Abstract
This study was designed to assess the influence of resistance training on salivary immunoglobulin A (IgA) levels and hormone profile in sedentary adults with Down syndrome (DS). A total of 40 male adults with DS were recruited for the trial through different community support groups for people with intellectual disabilities. All participants had medical approval for participation in physical activity. Twenty-four adults were randomly assigned to perform resistance training in a circuit with six stations, 3 days per week for 12 weeks. Training intensity was based on functioning in the eight-repetition maximum (8RM) test for each exercise. The control group included 16 age-, gender-, and BMI-matched adults with DS. Salivary IgA, testosterone, and cortisol levels were measured by ELISA. Work task performance was assessed using the repetitive weighted-box-stacking test. Resistance training significantly increased salivary IgA concentration (P=0.0120; d=0.94) and testosterone levels (P=0.0088; d=1.57) in the exercising group. Furthermore, it also improved work task performance. No changes were seen in the controls who had not exercised. In conclusion, a short-term resistance training protocol improved mucosal immunity response as well as salivary testosterone levels in sedentary adults with DS.
Keywords: Down syndrome, Resistance training, Saliva, Immunoglobulin A, Testosterone, Cortisol
Introduction
The increasing life expectancy of people with Down syndrome (DS) calls for knowledge of conditions that frequently occur in adults with the syndrome, and of which health personnel should be particularly aware (1).
This population group is particularly susceptible to infections of the respiratory and the gastrointestinal tracts, which may be explained, at least in part, by a severe impairment of immunoglobulin (Ig) secretion in the saliva (2). In this respect, Chaushu et al. (3) reported that detection of salivary Ig levels might serve as a predictor of the susceptibility of DS individuals to recurrent respiratory tract infections.
Recent studies have found that exercise at moderate intensity may have a positive impact on both the mucosal immune response and the salivary hormone levels (4,5). In a more detailed way, Akimoto et al. (4) reported that a 12-month mixed protocol based on endurance and resistance training improved salivary IgA levels in elderly subjects. Similarly, resistance training has significantly increased salivary testosterone levels in middle-aged, strength-trained men (6). However, the effect of regular exercise on the mucosal immune response has received no attention in people with DS. In this regard, the additional positive effects of resistance training on functional tasks of daily living and employability in this population group should be taken into consideration (7).
Thus, we hypothesized that resistance training may improve mucosal immunity response in sedentary adults with DS. Accordingly, the main objective of this research was to assess the influence of resistance training on the salivary hormone profile and IgA level in sedentary adults with DS.
Material and Methods
Participants
Forty male adults with DS (23.7±3.1 years, 26.2±2.8 kg/m2) were recruited through different community support groups for people with intellectual disabilities (ID). They had an intelligence quotient range of 60-69 determined by the Stanford-Binet Scale, and were diagnosed as having mild ID. Participants were excluded from the study if they met any of the following criteria: 1) atlantoaxial instability, 2) congenital heart disease, 3) thyroid disease, 4) toxic habits (smoking or alcohol), 5) participation in a training program in the 6 months prior to their participation in the trial to ensure that any effects could be attributed to the current intervention, and 6) not completing at least 90% of the training sessions. Twenty-four adults were randomly assigned to the exercising group to perform a short-term resistance training program. The control group included 16 age-, gender-, and BMI-matched adults with DS who did not take part in any training program.
Intervention program
The intervention consisted of a 12-week resistance circuit-training program performed 3 days per week (Table 1). It should be pointed out that training sessions were scheduled in the morning, taking into consideration that evenings and weekends are especially inactive time periods for participants with ID (8). The resistance training was performed in a six-station circuit including arm curl, leg extension, seated row, leg curl, triceps extension, and leg press stations. Training intensity (load) was based on functioning in the eight-repetition maximum (8RM) test for each exercise, given that it has been recommended for resistance training in patients with various diagnoses (9).
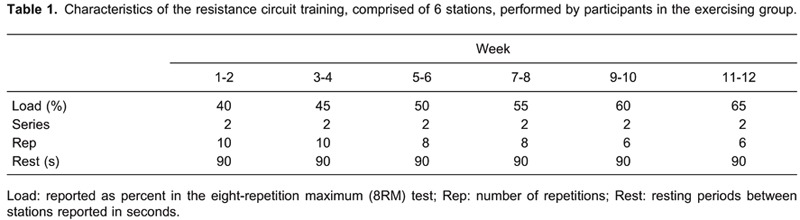
Each training session was performed in small groups of six participants and supervised by experienced physical therapists to ensure that participants used the correct technique and intensity.
Salivary evaluation
Whole saliva samples were collected after the mouth had been rinsed thoroughly with distilled water. Saliva production was stimulated by chewing a sterile cotton swab (Salivette; Sersted, Germany) at a frequency of 60/60 s. Saliva was separated from the cotton by centrifugation at 2500 g for 10 min. Collected samples were frozen at -80°C and stored until analysis.
Salivary IgA levels were determined by ELISA as described previously (5). Salivary levels of testosterone and cortisol were also measured by ELISA, according to the manufacturer's instructions (DiaMetra, Italy).
All individual outcomes were assessed first at baseline and again 72 h after the end of the intervention. Finally, it should be pointed out that biochemical outcomes were assessed after an overnight fast (10).
Functional assessment
Work task performance was assessed using the repetitive weighted-box-stacking test as recommended by the American College of Sports Medicine (11). Briefly, it requires the participants to repetitively lift 10-kg boxes, from the floor to a table 75 cm above the floor. The number of boxes stacked in 1 min was measured. Furthermore, it should be pointed out that all participants (n=40) underwent a preliminary training session to be familiar with the correct use of the test.
Ethics and statistics
Written informed consent was obtained from all parents or legal representatives. The study protocol was approved by the Ethics Committee of University of Malaga (Ref. No. 10-132). Results are reported as means±SD. The Shapiro-Wilk test was used to assess whether data were normally distributed.
To compare the mean values, a two-way repeated measures analysis of variance (ANOVA) followed by the Bonferroni post hoc test was used. Finally, Cohen's d statistics were used for determining mean effect sizes as follows: small, d≥0.2 and <0.5; medium, d≥0.5 and <0.8; large, d≥0.8.
Results
Resistance training improved the mucosal immunity response by increasing IgA concentration in the exercising group (P=0.0120; d=0.94). Similarly, the testosterone level was significantly increased (P=0.0088; d=1.57). Conversely, no significant changes were observed in cortisol concentration after the completion of the training program (P=0.572; d=0.12). These results are summarized in Table 2. The repetitive weighted-box-stacking test was also significantly increased (19.1±3.0 vs 23.3±2.7 boxes/min; P=0.0141) in the exercising group.
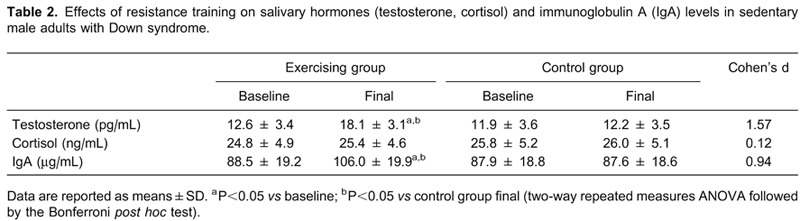
On the other hand, no significant changes in any assessed outcome were observed in those who had not exercised. Finally, neither sport-related injuries nor dropouts were reported during the study.
Discussion
This was the first study that demonstrated that resistance training improved mucosal immunity response, by increasing salivary IgA concentration, in sedentary adults with DS. The clinical relevance is related to the fact that Ig levels may serve as a predictor of the susceptibility of individuals with DS to recurrent respiratory tract infections (3). A previous study found that a 12-month mixed protocol based on endurance and resistance training improved salivary IgA levels in elderly subjects (4). Similarly, Martins et al. (12) concluded that 16 weeks of aerobic training significantly increased salivary IgA in older men and women. In this regard, it should be emphasized that our exercise intervention program lasted just 12 weeks and that continued activity by participants would be desirable. The importance of exercise as a life-long therapy should also be emphasized, given that people with DS are at risk of a life of inactivity that can result in a multitude of medical problems (13).
On the other hand, previous studies have reported that intensive training increased the incidence of upper respiratory tract infections in elite athletes by decreasing salivary IgA levels (14).
Furthermore, the current study showed that resistance training significantly increased salivary testosterone levels, indicating a strong effect size (d=1.57). Taking into consideration that salivary testosterone reflects the free bioavailable steroid hormone fraction found in blood circulation, the increase reported in the current study may have positive implications for adults with DS. This finding, coupled with no observed changes in cortisol levels, suggested an enhanced anabolic status in participants after the completion of the resistance training, as was recently reported by Hayes et al. (15) in sedentary aging men. In fact, Cadore et al. (16) reported that under exercise conditions, the salivary values of cortisol (r=0.52; P=0.005) and dehydroepiandrosterone (r=0.7; P<0.001) might offer a practical indicator for their serum concentrations. Similar results were found by Baillot et al. (17) in obese diabetic men during submaximal exercise.
A secondary finding was that resistance training improved work task performance. This finding was of particular interest for young adults with DS given that their workplace activities typically emphasize physical skills such as packing boxes of confectionery or assembling automotive parts, rather than cognitive skills (18). Furthermore, an improvement of muscle strength could potentially increase the amount of activity they undertake, which may ultimately give them the confidence to continue exercising after the trial concludes, thus reducing their risk of the secondary health consequences of inactivity in the long term (19).
Strengths of the current study included the excellent adherence rate as well as the homogeneous and large sample size. Conversely, previous studies that have focused on the influence of regular exercise in people with ID recruited mixed (male and female) groups in order to increase sample size with the aim of strengthening the research design. In addition, some studies have recruited participants with ID matched for intelligence quotient but with differing diagnoses. The presence of a control group, consisting of age- and gender-matched controls with DS, as in this study, may reduce the recruitment bias of healthy controls.
The present study had some limitations that should also be addressed. A major weakness was the relatively short duration of the exercise intervention. In fact, there was no follow-up to determine whether the positive effects induced by resistance training were maintained. Therefore, there is a clear need for long-term studies to determine whether the increased levels of salivary testosterone and IgA improve clinical outcomes of individuals with DS. Also, the use of weight-lifting machines may limit the reproducibility of this study in case the exercise equipment is not available. Accordingly, future studies evaluating the effect of circuits that utilize body-weight or free-weight exercises are also required to guarantee its reproducibility elsewhere.
In conclusion, a short-term resistance training protocol improved mucosal immunity response and salivary hormone profile in sedentary adults with DS. A secondary finding was that it also improved work task performance.
Footnotes
First published online March 31, 2014.
References
- 1.Malt EA, Dahl RC, Haugsand TM, Ulvestad IH, Emilsen NM, Hansen B, et al. Health and disease in adults with Down syndrome. Tidsskr Nor Laegeforen. 2013;133:290–294. doi: 10.4045/tidsskr.12.0390. [DOI] [PubMed] [Google Scholar]
- 2.Chaushu S, Yefenof E, Becker A, Shapira J, Chaushu G. Severe impairment of secretory Ig production in parotid saliva of Down Syndrome individuals. J Dent Res. 2002;81:308–312. doi: 10.1177/154405910208100504. [DOI] [PubMed] [Google Scholar]
- 3.Chaushu S, Yefenof E, Becker A, Shapira J, Chaushu G. A link between parotid salivary Ig level and recurrent respiratory infections in young Down's syndrome patients. Oral Microbiol Immunol. 2002;17:172–176. doi: 10.1034/j.1399-302X.2002.170306.x. [DOI] [PubMed] [Google Scholar]
- 4.Akimoto T, Akama T, Sugiura K. Alteration of local immunity in the oral cavity after endurance running. Jpn J Phys Fitness Sport Med. 1998;47:53–62. [Google Scholar]
- 5.Roschel H, Barroso R, Batista M, Ugrinowitsch C, Tricoli V, Arsati F, et al. Do whole-body vibration exercise and resistance exercise modify concentrations of salivary cortisol and immunoglobulin A? Braz J Med Biol Res. 2011;44:592–597. doi: 10.1590/S0100-879X2011007500059. [DOI] [PubMed] [Google Scholar]
- 6.Lusa Cadore E, Lhullier FL, Arias Brentano M, Marczwski DaSilva E, Bueno Ambrosini M, Spinelli R, et al. Salivary hormonal responses to resistance exercise in trained and untrained middle-aged men. J Sports Med Phys Fitness. 2009;49:301–307. [PubMed] [Google Scholar]
- 7.Cowley PM, Ploutz-Snyder LL, Baynard T, Heffernan KS, Jae SY, Hsu S, et al. The effect of progressive resistance training on leg strength, aerobic capacity and functional tasks of daily living in persons with Down syndrome. Disabil Rehabil. 2011;33:2229–2236. doi: 10.3109/09638288.2011.563820. [DOI] [PubMed] [Google Scholar]
- 8.Peterson JJ, Janz KF, Lowe JB. Physical activity among adults with intellectual disabilities living in community settings. Prev Med. 2008;47:101–106. doi: 10.1016/j.ypmed.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Taylor JD, Fletcher JP. Reliability of the 8-repetition maximum test in men and women. J Sci Med Sport. 2012;15:69–73. doi: 10.1016/j.jsams.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Allgrove JE, Geneen L, Latif S, Gleeson M. Influence of a fed or fasted state on the s-IgA response to prolonged cycling in active men and women. Int J Sport Nutr Exerc Metab. 2009;19:209–221. doi: 10.1123/ijsnem.19.3.209. [DOI] [PubMed] [Google Scholar]
- 11.Smail K, Horvat M. Relationship of muscular strength on work performance in high school students with mental retardation. Educ Train Dev Disabil. 2006;41:410–419. [Google Scholar]
- 12.Martins RA, Cunha MR, Neves AP, Martins M, Teixeira-Verissimo M, Teixeira AM. Effects of aerobic conditioning on salivary IgA and plasma IgA, IgG and IgM in older men and women. Int J Sports Med. 2009;30:906–912. doi: 10.1055/s-0029-1237389. [DOI] [PubMed] [Google Scholar]
- 13.Lotan M. Quality physical intervention activity for persons with Down syndrome. ScientificWorldJournal. 2007;7:7–19. doi: 10.1100/tsw.2007.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai ML, Chou KM, Chang CK, Fang SH. Changes of mucosal immunity and antioxidation activity in elite male Taiwanese taekwondo athletes associated with intensive training and rapid weight loss. Br J Sports Med. 2011;45:729–734. doi: 10.1136/bjsm.2009.062497. [DOI] [PubMed] [Google Scholar]
- 15.Hayes LD, Grace FM, Sculthorpe N, Herbert P, Ratcliffe JW, Kilduff LP, et al. The effects of a formal exercise training programme on salivary hormone concentrations and body composition in previously sedentary aging men. Springerplus. 2013;2:18. doi: 10.1186/2193-1801-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cadore E, Lhullier F, Brentano M, Silva E, Ambrosini M, Spinelli R, et al. Correlations between serum and salivary hormonal concentrations in response to resistance exercise. J Sports Sci. 2008;26:1067–1072. doi: 10.1080/02640410801919526. [DOI] [PubMed] [Google Scholar]
- 17.Baillot A, Vibarel-Rebot N, Thomasson R, Jollin L, Amiot V, Emy P, et al. Serum and saliva adrenocortical hormones in obese diabetic men during submaximal exercise. Horm Metab Res. 2011;43:148–150. doi: 10.1055/s-0030-1265222. [DOI] [PubMed] [Google Scholar]
- 18.Shields N, Taylor NF, Dodd KJ. Effects of a community-based progressive resistance training program on muscle performance and physical function in adults with Down syndrome: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89:1215–1220. doi: 10.1016/j.apmr.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 19.Shields N, Taylor NF, Fernhall B. A study protocol of a randomised controlled trial to investigate if a community based strength training programme improves work task performance in young adults with Down syndrome. BMC Pediatr. 2010;10:17. doi: 10.1186/1471-2431-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]


