinomial logistic regression analysis using STATA version 11.
For continual data (e.g., age), mean ± SD, median, and range were calculated. For category variables, absolute and relative frequencies were calculated. The data are purely descriptive. No other statistical analysis was carried out.
Results
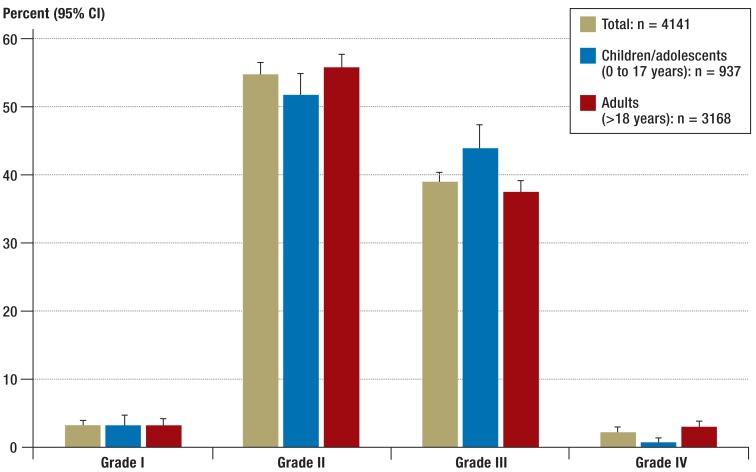
The results presented here include all cases reported to the registry from 1 January 2006 to 31 March 2013. A total of 4141 cases from Germany, Austria, and Switzerland were included (inclusion criteria: adequate severity of reaction no more than 12 months in the past). Of these, 2173 patients were female and 1968 were male. In the overall group, 3168 were adults (1840 female, 1328 male) and 973 were children (333 female and 640 male). Classification of the patients on the basis of their reported symptoms into Ring and Messmer severity grades (4) (Table 2) shows that in accordance with the inclusion criteria >85% of the reactions were equivalent to severity grade II or III. Grade IV reactions were rarely reported (n = 107): among adults, the percentage was 3.1% and among children it was 0.9% of cases (Figure 1).
Table 2. Severity scale for classification of anaphylactic reactions.
| Grade | Skin and subjective generalized symptoms | Abdomen | Respiratory tract | Cardiovascular |
|---|---|---|---|---|
| I | Itching Flushing Urticaria Angioedema |
– | – | – |
| II | Itching Flushing Urticaria Angioedema |
Nausea Cramps Vomiting |
Rhinorrhea Hoarseness Dyspnea |
Tachycardia (rise >20/min) Hypotension (fall >20 mmHg systolic) Arrhythmia |
| III | Itching Flushing Urticaria Angioedema |
Vomiting Diarrhea |
Laryngeal edema Bronchospasm Cyanosis |
Shock |
| IV | Itching Flushing Urticaria Angioedema |
Vomiting Diarrhea |
Respiratory arrest | Cardiovascular arrest |
(Modified from Ring and Messmer 1977 [4]); flushing = sudden erythema
Figure 1.
Distribution of severity grades (Ring and Messmer [4]) of cases reported to the anaphylaxis registry (n = 4141) (1 January 2006 to 31 March 2013); 95% CI, 95% confidence interval. Respiratory and/or cardiovascular symptoms are inclusion criteria for the registry, and therefore grade I is under-represented in this population
Trigger profile
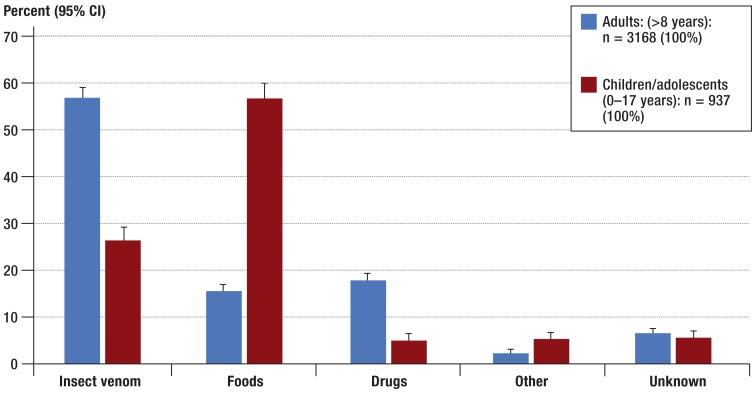
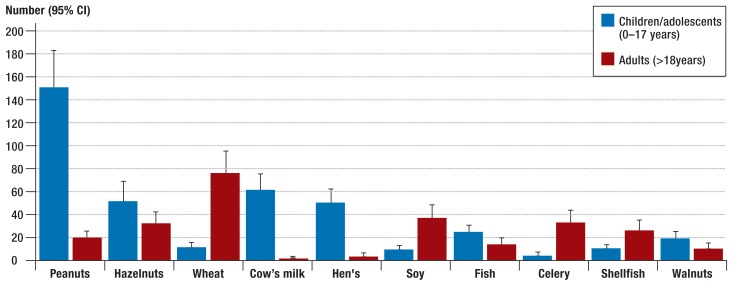
Among children (0 to 17 years), the most frequently reported triggers of severe allergic reactions were foods (Figure 2). Among adults (>18 years), insect venom was the most often reported trigger of severe allergic reactions. In terms of detailed trigger profile, further analyses showed that food allergens such as hen’s egg, cow’s milk, tree nut and peanut (14) were predominantly reported in children (Figure 3). In adults, foods ranked third as triggers of severe allergic reactions (Figure 2), when the data from the anaphylaxis registry were included. In this group, the most frequently reported food allergens were wheat, celery, soy, and shellfish (Figure 3). Table 3a shows individual foods as triggers of severe allergic reactions in the overall patient group.
Figure 2.
Relative percentages of the most frequent trigger groups related to the age of the patients reported in the anaphylaxis registry (n = 4141) (1 January 2006 to 31 March 2013); 95% CI, 95% confidence interval
Figure 3.
The ten most common food allergens as triggers in various age groups (n = 665, children and adolescents 0–17 years, adults >18 years) among cases reported in the anaphylaxis registry (1 January 2006 to 31 March 2013); 95% CI, 95% confidence interval
Table 3a. Foods triggering anaphylaxis (n = 1039, total study group = 4141).
| Food | n | % | 95% CI* |
|---|---|---|---|
| Legumes | 241 | 23.2 | 20.7–25.9 |
| Foods of animal origin | 225 | 21.7 | 19.2–24.3 |
| Nuts | 199 | 19.1 | 16.8–21.7 |
| Cereals | 101 | 9.6 | 8.0–11.7 |
| Fruit | 65 | 6.3 | 4.9–7.9 |
| Vegetables | 63 | 6.1 | 4.7–7.7 |
| Herbs/seeds/kernels | 55 | 5.3 | 4.0–6.8 |
| Food additives | 13 | 1.3 | 0.7–2.1 |
| Other | 77 | 7.4 | 5.9–9.2 |
*95% confidence interval calculated using the Clopper-Pearson interval
Drugs also triggered severe allergic reactions, especially nonsteroidal anti-inflammatory substances (Table 3b). Triggers in this group include diclofenac, acetylsalicylic acid, and ibuprofen (Table 3c). Another major drug group that triggered anaphylactic reactions was antibiotics, particularly penicillins and cephalosporins. Of the insect venoms, the most frequently reported trigger was wasp venom (1460 = 70.4%), less frequently bee venom (419 = 19.9%) or others (Table 3d).
Tabelle 3b. Drugs triggering anaphylaxis (n = 627, total study group = 4141).
| Drug group | n | % | 95% CI* |
|---|---|---|---|
| Painkillers | 277 | 44.2 | 40.2–48.2 |
| Antibiotics | 135 | 21.5 | 18.4–25.0 |
| Local anesthetics | 60 | 9.6 | 7.4–12.1 |
| X-ray contrast agents | 26 | 4.1 | 2.7–6.0 |
| Proton pump inhibitors | 20 | 3.2 | 2.0–4.9 |
| Muscle relaxants | 19 | 3.0 | 1.8–4.7 |
| Opiates | 15 | 2.4 | 1.3–3.9 |
| Corticosteroids | 8 | 1.3 | 0.6–2.5 |
| Cardiovascular drugs (ACE inhibitors, β-blockers) | 7 | 1.1 | 0.5–2.3 |
| Biologics | 5 | 0.8 | 0.3–1.9 |
| Volume replacement fluids | 5 | 0.8 | 0.3–1.9 |
| Chemotherapeutics | 4 | 0.6 | 0.2–1.6 |
| Vaccines | 3 | 0.5 | 0.1–1.4 |
| General anesthetics | 1 | 0.2 | 0.0–0.9 |
| Other drugs | 42 | 6.7 | 4.9–8.9 |
*95% confidence interval calculated using the Clopper-Pearson interval
Tabelle 3c. Painkillers triggering anaphylaxis (n = 277, total study group = 4141).
| Painkiller | n | % | 95% CI* |
|---|---|---|---|
| Diclofenac | 84 | 30.3 | 25.0–36.1 |
| Acetylsalicylic acid | 48 | 17.3 | 13.1–22.3 |
| Ibuprofen | 44 | 15.9 | 11.8–20.7 |
| Metamizole | 36 | 13.0 | 9.3–17.5 |
| Mefenaminic acid | 9 | 3.3 | 1.5–6.1 |
| Paracetamol | 6 | 2.2 | 0.8–4.7 |
| Propyphenazone | 2 | 0.7 | 0.1–2.6 |
| Painkiller (not further specified) | 48 | 17.3 | 13.1–22.3 |
*95% confidence interval calculated using the Clopper-Pearson interval
Tabelle 3d. Insect venoms triggering anaphylaxis (n = 2074, total study group = 4141).
| Insect venom | n | % | 95% CI* |
|---|---|---|---|
| Wasp | 1460 | 70.4 | 68.4–72.4 |
| Bee | 412 | 19.9 | 18.2–21.6 |
| Hornet | 93 | 4.5 | 3.6–5.5 |
| Bumble-bee | 5 | 0.2 | 0.1–0.6 |
| Horsefly | 4 | 0.2 | 0.1–0.5 |
| Mosquito, midge | 4 | 0.2 | 0.1–0.5 |
| Insect (nonspecific) | 96 | 4.6 | 3.8–5.6 |
*95% confidence interval calculated using the Clopper-Pearson interval
Emergency care
The emergency care data obtained from the anaphylaxis registry were generated from patient data; this did not always include details of emergency care protocols. For this reason, we compared the data from the anaphylaxis registry to primary data from the Berlin emergency physician service and the ADAC nationwide air rescue service.
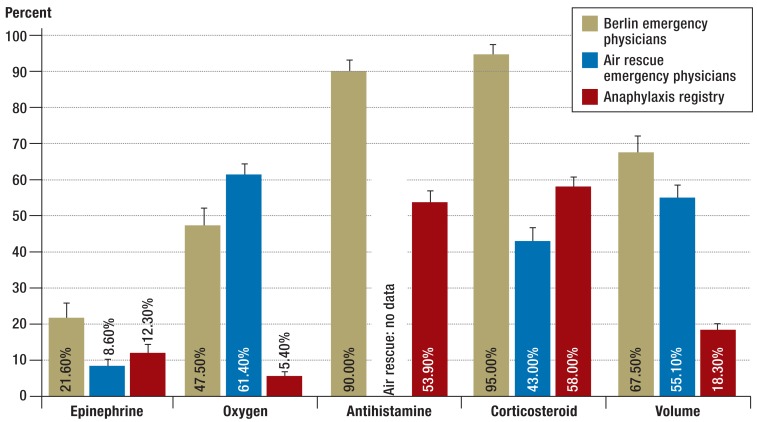
The results show that the majority of patients with anaphylactic reactions primarily received antihistamines and corticosteroids, and a smaller percentage received epinephrine, as part of their emergency treatment (Figure 4). It should be noted that the data from the anaphylaxis registry and the Berlin emergency physicians are results that include Ring and Messmer (4) severity grades II to IV. The air rescue data were evaluated in a retrospective manner that also included severity grade I. The frequency with which epinephrine was used as a first-line drug from severity grade II onwards in accordance with national and international guidelines varied among the severity grades. For severity grade III, the usage rate was 14.5% for the data from the anaphylaxis registry, 25% for the Berlin emergency physicians, and 19% for the air rescue service. For severity grade IV, the usage rates were 43.9% for the anaphylaxis registry data, 100% for the Berlin emergency physicians, and 78% for the air rescue service.
Figure 4.
Comparison of emergency treatments:
Berlin emergency physicians (ground-based) (n = 120), ADAC air rescue service (n = 994), anaphylaxis registry (n = 1123) (1 January 2010 to 31 December 2011)
Discussion
With the aid of the anaphylaxis registry, we have for the first time succeeded in collecting clinical epidemiological data on patients with anaphylaxis in the German-speaking countries using a standardized form of data collection. Data on the triggers of anaphylaxis show that their relative frequency depends on patient age. The leading triggers are foods, insect venoms, and medical drugs. Comparable data are available from other studies in Europe, e.g., France and Spain (15, 16).
In the literature, milk and hen’s egg are described as frequent triggers of anaphylaxis in young children (14), while in school-age children peanuts and tree nuts (hazel nut, walnut, cashew, Brazil nut, macadamia) frequently occur as triggers of a food allergy, not just in Europe, but also in the US (17). This also agrees with the results of the present study, in which these food allergens were predominantly reported as triggers of severe allergic reactions in children.
Food allergens as triggers of anaphylaxis are also determined by the eating habits of a given population; for example, shellfish are frequent triggers of anaphylactic reactions in Asia (18). Among adults in the German-speaking countries, the predominant trigger of anaphylaxis is insect venom. Here, the necessity of an appropriate allergological diagnostic work-up and initiation of therapy must be particularly emphasized, as specific immune therapy offers a possible causal treatment to build up strong protection against the case of another sting (19).
The data on medical drugs as triggers show high numbers of reactions, especially for painkillers and antibiotics. It should be taken into account that these drugs are in very widespread use. To what extent additional factors such as the presence of an active infection are significant for the triggering of an anaphylactic reaction needs to be investigated in future studies.
Even if foods or drugs triggered the anaphylaxis, according to the guidelines for the management of anaphylaxis, further allergological diagnostic tests should be carried out in all patients—including skin (preferably prick test), blood (specific serum IgE) and provocation tests (as appropriate)—to enable appropriate counseling (12). As up to a third of patients can have repeated severe allergic reactions (20), in addition to an allergy card recording the time, trigger, and symptoms of the reaction that has occurred, patients should be issued with an emergency kit consisting of an epinephrine autoinjector, an antihistamine, and corticosteroids, together with sufficient training in how to use them in an emergency situation (12).
For the insect venoms (bee and wasp), suitable skin test solutions and specific IgE tests are available, whereas for drugs (skin and IgE test) and foods (mainly skin test), only limited diagnostic procedures are available. Further diagnostic procedures after an anaphylactic reaction should always be carried out by appropriately qualified medical specialists.
Data on the emergency care of patients with severe allergic reactions show a discrepancy between the treatment recommendations given in current guidelines (epinephrine administration is recommended as a first line treatment from grade II upward) and the drugs actually given to patients (12, 21). This discrepancy is particularly obvious when the data are analyzed independently of severity grade.
In the overall group of patients, 12.3% from the anaphylaxis registry, 8.6% of those in the air rescue data, and 21.6% of those in the Berlin emergency physician records were given epinephrine. Among those with grade IV severity, however, the rate of epinephrine administration rises to 43.9% (anaphylaxis registry), 78% (air rescue service) and 100% (Berlin emergency physicians). Until now, seven deaths have been recorded in the anaphylaxis registry (six in Germany and one in Austria; the triggers were food in three cases, wasp venom in three cases, and in one case a medical drug). The statistics show a high survival rate; however, it needs to be kept in mind that the data reported to the anaphylaxis registry is generally obtained from patients who actively attend an allergy center for diagnosis and treatment.
The evidence that epinephrine administration has a positive influence on the survival of anaphylaxis patients is derived from earlier studies (13, 22, 23). On the basis of these study results, epinephrine is recommended as the drug of choice in the guidelines for the acute treatment of severe allergic reactions (12), even though there are no prospective controlled studies of the efficacy and tolerability of epinephrine in the treatment of anaphylaxis. It has been shown that patients who had received two doses of epinephrine before they reached the emergency room were less often hospitalized than patients who received the second or both doses in the emergency room. However, the case numbers in these cohorts were small (n = 58). Likewise, it was observed that persons who died as a result of an anaphylactic reaction had either received no or delayed epinephrine (23).
Various factors may explain the reluctance to use epinephrine in acute therapy, especially in patients with grade II and III reactions. The therapeutic window for epinephrine is narrow, and in the past cardiovascular complications were reported in patients with pre-existing cardiovascular conditions (24, 25). An overdose can also be fatal (23). On the other hand, in children and young adults the risk of unwanted cardiovascular effects such as tachyarrhythmia can be regarded as low.
Epinephrine was given by the Berlin emergency physicians more often than by the air rescue emergency physicians; in stage IV patients the Berlin physicians gave it in 100% of cases. A significant factor here might be that the project was presented to the Berlin emergency physicians before the data collection started, in a format similar to a training course, and therefore positive effects may have occurred in terms of the recognition of an anaphylactic reaction and the consequent use of epinephrine. Future activities will aim to train physicians and patients in the emergency care of anaphylaxis in a standardized way. Physicians in other medical specialties, such as general practitioners, radiologists, and oncologists, and other medical personnel (paramedics) likely to see patients experiencing anaphylaxis, should also be trained.
Limitations
A limitation of the present data is that the anaphylaxis registry contains only data submitted by allergy centers and/or allergology private practices. Since not all patients attend for specialist treatment after suffering a severe allergic reaction, this is a source of bias. For example, a different distribution of triggers was found in the primary data collection from emergency physicians (the most frequent possible triggers in the dataset from the emergency physicians was food, for both children and adults) (10).
Another analysis carried out among physicians in private practice has shown that the relative frequencies of triggers are to a significant extent determined by the specialty of the reporting physician (for example, chemotherapeutic drugs in reports from oncologists) (26).
Although a large number of the allergy centers are active in the anaphylaxis registry, not all severe allergic reactions seen in allergy centers in Germany are reflected by the presented data. Nevertheless, because of the large number of participating centers in the registry, a sentinel role may be assumed.
Summary
We hope that the data in the anaphylaxis registry, together with collaboration between the various medical societies and other specialist medical associations, and the establishment of standardized patient training, will enable the situation regarding the care of anaphylaxis patients with severe allergic reactions in Germany to be improved. This is necessary not least because what it is about is the most severe—potentially fatal—manifestation of a mast-cell-dependent reaction.
Key Messages.
Foods, insect venom, and medical drugs are the most common triggers of anaphylaxis.
The trigger profile in anaphylaxis is age-dependent.
Among food triggers of anaphylaxis, peanuts are common in children.
Nonsteroidal anti-inflammatory drugs (NSAIDs) and antibiotics are leading triggers of drug-induced anaphylaxis.
Emergency care in anaphylaxis should follow existing guidelines.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA
The authors are grateful to all participating centers for their active collaboration (see eBox 2 and www.anaphylaxie.net/index.php?id=968). This work was supported in part by an award from the Kanert-Stiftung and by NORA e.V. (Network for Online Registration of Anaphylaxis).
eBox 2. We are grateful to all participating centers for their active collaboration.
Germany:
Aachen University Hospital, Dermatology; Aachen University Hospital, Pediatrics; Vital Klinik Alzenau, Dermatology, Dermatology Clinic, Charité Allergy Center, Berlin; Berlin Spandau Hospital, Dermatology and Allergology; Pediatric Allergology, DRK Hospitals Berlin Westend; Ruhr-University Bochum, Dermatology; Ruhr-University Bochum, Pediatrics; St.-Marien-Hospital, Bonn, Adolescent Medicine; Protestant Hospitals in Bonn, Ear Nose and Throat Department; Bonn University Hospital, Dermatology; Bonn University Hospital, Pediatrics; Elbe Hospital Buxtehude, Dermatology Center; Dresden University Hospital, Adolescent Medicine; Dresden University Hospital, Dermatology; City Hospital, Dresden-Neustadt, Adolescent Medicine; Düsseldorf Protestant Hospital, Pediatric Cardiology, Adolescent Medicine; Barnim Hospital Eberswalde, Pediatric and Adolescent Medicine; Erlangen University Hospital, Dermatology; Essen University Hospital, Dermatology; Freiburg University, Adolescent Medicine; Fürth Hospital, Pediatric and Adolescent Medicine; University of Göttingen; University of Greifswald, Adolescent Medicine; University of Halle–Wittenberg, Dermatology; Wilhelmstift Catholic Children’s Hospital, Hamburg, Pediatric Dermatology; Hamburg University Hospital, Dermatology; Hannover Medical University, Dermatology; Hannover Medical University, Adolescent Medicine; University of Heidelberg, Dermatology; Heidelberg University Hospital, Pediatrics III; Saarland University Hospital at Homburg, Dermatology; University of Jena, Internal Medicine I; Schleswig-Holstein University Hospital, Kiel Campus, Dermatology; Schleswig-Holstein University Hospital, Lübeck Campus, Dermatology, Center for Pneumology; Schleswig-Holstein University Hospital, Lübeck Campus, Adolescent Medicine; Cologne University Hospital, Dermatology; Cologne University Hospital, Adolescent Medicine; Leipzig University Hospital, Pediatric Pneumology and Allergology, Pediatric and Adolescent Medicine; Leipzig University Hospital, Dermatology; University of Munich, Dermatology; Technical University of Munich, Dermatology; Friedrich Ebert Hospital, Neumünster, Adolescent Medicine; Oldenburg Hospital, Pediatric and Adolescent Medicine; Osnabrück Christian Children’s Hospital, Pediatric and Adolescent Medicine, Pneumology, Allergology; Kinderklinik III. Orden, Allergology and Pediatric Pneumology, Passau; St. Matthew’s Hospital, Rheine, Pediatric and Adolescent Medicine; Pediatric and Adolescent Medical Hospital, Rüsselsheim; Kloster Grafschaft Hospital, Schmallenberg; Asklepios Hospital Uckermark, Schwedt, Dermatology; St. John’s Hospital Treuenbritzen, Pneumology; Niederberg Hospital, Velbert, Pediatric and Adolescent Medicine; Wangen Hospital, Allgäu, Pediatric and Adolescent Medicine; Wiesbaden University Hospital, Rhinology; allergologists in practice in Aachen, Hamburg, Stade, Würzburg.
Austria:
Graz University Hospital, Dermatology; Graz University Hospital, Adolescent Medicine; Innsbruck Medical University, Dermatology; Salzburg Private University, Dermatology; Vienna Allergy and Clinical Immunology Outpatients Department; Vienna Medical University, Dermatology; Vienna Medical University, Pediatric and Adolescent Medicine.
Switzerland:
Aarau Children’s Hospital, Pediatric Allergology and Pneumology; Basel University Hospital, Allergology Polyclinic; Lucerne Children’s Hospital, Allergology Polyclinic, Pediatrics, Pediatric Allergology and Pneumology; University of Zurich Children’s Hospital; Zurich University Hospital, Dermatology; Triemli Hospital, Zurich, Pediatrics.
Footnotes
Conflict of interest statement
Margitta Worm has received consultancy fees from ALK-Abello, Allergopharma, Bencard, Hal Allergy, Meda Pharma, Novartis, and Thermo Fisher Scientific. She has received lecture fees from Allergopharma, ALK-Abello und Meda Pharma. She has received (third party) research support from ALK-Abello and Meda Pharma.
Kirsten Beyer has received consultancy and lecture fees and reimbursement of conference fees and travel expenses from ALK-Abello and Meda Pharma.
Thomas Hawranek has received consultancy fees from ALK-Abello. He has received lecture fees and reimbursement of conference fees and travel expenses from ALK-Abello and Meda Pharma.
Stephanie Hompes has received reimbursement of travel costs from Meda Pharma.
Alice Koehli has received consultancy (advisory board) fees and reimbursement of conference fees and travel expenses from ALK-Abello. She has received lecture fees from Meda Pharma.
Katja Nemat has received lecture fees from Thermo Fisher Scientific, Nutricia, Novartis, and Hal Allergy.
Claudia Pföhler has received lecture fees from Bencard, Hal Allergy, ALK-Abello, and Stallergenes.
Uta Rabe has received lecture fees from Meda Pharma.
Ernst Rietschel has received consultancy (advisory board) and lecture fees from Meda Pharma.
Regina Treudler has received lecture fees and reimbursement of conference fees and travel expenses from ALK-Abello and Meda Pharma. She has received research funding from ALK-Abello and Thermo Fisher Scientific.
Vera Mahler has received lecture fees from Meda Pharma.
Franziska Ruëff has received reimbursement of conference fees from ALK-Abello. She has had travel costs reimbursed by ALK-Abello, HAL Allergy, and Bencard. She has received lecture fees from ALK-Abello, HAL Allergy, Bencard, Novartis, Interplan, and Agentur Herzberg. She has received research funding from ALK-Abello, HAL Allergy, Pierre Fabre, Bencard, and Thermo Fisher Scientific.
Oliver Eckermann, Sabine Dölle, Werner Aberer, Bodo Niggemann, Angelika Reissig, and Kathrin Scherer declare that no conflict of interest exists.
References
- 1.Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 2.Ring J, Grosber M, Mohrenschlager M, Brockow K. Anaphylaxis: acute treatment and management. Chem Immunol Allergy. 2010;95:201–210. doi: 10.1159/000315953. [DOI] [PubMed] [Google Scholar]
- 3.Simons FE, Ardusso LR, Bilo MB, et al. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. 2011;127:587–593. doi: 10.1016/j.jaci.2011.01.038. e1-22. [DOI] [PubMed] [Google Scholar]
- 4.Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1:466–469. doi: 10.1016/s0140-6736(77)91953-5. [DOI] [PubMed] [Google Scholar]
- 5.Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report-Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 6.Panesar SS, Javad S, de Silva D, et al. The epidemiology of anaphylaxis in Europe: a systematic review. Allergy. 2013;68:1353–1361. doi: 10.1111/all.12272. [DOI] [PubMed] [Google Scholar]
- 7.Worm M. Epidemiologie der Anaphylaxie. Hautarzt. 2013;64:88–92. doi: 10.1007/s00105-012-2449-1. [DOI] [PubMed] [Google Scholar]
- 8.Decker WW, Campbell RL, Manivannan V, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008;122:1161–1165. doi: 10.1016/j.jaci.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheikh A, Hippisley-Cox J, Newton J, Fenty J. Trends in national incidence, lifetime prevalence and adrenaline prescribing for anaphylaxis in England. J R Soc Med. 2008;101:139–143. doi: 10.1258/jrsm.2008.070306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyer K, Eckermann O, Hompes S, Grabenhenrich L, Worm M. Anaphylaxis in an emergency setting—elicitors, therapy and incidence of severe allergic reactions. Allergy. 2012;67:1451–1456. doi: 10.1111/all.12012. [DOI] [PubMed] [Google Scholar]
- 11.Poulos LM, Waters AM, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993-1994 to 2004-2005. J Allergy Clin Immunol. 2007;120:878–884. doi: 10.1016/j.jaci.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 12.Ring J, Beyer K, Biedermann T, et al. Akuttherapie und Management der Anaphylaxie. Leitlinie der Deutschen Gesellschaft für Allergologie und klinische Immunologie (DGAKI), des Ärzteverbands Deutscher Allergologen (AeDA), der Gesellschaft für Pädiatrische Allergologie und Umweltmedizin (GPA), der Deutschen Akademie für Allergologie und Umweltmedizin (DAAU), des Berufsverbands der Kinder- und Jugendärzte Deutschlands (BVKJ), der Österreichischen Gesellschaft für Allergologie und Immunologie (ÖGAI), der Schweizerischen Gesellschaft für Allergologie und Immunologie (SGAI), der Deutschen Gesellschaft für Anästhesiologie und Intensivmedizin (DGAI), der Deutschen Gesellschaft für Pharmakologie (DGP), der Deutschen Gesellschaft für Psychosomatische Medizin (DGPM), der Arbeitsgemeinschaft Anaphylaxie Training und Edukation (AGATE) und der Patientenorganisation Deutscher Allergie- und Asthmabund (DAAB) Allergo J. 2014;23 doi: 10.1007/s15007-020-4750-0. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhami S, Panesar SS, Roberts G, et al. Management of anaphylaxis: a systematic review. Allergy. 2014;69:168–175. doi: 10.1111/all.12318. [DOI] [PubMed] [Google Scholar]
- 14.Dölle S, Hompes S, Grünhagen J, Worm M. Nahrungsmittelassoziierte Anaphylaxie. Hautarzt. 2012;63:294–298. doi: 10.1007/s00105-011-2265-z. [DOI] [PubMed] [Google Scholar]
- 15.Moneret-Vautrin DA, Kanny G, Morisset M, Rance F, Fardeau MF, Beaudouin E. Severe food anaphylaxis: 107 cases registered in 2002 by the Allergy Vigilance Network. Eur Ann Allergy Clin Immunol. 2004;36:46–51. [PubMed] [Google Scholar]
- 16.Tejedor Alonso MA, Moro Moro M, Mugica Garcia MV, et al. Incidence of anaphylaxis in the city of Alcorcon (Spain): a population-based study. Clin Exp Allergy. 2012;42:578–589. doi: 10.1111/j.1365-2222.2012.03930.x. [DOI] [PubMed] [Google Scholar]
- 17.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Lee AJ, Thalayasingam M, Lee BW. Food allergy in Asia: how does it compare? Asia Pac Allergy. 2013;3:3–14. doi: 10.5415/apallergy.2013.3.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przybilla B, Rueff F, Walker A, et al. Diagnose und Therapie der Bienen- und Wespengiftallergie. Leitlinie der Deutschen Gesellschaft für Allergologie und klinische Immunologie (DGAKI), des Ärzteverbandes Deutscher Allergologen (ÄDA), der Gesellschaft für Pädiatrische Allergologie und Umweltmedizin (GPA), der Deutschen Dermatologischen Gesellschaft (DDG) und der Deutschen Gesellschaft für Kinder- und Jugendmedizin (DGKJ) in Zusammenarbeit mit der Österreichischen Gesellschaft für Allergologie und Immunologie (ÖGAI) und der Schweizerischen Gesellschaft für Allergologie und Immunologie (SGAI) Allergo J. 2011;20:318–339. [Google Scholar]
- 20.Vetander M, Ly DH, Hakansson N, et al. Recurrent reactions to food among children at paediatric emergency departments: epidemiology of allergic disease. Clin Exp Allergy. 2014;44:113–120. doi: 10.1111/cea.12203. [DOI] [PubMed] [Google Scholar]
- 21.Grabenhenrich L, Hompes S, Gough H, et al. Implementation of anaphylaxis management guidelines: a register-based study. PloS one. 2012;7 doi: 10.1371/journal.pone.0035778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang F, Chawla K, Jarvinen KM, Nowak-Wegrzyn A. Anaphylaxis in a New York City pediatric emergency department: triggers, treatments, and outcomes. J Allergy Clin Immunol. 2012;129:162–168. e1–e3. doi: 10.1016/j.jaci.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pumphrey RS, Gowland MH. Further fatal allergic reactions to food in the United Kingdom, 1999-2006. J Allergy Clin Immunol. 2007;119:1018–1019. doi: 10.1016/j.jaci.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Khoueiry G, Abi Rafeh N, Azab B, et al. Reverse Takotsubo cardiomyopathy in the setting of anaphylaxis treated with high-dose intravenous epinephrine. J Emerg Med. 2013;44:96–69. doi: 10.1016/j.jemermed.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 2001;108:871–873. doi: 10.1067/mai.2001.119409. [DOI] [PubMed] [Google Scholar]
- 26.Worm M, Kostev K, Hompes S, Zuberbier T. Versorgungsprofil von Patienten mit schweren allergischen Reaktionen. Allergologie. 2011;34:285–393. [Google Scholar]