Abstract
Plant glutamate receptor-like genes (GLRs) are homologous to the genes for mammalian ionotropic glutamate receptors (iGluRs), after which they were named, but in the 16 years since their existence was first revealed, progress in elucidating their biological role has been disappointingly slow. Recently, however, studies from a number of laboratories focusing on the model plant species Arabidopsis thaliana (L.) have thrown new light on the functional properties of some members of the GLR gene family. One important finding has been that plant GLR receptors have a much broader ligand specificity than their mammalian iGluR counterparts, with evidence that some individual GLR receptors can be gated by as many as seven amino acids. These results, together with the ubiquity of their expression throughout the plant, open up the possibility that GLR receptors could have a pervasive role in plants as non-specific amino acid sensors in diverse biological processes. Addressing what one of these roles could be, recent studies examining the wound response and disease susceptibility in GLR knockout mutants have provided evidence that some members of clade 3 of the GLR gene family encode important components of the plant's defence response. Ways in which this family of amino acid receptors might contribute to the plant's ability to respond to an attack from pests and pathogens are discussed.
Introduction
iGluRs are ligand-gated ion channels best known for their role in fast excitatory neurotransmission in the mammalian central nervous system. Therefore, the discovery in 1998 that plants have a large family of iGluR-like genes (the GLR genes) presented something of an enigma [1]. What could this gene family be doing in an organism with no nervous system? Despite the excitement that their discovery provoked, GLR genes have been slow to relinquish their secrets, and it is only very recently that efforts to understand their biological functions have really begun to bear fruit. The structure and evolutionary origins of the plant GLRs have been the subject of two detailed reviews [2,3], and here we focus only on the most recent advances. Amongst the most important recent findings are that plant GLRs appear to have a much broader ligand specificity than their mammalian homologues and that some members of the GLR family in Arabidopsis have a role in the innate immune response.
Ligand promiscuity of the plant glutamate-like receptors
Once the Arabidopsis thaliana genome had been fully sequenced, it was found that this model plant species has 20 GLR genes (Arabidopsis thaliana glutamate-like receptor [AtGLRs]) that can be grouped into three clades [4]. The plant GLRs are predicted to have the same modular structure as their mammalian homologues, with an amino-terminal domain, a ligand-binding domain, a transmembrane domain that includes the pore region, and a carboxyl-terminal domain [4]. Functionally, mammalian iGluRs are glutamate-gated cation channels, selective for Na+, K+, and Ca2+ ions. Aspartate is also an iGluR agonist, but a weak one, and glycine and D-serine act as co-agonists with glutamate for some iGluRs [5].
In contrast to the ligand specificity of the iGluRs, in planta studies using GLR knockout mutants [6-8] recently supported by heterologous expression experiments [9,10] indicate that, collectively, the GLR receptors in Arabidopsis are gated by a broad spectrum of amino acids. The data for the three AtGLR genes that have been studied in most detail (summarised in Table 1) suggest that at least 12 of the 20 proteinogenic amino acids, as well as the tripeptide glutathione (GSH), can serve as GLR agonists. Although it is possible that the phenotype of GLR knockout mutants could be influenced by pleiotropic effects on other channels, the heterologous expression experiments found that AtGLR1.4 was gated by seven amino acids and AtGLR3.4 by three (different) amino acids [9,10]. Significantly, glutamate was not amongst the group of agonists identified for either AtGLR1.4 or AtGLR3.4 in these experiments. The heterologous expression studies also found that AtGLR1.4, like mammalian iGluRs, acted as a non-selective, Ca2+-permeable cation channel [9] but that AtGLR3.4 was highly selective for Ca2+ over Na+ [10]. These findings are consistent with in planta evidence that glutamate and other amino acids trigger membrane depolarisation and Ca2+ influx in a GLR-dependent manner (reviewed in [3]).
Table 1. Summary of data indicating the broad ligand specificity of products of the Arabidopsis GLR gene family.
| GLR gene | Assay system | Agonist(s) | Non-agonist(s) | Reference |
|---|---|---|---|---|
| AtGLR1.4 | Xenopus oocytes/ patch clamp |
Met, Trp, Phe, Leu, Tyr, Asn, Thr | Other proteinogenic amino acids | [9] |
| AtGLR1.4 | Knockout mutant/ cotyledons/ membrane potential measurements |
Met | L-Glu | [9] |
| AtGLR3.3 | Knockout mutant/roots/ membrane potential measurements |
GSH, L-Ala, Asn, Cys, L-Glu, Gly, L-Ser | Other proteinogenic amino acids, D-Glu, D-Ser, D-Ala, GABA, NMDA | [6] |
| AtGLR3.3/AtGLR3.4 | Knockout mutants/ hypocotyls/ membrane potential measurements |
L-Glu, L-Ala, Asn, Cys, Gly, L-Ser | Other proteinogenic amino acids | [7] |
| AtGLR3.4 | HEK293 cells/ patch clamp |
Asn, L-Ser, Gly | L-Glu, L-Ala, Cys, Phe | [10] |
GABA, gamma-aminobutyric acid; GLR, glutamate-like receptor; GSH, glutathione (reduced); NMDA, N-methyl D-aspartate.
On the basis of the homology modelling of their ligand-binding domains, it has been concluded that all members of the AtGLR family are likely to be gated by amino acid residues and that the broad ligand specificity observed experimentally is consistent with the high degree of sequence diversity found within the region of the ligand-binding domain that is predicted to bind the agonist side chain [9]. Therefore, even though only a few of the 20 AtGLRs have so far been characterised, it seems safe to conclude that collectively the plant GLR family is likely to be gated by a significant proportion of the 20 proteinogenic amino acids as well as by an unknown number of related molecules, such as small peptides like GSH. In the N-methyl D-aspartate (NMDA) subgroup of mammalian iGluRs (the subgroup most closely related to plant GLRs), the amino-terminal domain contains sequences related to the bacterial periplasmic-binding domain that binds a variety of small molecules and ions and that plays an important role in both negative and positive allosteric regulation of the receptor [11]. Plant GLRs possess a similar conserved sequence in their amino-terminal domain [12,13], suggesting the potential for additional sensory complexity arising from allosteric interactions between this domain and as-yet-unknown regulatory molecules.
Role of plant glutamate-like receptors in the immune response
The first indication that GLRs might have a role in the plant defence response came from the finding that transgenic Arabidopsis plants overexpressing a radish GLR complementary DNA (RsGluR) showed an increased expression of a number of defence-related genes and enhanced the resistance to a necrotic fungal pathogen (Botrytis cinerea) [14]. Two later pharmacological studies reported that antagonists of mammalian iGluRs were able to interfere with aspects of the immune response in tobacco suspension culture cells [15] and Arabidopsis seedlings [16]. These early findings have now been backed up by genetic evidence from the study of a number of AtGLR knockout mutants [17,18]. Analysis of the immune response in atglr3.3 mutants found increased susceptibility to the bacterial pathogen Pseudomonas syringae, which was correlated with a defect in the activation of defence gene expression in response to infection [17]. The same study demonstrated that the abilities of GSH to activate defence gene expression and to enhance the immunity to P. syringae were also dependent on AtGLR3.3. This aspect of the disease resistance phenotype was specific to a small subset of AtGLR3.3 agonists, since of the ligands able to elicit AtGLR3.3-dependent membrane depolarisation in roots [6], only GSH and cysteine were able to suppress bacterial growth in an AtGLR3.3-dependent manner [17]. In an independent study, AtGLR3.3 was also found to be required for basal resistance to downy mildew disease, caused by the oomycete pathogen, Hyaloperonospora arabidopsidis [18]. Again, atglr3.3 mutants failed to activate defence gene expression in response to infection. Both P. syringae and H. arabidopsidis are biotrophic pathogens (they do not directly kill plant tissues), but when atglr3.3 mutants were infected with the necrotrophic fungal pathogen Botrytis cinerea, no difference in resistance was detected [18]. This may suggest that AtGLR3.3 is required for the full activation of salicylic acid-dependent plant defences, which are typically associated with resistance against biotrophic, but not necrotrophic, pathogens. So far, all GLR genes implicated in the defence response (including RsGluR) belong to clade 3 of the GLR family. The finding that mutations in four other clade 3 GLRs (AtGLR3.1, AtGLR3.4, AtGLR3.5, and AtGLR3.7) had no impact on the resistance to either H. arabidopsidis or B. cinerea [18] suggests either that roles in pathogen resistance are restricted to a subset of clade 3 GLRs or that there is functional redundancy amongst some of these AtGLR genes.
Basal resistance to virulent pathogens, such as P. syringae and H. arabidopsidis, is conferred principally by so-called PAMP (pathogen-associated molecular patterns)-triggered immunity (PTI), which is activated by the recognition of PAMPs [19]. iGluR-related receptors were previously suggested to have a role in PAMP-mediated signaling in Arabidopsis, based on the ability of the antagonists of mammalian iGluRs to block cytoplasmic Ca2+ transients triggered by two common PAMPs perceived by plants: the peptides flg22 and efl18 [16]. Although the specificity and precise targets of iGluR antagonists in plants are still unclear, these findings suggest the possibility that plant GLRs mediate PTI by acting as Ca2+ channels downstream of PAMP perception by pattern-recognition receptors (PRRs).
AtGLR3.3 has also been implicated in the defence response to mechanical wounding [20], which has many features in common with the defence response to feeding by insects and related pests [21]. Wounding and herbivore feeding elicit responses both in the injured leaf and, systemically, in undamaged parts of the plant. Several long-distance signals, including chemical, hydraulic, and electrical signals, have previously been suggested to carry information to systemic leaves [21]. The concept of electrical signalling in plants often generates much interest but with the exception of a few specific responses, such as rapid movements in plants such as the Venus flytrap [22], it is relatively poorly defined. However, Mousavi and colleagues identified several GLRs from amongst a panel of membrane transporters as proteins required for systemic transmission of a wound-induced electrical signal in Arabidopsis [20]. Importantly, systemic wound-induced gene expression was tightly correlated with electrical signal transmission, and both responses were eliminated in atglr3.3 atglr3.6 double mutants. Although the wound-induced electrical signal moves as a wave of plasma membrane depolarisation [20], which would be consistent with GLR activity as an inward cation channel, it has yet to be established whether GLRs are directly responsible for the propagation of the electrical signal, or whether they act indirectly, in the upstream signalling process that generates the initial signal. The data supporting the role of GLRs in plant defence responses are summarised in Table 2.
Table 2. Summary of data supporting a role for Arabidopsis GLR genes in the defense response.
| Gene(s) | Manipulation | Effect on disease resistance |
Other effects on the defense response |
Reference |
|---|---|---|---|---|
| RsGluR | Constitutive overexpression in Arabidopsis (35S promoter) | Increased basal resistance to Botrytis cinerea | Increased expression of defense-related genes (e.g. defensins, jasmonic acid biosynthetic genes) | [14] |
| AtGLR3.3 | Knockout mutant | Increased susceptibility to Pseudomonas syringae | Attenuation of P. syringae- elicited induction of defence-related genes Loss of ability of GSH pre-treatment to confer resistance to P. syringae |
[17] |
| AtGLR3.3 | Knockout mutant | Increased susceptibility to Hyaloperonospora arabidopsidis; No effect on susceptibility to B. cinerea |
Attenuation of oligogalacturonide-elicited increase in reactive oxygen species (ROS) and nitric oxide (NO) production; Attenuation of oligogalacturonide-elicited and H. arabidopsidis-elicited effects on defence gene expression |
[18] |
| AtGLR3.3, AtGLR3.4, AtGLR3.6 | Knockout mutants | Not tested | Attenuation of wound- induced surface potential changes; Attenuation of long-distance wound-stimulated expression of JAZ (jasmonate-signalling) genes |
[20] |
GSH, glutathione (reduced).
Concluding remarks
Had the plant GLR gene family been characterised before their mammalian iGluR homologues, it now seems likely they would have been referred to as general or non-specific amino acid receptors rather than glutamate receptors. In the breadth of their ligand specificity, they are analogous to other general amino acid sensors like the yeast Ssy1p transceptor [23] and some members of the mammalian Ca2+-sensing receptor superfamily [24]. The ubiquity of their expression in the plant [2,25] and their potential localisation on both the plasma membrane and the chloroplast inner membrane [9,26,27] place the GLRs in an ideal location to serve multiple roles in sensing amino acids (and related molecules) endogenously, intercellularly, and in the soil environment. In their potential role as sensors of both internal and environmental chemical cues, they resemble the recently uncovered family of ionotropic receptors (IRs) in Drosophila, which is a variant subfamily of the iGluRs that function as odorant and taste receptors for a diverse range of molecules [28]. Root growth and branching are known to be sensitive to signals from external glutamate [29] and many biological processes in plants are potentially regulated by amino acid signalling [30].
At least in the case of AtGLR3.3 and perhaps other members of GLR clade 3, there is now strong evidence for a role in the innate immune response but what might this role be? Like animals, plants use conserved molecules of their enemies as PAMPs (or HAMPs in the case of herbivore-associated molecular patterns) to stimulate defence responses [19,31], but it is becoming increasingly clear that they also use certain molecules released by their own damaged cells (called damage-associated molecular patterns, or DAMPs) for the recognition of “damaged-self” [32]. It has been hypothesised that these DAMPs can act in concert with PAMPs to help the host differentiate between beneficial or harmless microbes and those that are causing pathological damage [32]. For example, extracellular ATP released from damaged cells is sensed by neighbouring intact cells to stimulate defence responses in both animals and plants [33,34]. It is possible that the ability of AtGLR3.3 and other clade 3 GLRs to act as sensors of changes in the extracellular amino acid concentration provides an important additional signal to confirm that tissue damage has taken place (e.g. after wounding or herbivore activity). In the case of pathogen attack, increased concentrations of extracellular amino acids have been found in infected tissues [35], and PAMP-induced exocytosis of glutamate has been reported in tobacco cells [14].
In Figure 1, we present a speculative model that attempts to integrate the evidence that GLRs have a role in plant defence, with what is known of other pathways by which plants activate their defence responses. The model incorporates the idea that GLR ligands might in some cases function as DAMPs and suggests that the sensing of different combinations of host- and non-host-derived cues by GLRs and other receptors may be integrated to differentially regulate alternative plant defence pathways. In the model, jasmonic acid-dependent defences are activated by increased amino acid concentrations (perceived by the GLR receptors [20]), by increased extracellular ATP (eATP) concentrations (perceived by the newly identified DORN1 [does not respond to nucleotides 1] eATP receptor [36]), and by specific herbivore-derived elicitors (HAMPs) perceived by PRRs [31]. Salicylic acid-mediated defence activated following perception of PAMPs by their cognate PRRs, may also be partly dependent on GLR signalling activated by pathogen-induced increases in apoplastic amino acid concentrations [15,35], and on DORN1 signalling in response to reduced levels of eATP [37].
Figure 1. Speculative model for the role of glutamate-like receptors in the regulation of plant defence responses.
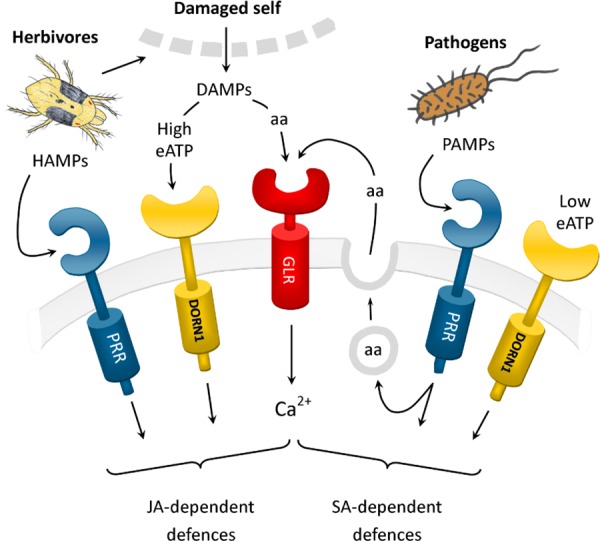
In this model, glutamate-like receptors (GLRs) are acting as amino acid-gated Ca2+ channels to perceive changes in apoplastic amino acid concentrations resulting either from cell damage or from PAMP-induced exocytosis [15,35]. The model shows them acting in parallel with other receptors (such as DORN1 [does not respond to nucleotides 1] and pattern-recognition receptor [PRRs]) to activate jasmonic acid (JA)-dependent defences (in the case of herbivore attack) or salicylic acid (SA)-mediated defences (in the case of pathogen attack). See text for further details.
Establishing whether amino acids (and other potential GLR ligands) do indeed function as DAMPs will require a much better understanding of the positioning of GLRs in defence signalling pathways and, more importantly, a clearer definition of which of the potential ligands are biologically relevant for defence responses. Given the ligand promiscuity of plant GLRs, the number of candidates for the biologically relevant ligands may be very large.
Finally, it is worth noting that in the intervening years since the GLRs were discovered in plants, the presence of iGluRs in a variety of cell types beyond the synapse has been recognised (even if their functions in those cells are not necessarily very clear) [38], so that even in mammals, iGluRs are no longer thought of as strictly being associated with the nervous system. Perhaps significantly, in the context of the new research on plant GLRs discussed here, iGluRs are abundant on the surface of T cells and other cells of the mammalian immune system and glutamate has been identified as an important immunomodulator [39,40]. Given the ancient evolutionary origins of the innate immune system and the homologies that exist between some of its key components in plants and animals [41], perhaps parallels will yet be found between the roles of iGluRs and GLRs in their respective defence response systems.
Acknowledgments
Research on plant defence signalling in Michael R. Roberts' lab is supported by the Biotechnology and Biological Sciences Research Council grant BB/L008939/1.
Abbreviations
- AtGLR
Arabidopsis thaliana glutamate-like receptor
- DAMP
damage-associated molecular pattern
- DORN1
does not respond to nucleotides 1
- eATP
extracellular ATP
- GLR
glutamate-like receptor
- GSH
glutathione (reduced)
- HAMP
herbivore-associated molecular pattern
- iGluR
ionotropic glutamate receptor
- PAMP
pathogen-associated molecular pattern
- PRR
pattern-recognition receptor
- PTI
PAMP-triggered immunity
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/06/37
References
- 1.Lam HM, Chiu J, Hsieh MH, Meisel L, Oliveira IC, Shin M, Coruzzi G. Glutamate-receptor genes in plants. Nature. 1998;396:125–6. doi: 10.1038/24066. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718385998
- 2.Dietrich P, Anschütz U, Kugler A, Becker D. Physiology and biophysics of plant ligand-gated ion channels. Plant Biol (Stuttg) 2010;12(Suppl 1):80–93. doi: 10.1111/j.1438-8677.2010.00362.x. [DOI] [PubMed] [Google Scholar]
- 3.Price MB, Jelesko J, Okumoto S. Glutamate receptor homologs in plants: functions and evolutionary origins. Front Plant Sci. 2012;3:235. doi: 10.3389/fpls.2012.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davenport R. Glutamate receptors in plants. Annals of Botany. 2002;90:549–57. doi: 10.1093/aob/mcf228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores-Soto ME, Chaparro-Huerta V, Escoto-Delgadillo M, Vazquez-Valls E, González-Castañeda RE, Beas-Zarate C. Structure and function of NMDA-type glutamate receptor subunits. Neurologia. 2012;27:301–10. doi: 10.1016/j.nrl.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Qi Z, Stephens NR, Spalding EP. Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol. 2006;142:963–71. doi: 10.1104/pp.106.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718386000
- 7.Stephens NR, Qi Z, Spalding EP. Glutamate receptor subtypes evidenced by differences in desensitization and dependence on the GLR3.3 and GLR3.4 genes. Plant Physiol. 2008;146:529–38. doi: 10.1104/pp.107.108134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu L, Obermeyer G, Feijó JA. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science. 2011;332:434–7. doi: 10.1126/science.1201101. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/10038956
- 9.Tapken D, Anschütz U, Liu L, Huelsken T, Seebohm G, Becker D, Hollmann M. A plant homolog of animal glutamate receptors is an ion channel gated by multiple hydrophobic amino acids. Sci Signal. 2013;6:ra47. doi: 10.1126/scisignal.2003762. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718018894
- 10.Vincill ED, Bieck AM, Spalding EP. Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiol. 2012;159:40–6. doi: 10.1104/pp.112.197509. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/715898018
- 11.Kumar J, Mayer ML. Functional insights from glutamate receptor ion channel structures. Annu Rev Physiol. 2013;75:313–37. doi: 10.1146/annurev-physiol-030212-183711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acher FC, Bertrand H. Amino acid recognition by Venus flytrap domains is encoded in an 8-residue motif. Biopolymers. 2005;80:357–66. doi: 10.1002/bip.20229. [DOI] [PubMed] [Google Scholar]
- 13.Turano FJ, Panta GR, Allard MW, van Berkum P. The putative glutamate receptors from plants are related to two superfamilies of animal neurotransmitter receptors via distinct evolutionary mechanisms. Mol Biol Evol. 2001;18:1417–20. doi: 10.1093/oxfordjournals.molbev.a003926. [DOI] [PubMed] [Google Scholar]
- 14.Kang S, Kim HB, Lee H, Choi JY, Heu S, Oh CJ, Kwon SI, An CS. Overexpression in Arabidopsis of a plasma membrane-targeting glutamate receptor from small radish increases glutamate-mediated Ca2+ influx and delays fungal infection. Mol Cells. 2006;21:418–27. [PubMed] [Google Scholar]; http://f1000.com/prime/718386015
- 15.Vatsa P, Chiltz A, Bourque S, Wendehenne D, Garcia-Brugger A, Pugin A. Involvement of putative glutamate receptors in plant defence signaling and NO production. Biochimie. 2011;93:2095–101. doi: 10.1016/j.biochi.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Kwaaitaal M, Huisman R, Maintz J, Reinstädler A, Panstruga R. Ionotropic glutamate receptor (iGluR)-like channels mediate MAMP-induced calcium influx in Arabidopsis thaliana. Biochem J. 2011;440:355–65. doi: 10.1042/BJ20111112. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Wang J, Ma C, Zhao Y, Wang Y, Hasi A, Qi Z. Glutamate receptor-like channel3.3 is involved in mediating glutathione-triggered cytosolic calcium transients, transcriptional changes, and innate immunity responses in Arabidopsis. Plant Physiol. 2013;162:1497–509. doi: 10.1104/pp.113.217208. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718008788
- 18.Manzoor H, Kelloniemi J, Chiltz A, Wendehenne D, Pugin A, Poinssot B, Garcia-Brugger A. Involvement of the glutamate receptor AtGLR3.3 in plant defense signaling and resistance to Hyaloperonospora arabidopsidis. Plant J. 2013;76:466–80. doi: 10.1111/tpj.12311. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718078863
- 19.Zipfel C. Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol. 2008;20:10–6. doi: 10.1016/j.coi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Mousavi Seyed AR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature. 2013;500:422–6. doi: 10.1038/nature12478. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718082760
- 21.de Bruxelles, Guy L, Roberts MR. Signals regulating multiple responses to wounding and herbivores. Critical Reviews in Plant Sciences. 2001;20:487–521. doi: 10.1080/07352689.2001.10131828. [DOI] [Google Scholar]
- 22.Volkov AG, Adesina T, Markin VS, Jovanov E. Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiol. 2008;146:694–702. doi: 10.1104/pp.107.108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iraqui I, Vissers S, Bernard F, de Craene, J O, Boles E, Urrestarazu A, André B. Amino acid signaling in Saccharomyces cerevisiae: a permease-like sensor of external amino acids and F-Box protein Grr1p are required for transcriptional induction of the AGP1 gene, which encodes a broad-specificity amino acid permease. Mol Cell Biol. 1999;19:989–1001. doi: 10.1128/mcb.19.2.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conigrave AD, Franks AH, Brown EM, Quinn SJ. L-amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism? Eur J Clin Nutr. 2002;56:1072–80. doi: 10.1038/sj.ejcn.1601463. [DOI] [PubMed] [Google Scholar]
- 25.Chiu JC, Brenner ED, DeSalle R, Nitabach MN, Holmes TC, Coruzzi GM. Phylogenetic and expression analysis of the glutamate-receptor-like gene family in Arabidopsis thaliana. Mol Biol Evol. 2002;19:1066–82. doi: 10.1093/oxfordjournals.molbev.a004165. [DOI] [PubMed] [Google Scholar]
- 26.Teardo E, Formentin E, Segalla A, Giacometti GM, Marin O, Zanetti M, Lo Schiavo F, Zoratti M, Szabò I. Dual localization of plant glutamate receptor AtGLR3.4 to plastids and plasmamembrane. Biochim Biophys Acta. 2011;1807:359–67. doi: 10.1016/j.bbabio.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Vincill ED, Clarin AE, Molenda JN, Spalding EP. Interacting glutamate receptor-like proteins in phloem regulate lateral root initiation in Arabidopsis. Plant Cell. 2013;25:1304–13. doi: 10.1105/tpc.113.110668. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718001374
- 28.Rytz R, Croset V, Benton R. Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem Mol Biol. 2013;43:888–97. doi: 10.1016/j.ibmb.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Forde BG. Glutamate signalling in roots. J Exp Bot. 2014;65:779–87. doi: 10.1093/jxb/ert335. [DOI] [PubMed] [Google Scholar]
- 30.Tegeder M. Transporters for amino acids in plant cells: some functions and many unknowns. Curr Opin Plant Biol. 2012;15:315–21. doi: 10.1016/j.pbi.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Mithöfer A, Boland W. Recognition of herbivory-associated molecular patterns. Plant Physiol. 2008;146:825–31. doi: 10.1104/pp.107.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heil M, Ibarra-Laclette E, Adame-Álvarez RM, Martínez O, Ramirez-Chávez E, Molina-Torres J, Herrera-Estrella L. How plants sense wounds: damaged-self recognition is based on plant-derived elicitors and induces octadecanoid signaling. PLoS ONE. 2012;7:e30537. doi: 10.1371/journal.pone.0030537. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/14264205
- 33.Khakh BS, Burnstock G. The double life of ATP. Sci Am. 2009;301:84–90. 92. doi: 10.1038/scientificamerican1209-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka K, Gilroy S, Jones AM, Stacey G. Extracellular ATP signaling in plants. Trends Cell Biol. 2010;20:601–8. doi: 10.1016/j.tcb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon PS, Oliver RP. The nitrogen content of the tomato leaf apoplast increases during infection by Cladosporium fulvum. Planta. 2001;213:241–9. doi: 10.1007/s004250000500. [DOI] [PubMed] [Google Scholar]
- 36.Choi J, Tanaka K, Cao Y, Qi Y, Qiu J, Liang Y, Lee SY, Stacey G. Identification of a plant receptor for extracellular ATP. Science. 2014;343:290–4. doi: 10.1126/science.343.6168.290. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718241915
- 37.Chivasa S, Murphy AM, Hamilton JM, Lindsey K, Carr JP, Slabas AR. Extracellular ATP is a regulator of pathogen defence in plants. Plant J. 2009;60:436–48. doi: 10.1111/j.1365-313X.2009.03968.x. [DOI] [PubMed] [Google Scholar]
- 38.Hinoi E, Takarada T, Ueshima T, Tsuchihashi Y, Yoneda Y. Glutamate signaling in peripheral tissues. Eur J Biochem. 2004;271:1–13. doi: 10.1046/j.1432-1033.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 39.Boldyrev AA, Bryushkova EA, Vladychenskaya EA. NMDA receptors in immune competent cells. Biochemistry Mosc. 2012;77:128–34. doi: 10.1134/S0006297912020022. [DOI] [PubMed] [Google Scholar]
- 40.Ganor Y, Levite M. Glutamate in the Immune System: Glutamate Receptors in Immune Cells, Potent Effects, Endogenous Production and Involvement in Disease. In: Levite M, editor. In Nerve-Driven Immunity. Vienna: Springer Vienna; 2012. pp. 121–61. [DOI] [Google Scholar]
- 41.Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]


