Abstract
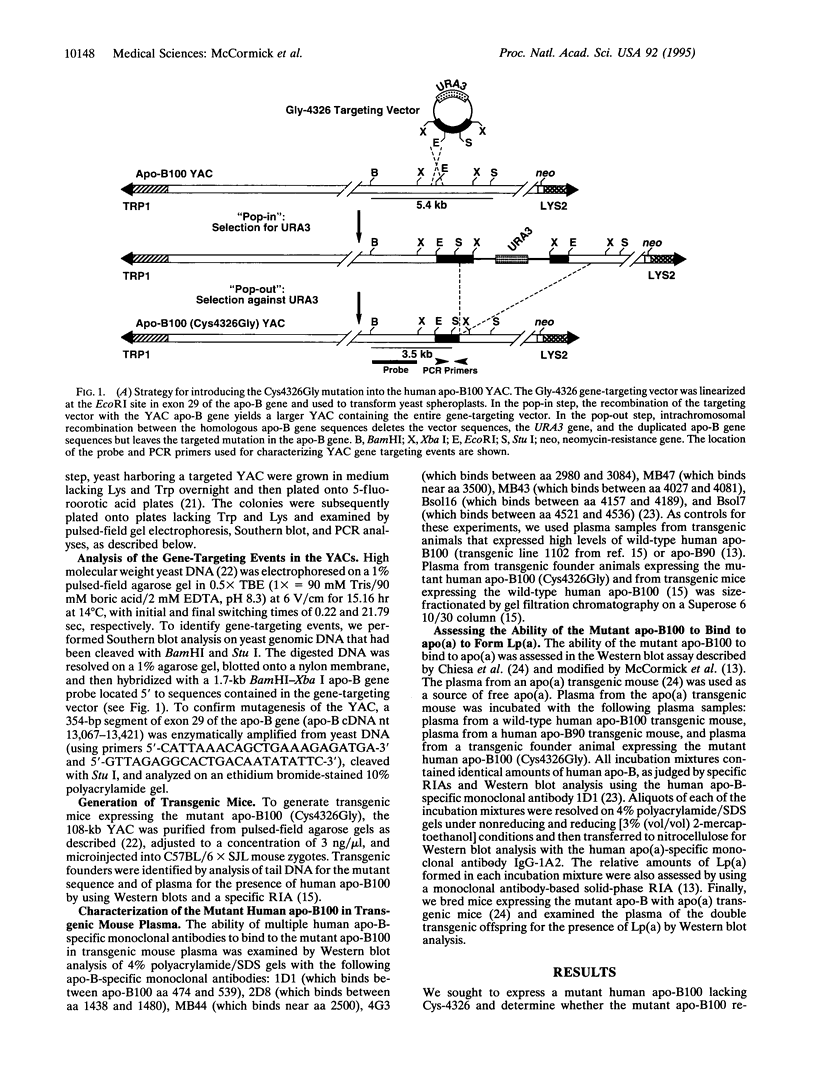
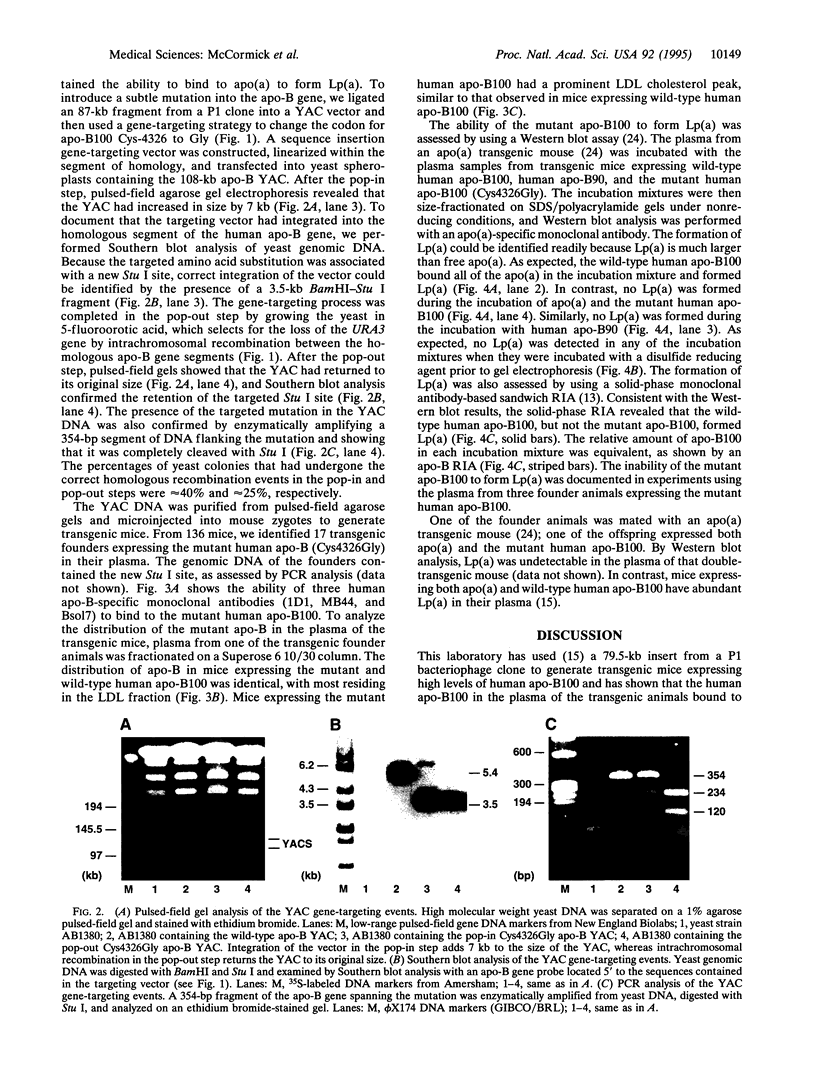
Lipoprotein(a) [Lp(a)] is a lipoprotein formed by the disulfide linkage of apolipoprotein (apo) B100 of a low density lipoprotein particle to apolipoprotein(a). Prior studies have suggested that one of the C-terminal Cys residues of apo-B100 is involved in the disulfide linkage of apo-B100 to apo(a). To identify the apo-B100 Cys residue involved in the formation of Lp(a), we constructed a yeast artificial chromosome (YAC) spanning the human apo-B gene and used gene-targeting techniques to change Cys-4326 to Gly. The mutated YAC DNA was used to generate transgenic mice expressing the mutant human apo-B100 (Cys4326Gly). Unlike the wild-type human apo-B100, the mutant human apo-B100 completely lacked the ability to bind to apo(a) and form Lp(a). This study demonstrates that apo-B100 Cys-4326 is required for the assembly of Lp(a) and shows that gene targeting in YACs, followed by the generation of transgenic mice, is a useful approach for analyzing the structure of large proteins coded for by large genes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackhart B. D., Yao Z. M., McCarthy B. J. An expression system for human apolipoprotein B100 in a rat hepatoma cell line. J Biol Chem. 1990 May 25;265(15):8358–8360. [PubMed] [Google Scholar]
- Brunner C., Kraft H. G., Utermann G., Müller H. J. Cys4057 of apolipoprotein(a) is essential for lipoprotein(a) assembly. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11643–11647. doi: 10.1073/pnas.90.24.11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. T., Olson M. V. Preparation of clone libraries in yeast artificial-chromosome vectors. Methods Enzymol. 1991;194:251–270. doi: 10.1016/0076-6879(91)94020-d. [DOI] [PubMed] [Google Scholar]
- Chiesa G., Hobbs H. H., Koschinsky M. L., Lawn R. M., Maika S. D., Hammer R. E. Reconstitution of lipoprotein(a) by infusion of human low density lipoprotein into transgenic mice expressing human apolipoprotein(a). J Biol Chem. 1992 Dec 5;267(34):24369–24374. [PubMed] [Google Scholar]
- Chiesa G., Johnson D. F., Yao Z., Innerarity T. L., Mahley R. W., Young S. G., Hammer R. H., Hobbs H. H. Expression of human apolipoprotein B100 in transgenic mice. Editing of human apolipoprotein B100 mRNA. J Biol Chem. 1993 Nov 15;268(32):23747–23750. [PubMed] [Google Scholar]
- Coleman R. D., Kim T. W., Gotto A. M., Jr, Yang C. Y. Determination of cysteine on low-density lipoproteins using the fluorescent probe, 5-iodoacetamidofluoresceine. Biochim Biophys Acta. 1990 Jan 19;1037(1):129–132. doi: 10.1016/0167-4838(90)90111-r. [DOI] [PubMed] [Google Scholar]
- Dahlen G. H., Guyton J. R., Attar M., Farmer J. A., Kautz J. A., Gotto A. M., Jr Association of levels of lipoprotein Lp(a), plasma lipids, and other lipoproteins with coronary artery disease documented by angiography. Circulation. 1986 Oct;74(4):758–765. doi: 10.1161/01.cir.74.4.758. [DOI] [PubMed] [Google Scholar]
- Duff K., McGuigan A., Huxley C., Schulz F., Hardy J. Insertion of a pathogenic mutation into a yeast artificial chromosome containing the human amyloid precursor protein gene. Gene Ther. 1994 Jan;1(1):70–75. [PubMed] [Google Scholar]
- Gabel B., Yao Z., McLeod R. S., Young S. G., Koschinsky M. L. Carboxyl-terminal truncation of apolipoproteinB-100 inhibits lipoprotein(a) particle formation. FEBS Lett. 1994 Aug 15;350(1):77–81. doi: 10.1016/0014-5793(94)00737-3. [DOI] [PubMed] [Google Scholar]
- Guevara J., Jr, Spurlino J., Jan A. Y., Yang C. Y., Tulinsky A., Prasad B. V., Gaubatz J. W., Morrisett J. D. Proposed mechanisms for binding of apo[a] kringle type 9 to apo B-100 in human lipoprotein[a]. Biophys J. 1993 Mar;64(3):686–700. doi: 10.1016/S0006-3495(93)81428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann T., Metzger S., Fisher E. A., Breslow J. L., Huang L. S. Alternative polyadenylation of apolipoprotein B RNA is a major cause of B-48 protein formation in rat hepatoma cell lines transfected with human apoB-100 minigenes. J Lipid Res. 1994 Dec;35(12):2200–2211. [PubMed] [Google Scholar]
- Jauhiainen M., Koskinen P., Ehnholm C., Frick M. H., Mänttäri M., Manninen V., Huttunen J. K. Lipoprotein (a) and coronary heart disease risk: a nested case-control study of the Helsinki Heart Study participants. Atherosclerosis. 1991 Jul;89(1):59–67. doi: 10.1016/0021-9150(91)90007-p. [DOI] [PubMed] [Google Scholar]
- Koschinsky M. L., Côté G. P., Gabel B., van der Hoek Y. Y. Identification of the cysteine residue in apolipoprotein(a) that mediates extracellular coupling with apolipoprotein B-100. J Biol Chem. 1993 Sep 15;268(26):19819–19825. [PubMed] [Google Scholar]
- Kostner G. M., Avogaro P., Cazzolato G., Marth E., Bittolo-Bon G., Qunici G. B. Lipoprotein Lp(a) and the risk for myocardial infarction. Atherosclerosis. 1981 Jan-Feb;38(1-2):51–61. doi: 10.1016/0021-9150(81)90103-9. [DOI] [PubMed] [Google Scholar]
- Linton M. F., Farese R. V., Jr, Chiesa G., Grass D. S., Chin P., Hammer R. E., Hobbs H. H., Young S. G. Transgenic mice expressing high plasma concentrations of human apolipoprotein B100 and lipoprotein(a). J Clin Invest. 1993 Dec;92(6):3029–3037. doi: 10.1172/JCI116927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S. P., Linton M. F., Hobbs H. H., Taylor S., Curtiss L. K., Young S. G. Expression of human apolipoprotein B90 in transgenic mice. Demonstration that apolipoprotein B90 lacks the structural requirements to form lipoprotein. J Biol Chem. 1994 Sep 30;269(39):24284–24289. [PubMed] [Google Scholar]
- McLean J. W., Tomlinson J. E., Kuang W. J., Eaton D. L., Chen E. Y., Fless G. M., Scanu A. M., Lawn R. M. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987 Nov 12;330(6144):132–137. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- Pease R. J., Milne R. W., Jessup W. K., Law A., Provost P., Fruchart J. C., Dean R. T., Marcel Y. L., Scott J. Use of bacterial expression cloning to localize the epitopes for a series of monoclonal antibodies against apolipoprotein B100. J Biol Chem. 1990 Jan 5;265(1):553–568. [PubMed] [Google Scholar]
- Peterson K. R., Clegg C. H., Huxley C., Josephson B. M., Haugen H. S., Furukawa T., Stamatoyannopoulos G. Transgenic mice containing a 248-kb yeast artificial chromosome carrying the human beta-globin locus display proper developmental control of human globin genes. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7593–7597. doi: 10.1073/pnas.90.16.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. R., Li Q. L., Clegg C. H., Furukawa T., Navas P. A., Norton E. J., Kimbrough T. G., Stamatoyannopoulos G. Use of yeast artificial chromosomes (YACs) in studies of mammalian development: production of beta-globin locus YAC mice carrying human globin developmental mutants. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5655–5659. doi: 10.1073/pnas.92.12.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtell C., Maeda N., Ebert D. L., Kaiser M., Lund-Katz S., Sturley S. L., Kodoyianni V., Grunwald K., Nevin D. N., Aiello R. J. Nucleotide sequence encoding the carboxyl-terminal half of apolipoprotein B from spontaneously hypercholesterolemic pigs. J Lipid Res. 1993 Aug;34(8):1323–1335. [PubMed] [Google Scholar]
- Reuben M. A., Svenson K. L., Doolittle M. H., Johnson D. F., Lusis A. J., Elovson J. Biosynthetic relationships between three rat apolipoprotein B peptides. J Lipid Res. 1988 Oct;29(10):1337–1347. [PubMed] [Google Scholar]
- Ridker P. M., Hennekens C. H., Stampfer M. J. A prospective study of lipoprotein(a) and the risk of myocardial infarction. JAMA. 1993 Nov 10;270(18):2195–2199. [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Srivastava A. K., Schlessinger D. Vectors for inserting selectable markers in vector arms and human DNA inserts of yeast artificial chromosomes (YACs). Gene. 1991 Jul 15;103(1):53–59. doi: 10.1016/0378-1119(91)90390-w. [DOI] [PubMed] [Google Scholar]
- Utermann G., Menzel H. J., Kraft H. G., Duba H. C., Kemmler H. G., Seitz C. Lp(a) glycoprotein phenotypes. Inheritance and relation to Lp(a)-lipoprotein concentrations in plasma. J Clin Invest. 1987 Aug;80(2):458–465. doi: 10.1172/JCI113093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermann G. The mysteries of lipoprotein(a). Science. 1989 Nov 17;246(4932):904–910. doi: 10.1126/science.2530631. [DOI] [PubMed] [Google Scholar]
- Young S. G., Farese R. V., Jr, Pierotti V. R., Taylor S., Grass D. S., Linton M. F. Transgenic mice expressing human apoB100 and apoB48. Curr Opin Lipidol. 1994 Apr;5(2):94–101. doi: 10.1097/00041433-199404000-00005. [DOI] [PubMed] [Google Scholar]