Abstract
The purpose of this study was to investigate the effect of supplementary vitamin D therapy in addition to amitriptyline on the frequency of migraine attacks in pediatric migraine patients. Fifty-three children 8-16 years of age and diagnosed with migraine following the International Headache Society 2005 definition, which includes childhood criteria, were enrolled. Patients were classified into four groups on the basis of their 25-hydroxyvitamin D [25(OH)D] levels. Group 1 had normal 25(OH)D levels and received amitriptyline therapy alone; group 2 had normal 25(OH)D levels and received vitamin D supplementation (400 IU/day) plus amitriptyline; group 3 had mildly deficient 25(OH)D levels and received amitriptyline plus vitamin D (800 IU/day); and group 4 had severely deficient 25(OH)D levels and was given amitriptyline plus vitamin D (5000 IU/day). All groups were monitored for 6 months, and the number of migraine attacks before and during treatment was determined. Calcium, phosphorus alkaline phosphatase, parathormone, and 25(OH)D levels were also determined before and during treatment. Results were compared between the groups. Data obtained from the groups were analyzed using one-way analysis of variance. The number of pretreatment attacks in groups 1 to 4 was 7±0.12, 6.8±0.2, 7.3±0.4, and 7.2±0.3 for 6 months, respectively (all P>0.05). The number of attacks during treatment was 3±0.25, 1.76±0.37 (P<0.05), 2.14±0.29 (P<0.05), and 1.15±0.15 (P<0.05), respectively. No statistically significant differences in calcium, phosphorus, alkaline phosphatase, or parathormone levels were observed (P>0.05). Vitamin D given in addition to anti-migraine treatment reduced the number of migraine attacks.
Keywords: Children, Migraine, Vitamin D
Introduction
Headache is one of the most common problems worldwide, and migraines represent a significant proportion of primary headaches. In childhood, migraine causes frequent, chronic, progressive, and recurring headache (1). There has been a significant increase in the prevalence of migraine in children over the last 20 years. Although its pathophysiology is still not yet fully understood, migraine is thought to be a neurovascular reaction to sudden changes in the external and internal environments (2). Cerebral blood flow in migraine attacks is regulated by serotonergic and aminergic signals, and cortical neuron stimulability can be affected. Neurogenic inflammation and vasodilation that occur following the effects of neurotransmitters on the trigeminovascular system play a role in pain development. The ability to identify factors that predispose to inflammation in migraine is important for both prophylaxis and strategies for treating acute attacks. The search for markers of biological disease and prognosis that can be used in migraine is ongoing (3,4).
While a large number of drugs can be used for prophylaxis and acute treatment in adults, the number of agents specifically indicated for pediatric patients is inadequate, and the use of alternative therapies is widespread (5).
Vitamin D is a steroid produced by the effect of ultraviolet light on 7-dehydrocholesterol in the skin. Up to 95% of vitamin D requirements is synthesized in the skin by exposure to sunlight. The effect of vitamin D takes place, as with other steroid hormones, either directly (over hours or days) by regulating gene transcription via nuclear vitamin D receptors (VDRs; genomic effect), or by altering calcium and chloride membrane transport via VDRs on the cell membrane (occurring in a shorter period), or by activating intracellular signal pathway activities (cAMP, PKA, PLC, PI-3 kinase, and MAP kinase; non-genomic effect). VDRs are also found in T lymphocytes in many organs and tissues, such as the brain, prostate, pancreas, gonads, breast tissue, colon, and muscles (6-10).
The effectiveness of vitamin D is not limited to the maintenance of bone health by regulating calcium homeostasis; it is also known to possess apoptotic, anti-inflammatory and immunomodulatory properties (7,8).
Studies have shown the presence of enzymes involved in the metabolism of vitamin D and VDRs in all regions of the brain. The 1,25-dihydroxyvitamin D3 receptor and the enzyme 1α-hydroxylase, which catalyzes the conversion of vitamin D into its active form, are present in both neurons and glial cells. While VDRs are present in the nucleus, 1α-hydroxylase is distributed in cytoplasm. This distribution suggests that vitamin D is a neurosteroid with autocrine/paracrine properties (11). Vitamin D has been shown in vitro to have a neuroprotective effect on brain cells. Dysregulation of the synthesis of several proteins involved in cellular processes in the brain develops in rats with vitamin D deficiency in the perinatal period. These dysregulated proteins have been reported to be possibly involved in the etiopathology of autism, depression, schizophrenia, and diseases affecting the central nervous system (CNS), such as multiple sclerosis (MS) (11-15).
The fact that vitamin D plays a role in the secretion of mediators in the CNS that are involved in the pathophysiology of migraine and the neuroprotective and antioxidant effects of vitamin D in the CNS suggest that vitamin D and migraine may be related (16). The few published studies in adults report that vitamin D supplementation reduces the frequency of migraine attacks (17,18). This study examined the effect of vitamin D in addition to amitriptyline therapy on the number of childhood migraine attacks.
Material and Methods
This prospective trial was conducted at the Department of Pediatric Neurology, Ataturk University, Erzurum, Turkey, between June 2011 and June 2012. The latitude of the city is 41°17′ E and this location is at a relatively high altitude in Turkey. Written informed consent was obtained from the parents of all children included during the first screening visit. The study was approved by the Ethics Committee of Ataturk University.
Only children with a history of migraine, and no other known neurological or psychiatric condition, and who had experienced at least five migraine attacks in the previous 3 months were included in the study. The migraine group consisted of 61 ambulatory children aged 8-16 years visiting the hospital's Department of Pediatric Neurology. There were no significant differences in terms of age or gender. Diagnosis of migraine (without aura) was based on the 2005 definition by the International Headache Society, which includes childhood criteria (19).
Of the 61 patients included at the start of the study, five were excluded for irregular checkup attendance and three for noncompliance with medication. All patients were given full physical and neurological examinations and were assessed by the same pediatric neurology specialist. The patients were given a structured interview concerning the characteristics of their headaches, associated symptoms, and medications used. Patients were also given baseline laboratory screening tests. A migraine checklist, including demographic and clinical details such as frequency, duration, and intensity of headache, was filed for each subject. The migraine disability assessment (MIDAS) questionnaire was used in all groups before and during treatment to assess the severity of symptoms (20). None of the patients had taken prophylactic medication or any other regular medication for at least 6 months prior to recruitment, or had any psychiatric disorder or neurological disorder including a form of headache other than migraine without aura.
Exclusion criteria consisted of chronic neurological and psychiatric problems (epilepsy, altered behavior and school performance, mental retardation, focal neurological deficits), neuroendocrine tumor, traumatic brain injuries, stroke, multisystemic trauma, chronic systemic disease, hypertension, anemia, other types of chronic headaches, and changes in behavior and school performance.
The study was carried out in three stages. In the first stage, patients diagnosed with migraine were enrolled. In the second stage, the included patients were divided into four groups based on their 25-hydroxyvitamin D [25(OH)D] levels as described below, and their treatment protocols were established. In the final stage, patients were invited to monthly checkups for 6 months, when general examinations were performed and data concerning drug use and number of attacks were recorded. Patients not using the amitriptyline medication and/or vitamin D supplementation given throughout the study or failing to attend the regular checkups were excluded. All patients were started on amitriptyline therapy (1 mg/kg); the efficacy of it for treating childhood migraine has already been demonstrated, in accordance with the literature (21).
Serum 25(OH)D levels above 20 ng/mL were regarded as normal, 15-20 ng/mL were regarded as vitamin D insufficiency, and levels below 15 ng/mL were regarded as vitamin D deficiency (8,22,23). Patients were given oral vitamin D, in a manner compatible with the literature based on their vitamin D levels, in addition to anti-migraine treatment (8,22,23).
Group 1 patients (11.8±0.58 years old; 10M/3F) had normal 25(OH)D levels and received only amitriptyline therapy. Group 2 patients (12±0.69 years old; 8M/F5) had normal 25(OH)D levels and received vitamin D 400 IU/day plus amitriptyline. Group 3 patients (12.7±0.62 years old; 9M/5F) had mild 25(OH)D deficiency and received vitamin D 800 IU/day plus amitriptyline. Group 4 patients (12.3±0.52 years old; 6M/7F) had severe 25(OH)D deficiency and received vitamin D 5000 IU/day plus amitriptyline. Patients were monitored for 6 months after commencement of treatment.
The total number of attacks throughout the course of the study, 25(OH)D, parathormone (PTH), calcium (Ca), phosphorus (P), and alkaline phosphatase (ALP) levels at the beginning and end of the study were determined separately for each group and were compared only with group 1, the amitriptyline group.
Biochemical analysis
In accordance with laboratory reference values, normal serum values were defined as 15-65 pg/mL for PTH, 8.8-10.8 mg/dL for Ca, 2.8-6.0 mg/dL for P, and 75-400 U/L for ALP. Blood samples were collected from patients between 8:00 and 10:00 am after 12-h fasting to avoid diurnal variations. All blood samples were stored at -80°C until analysis. All tests were performed according to the manufacturers' instructions. 25(OH)D levels (ng/mL) were determined by electrochemiluminescence (E-170 system, Roche, Japan), and PTH (pg/mL) was measured by a chemiluminescent enzyme immunoassay using an Immulite autoanalyzer (DPC Co., USA). Serum Ca, P, and ALP levels were determined using a Roche Cobas 8000 modular analyzer system and Roche Diagnostics kits.
Statistical analyses
The Pearson chi-square test and one-way analysis of variance were used to establish the significance of study parameters between groups of samples. The post hoc least significant difference test was used to evaluate pairwise differences using the Statistical Package for Social Sciences 18.0 (SSPS, USA) software. Significance was set at P≤0.05. Results are reported as means±SE.
Results
25(OH)D levels at the beginning of the study in groups 1 to 4 were 32.4±2, 28.1±1.8, 17.2±0.3, and 10.9±0.6 ng/mL, respectively (all P<0.05). At the end of the study, only groups 3 and 4 were significantly different (25.6±1.1 and 22.3±1.9 ng/mL, respectively) compared to the beginning of the study (P<0.05; Table 1). There were no significant differences in the levels of PTH, Ca, P, or ALP comparing the beginning to the end of the study in any group (P>0.05; Table 1).
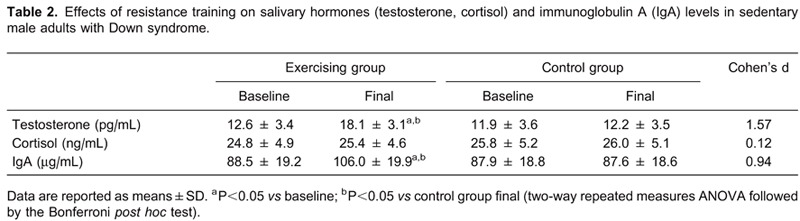
Pairwise comparisons of the number of migraine attacks before and after therapy in the groups receiving vitamin D supplementation with the group not receiving vitamin D support (group 1) are shown in Table 2.
Discussion
Vitamin D deficiency is a widespread public health problem. It has been shown to be associated with musculoskeletal disorders, various malignant diseases, autoimmune diseases, cardiovascular diseases, and skin disorders (6-8).
In the present study, there was a significant decrease in migraine attacks in the groups starting treatment with a diagnosis of migraine and receiving vitamin D compared with the group receiving migraine treatment alone. Vitamin D is known to have a neuroprotective effect on the CNS. It manifests this effect through its antioxidative mechanism, neuronal calcium modulation, and detoxification mechanisms (11,24). There have been several studies of the association between vitamin D and MS in particular. A correlation was established with vitamin D since MS demonstrates geographical and seasonal variation, and this was confirmed when a decrease in the incidence of MS was seen following vitamin D supplementation (15,25). Vitamin D has been shown to be associated with chronic pain. Levels of the neuroexcitator mediator, substance P, calcitonin gene-related peptide (GCRP), and nitric oxide (NO) increase in chronic pain and migraine attacks (26,27). A decrease in these mediators involved in oxidative stress has been shown to take place with vitamin D therapy. Lomerizine, one of a new group of drugs claimed to be effective for use in migraine prophylaxis, acts by reducing oxidative stress (27-29).
Vitamin D deficiency is known to be associated with interleukin (IL)-1, IL-6, tumor necrosis factor, and NO production. It also plays a role in mast cell development. These facts support the idea of using the anti-inflammatory effect of vitamin D in inflammatory diseases such as migraine, where mast cells contribute to the pathophysiology. Some studies have reported an increase in migraine attacks in children in fall and winter, or a seasonal variation in vitamin D parallel to an increase in migraine attacks, and decreased levels in winter, in particular. Prevalence of both migraines and vitamin D deficiency increases at high altitudes far from the equator (30-34).
The prevalence of childhood migraine in Turkey is 10% in regions with a low altitude, but more than 20% in high-altitude regions (35,36). Our patients lived in the highest altitudes of Turkey. There is a known powerful correlation between vitamin D deficiency and altitude. Prakash et al. (32) demonstrated a correlation between altitude and headache. Yang et al. (33) also suggested a potential correlation between headache and vitamin D deficiency. Motaghi et al. (37) showed that VDR gene polymorphisms are associated with migraine patients. Kjaergaard et al. (38) found an association between headache and vitamin D, but did not determine a correlation between migraine and vitamin D.
We think that by exerting a similar effect, vitamin D can become a treatment option for migraine. Since not all patients benefited, even though a considerable decrease in attack frequency was determined with treatment, the effect of vitamin D may be dose-dependent. The administration of vitamin D in higher doses, as in MS and postherpetic neuralgia, may increase the effectiveness of treatment (39,40).
The small number of patients in the treatment and control groups, the short observation period, the fact that 25(OH)D levels were not compared between patients diagnosed with migraine and a healthy control group, 25(OH)D levels not being investigated over all four seasons, and the fact that patients' dietary habits were not examined represent study limitations.
In conclusion, there was a correlation between vitamin D and migraine. Thus, vitamin D therapy represents grounds for hope in the treatment of migraine. In order for the use of vitamin D in migraine to be conclusively established, the optimal dose of vitamin D must be determined. For this relationship to be corroborated, further multicenter studies with large numbers of patients and controlling altitude and seasonal differences should be performed.
Footnotes
First published online April 7, 2014.
References
- 1.Lewis DW. Pediatric migraine. Neurol Clin. 2009;27:481–501. doi: 10.1016/j.ncl.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Teepker M, Munk K, Mylius V, Haag A, Moller JC, Oertel WH, et al. Serum concentrations of s100b and NSE in migraine. Headache. 2009;49:245–252. doi: 10.1111/j.1526-4610.2008.01228.x. [DOI] [PubMed] [Google Scholar]
- 3.Goadsby PJ. Pathophysiology of migraine. Neurol Clin. 2009;27:335–360. doi: 10.1016/j.ncl.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Kesby JP, Cui X, Ko P, McGrath JJ, Burne TH, Eyles DW. Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain. Neurosci Lett. 2009;461:155–158. doi: 10.1016/j.neulet.2009.05.070. [DOI] [PubMed] [Google Scholar]
- 5.Esposito M, Ruberto M, Pascotto A, Carotenuto M. Nutraceutical preparations in childhood migraine prophylaxis: effects on headache outcomes including disability and behaviour. Neurol Sci. 2012;33:1365–1368. doi: 10.1007/s10072-012-1019-8. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D: extraskeletal health. Endocrinol Metab Clin North Am. 2010;39:381–400, table. doi: 10.1016/j.ecl.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozkan B. Nutritional rickets. J Clin Res Pediatr Endocrinol. 2010;2:137–143. doi: 10.4274/jcrpe.v2i4.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mutlu A, Mutlu GY, Ozsu E, Cizmecioglu FM, Hatun S. Vitamin D deficiency in children and adolescents with type 1 diabetes. J Clin Res Pediatr Endocrinol. 2011;3:179–183. doi: 10.4274/jcrpe.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cizmecioglu FM, Etiler N, Gormus U, Hamzaoglu O, Hatun S. Hypovitaminosis D in obese and overweight schoolchildren. J Clin Res Pediatr Endocrinol. 2008;1:89–96. doi: 10.4008/jcrpe.v1i2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Balden R, Selvamani A, Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology. 2012;153:2420–2435. doi: 10.1210/en.2011-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGrath J, Eyles D, Mowry B, Yolken R, Buka S. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003;63:73–78. doi: 10.1016/S0920-9964(02)00435-8. [DOI] [PubMed] [Google Scholar]
- 14.Oudshoorn C, Mattace-Raso FU, van der Velde N, Colin EM, van der Cammen TJ. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2008;25:539–543. doi: 10.1159/000134382. [DOI] [PubMed] [Google Scholar]
- 15.Soilu-Hanninen M, Airas L, Mononen I, Heikkila A, Viljanen M, Hanninen A. 25-Hydroxyvitamin D levels in serum at the onset of multiple sclerosis. Mult Scler. 2005;11:266–271. doi: 10.1191/1352458505ms1157oa. [DOI] [PubMed] [Google Scholar]
- 16.Molinari C, Uberti F, Grossini E, Vacca G, Carda S, Invernizzi M, et al. 1α,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell Physiol Biochem. 2011;27:661–668. doi: 10.1159/000330075. [DOI] [PubMed] [Google Scholar]
- 17.Thys-Jacobs S. Vitamin D and calcium in menstrual migraine. Headache. 1994;34:544–546. doi: 10.1111/j.1526-4610.1994.hed3409544.x. [DOI] [PubMed] [Google Scholar]
- 18.Thys-Jacobs S. Alleviation of migraines with therapeutic vitamin D and calcium. Headache. 1994;34:590–592. doi: 10.1111/j.1526-4610.1994.hed3410590.x. [DOI] [PubMed] [Google Scholar]
- 19.Olesen J. The international classification of headache disorders. 2nd edition (ICHD-II) Rev Neurol. 2005;161:689–691. doi: 10.1016/S0035-3787(05)85119-7. [DOI] [PubMed] [Google Scholar]
- 20.Lipton RB, Stewart WF, Sawyer J, Edmeads JG. Clinical utility of an instrument assessing migraine disability: the Migraine Disability Assessment (MIDAS) questionnaire. Headache. 2001;41:854–861. doi: 10.1046/j.1526-4610.2001.01156.x. [DOI] [PubMed] [Google Scholar]
- 21.Hershey AD, Powers SW, Coffey CS, Eklund DD, Chamberlin LA, Korbee LL. Childhood and Adolescent Migraine Prevention (CHAMP) study: a double-blinded, placebo-controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo in the prevention of childhood and adolescent migraine. Headache. 2013;53:799–816. doi: 10.1111/head.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 23.Andiran N, Celik N, Akca H, Dogan G. Vitamin D deficiency in children and adolescents. J Clin Res Pediatr Endocrinol. 2012;4:25–29. doi: 10.4274/jcrpe.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med. 2008;29:415–422. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol. 2003;134:128–132. doi: 10.1016/S0165-5728(02)00396-X. [DOI] [PubMed] [Google Scholar]
- 26.Kragstrup TW. Vitamin D supplementation for patients with chronic pain. Scand J Prim Health Care. 2011;29:4–5. doi: 10.3109/02813432.2011.584717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goncalves FM, Martins-Oliveira A, Speciali JG, Luizon MR, Izidoro-Toledo TC, Silva PS, et al. Endothelial nitric oxide synthase haplotypes associated with aura in patients with migraine. DNA Cell Biol. 2011;30:363–369. doi: 10.1089/dna.2010.1152. [DOI] [PubMed] [Google Scholar]
- 28.Ishii M, Iizuka R, Kiuchi Y, Mori Y, Shimizu S. Neuroprotection by lomerizine, a prophylactic drug for migraine, against hydrogen peroxide-induced hippocampal neurotoxicity. Mol Cell Biochem. 2011;358:1–11. doi: 10.1007/s11010-011-0913-3. [DOI] [PubMed] [Google Scholar]
- 29.Nair-Shalliker V, Armstrong BK, Fenech M. Does vitamin D protect against DNA damage? Mutat Res. 2012;733:50–57. doi: 10.1016/j.mrfmmm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Arantes HP, Kulak CA, Fernandes CE, Zerbini C, Bandeira F, Barbosa IC, et al. Correlation between 25-hydroxyvitamin D levels and latitude in Brazilian postmenopausal women: from the Arzoxifene Generations Trial. Osteoporos Int. 2013;24:2707–2712. doi: 10.1007/s00198-013-2366-x. [DOI] [PubMed] [Google Scholar]
- 31.Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008;9:107–118. doi: 10.1517/14656566.9.1.107. [DOI] [PubMed] [Google Scholar]
- 32.Prakash S, Mehta NC, Dabhi AS, Lakhani O, Khilari M, Shah ND. The prevalence of headache may be related with the latitude: a possible role of vitamin D insufficiency? J Headache Pain. 2010;11:301–307. doi: 10.1007/s10194-010-0223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Zhang HL, Wu J. Is headache related with vitamin D insufficiency? J Headache Pain. 2010;11:369. doi: 10.1007/s10194-010-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soriani S, Fiumana E, Manfredini R, Boari B, Battistella PA, Canetta E, et al. Circadian and seasonal variation of migraine attacks in children. Headache. 2006;46:1571–1574. doi: 10.1111/j.1526-4610.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- 35.Celik Y, Ekuklu G, Tokuc B, Utku U. Migraine prevalence and some related factors in Turkey. Headache. 2005;45:32–36. doi: 10.1111/j.1526-4610.2005.05007.x. [DOI] [PubMed] [Google Scholar]
- 36.Kececi H, Dener S. Epidemiological and clinical characteristics of migraine in Sivas, Turkey. Headache. 2002;42:275–280. doi: 10.1046/j.1526-4610.2002.02080.x. [DOI] [PubMed] [Google Scholar]
- 37.Motaghi M, Haghjooy JS, Haghdoost F, Tajadini M, Saadatnia M, Rafiee L, et al. Relationship between vitamin D receptor gene polymorphisms and migraine without aura in an Iranian population. Biomed Res Int. 2013;2013: doi: 10.1155/2013/351942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kjaergaard M, Eggen AE, Mathiesen EB, Jorde R. Association between headache and serum 25-hydroxyvitamin D; the Tromso Study: Tromso 6. Headache. 2012;52:1499–1505. doi: 10.1111/j.1526-4610.2012.02250.x. [DOI] [PubMed] [Google Scholar]
- 39.Bartley J. Post herpetic neuralgia, schwann cell activation and vitamin D. Med Hypotheses. 2009;73:927–929. doi: 10.1016/j.mehy.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 40.Simpson S, Jr, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68:193–203. doi: 10.1002/ana.22043. [DOI] [PubMed] [Google Scholar]


