Abstract
Background
Attention-deficit/hyperactivity disorder (ADHD) is often comorbid with cocaine abuse. Controversy exists regarding long-term consequences of ADHD medications on cocaine abuse liability. Whereas childhood methylphenidate treatment may be preventative, methylphenidate in teens appears to further increase later cocaine abuse risk. In rodents, adolescent methylphenidate treatment further increases adult cocaine self-administration in the Spontaneously Hypertensive Rat (SHR) model of ADHD, whereas adolescent atomoxetine treatment does not. Effects of ADHD medications on cocaine cue reactivity, a critical component of addiction, are unknown.
Methods
To investigate this, SHR, Wistar-Kyoto (inbred control) and Wistar (outbred control) rats received therapeutically relevant doses of methylphenidate (1.5 mg/kg, oral) and atomoxetine (0.3 mg/kg, intraperitoneal), or respective vehicles from post-natal day 28–55. Cocaine seeking, reflecting cue reactivity, was measured in adulthood during self-administration maintenance and cue-induced reinstatement tests conducted under a second-order schedule.
Results
Compared to control strains, SHR earned more cocaine infusions, emitted more cocaine-seeking responses during maintenance and reinstatement testing, and required more sessions to reach the extinction criterion. Compared to vehicle, adolescent methylphenidate, but not atomoxetine, further increased cocaine intake during maintenance testing in SHR. Adolescent atomoxetine, but not methylphenidate, decreased cocaine seeking during reinstatement testing in SHR. Neither medication had effects on cocaine intake or cue reactivity in control strains.
Conclusions
The SHR successfully model ADHD and cocaine abuse comorbidity and show differential effects of adolescent ADHD medications on cocaine intake and cue reactivity during adulthood. Thus, SHR have heuristic value for assessing neurobiology underlying the ADHD phenotype and for evaluating pharmacotherapeutics for ADHD.
Keywords: Addiction, Attention-deficit/hyperactivity disorder, Cocaine, Methylphenidate, Atomoxetine, Spontaneously Hypertensive Rat
1. INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent neurodevelopmental condition. Diagnoses have risen 41% over the past decade, with rates escalating fastest in boys aged 14–17 (Visser et al., 2010; Schwarz and Cohen, 2013). ADHD is highly comorbid with substance abuse, including cocaine (van Emmerik-van Oortmerssen et al., 2012). Children with ADHD are 2–3 times more likely to abuse cocaine in adulthood compared to children without an ADHD diagnosis (Lee et al., 2011).
Controversy exists regarding long-term consequences of ADHD medications on cocaine abuse liability. Approximately two-thirds of U.S. children and adolescents diagnosed with ADHD are prescribed a stimulant medication, such as methylphenidate (Schwarz and Cohen, 2013). Methylphenidate, like cocaine, inhibits dopamine and norepinephrine transporters (DAT and NET, respectively). Because adolescence represents a period of elevated plasticity in the mesocorticolimbic dopamine system, stimulant exposure during this period may have unique long-term effects on reward responsivity (Andersen, 2005). Whereas childhood methylphenidate treatment is protective against an increase in later cocaine abuse (Wilens et al., 2003; Humphreys et al., 2013), adolescent methylphenidate treatment can increase later abuse of cocaine and other drugs (Lambert and Hartsough, 1998; Mannuzza et al., 2008, Dalsgaard et al., 2014). Although some studies reported protective effects of adolescent stimulant treatment (e.g., Biederman et al., 1999), these studies often fail to distinguish actively medicated participants from those who discontinued treatment at assessment. As cocaine use may be a form of self-medication for untreated ADHD (Gudjonsson et al., 2012), ongoing methylphenidate treatment may compromise detecting increased cocaine abuse, as suggested by animal studies (Schenk and Izenwasser, 2002). Further, many clinical studies employ a limited follow-up period into adulthood. Because cocaine abuse generally develops later than abuse of other substances (Degenhardt et al., 2008), participants evaluated in their late teens and early twenties may not have surpassed the risk period for initiating cocaine use.
Preclinical models can address clinically relevant questions concerning ADHD. Typically used is the Spontaneously Hypertensive Rat (SHR), whose behavioral and cognitive deficits model the ADHD combined subtype and are unrelated to hypertension (Wyss et al., 2003; Sagvolden et al., 2005; Russell et al., 2005; Kantak et al., 2008). Furthermore, SHR exhibit elevated cocaine self-administration compared to Wistar-Kyoto (WKY; inbred progenitor of SHR) or Wistar (WIS; outbred common ancestor to SHR and WKY) control strains (Harvey et al., 2011; Somkuwar/Jordan et al., 2013). Using a therapeutically relevant dose (Kuczenski and Segal, 2002), we demonstrated that adolescent treatment with 1.5 mg/kg oral methylphenidate further enhanced the speed to acquire cocaine self-administration and the efficacy and motivating influence of cocaine reinforcement in adult SHR, but not in adult WKY or WIS (Harvey et al., 2011).
Atomoxetine, a non-stimulant ADHD medication, is a viable alternative to methylphenidate for adolescents with ADHD in whom drug abuse is a concern (Kratochvil et al., 2002). At therapeutic doses, atomoxetine selectively inhibits NET to increase extracellular norepinephrine and dopamine in prefrontal cortex (PFC; Bymaster et al., 2002). We recently demonstrated that adolescent treatment with 0.3 mg/kg atomoxetine did not further enhance the speed to acquire cocaine self-administration or the efficacy and motivating influence of cocaine reinforcement in adult SHR or WIS, but did facilitate acquisition of cocaine self-administration in adult WKY (Somkuwar/Jordan et al., 2013).
Environmental cues associated with cocaine use play a major role in compulsive drug seeking and relapse, and are linked to changes in dopamine-mediated neurotransmission in cortical sites such as medial prefrontal cortex (mPFC) and orbitofrontal cortex (OFC) (Ciccocioppo et al., 2001; Di Pietro et al., 2008). DAT function in mPFC and OFC also is affected by adolescent ADHD medications (Somkuwar/Jordan et al., 2013; Somkuwar et al., 2013). Unknown is whether ADHD influences reactivity to cocaine-related cues, and if medications prescribed for teens with ADHD alter cue reactivity in adulthood after treatment discontinuation. Cocaine cue reactivity is a fundamentally different issue than those addressed in our previous studies, which focused instead on the efficacy and motivating influence of cocaine reinforcement through the use of fixed-ratio (FR) and progressive-ratio (PR) schedules of cocaine delivery (Harvey et al., 2011; Somkuwar/Jordan et al., 2013). To address these new clinically relevant questions, we assessed strain differences in cocaine cue reactivity among SHR, WKY and WIS rats, and determined whether adolescent methylphenidate or atomoxetine influenced cocaine cue reactivity during adulthood after adolescent treatment was discontinued. A second-order schedule of cocaine delivery and cue presentation was used so that cocaine seeking, reflecting cue reactivity, could be measured when cocaine was (maintenance) and was not (reinstatement) available for self-administration (Kantak et al., 2002).
2. MATERIALS AND METHODS
2.1 Subjects
Male WKY/Cr, WIS/Cr, and SHR/Cr rats (Charles River Laboratories, USA) arrived on postnatal day 25 (P25). Rats had free access to water. Food was restricted to ~90% of a growth-adjusted free-feeding body weight until P55 to mimic conditions of past comparator studies (Harvey et al., 2011, 2013; Somkuwar/Jordan et al., 2013; Somkuwar et al., 2013). Rats in Experiment 1 were utilized previously to measure strategy set shifting performance during adolescence (Harvey et al., 2013), whereas rats in Experiment 2 were experimentally naïve to behavioral testing. Procedures were approved by the Institutional Animal Care and Use Committee at Boston University and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Drugs
To mimic clinical practice of medication-free holidays on weekends (Martins et al., 2004), (±)-methylphenidate hydrochloride (Sigma-Aldrich; St. Louis, MO) and atomoxetine hydrochloride (Tocris Biosciences; Ellisville, MO) treatments were administered during the light phase Monday–Friday from P28–P55, constituting the rat adolescent period (Spear, 2000). The chosen dose (1.5 mg/kg) and oral route of methylphenidate administration produces therapeutically relevant plasma drug levels that mimic clinical oral dosing (Kuczenski and Segal, 2002). The chosen dose (0.3 mg/kg) and intraperitoneal (i.p.) route of atomoxetine administration selectively increases extracellular norepinephrine and dopamine in PFC (Bymaster et al., 2002). Atomoxetine was injected i.p. due to poor oral bioavailability in rats (Mattiuz et al., 2003). Methylphenidate was dissolved in water (1.5 mg/ml). To attain a dose of 1.5 mg/kg, 1 ml/kg was injected into an oyster cracker for oral consumption. Oyster crackers injected with water (1 ml/kg) were used for vehicle control. Atomoxetine was dissolved in 0.9% sterile saline (0.15 mg/ml) and injected intraperitoneally (i.p.) in a volume of 2 ml/kg to attain a dose of 0.3 mg/kg. Injections of 0.9% sterile saline (2 ml/kg) were used for vehicle control. Cocaine hydrochloride (NIDA, Bethesda, MD) was mixed in 0.9% sterile saline containing 3 IU of heparin/ml and was self-administered at a dose of 0.3 mg/kg via catheters implanted into the right femoral vein on P67. A 0.8 mg/ml solution of cocaine was infused at a rate of 1.8 ml/min. The infusion duration was adjusted for each animal’s daily body weight (1.2 s/100 g) to attain a dose of 0.3 mg/kg. Details for surgery and the testing environment are described in Supplementary Materials1. The number of animals that survived surgery and completed all phases of testing with intact catheters is indicated below.
2.3 Experiment 1: Effects of adolescent methylphenidate on adult behavior
2.3.1. Maintenance testing
On P77, vehicle- and methylphenidate-treated WKY (n=10 and 7, respectively), vehicle- and methylphenidate-treated WIS (n=10 and 10, respectively), and vehicle- and methylphenidate-treated SHR (n=9 and 7, respectively) began cocaine self-administration training for delivery of 0.3 mg/kg cocaine under an FR 1 schedule. Testing was conducted during the light phase at the same time each day throughout all phases of the experiments. Illumination of the house light signaled the onset of each session. Drug delivery coincided with onset of the cue light and accompanying pump sound. Infusions were followed by a 20-sec timeout for which the cue light remained illuminated while the house light was extinguished. The house light was re-illuminated following the 20-sec timeout period. Rats were trained incrementally to a terminal fixed-interval (FI)-based second-order schedule designated FI 5-min [FR5:S]. The cue light (S) was presented under an FR 5 contingency and was illuminated for 2-sec upon completion of each FR 5 during the FI 5-min. The house light was not extinguished during 2-sec cue light presentations. After the FI elapsed, cocaine was delivered upon completion of an FR 5, and coincided with 20-sec cue light presentation and termination of the house light. After the 20-sec timeout, the house light was re-illuminated and the FI component was again in effect. Self-administration sessions were conducted once daily, Monday–Friday during the light phase for 2-hr. Training continued until rats achieved stable levels of responding (≤15% variation in active lever responding, and ≤33% of total responses on the inactive lever) for a minimum of 5 sessions, designated as the maintenance baseline. A dose of 0.3 mg/kg cocaine was selected because it produces the highest rate of responding under an FI 5-min [FR5:S] schedule of cocaine delivery in rats (Kantak et al., 2009).
2.3.2. Extinction training
Following maintenance testing, rats underwent response extinction training. Sessions were conducted once daily, Monday–Friday, for 2-hr durations. Rats received a minimum of 10 extinction sessions; criterion was defined as active lever responding ≤10% of the maintenance baseline for 3 consecutive days. A maximum of 21 extinction sessions was employed if criterion was not met.
2.3.3. Reinstatement testing
During reinstatement, discrete and contextual sound and light cues were presented under second-order schedule contingencies. Experimental conditions were identical to maintenance testing, except cocaine was not delivered. Animals underwent seven 1-hr daily sessions of reinstatement testing.
2.4 Experiment 2: Effects of adolescent atomoxetine on adult behavior
On P77, vehicle- and atomoxetine-treated WKY (n=8 and 8, respectively), vehicle- and atomoxetine-treated WIS (n=9 and 8, respectively), and vehicle- and atomoxetine-treated SHR (n=8 and 8, respectively) began cocaine self-administration training for delivery of 0.3 mg/kg cocaine under an FR 1 schedule, followed by second-order schedule training. All rats received maintenance testing, extinction training, and reinstatement testing as described in Experiment 1.
2.5 Data analysis
Dependent measures were number of cocaine infusions, active and inactive lever responses, and number of sessions to reach the extinction criterion. Measures were analyzed using separate two-factor (strain × treatment) or three-factor (strain × treatment × phase) ANOVAs, with repeated measures for phase. Tukey tests were used for post-hoc comparisons following significant main effects and interactions. Based on prior work, corrected Bonferroni t-tests were used for planned comparisons examining differences between vehicle and drug treatments. Both multiple-comparison procedures control for type-1 error.
3. RESULTS
3.1 Experiment 1: Effects of adolescent methylphenidate on adult behavior
3.1.1. Maintenance testing
Cocaine intake during maintenance testing under the second-order schedule is shown in Fig. 1a. Strains differed in number of cocaine infusions [F(2,47) = 11.3; p ≤ 0.001], with adult SHR earning more infusions than WKY and WIS (p ≤ 0.001 and 0.01, respectively). Main and interaction effects of treatment were not significant, but Bonferroni analysis revealed treatment differences in adult SHR, with more cocaine infusions earned after adolescent methylphenidate than vehicle treatment (p ≤ 0.014). In adult WKY or WIS, adolescent methylphenidate did not significantly alter cocaine intake compared to vehicle treatment during maintenance testing.
Fig. 1.
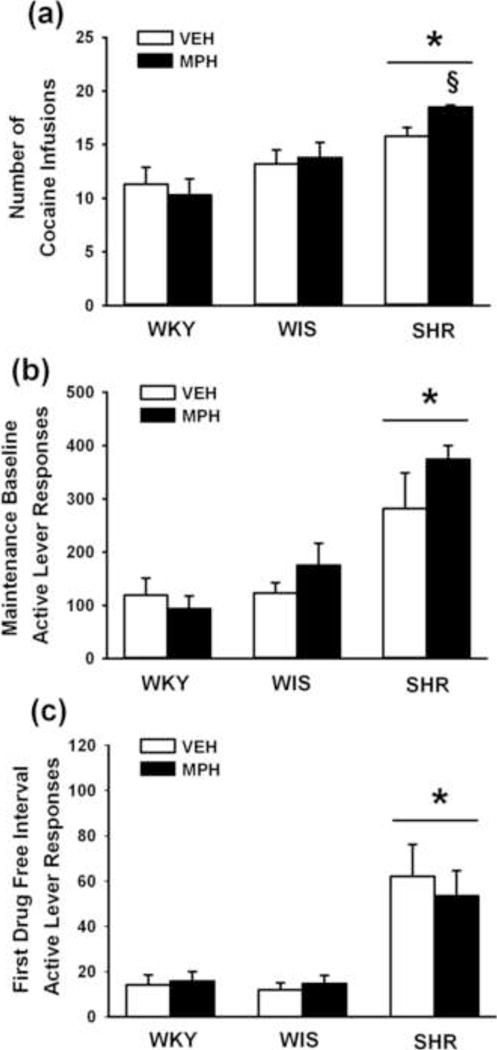
Effects of adolescent methylphenidate treatment during maintenance testing under a second-order schedule. (a) Cocaine intake averaged across a five-day baseline; (b) Active lever responses averaged across a five-day baseline; (c) Active lever responses during the first drug-free interval of the final maintenance testing session. Experiments were conducted in adult Wistar-Kyoto (WKY), Wistar (WIS), and Spontaneously Hypertensive (SHR) rats after adolescent methylphenidate (MPH) or vehicle (VEH) treatment was discontinued (n = 7–10 per strain and treatment). Values are presented as mean ± SEM. *p ≤ 0.01 compared to WKY and WIS (main effect of strain). § p ≤ 0.014 compared to vehicle-treated SHR.
Active lever responses during maintenance testing and for the first drug-free interval of the final maintenance testing session are shown in Fig. 1b–c. This interval represents the period prior to delivery of the first cocaine infusion of the session when responding is maintained exclusively by cocaine-paired cues. Strains differed during maintenance testing [F(2,47) = 16; p ≤ 0.001] and for the first drug-free interval [F(2,47) = 20.3; p ≤ 0.001], with adult SHR making more active lever responses than WKY and WIS in each analysis (p ≤ 0.001). Analysis of inactive lever responses also revealed strain differences [F(2,47) = 17.3; p ≤ 0.001], with SHR making more inactive lever responses (62±7) than WKY (11±7) and WIS (19±6) strains (p ≤ 0.001). Adolescent methylphenidate did not significantly alter active or inactive lever responses compared to vehicle treatment in any strain during maintenance testing or the first drug-free interval.
3.1.2. Extinction training
The number of sessions to reach the extinction criterion is shown in Fig. 2a. Strains differed in number of sessions [F(2,47) = 4.7; p ≤ 0.01], with adult WIS requiring fewer sessions than SHR (p ≤ 0.01), but not WKY. SHR and WKY did not differ. Analysis of the extinction baseline (averaged over the last three sessions and expressed as the percentage of the self-administration maintenance baseline) revealed that the relative degree of extinguished responding was not significantly different between treatments and across strains (Fig. 2b). Inactive lever responses differed by strain [F(2,47) = 13.7; p ≤ 0.001], with SHR making more inactive lever responses (22±2) than WKY (9±2) and WIS (7±2) strains (p ≤ 0.001). Adolescent methylphenidate did not significantly alter extinction behavior in any strain compared to vehicle treatment during extinction training.
Fig. 2.
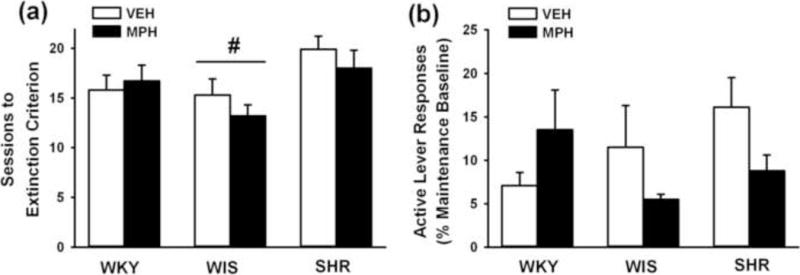
Effects of adolescent methylphenidate treatment during extinction training. (a) Number of sessions to reach extinction criterion; (b) Active lever responding averaged across the last three sessions of extinction and expressed as the percentage of the self-administration maintenance baseline. Experiments were conducted in adult Wistar-Kyoto (WKY), Wistar (WIS), and Spontaneously Hypertensive (SHR) rats after adolescent methylphenidate (MPH) or vehicle (VEH) treatment was discontinued (n = 7–10 per strain and treatment). Values are presented as mean ± SEM. # p ≤ 0.01 compared to SHR (main effect of strain).
3.1.3. Reinstatement testing
The number of active lever responses during reinstatement testing and, for comparison, the first hour of the extinction baseline is shown in Fig. 3. Three-factor ANOVA revealed main effects of phase [F(1,47) = 99.8; p ≤ 0.001] and strain [F(2,47) = 30.1; p ≤ 0.001], and a strain × phase interaction [F(2,47) = 20.4; p ≤ 0.001]. Post-hoc testing of the interaction indicated that cue re-exposure during the reinstatement phase reinstated cocaine-seeking responses above extinction levels in each group (p ≤ 0.02) and that adult SHR reinstated more cocaine-seeking responses and emitted more responses during the first hr of the extinction baseline than WKY or WIS (p ≤ 0.001). Main and interaction effects of treatment were not significant, and Bonferroni analysis confirmed that adolescent methylphenidate did not significantly alter cocaine-seeking responses compared to vehicle treatment in any strain during reinstatement testing. Inactive lever responses differed by strain during reinstatement testing [F(2,47) = 26.1; p ≤ 0.001], with SHR making more inactive lever responses (19±1) than WKY (7±1) and WIS (7±1) strains (p ≤ 0.001).
Fig. 3.
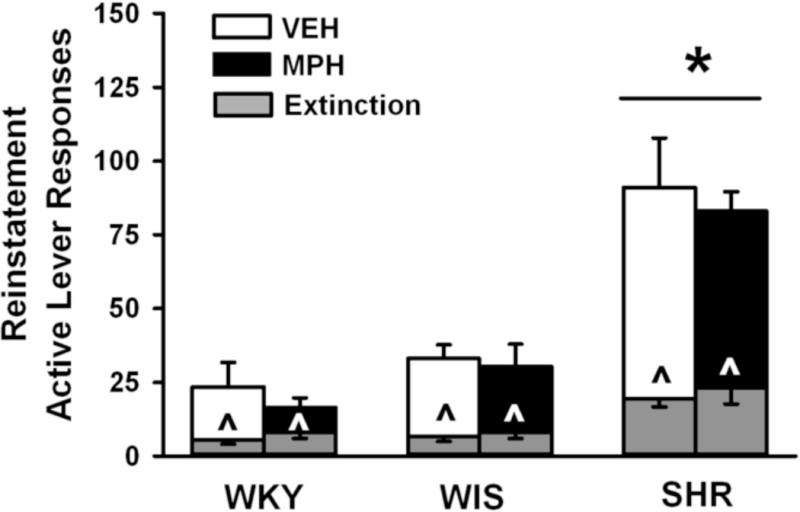
Effects of adolescent methylphenidate treatment during reinstatement testing. Active lever responses during reinstatement testing were averaged across the seven 1-hr sessions. For comparison, active lever responses during the first hour of extinction training were averaged across the last three sessions of extinction training and are depicted by the gray bars. Experiments were conducted in adult Wistar-Kyoto (WKY), Wistar (WIS), and Spontaneously Hypertensive (SHR) rats after adolescent methylphenidate (MPH) or vehicle (VEH) treatment was discontinued (n = 7–10 per strain and treatment). Values are presented as mean ± SEM. *p ≤ 0.001 compared to WKY and WIS (main effect of strain). ^p ≤ 0.023 compared to reinstatement responses.
3.2 Experiment 2: Effects of adolescent atomoxetine on adult behavior
3.2.1. Maintenance testing
Cocaine intake during maintenance testing under the second-order schedule is shown in Fig. 4a. Consistent with Experiment 1, strains differed in number of cocaine infusions [F(2,42) = 16; p ≤ 0.001], with adult SHR earning more infusions than WKY and WIS (p ≤ 0.001). Main and interaction effects of treatment were not significant, and Bonferroni analysis confirmed that adolescent atomoxetine did not significantly alter cocaine intake compared to vehicle treatment in any strain during maintenance testing.
Fig. 4.
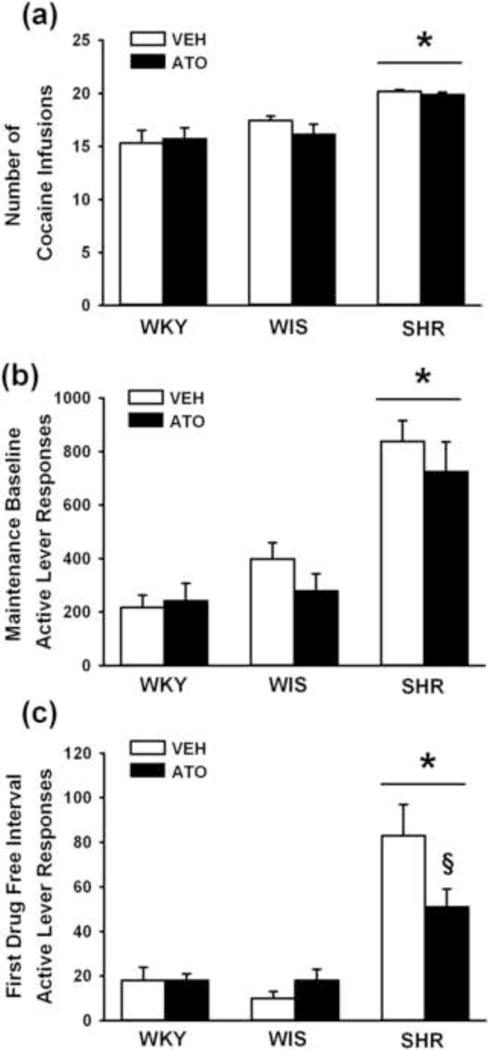
Effects of adolescent atomoxetine treatment during maintenance testing under a second-order schedule. (a) Cocaine intake averaged across a five-day baseline; (b) Active lever responses averaged across a five-day baseline; (c) Active lever responses during the first drug-free interval of the final maintenance testing session. Experiments were conducted in adult Wistar-Kyoto (WKY), Wistar (WIS), and Spontaneously Hypertensive (SHR) rats after adolescent atomoxetine (ATO) or vehicle (VEH) treatment was discontinued (n = 7–9 per strain and treatment). Values are presented as mean ± SEM. * p ≤ 0.001 compared to WKY and WIS (main effect of strain). § p ≤ 0.005 compared to vehicle-treated SHR.
Active lever responses during maintenance testing and for the first drug-free interval of the final maintenance testing session are shown in Fig. 4b–c. During maintenance testing, strains differed [F(2,42) = 29.6; p ≤ 0.001], with adult SHR making more active lever responses than WKY and WIS (p ≤ 0.001). Adolescent atomoxetine treatment did not significantly alter active lever responses during 1-hr maintenance tests in any strain. However, during the first drug-free interval there was a main effect of strain [F(2,42) = 31.4; p ≤ 0.001] and a strain × treatment interaction [F(2,42) = 4.1; p ≤ 0.02]. Overall, adult SHR made more active lever responses than WKY and WIS during the first drug-free interval (p ≤ 0.001). Post-hoc testing of the interaction revealed that adolescent atomoxetine reduced active lever responses compared to vehicle treatment in SHR during the first drug-free interval (p ≤ 0.005). In contrast, adolescent atomoxetine did not significantly alter active lever responses compared to vehicle treatment during the first drug-free interval in WKY or WIS. There were no significant strain or treatment differences in inactive lever responding during maintenance testing (SHR 85±34, WKY 39±15, WIS 69±28).
3.2.2. Extinction training
The number of sessions to reach the extinction criterion is shown in Fig. 5a. There were strain differences in number of sessions [F(2,42) = 6.1; p ≤ 0.005], with adult WIS requiring fewer sessions than SHR and WKY (p ≤ 0.005 and p ≤ 0.04, respectively). SHR and WKY did not differ. A strain × treatment interaction also was found [F(2,42) = 3.9; p ≤ 0.02] and post-hoc testing revealed that SHR receiving adolescent atomoxetine required more sessions to reach the extinction criterion than SHR receiving vehicle (p ≤ 0.006). Treatments did not significantly differ in WKY and WIS. Analysis of the extinction baseline revealed that the relative degree of extinguished responding (values expressed as percentage of the maintenance baseline) was not different between treatments and across strains (Fig. 5b). Inactive lever responses differed by strain [F(2,42) = 3.7; p ≤ 0.03], with SHR making more inactive lever responses (24±5) than WIS (11±3; p ≤ 0.02), but not WKY (15±4).
Fig. 5.
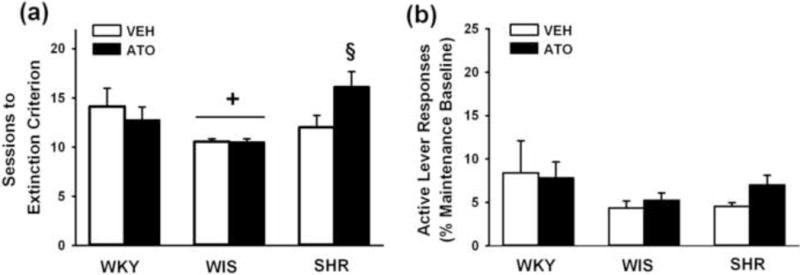
Effects of adolescent atomoxetine treatment during extinction training. (a) Number of sessions to reach extinction criterion; (b) Active lever responding averaged across the last three sessions of extinction and expressed as the percentage of the self-administration maintenance baseline. Experiments were conducted in adult Wistar-Kyoto (WKY), Wistar (WIS), and Spontaneously Hypertensive (SHR) rats after adolescent atomoxetine (ATO) or vehicle (VEH) treatment was discontinued (n = 7–9 per strain and treatment). Values are presented as mean ± SEM. + p ≤ 0.04 compared to SHR and WKY (main effect of strain). § p ≤ 0.006 compared to vehicle-treated SHR.
3.2.3. Reinstatement testing
The number of active lever responses during reinstatement testing and, for comparison, the first hour of the extinction baseline is shown in Fig. 6. Three-factor ANOVA revealed main effects of phase [F(1,42) = 213.9; p ≤ 0.001] and strain [F(2,42) = 77.1; p ≤ 0.001], and a strain × treatment × phase interaction [F(2,42) = 3.1; p ≤ 0.05]. Post-hoc testing of the interaction indicated that cue re-exposure during the reinstatement phase reinstated cocaine-seeking responses above extinction levels in each group (p ≤ 0.002) and that adult SHR reinstated more cocaine-seeking responses and emitted more responses during the first hr of the extinction baseline than WKY or WIS (p ≤ 0.001). During reinstatement testing, adolescent atomoxetine treatment attenuated cocaine-seeking responses compared to vehicle only in SHR (p ≤ 0.032). Inactive lever responses did not significantly differ between treatments and across strains during reinstatement testing (SHR 16±3, WKY 12±2, WIS 10±2).
Fig. 6.
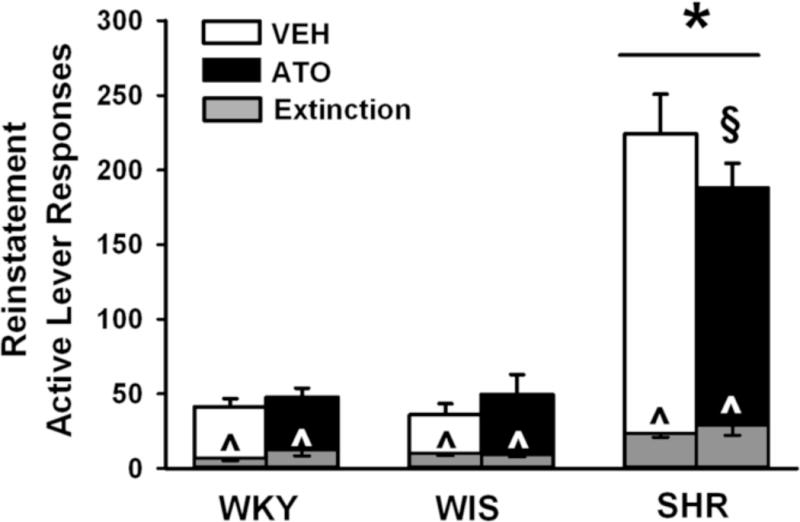
Effects of adolescent atomoxetine treatment during reinstatement testing. Active lever responses during reinstatement testing were averaged across the seven 1-hr sessions. For comparison, active lever responses during the first hour of extinction training were averaged across the last three sessions of extinction training and are depicted by the gray bars. Experiments were conducted in adult Wistar-Kyoto (WKY), Wistar (WIS), and Spontaneously Hypertensive (SHR) rats after adolescent atomoxetine (ATO) or vehicle (VEH) treatment was discontinued (n = 7–9 per strain and treatment). Values are presented as mean ± SEM. *p ≤ 0.001 compared to WKY and WIS (main effect of strain). ^p ≤ 0.002 compared to reinstatement responding. § p ≤ 0.032 compared to vehicle-treated SHR.
4. DISCUSSION
4.1 Strain differences in cocaine-seeking and cocaine-taking behavior
The current study replicates and extends previous research suggesting that SHR are an excellent model of comorbid ADHD and cocaine abuse (Harvey et al., 2011; Somkuwar/Jordan et al., 2013). Consistent with prior studies, cocaine intake was greater in SHR than WKY or WIS. The current study also revealed that SHR extinguish responding to criterion levels within the same timeframe as WKY, but more slowly than WIS, and that SHR emit more cocaine-seeking responses than WKY or WIS. High levels of cocaine seeking by SHR when cocaine was (maintenance testing) and was not (reinstatement testing) available for self-administration, as well as during the first drug-free interval of maintenance testing, suggest SHR exhibit heightened cocaine cue reactivity compared to WKY or WIS. The observation that SHR exhibit similar rates of extinction compared to WKY suggests that extinction learning is not impaired in SHR, but rather may be faster in WIS. High levels of cocaine seeking by SHR during reinstatement testing are therefore not likely due to impaired extinction learning, but rather further reflect heightened cocaine cue reactivity in this strain. SHR also often made more inactive lever responses than WKY and WIS, which may relate to hyperactivity in SHR when reinforcers are infrequent (Sagvolden et al., 2005). Nonetheless, SHR remained goal-directed, as active lever responding was substantially greater than inactive lever responding throughout maintenance and reinstatement testing. Although both vehicle- and drug-treated rats of each strain exhibited an approximately 2-fold higher rate of active lever responding in Experiment 2 compared to Experiment 1, the same relative magnitude of differences between WKY and WIS vs. SHR was maintained in both experiments. Differences in the absolute number of responses across experiments may be due to prior experimental history of rats in Experiment 1, or potentially to subjects in Experiments 1 and 2 being tested by different investigators, as experimenter identity has a strong effect on variance in rodent behavior (Chesler et al., 2002).
Sign tracking, a Pavlovian conditioned approach behavior associated with drug abuse (Tomie et al., 2008; for review), may contribute to heightened incentive salience of cocaine-associated cues in animals especially vulnerable to cocaine addiction (Yager and Robinson, 2013). Whereas goal tracking involves approach to an unconditioned stimulus, sign tracking involves compulsive approach to reward-related cues. Although Pavlovian conditioned approach behavior has not been evaluated directly in SHR, ADHD-related characteristics such as impulsivity and poor sustained attention are observed in SHR as well as in sign tracking rats (Sagvolden et al., 2005; Tomie et al., 2008; Wooters and Bardo, 2011). Thus, it is possible that SHR also have a sign tracking phenotype, given their high degree of cocaine cue reactivity. If the current findings are translational, substance-dependent individuals with ADHD may therefore uniquely benefit from cue-exposure therapy (Mitchell et al., 2014), which is most effective in individuals with initially high cue reactivity (Unrod et al., 2013). Research on animal models of pharmacotherapy-assisted cue-exposure therapy for enhancing extinction of cocaine-seeking behavior (e.g., Achat-Mendes et al., 2012) may therefore have relevance to ADHD treatment.
4.2 Effects of adolescent methylphenidate
In adult SHR, cocaine intake under the second-order schedule was increased by adolescent methylphenidate, extending our earlier observations under FR and PR schedules (Harvey et al., 2011). These findings are consistent with clinical studies demonstrating that methylphenidate treatment in teens with ADHD is associated with increased cocaine abuse risk during adulthood, a relationship that may (Barkley et al., 2003; Mannuzza et al., 2008) or may not (Lambert and Hartsough, 1998) be linked to comorbid conduct disorder.
The long-term effects of adolescent methylphenidate treatment on DAT may help explain increased cocaine intake in adult SHR. The same adolescent methylphenidate treatment regimen used herein selectively increased DAT function (Vmax for [3H]dopamine uptake at DAT) in mPFC of adult SHR (Somkuwar et al., 2013), inferring faster clearance of dopamine. Faster clearance leads to lower basal dopamine tone in mesocortical neurons (Zahnisher and Sorkin, 2004), resulting in greater post-synaptic responses to phasically released dopamine (Grace, 2001), such as when cocaine is self-administered. We propose this mechanism contributes to the further increase in cocaine intake in adult SHR. In adult WKY and WIS, adolescent methylphenidate did not further increase cocaine self-administration (present findings; Harvey et al., 2011) and did not increase DAT function in mPFC (Somkuwar et al., 2013).
In contrast to effects on cocaine intake, adolescent methylphenidate did not alter cocaine seeking during maintenance or reinstatement testing, or the number of sessions required to reach the extinction criterion in SHR. Because adolescent methylphenidate further increased cocaine intake in SHR, it might be expected that cue reactivity also would be further increased. However, our results are consistent with prior findings in outbred rats showing that acute administration of methylphenidate did not alter cocaine seeking under a second-order schedule (Economidou et al., 2011). Notably, in outbred rats, NET plays an important role in regulating saliency of drug-associated cues (Economidou et al., 2011; Janak et al., 2012), and therefore be a critical regulator of cue reactivity in SHR.
4.3 Effects of adolescent atomoxetine
Adolescent atomoxetine treatment did not further increase cocaine intake or cocaine seeking during maintenance testing under a second-order schedule in adult SHR or control strains. These findings are consistent with studies showing that adolescent atomoxetine in SHR, WKY and WIS did not further increase cocaine intake or lever responding under FR or PR schedules (Somkuwar/Jordan et al., 2013). Although a previous study reported attenuated cocaine seeking during second-order maintenance testing in an outbred rat strain following acute atomoxetine administration (Economidou et al., 2011), these effects of atomoxetine were only observed with acute doses that were 3- to 10-fold higher (1 or 3 mg/kg) than the therapeutically relevant 0.3 mg/kg dose administered chronically in the present study. We previously reported that 0.3 mg/kg atomoxetine administered during adolescence did not affect DAT function and cell surface distribution in mPFC of adult SHR, WKY, or WIS rats, and decreased DAT function and cell surface distribution in OFC of adult SHR (Somkuwar/Jordan et al., 2013). The failure to increase DAT function in mPFC may explain why adolescent atomoxetine, unlike methylphenidate, did not increase cocaine intake in adult SHR. Further, although not tested directly in the current study, the previously observed decrease in DAT function and cell surface distribution in OFC following an identical adolescent atomoxetine treatment regimen may contribute to the reduced cocaine seeking observed in atomoxetine-treated SHR in the present work.
It is unclear why adolescent atomoxetine increased the number of sessions to reach the extinction criterion in adult SHR. However, this action did not translate into increased cocaine abuse risk, as atomoxetine attenuated the heightened cocaine seeking in SHR observed during reinstatement testing as well as during the first drug-free interval of maintenance testing, which occurred prior to extinction training. This suggests that atomoxetine reduces cocaine cue reactivity in SHR when cocaine is not available. Effects of atomoxetine on NET function and expression in OFC may help explain these findings. A past study demonstrated that daily atomoxetine (1 mg/kg) during late adolescence (P40–54) increased NET mRNA in OFC of adult outbred rats, without affecting NET mRNA in mPFC or nucleus accumbens (Sun et al., 2012). This treatment regimen also decreased synaptic plasticity markers in OFC, inferring an inhibitory effect of low basal NE tone on OFC signaling. While it remains to be determined if adolescent treatment with 0.3 mg/kg atomoxetine increases NET function or expression in OFC of adult SHR, this mechanism could contribute to the decrease in cocaine cue reactivity observed in adult SHR. OFC activation is necessary for cocaine seeking under a second-order schedule in rats (Kantak et al., 2009). In non-human primates, association of a visual cue with intravenous cocaine infusions led to activation of the ventral OFC (Nelissen et al., 2012), supporting a role for OFC in cocaine cue reactivity. More broadly, the OFC is thought to be involved in the integration of multimodal sensory input, processing of reinforcer value and punishment, regulation of motivation and goal-directed behavior, and response inhibition or reversal learning (see Kringelbach, 2005, for review; Ghods-Sharifi et al., 2008). Abnormal OFC function has been observed both in cocaine-dependent individuals (Bolla et al., 2003) as well as in individuals with conduct disorder (Rubia et al., 2009), a condition that is often comorbid with ADHD and is associated with increased risk for substance use disorders (Wilens et al., 2011).
4.4 Conclusions
There are limitations to every animal model of human disease. Nonetheless, SHR exhibit behavioral and cognitive deficits (Wyss et al., 2003; Sagvolden et al., 2005; Russell et al., 2005; Kantak et al., 2008) as well as neurochemical and genetic differences (Mill et al., 2005; Roessner et al., 2010) reflecting those observed in ADHD. Thus, SHR have heuristic value for assessing the neurobiology underlying the ADHD phenotype and for evaluating pharmacotherapeutics for ADHD. Questions regarding illegal substances of abuse are difficult to approach systematically in minors, and our work may provide important leads for targeted research in teens with ADHD. The current findings suggest that initiation of methylphenidate treatment in adolescence may increase cocaine abuse risk if treatment is discontinued before adulthood. This finding is of particular importance given a recent epidemiological study from the U.K. reporting that up to 57% of ADHD patients who began treatment as teenagers discontinued their ADHD medication by early adulthood (McCarthy et al., 2012). Our work further suggests that atomoxetine may represent a viable alternative to methylphenidate for teenagers who are first beginning treatment, as atomoxetine does not appear to increase cocaine abuse risk and may even be protective against increased cocaine cue reactivity, a trait which can increase the risk of relapse.
Implications of our research become more critical in light of the recent Centers for Disease Control report showing that 1 in 5 teenage boys is currently diagnosed with ADHD (Schwarz and Cohen, 2013). This report raises several important questions that remain unanswered, such as: Are all these teenage boys accurately diagnosed? When and what medication was initiated in this cohort? What are the long-term consequences of taking ADHD medications during the teenage years in terms of cocaine abuse outcomes and neurobiological changes? The SHR model of ADHD has begun to help address these questions comprehensively and more appropriately than studies using only outbred rats.
Supplementary Material
Acknowledgments
The authors thank Angelica DellaMorte and Katherine Rodriguez for technical assistance.
Role of funding source
This study was funded by grant NIH R01 DA011716. NIH had no further role in study design; in data collection, analysis and interpretation; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors
CJ was responsible for data collection, data analysis and writing the report. KK and LD were responsible for study concept and design, and provided important intellectual content in the writing of the report. RH and BB were involved in data collection. All authors contributed to and have approved the final version of the manuscript.
Conflict of interest
All authors declare no conflict of interest.
References
- Achat-Mendes C, Nic Dhonnchadha BÁ, Platt DM, Kantak KM, Spealman RD. Glycine transporter-1 inhibition preceding extinction training inhibits reacquisition of cocaine seeking. Neuropsychopharmacology. 2012;37:2837–2845. doi: 10.1038/npp.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111:97–109. doi: 10.1542/peds.111.1.97. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:1–5. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Influences of laboratory environment on behavior. Nat Neurosci. 2002;5:1101–1102. doi: 10.1038/nn1102-1101. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Paolo Sanna P, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D1 antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH. ADHD, stimulant treatment in childhood and subsequent substance abuse in adulthood – a naturalistic long-term follow-up study. Addict Behav. 2014;39:325–328. doi: 10.1016/j.addbeh.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, Bruffaerts R, de Girolamo G, Gureje O, Huang Y, Karam A, Kostyuchenko S, Lepine JP, Mora ME, Neumark Y, Ormel JH, Pinto-Meza A, Posada-Villa J, Stein DJ, Takeshima T, Wells JE. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Health Mental Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro NC, Mashhoon Y, Heaney C, Yager LM, Kantak KM. Role of dopamine D1 receptors in the prefrontal dorsal agranular insular cortex in mediating cocaine self-administration in rats. Psychopharmacology. 2008;200:81–91. doi: 10.1007/s00213-008-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Dalley JW, Everitt BJ. Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol Psychiatry. 2011;69:266–274. doi: 10.1016/j.biopsych.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobio Learn Mem. 2012;89:567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Gudjonsson GH, Sigurdsson JF, Sigfusdottir ID, Young S. An epidemiological study of ADHD symptoms among young persons and the relationship with cigarette smoking, alcohol consumption, and illicit drug use. J Child Psychol Psychiatry. 2012;53:304–312. doi: 10.1111/j.1469-7610.2011.02489.x. [DOI] [PubMed] [Google Scholar]
- Grace AA. Psychostimulant actions on dopamine and limbic system function: relevance to the pathophysiology and treatment of ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. Oxford University Press; London, United Kingdom: 2001. pp. 134–157. [Google Scholar]
- Harvey RC, Jordan CJ, Tassin DH, Moody KR, Dwoskin LP, Kantak KM. Performance on a strategy set shifting task during adolescence in a genetic model of attention deficit/hyperactivity disorder: methylphenidate vs. atomoxetine treatments. Behav Brain Res. 2013;244:38–47. doi: 10.1016/j.bbr.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RC, Sen S, Deaciuc A, Dwoskin LP, Kantak KM. Methylphenidate treatment in adolescent rats with an attention deficit/hyperactivity disorder phenotype: cocaine addiction vulnerability and dopamine transporter function. Neuropsychopharmacology. 2011;36:837–847. doi: 10.1038/npp.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Eng T, Lee SS. Stimulant medication and substance use outcomes: a meta-analysis. JAMA Psychiatry. 2013;70:740–749. doi: 10.1001/jamapsychiatry.2013.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Bowers MS, Corbit LH. Compound stimulus presentation and the norepinephrine reuptake inhibitory atomoxetine enhance long-term extinction of cocaine-seeking behavior. Neuropsychopharmacology. 2012;37:975–985. doi: 10.1038/npp.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Mashhoon Y, Silverman DN, Janes AC, Goodrich CM. Role of the orbitofrontal cortex and dorsal striatum in regulating the dose-related effects of self-administered cocaine. Behav Brain Res. 2009;201:128–136. doi: 10.1016/j.bbr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Singh T, Kerstetter KA, Dembro KA, Mutebi MM, Harvey RC, Deschepper CF, Dwoskin LP. Advancing the spontaneous hypertensive rat model of attention deficit/hyperactivity disorder. Behav Neurosci. 2008;122:340–357. doi: 10.1037/0735-7044.122.2.340. [DOI] [PubMed] [Google Scholar]
- Kratochvil CJ, Heiligenstein JH, Dittman R, Spencer TJ, Biederman J, Wernicke J, Newcorn JH, Casat C, Milton D, Michelson D. Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry. 2002;41:776–784. doi: 10.1097/00004583-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracelluar norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabilities. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Truong NL, Moulton JL, III, Roizen ER, Howell KH, Castellanos FX. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am J Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins S, Tramontina S, Polanczyk G, Eizirik M, Swanson JM, Rohde LA. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharm. 2004;14:195–205. doi: 10.1089/1044546041649066. [DOI] [PubMed] [Google Scholar]
- Mattiuz EL, Ponsler GD, Barbuch RJ, Wood PG, Mullen JH, Shugert RL, Li Q, Wheeler WJ, Kuo F, Conrad PC, Sauer JM. Disposition and metabolic fate of atomoxetine hydrochloride: pharmacokinetics, metabolism, and excretion in the Fischer 344 rat and beagle dog. Drug Metab Dispos. 2003;31:88–97. doi: 10.1124/dmd.31.1.88. [DOI] [PubMed] [Google Scholar]
- McCarthy S, Wilton L, Murray ML, Hodgkins P, Asherson P, Wong ICK. Persistence of pharmacological treatment into adulthood, in UK primary care, for ADHD patients who started treatment in childhood or adolescence. BMC Psychiatry. 2012;12:219–227. doi: 10.1186/1471-244X-12-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Sagvolden T, Asherson P. Sequence analysis of Drd2, Drd4, and Dat1 in SHR and WKY rat strains. Behav Brain Func. 2005;1:24. doi: 10.1186/1744-9081-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JT, McIntyre EM, McClernon FJ, Kollins SH. Smoking motivation in adults with attention-deficit/hyperactivity disorder using the Wisconsin Inventory of Smoking Dependence Motives. Nicotine Tob Res. 2014;16:120–125. doi: 10.1093/ntr/ntt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morón JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in regions with low levels of the dopamine transporter: Evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen K, Jarraya B, Arsenault JT, Rosen BR, Wald LL, Mandeville JB, Marota JJ, Vanduffel W. Neural correlates of the formation and retention of cocaine-induced stimulus-reward associations. Biol Psychiatry. 2012;72:422–428. doi: 10.1016/j.biopsych.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Roessner V, Sagvolden T, DasBanerjee T, Middleton FA, Faraone SV, Walaas SI, Becker A, Rothenberger A, Bock N. Methylphenidate normalizes elevated dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neuroscience. 2010;167:1183–1191. doi: 10.1016/j.neuroscience.2010.02.073. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, Brammer MJ. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Russell VA, Sagvolden T, Borgå Johansen E. Animal models of attention-deficit hyperactivity disorder. Behav Brain Func. 2005;1:9. doi: 10.1186/1744-9081-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Schenk S, Izenwasser S. Pretreatment with methylphenidate sensitizes rats to the reinforcing effects of cocaine. Pharmacol Biochem Behav. 2002;72:651–657. doi: 10.1016/s0091-3057(02)00735-9. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Cohen S. A.D.H.D. seen in 11% of U.S. children as diagnoses rise. NY Times. 2013 Available at http://www.nytimes.com/2013/04/01/health/more-diagnoses-of-hyperactivity-causing-concern.html. Accessed 12 March 2014.
- Somkuwar SS, Darna M, Kantak KM, Dwoskin LP. Adolescence methylphenidate treatment in a rodent model of attention deficit/hyperactivity disorder: dopamine transporter function and cellular distribution in adulthood. Biochem Pharmacol. 2013;86:309–316. doi: 10.1016/j.bcp.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS*, Jordan CJ*, Kantak KM, Dwoskin LP. Adolescent atomoxetine treatment in a rodent model of ADHD: effects on cocaine self-administration and dopamine transporters in frontostriatal regions. Neuropsychopharmacology. 2013;38:2588–2597. doi: 10.1038/npp.2013.163. *Co-first authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sun H, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology (Berl) 2012;219:285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Poherecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unrod M, Drobes DJ, Stasieqicz PR, Ditre JW, Heckman B, Miller RR, Sutton SK, Brandon TH. Decline in cue-provoked craving during cue exposure therapy for smoking cessation. Nicotine Tob Res. 2013;16:306–315. doi: 10.1093/ntr/ntt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Emmerik-van Oortmerssen K, van de Glind G, van den Brink W, Smit F, Crunelle CL, Swets M, Schoevers RA. Prevalence of attention-deficit hyperactivity disorder in substance use disorder patients: a meta-analysis and meta-regression analysis. Drug Alcohol Depend. 2012;122:11–19. doi: 10.1016/j.drugalcdep.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Visser SN, Bitsko RH, Danielson ML, Perou R, Blumberg SJ. Increasing Prevalence of Parent-Reported Attention-Deficit/Hyperactivity Disorder Among Children — United States, 2003 and 2007. Center for Disease Control and Prevention; 2010. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5944a3.htm. Accessed 12 March 2014. [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substane abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C, Biederman J. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adol Psychiatry. 2011;50:543–553. doi: 10.1016/j.jaac.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT. Methylphenidate and fluphenazine, but not amphetamine, differentially affect impulsive choice in Spontaneously Hypertensive, Wistar-Kyoto and Sprague-Dawley rats. Brain Res. 2011;1396:45–53. doi: 10.1016/j.brainres.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM, Kadish I, van Groen T. Age-related decline in spatial learning and memory: attenuation by captopril. Clin Exp Hypertension. 2003;25:455–474. doi: 10.1081/ceh-120024988. [DOI] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2013;226:217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology. 2004;47:80–91. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


