Abstract
Chronotype is an established concept designed to identify distinct phase relationships between the expression of circadian rhythms and external synchronizers in humans. Although it has been widely accepted that chronotype is subjected to ontogenetic modulation, there is no consensus on the interaction between age and gender. This study aimed to determine the relationship between age- and gender-related changes in the morningness-eveningness character in a large sample of people. A total of 14,650 volunteers were asked to complete the Brazilian version of the Horne and Östberg chronotype questionnaire. The data demonstrated that, on average, women were more morning-oriented than men until the age of 30 and there were no significant differences between men and women from 30 to 45 years of age. In contrast to the situation observed until the age of 30, women older than 45 years were more evening-oriented than men. These results suggest that the ontogenetic development of the circadian timekeeping system is more plastic in men, as represented by the larger amplitude of chronotype changes throughout their aging process. The phase delay of adolescence and phase advance of the elderly seem to be phenomena that are more markedly present in men than in women. Thus, our data, for the first time, provide support that sharply opposes the view that there is a single path toward morningness as a function of age, regardless of gender.
Keywords: Chronotype, Gender, Ontogeny, Circadian rhythms, Sleep timing
Introduction
An important aspect of the phase differences of circadian rhythms is called chronotype or degree of morningness-eveningness. Ontogenetic traits in chronotype have been widely reported. In general, it is plausible to state that younger individuals have a strong tendency toward eveningness, whereas aging is strongly associated with morningness (1,2).
Previous studies on the theme of morningness-eveningness have reported mixed evidence concerning the interaction of age and gender. Some authors report that no correlations can be made between the scores from chronotype questionnaires and gender (3-6), while others point to differences in mean scores between men and women (2,7,8).
Christopher Randler (9) conducted a large sample study aiming to clarify the interaction between age and gender during adolescence and reported that male subjects are, on average, more evening-oriented than female adolescents. In addition, he reported that it was not only the morningness-eveningness score or orientation that changes during adolescence, thus exposing gender-related differences, but also that the dynamics of the transition from eveningness, during puberty, toward morningness, in adulthood, is gender-sensitive. Specifically, it was reported that females reach their peak of eveningness earlier than males (9,10). With regard to adulthood, Paine et al. (11) found no gender-related differences when they analyzed chronotype orientation in a population ranging in age from 30 to 48 years. However, Caci et al. (12), exploring the use of distinct chronotype questionnaires, were able to report a very strong effect of age on morningness-eveningness during adulthood. Despite the lack of statistical significance, these authors reported that the chronotype scores were skewed toward morningness in females, suggesting the presence of a gender effect that was not revealed by comparing the mean scores.
In elderly individuals, who are healthy and without sleep disorders, the circadian rhythm parameters of melatonin levels (nocturnal peak), core body temperature (acrophase), and cortisol levels (acrophase) occur earlier in the day compared to young adults (13,14). Other changes in the elderly include a reduction in the amplitude of circadian rhythms and reduced tolerance to abrupt phase changes (15). It has been suggested that chronotype remains relatively stable until around the age of 35 and, thereafter, morningness increases (12,16). Considering that the dynamics of the age-related transition from eveningness to morningness appears to be gender-sensitive, gender-driven changes in the expression of chronotype seem to not be significant after the age of 50. According to Roenneberg et al. (17), both male and female subjects over 50 years of age follow the same path toward morningness.
The apparent contradiction among the results of these studies appears to arise from discrepancies in the age and size of the studied populations. In general, however, the consensus in the field of chronobiology appears to be that women are more morning-oriented than men (7,17). Additionally, a small number of studies have focused on the ontogenetic traits of morningness-eveningness, especially because of the need for large samples and the misconception that chronotype is a fixed construct that does not change throughout the course of a life, as recently noted by Di Milia and Randler (18).
Here, we further explore the interaction between gender and age to better understand their relationship to chronotypes. This study aimed to determine the relationship between age- and gender-related changes in the morningness-eveningness characteristics in humans.
Material and Methods
The study was reviewed and approved by the Ethics Committee of Instituto de Ciências Biomédicas, Universidade de São Paulo. A sample of 14,650 Brazilian volunteers completed the Brazilian Portuguese validated version of the Horne and Östberg (HO) Morningness-Eveningness Questionnaire (19).
The chronotype questionnaire consisted of 19 multiple choice questions that simulated different daily situations, and individuals had to declare their preferred schedule for implementing the proposed activities. A score for each question and the total score resulted in a value ranging from 16 (greater eveningness) to 86 (highest morningness). The electronic version of this questionnaire can be accessed at http://www.each.usp.br/crono. The dependent variable of the study was the score from the chronotype questionnaire (HO score), and the independent variables were gender (categorized into male and female) and age.
To compare the mean scores from the questionnaires according to the categories male and female, a parametric Z-test for means was applied. ANOVA was carried out using age groups, gender, and the score of the chronotype questionnaire as independent factors. Post hoc comparisons were performed using the Bonferroni test. The statistical significance level was set at P=0.05.
Results
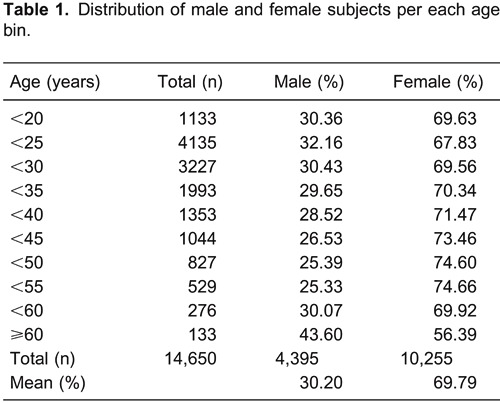
The mean score from the chronotype questionnaire was 46.12 (SD=12.21) for men and 46.63 (SD=12.27) for women. The overall comparison revealed that men on average scored slightly higher than women according to the Z-test (Z=2.53, P=0.01). For the analysis of age associated with gender, we arbitrarily divided the groups of men and women into 10 subpopulations as follows: less than 20, 20 to <25, 25 to <30, 30 to <35, 35 to <40, 40 to <45, 45 to <50, 50 to <55, 55 to <60, and 60 years and older. Table 1 summarizes the number of volunteers and the proportions of male and female subjects for each age bin. Applying ANOVA and the Bonferroni post hoc test, we compared the mean scores of men and women in each subpopulation group. The effect of gender was statistically significant (P<0.05) for all ages except those between 30 and 44 years. While men display, on average, later chronotypes in earlier age groups, this situation was reversed after the age of 45 years.
Discussion
Our present results showed that the ontogeny of chronotype was different in men and women. We observed that men displayed, on average, a later chronotype during younger ages and that this situation was reversed during older ages, when men became more morning-oriented than women.
These results suggest that the ontogenetic development of the circadian timekeeping system is more plastic in men, characterized by a larger amplitude of chronotype changes throughout the aging process. The phase delay of adolescents and the phase advance of the elderly seem to be phenomena that are markedly more present in men than in women.
Several studies have reported differences in circadian properties when comparing men and women. The classical experiments of Wever (20) that investigated internal desynchronization under isolation conditions showed that men had faster desynchronization rates than women. Cain et al. (21) explored gender differences in the phase angle of entrainment in humans. They found that, compared with men, the circadian phases of women occur at an earlier circadian phase of core body temperature and melatonin relative to their habitual wake time, despite the lack of significant gender-related differences in the chronotype score. This reveals a distinct pattern of sensitivity to the phase-shifting effects of light according to gender. Taken together, these results emphasize that the expression of the circadian timekeeping system in humans can exhibit gender-specific traits, which is in line with the findings of Davis et al. (22) and Goel and Lee (23), who reported sex differences in the light sensitivity of rodents.
Previous studies have indicated that large samples are needed to detect age and gender differences in morningness-eveningness scores (24,25). To provide an epidemiological portrait of chronotype, Roenneberg et al. (17) conducted a questionnaire-based survey that had the participation of more than 55,000 volunteers. One of their objectives was to confirm the gender differences observed throughout ontogeny. They provided evidence for a gender-related effect on chronotype, reporting that females reach the maximum of their eveningness sooner than males and that this gender effect disappears around the age of 50, the average age of menopause. Therefore, these authors attribute the disappearance of the phase differences between men and women to the influence of menopause. Our results, however, contrast sharply with the view that there is a single path toward morningness as a function of age. Indeed, here we described a distinct dynamic in the plasticity of the circadian timekeeping system according to gender in a sample from the Brazilian population over the age of 45 years.
The different dynamics of the changes in chronotype observed in men and women during ontogeny may indicate that endocrine factors are directly or indirectly involved in this phenomenon. Support for such interactions is indicated by the presence of sex hormone receptors in the suprachiasmatic nuclei in both humans (26) and rodents (27). The hormonal influence on gender differences of chronotype during puberty and in the transition to adulthood has been explored. Randler et al. (28) described a positive association between the level of salivary testosterone and evening orientation in young male university students. Although it was the first description of such an association in humans, it is well known that sex hormones, particularly testosterone, play a role in chronotype changes observed during puberty in mammals (29). In addition, it has been suggested that, in females, there is an association between development during puberty and the changes that occur in sleep phase preferences. Specifically, the 5-year period after menarche has been implicated as an important marker of the stability of sleep phase preferences, and there is a return to a morning orientation in females (30). Moreover, female menopause and male andropause are two major ontogenetic challenges, and their possible impacts on the sleep/wake cycle and sleep preferences are mediated by hormonal changes. Peri- and postmenopausal women experience decreased estrogen release (31), while male andropause consists of a myriad of signs and signals related to an age-dependent progressive decrease of free testosterone levels (32). Taking into account the roles of estrogen and testosterone in young female and male subjects, the decreased expression of these sex hormones would imply a shift toward morningness in older people, which is in accordance with our data. However, the underlying processes responsible for the distinct sex-driven implications of hormonal changes on the circadian timekeeping system still remain elusive.
Even though our results confirm the already described lack of differences in chronotype between middle-aged men and women (17), we go further and provide, for the first time, evidence that contrasts sharply with the view that there is a single path toward morningness as a function of age. Indeed, we describe a distinct dynamic in the ontogeny of the circadian timekeeping system toward morningness at older ages according to gender in a sample of the Brazilian population. An additional strength of our study is that we not only described the gender differences but also reinforced the evidence that chronotype should not be considered fixed throughout life.
The biological substrates associated with the physiological regulation of these chronotype differences related to gender described here are not yet known. Further investigation and a better understanding of the role of these biological substrates may be useful to help older people cope with well-known sleep and circadian problems related to aging.
Figure 1. Means±SE of the Horne and Östberg (HO) score of males and females as a function of the selected age groups. *P<0.05, Bonferroni test.
Footnotes
First published online April 7, 2014.
References
- 1.Andrade MM, Benedito-Silva AA, Menna-Barreto L. Correlations between morningness-eveningness character, sleep habits and temperature rhythm in adolescents. Braz J Med Biol Res. 1992;25:835–839. [PubMed] [Google Scholar]
- 2.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, et al. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 3.Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11:191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 4.Posey TB, Ford JA. The morningness-eveningness preference of college students as measured by the Horne and Ostberg questionnaire. Int J Chronobiol. 1981;7:141–144. [Google Scholar]
- 5.Neubauer AC. Psychometric comparison of two circadian rhythm questionnaires and their relationship with personality. Personal Individ Differences. 1992;13:125–131. doi: 10.1016/0191-8869(92)90035-N. [DOI] [Google Scholar]
- 6.Ishihara K, Miyake S, Miyasita A, Miyata Y. Comparisons of sleep-wake habits of morning and evening types in Japanese worker sample. J Hum Ergol. 1988;17:111–118. [PubMed] [Google Scholar]
- 7.Chelminski I, Ferraro FR, Petros T, Plaud JJ. Horne and Ostberg questionnaire: A score distribution in a large sample of young adults. Personal Individ Differences. 1997;23:647–652. doi: 10.1016/S0191-8869(97)00073-1. [DOI] [Google Scholar]
- 8.Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiol Int. 2002;19:709–720. doi: 10.1081/CBI-120005390. [DOI] [PubMed] [Google Scholar]
- 9.Randler C. Age and gender differences in morningness-eveningness during adolescence. J Genet Psychol. 2011;172:302–308. doi: 10.1080/00221325.2010.535225. [DOI] [PubMed] [Google Scholar]
- 10.Tonetti L, Fabbri M, Natale V. Sex difference in sleep-time preference and sleep need: a cross-sectional survey among Italian pre-adolescents, adolescents, and adults. Chronobiol Int. 2008;25:745–759. doi: 10.1080/07420520802394191. [DOI] [PubMed] [Google Scholar]
- 11.Paine SJ, Gander PH, Travier N. The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30-49 years) J Biol Rhythms. 2006;21:68–76. doi: 10.1177/0748730405283154. [DOI] [PubMed] [Google Scholar]
- 12.Caci H, Deschaux O, Adan A, Natale V. Comparing three morningness scales: age and gender effects, structure and cut-off criteria. Sleep Med. 2009;10:240–245. doi: 10.1016/j.sleep.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Carrier J, Monk TH, Buysse DJ, Kupfer DJ. Sleep and morningness-eveningness in the ‘middle’ years of life (20-59 y) J Sleep Res. 1997;6:230–237. doi: 10.1111/j.1365-2869.1997.00230.x. [DOI] [PubMed] [Google Scholar]
- 14.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275:R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 15.Monk TH, Buysse DJ, Reynolds CF, III, Kupfer DJ, Houck PR. Circadian temperature rhythms of older people. Exp Gerontol. 1995;30:455–474. doi: 10.1016/0531-5565(95)00007-4. [DOI] [PubMed] [Google Scholar]
- 16.Cofer LF, Grice JP, Sethre-Hofstad L, Radi CJ, Zimmermann LK, Palmer-Seal D, et al. Developmental perspectives on morningness-eveningness and social interactions. Human Develop. 1999;42:169–198. doi: 10.1159/000022623. [DOI] [Google Scholar]
- 17.Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Di Milia L, Randler C. The stability of the morning affect scale across age and gender. Personal Individ Differences. 2013;54:298–301. doi: 10.1016/j.paid.2012.08.031. [DOI] [Google Scholar]
- 19.Benedito-Silva AA, Menna-Barreto L, Marques N, Tenreiro S. A self-assessment questionnaire for the determination of morningness-eveningness types in Brazil. Prog Clin Biol Res. 1990;341B:89–98. [PubMed] [Google Scholar]
- 20.Wever RA. Characteristics of circadian rhythms in human functions. J Neural Transm Suppl. 1986;21:323–373. [PubMed] [Google Scholar]
- 21.Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SB, Santhi N, et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25:288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol. 1983;244:R93–R105. doi: 10.1152/ajpregu.1983.244.1.R93. [DOI] [PubMed] [Google Scholar]
- 23.Goel N, Lee TM. Sex differences and effects of social cues on daily rhythms following phase advances in Octodon degus . Physiol Behav. 1995;58:205–213. doi: 10.1016/0031-9384(95)00051-J. [DOI] [PubMed] [Google Scholar]
- 24.Mateo MJC, Diaz-Morales JF, Escribano Barreno C, Delgado Prieto P, Randler C. Morningness-eveningness and sleep habits among adolescents: age and gender differences. Psicothema. 2012;24:410–415. [PubMed] [Google Scholar]
- 25.Randler C. Gender differences in morningness-eveningness assessed by self-report questionnaires: A meta-analysis. Personal Individ Differences. 2007;43:1667–1675. doi: 10.1016/j.paid.2007.05.004. [DOI] [Google Scholar]
- 26.Kruijver FP, Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology. 2002;75:296–305. doi: 10.1159/000057339. [DOI] [PubMed] [Google Scholar]
- 27.Lee TM, Hummer DL, Jechura TJ, Mahoney MM. Pubertal development of sex differences in circadian function: an animal model. Ann N Y Acad Sci. 2004;1021:262–275. doi: 10.1196/annals.1308.031. [DOI] [PubMed] [Google Scholar]
- 28.Randler C, Ebenhoh N, Fischer A, Hochel S, Schroff C, Stoll JC, et al. Chronotype but not sleep length is related to salivary testosterone in young adult men. Psychoneuroendocrinology. 2012;37:1740–1744. doi: 10.1016/j.psyneuen.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Hagenauer MH, Ku JH, Lee TM. Chronotype changes during puberty depend on gonadal hormones in the slow-developing rodent, Octodon degus . Horm Behav. 2011;60:37–45. doi: 10.1016/j.yhbeh.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frey S, Balu S, Greusing S, Rothen N, Cajochen C. Consequences of the timing of menarche on female adolescent sleep phase preference. PLoS One. 2009;4: doi: 10.1371/journal.pone.0005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okatani Y, Morioka N, Wakatsuki A. Changes in nocturnal melatonin secretion in perimenopausal women: correlation with endogenous estrogen concentrations. J Pineal Res. 2000;28:111–118. doi: 10.1034/j.1600-079X.2001.280207.x. [DOI] [PubMed] [Google Scholar]
- 32.Samaras N, Frangos E, Forster A, Lang PO, Samaras D. Andropause: A review of the definition and treatment. Eur Geriat Med. 2012;3:368–373. doi: 10.1016/j.eurger.2012.08.007. [DOI] [Google Scholar]


