Abstract
Rho guanine exchange factors (GEFs) are a large, diverse family of proteins defined by their ability to catalyze the exchange of GDP for GTP on small GTPase proteins such as Rho family members. GEFs act as integrators from varied intra- and extracellular sources to promote spatiotemporal activity of Rho GTPases that control signaling pathways regulating cell proliferation and movement. Here we review recent studies elucidating roles of RhoGEF proteins in cell motility. Emphasis is placed on Dbl-family GEFs and connections to development, integrin signaling to Rho GTPases regulating cell adhesion and movement, and how these signals may enhance tumor progression. Moreover, RhoGEFs have additional domains that confer distinctive functions or specificity. We will focus on a unique interaction between Rgnef (also termed Arhgef28 or p190RhoGEF) and focal adhesion kinase (FAK), a non-receptor tyrosine kinase that controls migration properties of normal and tumor cells. This Rgnef-FAK interaction activates canonical GEF-dependent RhoA GTPase activity to govern contractility and also functions as a scaffold in a GEF-independent manner to enhance FAK activation. Recent studies have also brought to light the importance of specific regions within the Rgnef pleckstrin homology (PH) domain for targeting the membrane. As revealed by ongoing Rgnef-FAK investigations, exploring GEF roles in cancer will yield fundamental new information on the molecular mechanisms promoting tumor spread and metastasis.
Keywords: cell motility, Dbl-related GEF, FAK, integrin signaling, Rgnef/ARHGEF28, RhoGTPase
INTRODUCTION
Cell motility is a complex process that involves cellular interactions with the environment leading to intracellular changes that modulate protein function and gene expression [1, 2]. Communication between the outside and inside of cells is relayed from the extracellular matrix (ECM) via integrins to the actin cytoskeleton [3, 4]. Signals initiated from inside cells can also alter integrin activation states to modulate cell adhesion to the ECM [5]. All of these changes must be coordinated in time and space within cells in order to initiate and maintain directional movement [6].
The Rho family of GTPases are small ubiquitous (~21 kDa) signaling G proteins (guanine nucleotide-binding proteins) that bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). Canonical members include RhoA, Rac1, and Cdc42 [7]. Rho-family GTPases act as switches; when they bind GTP, they are active, and, when they bind GDP, they are inactive. When bound to GTP, Rho-family GTPases associate with a variety of target proteins that regulate many aspects of intracellular actin dynamics needed for cell movement [8]. Since basal nucleotide exchange and intrinsic hydrolysis are slow, the Rho-family GTPase activation cycle is controlled in part by GTPase activating proteins (GAPs) that stimulate GTP hydrolysis and guanine-nucleotide exchange factor (GEFs) that promote the exchange of GDP for GTP [9]. The large number of GEFs and GAPs (>70 members each) far outnumber Rho GTPase targets and this likely reflects signaling diversity in Rho GTPase regulation [10]. The molecular regulation of various GEFs or GAPs contains both conserved and unique protein-specific elements. There have been recent reviews on GAPs in signal termination [11] and in the regulation of membrane traffic [12]. Herein, we will focus on GEFs.
There are two distinct GEF families for Rho proteins: those of the diffuse B-cell lymphoma (Dbl) and dedicator of cytokinesis (Dock) families [10, 13, 14]. In the interest of space and to provide a focused review, emphasis will be on the Dbl GEFs. The Dbl-homology (DH) domain (~200 amino acids) comprises a region with GEF activity and there are more than 70 human DH-containing proteins (Table I) [15]. The DH domain may have considerable amino acid divergence between GEFs, but it comprises a related three-dimensional structure [16]. The majority of Dbl family proteins have a DH domain followed by a pleckstrin homology (PH) domain (~100 amino acids) that binds phospholipids and other proteins [17, 18]. The conservation of the tandem DH-PH organization implies a conserved function within GEFs, but the PH domain is also found in many other human proteins [19]. In a small subset of Dbl members, the DH domain is followed by a BAR (Bin–Amphiphysin–Rvs) domain that can promote either protein dimerization or membrane binding [15]. Outside of the DH-PH region, GEFs encompass a diverse range of sequence motifs and domains that can connect GEFs to various subcellular sites or signaling pathways. The fact that there are greater numbers of GEFs than RhoGTPases suggests that signal integration and specificity for Rho activation may be regulated by GEF activity. Many GEFs have distinct domains that may allow for additional functional specificity. In the following discussion, we will emphasize those GEFs that contribute to the complex process of cell migration. In particular, we highlight Rgnef, a Dbl family RhoGEF that uniquely binds FAK, a well-known mediator of cell motility.
Table 1.
Known human Dbl family RhoGEFs and their roles in development
| Dbl protein | Other Names |
Acc # | aa # | Target GTPases |
Defect | Reference |
|---|---|---|---|---|---|---|
| α-Pix | ARHGEF6; Cool-2 | Q15052 | 776 | Rac1, Cdc42 | Viable and fertile; deficient immune response, lower mature lymphocyte population, impaired spatial and complex learning | [98, 99] |
| β-Pix | ARHGEF7; Cool-1 | Q14155 | 803 | Rac1, Cdc42, Lrrk2 | Embryonic lethal | [98, 100] |
| Abr | Q12979 | 859 | RhoA, Rac1, Cdc42 | Viable and fertile; cerebellar and vestibular defects with combined Bcr loss | [101, 102] | |
| AKAP13 | ARHGEF13; Lbc | Q12802 | 2813 | RhoA | Early embryonic lethality with heart development defects | [50] |
| ALS2 | Alsin | Q96Q42 | 1657 | Rac1, Rab5 | Viable and fertile; hypoactive behavior, shorter lifespan in some genetic backgrounds | [103, 104] |
| ARHGEF4 | XPLN; STA3 | Q9NR81 | 526 | RhoA/B | In zebrafish: cytopenia, abnormal vascular development | [105, 106] |
| ARHGEF10 | RhoGEF10 | O15013 | 1369 | RhoA | unknown | [107] |
| ARHGEF10L | GrinchGEF | Q9HCE6 | 1279 | RhoA/B/C | unknown | [108] |
| ARHGEF16 | Ephexin-4 | Q5VV41 | 709 | RhoG, Cdc42 | unknown | [109, 110] |
| ARHGEF33 | FLJ41381 | A8MVXO | 844 | unknown | unknown | |
| ARHGEF37 | FLJ41603 | A1IGU5 | 675 | unknown | unknown | |
| ARHGEF38 | FLJ20184 | Q9NXL2 | 219 | unknown | unknown | |
| Asef1 | ARHGEF4; ASEF | Q9NR80 | 690 | Rac1, Cdc42 | Viable and fertile; impaired retinal angiogenesis | [111] |
| Asef2 | ARHGEF29; SPATA13 | Q96N96 | 652 | RhoA, Rac1, Cdc42 | Viable and fertile | [112, 113] |
| BCR | P11274 | 1271 | Rac1, Cdc42 | Viable and fertile; increase in neutrophil respiratory burst | [114] | |
| C9orf100 | ARHGEF39; FLJ14642 | Q8N4T4 | 335 | unknown | unknown | [115] |
| Dbl | ARHGEF21; MCF2 | P10911 | 925 | RhoA/B/C/G, Rac1, Cdc42 | Viable and fertile; dendrite elongation defect | [116] |
| Dbs | ARHGEF14; MCF2L | O15068 | 1137 | RhoA, Rac1, Cdc42 | Viable and fertile; lower B cell count and cholesterol, increased grip strength, hyperphosphatemia (males only) | [117, 118] |
| DNMBP | ARHGEF36; TUBA | Q6XZF7 | 1577 | Cdc42 | unknown | [119] |
| Ect2 | ARHGEF31 | Q9H8V3 | 914 | RhoA/B, Rac1, Cdc42 | Peri-implantation lethality | [52, 120] |
| Ect2L | ARHGEF32 | Q008S8 | 904 | unknown | unknown | |
| Ephexin-1 | ARHGEF27; WGEF | Q8N5V2 | 710 | RhoA, Rac1, Cdc42 | Viable and fertile; severe muscle weakness in adults | [121] |
| FARP1 | CDEP | Q9Y4F1 | 1045 | RhoA, Rac1 | unknown | [122, 123] |
| FARP2 | FIR, FRG | O94887 | 1054 | Rac1, Cdc42 | Viable | [124–126] |
| FGD1 | FGDY; ZFYVE3 | P98174 | 961 | Cdc42 | Human genomic deletions cause Aarskog-Scott syndrome | [127] |
| FGD2 | ZFYVE4 | Q7Z6J4 | 655 | Cdc42 | unknown | [128] |
| FGD3 | ZFYVE5 | Q5JSP0 | 725 | Cdc42 | unknown | [129] |
| FGD4 | CMT4H; Frabin; ZFYVE5 | Q96M96 | 766 | Cdc42 | Viable and fertile; myelin abnormalities | [130] |
| FGD5 | ZFYVE23 | Q6ZNL6 | 1462 | Cdc42 | unknown | [131] |
| FGD6 | ZFYVE24 | Q6ZV73 | 1430 | unknown | unknown | |
| GEF-H1 | ARHGEF2; Lfc | Q92974 | 986 | RhoA, RhoB | unknown | [132, 133] |
| hPEM-2 | ARHGEF9; Collybistin | O43307 | 516 | Cdc42 | Loss of function in humans causes mental retardation and epilepsy | [134, 135] |
| Intersectin-1 | ITSN1 | Q15811 | 1721 | Cdc42 | Some early postnatal fatality; fertile, dysregulated neuronal vesicle trafficking | [136, 137] |
| Intersectin-2 | ITSN2 | Q9NZM3 | 1697 | Cdc42 | unknown | [138] |
| Kalirin | ARHGEF24; Duet, Duo | O60229 | 2985 | Rac1 | Viable and fertile; reduced cortex and hippocampal size, locomotor hyperactivity, memory impairment, abnormal social behavior | [139] |
| LARG | ARHGEF12 | Q9NZN5 | 1544 | RhoA | Viable and fertile; smooth muscle hypertension defects | [140] |
| MCF2L2 | ARHGEF22 | Q86YR7 | 1114 | unknown | unknown | |
| MyoGEF | PLEKHG6 | Q3KR16 | 790 | RhoA/C/G, Rac1 | unknown | [141, 142] |
| NET1 | ARHGEF8 | Q7Z628 | 596 | RhoA/B/C | unknown | [120, 143, 144] |
| Obscurin | ARHGEF30; OBSCN | Q5VST9 | 7968 | RhoA/Q | Viable and fertile; muscle weakness, mild age-dependent muscular myopathy | [145–148] |
| P-Rex1 | Q8TCU6 | 1659 | Rac1/2 | Viable and fertile; reduced lung permeability, platelet secretion and aggregation, and neutrophil recruitment | [149–152] | |
| P-Rex2 | Q70Z35 | 1606 | Rac1 | Viable and fertile; altered Purkinje cell morphology, impaired motor coordination | [153, 154] | |
| p114RhoGEF | ARHGEF18 | Q6ZSZ5 | 1173 | RhoA, Rac1 | unknown | [155] |
| p115RhoGEF | ARHGEF1; LSC | Q6NX52 | 948 | RhoA | Viable and fertile; leukocyte homeostasis defects, gastrointestinal motor dysfunctions | [56, 57, 156] |
| p164-RhoGEF | ARHGEF17; TEM4 | Q96PE2 | 2063 | RhoA/B/C | unknown | [157, 158] |
| p63RhoGEF | ARHGEF25; GEFT | Q86VW2 | 580 | RhoA | unknown | [159] |
| PDZ-RhoGEF | ARHGEF11; PRG | O15085 | 1522 | RhoA | Viable and fertile | [156, 160] |
| PLEKHG1 | ARHGEF41 | Q9ULL1 | 1385 | unknown | Decreased granulocytes, decreased susceptibility to bacterial infection | |
| PLEKHG2 | ARHGEF42; FLJ00018 | Q9H7P9 | 1386 | Rac1, Cdc42 | unknown | [161] |
| PLEKHG3 | ARHGEF43 | A1L390 | 1219 | unknown | Deleted in some human autism cases, learning difficulties | [162, 163] |
| PLEKHG4 | ARHGEF44; SCA4 | Q58EX7 | 1191 | unknown | Human genetic mutations associated with spinocerebellar ataxia | [164] |
| PLEKHG4B | KIAA1909 | Q96PX9 | 1271 | unknown | unknown | |
| PLEKHG5 | DSMA4; GEF720 | O94827 | 1062 | RhoA | Human genetic mutations associated with distal spinal muscular atrophy | [165, 166] |
| PLEKHG7 | Q6ZR37 | 379 | unknown | unknown | ||
| RasGRF1 | CDC25; GRF1 | Q13972 | 1275 | Ras, Rac1 | Viable and fertile; reduced body weight and impaired growth, glucose homeostasis and retinal defects, impaired long-term memory, longer lifespan | [167–172] |
| RasGRF2 | GRF2 | O14827 | 1237 | Ras, Rac1 | Viable and fertile; impaired T cell signaling | [173, 174] |
| Rgnef | ARHGEF28; p190RhoGEF | Q8N1W1 | 1705 | RhoA/C | Partial embryonic lethality; fertile, decreased size at birth | [22, 44] |
| SGEF | ARHGEF26 | Q96DR7 | 871 | RhoG | Viable and fertile | [175, 176] |
| Solo | ARHGEF40; Scambio | Q8TER5 | 1519 | RhoA/C | unknown | [177] |
| SOS1 | GF1 | Q07889 | 1333 | Ras, Rac1 | Embryonic lethal | [51, 178, 179] |
| SOS2 | Q07890 | 1332 | Ras, Rac1 | Viable and fertile | [179, 180] | |
| Tiam1 | Q13009 | 1591 | Rac1, Cdc42, RhoA | Partial embryonic lethality; fertile, smaller brain size, some anencephaly and exencephaly | [181, 182] | |
| Tiam2 | STEF | Q8IVF5 | 1701 | Rac1 | unknown | [183] |
| TIM-1 | ARHGEF5; Ephexin-3 | Q12774 | 1597 | RhoA/B/C/G | Viable and fertile; decrease in dendritic cell migration | [184] |
| Trio | ARHGEF23 | O75962 | 3038 | RhoA/G, Rac1 | Embryonic lethal; muscle and neural tissue defects | [53] |
| VAV1 | VAV | P15498 | 845 | RhoA/G, Rac1, Cdc42 | Viable and fertile; T cell development defects | [185, 186] |
| VAV2 | P52735 | 878 | RhoA/G, Rac1, Cdc42 | Viable and fertile; cardiovascular remodeling, renal dysfunction | [187, 188] | |
| VAV3 | Q9UKW4 | 847 | RhoA/G, Rac1, Cdc42 | Viable and fertile; large bones, cardiovascular remodeling, tachycardia, hypertension, renal dysfunction, cerebellar defects | [189, 190] | |
| Vsm-RhoGEF | ARGEF15; Ephexin-5 | O94989 | 841 | Cdc42 | Viable and fertile; reduced retinal vasculature growth | [191] |
| WGEF | ARHGEF19; Ephexin-2 | Q8IW93 | 802 | RhoA, Cdc42, Rac1 | unknown | [192] |
Acc #, human protein accession number; aa #, protein amino acid length; Defects as determined by human pathology or targeting appropriate GEF homolog in other animal species.
RGNEF (p190RHOGEF/ARHGEF28) AND FAK
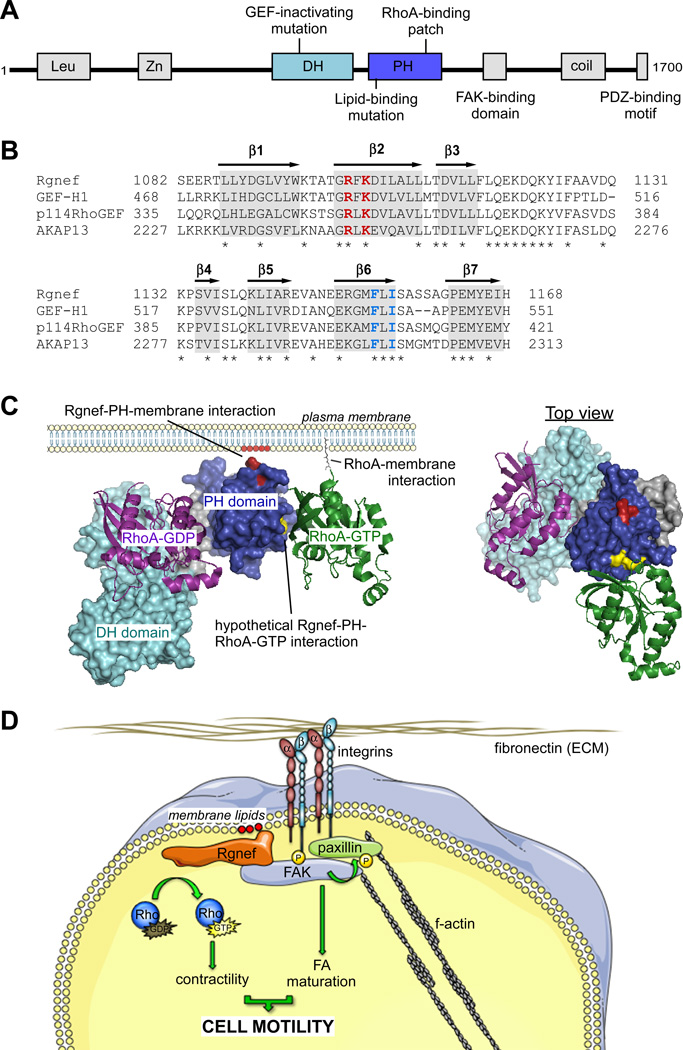
Rgnef (previously named p190RhoGEF for its 190 kDa molecular weight, gene name Rgnef recently changed to Arhgef28) is a ubiquitously-expressed DH-PH-containing GEF [20] that can activate RhoA and RhoC in cells [21, 22]. Rgnef is most highly related to p114 (ARHGEF18), Lbc (ARHGEF13), and GEFH1 (ARHGEF2). Rgnef contains several potential regulatory motifs (Fig. 1A), including an N-terminal leucine-rich region, a cysteine-rich zinc finger domain. The large C-terminal region of Rgnef contains a potential coiled-coil domain that can bind microtubules [21], the 3'-untranslated region of neurofilament mRNA [23], and phosphorylation independent associations with 14-3-3 [24] or c-Jun amino-terminal kinase interacting protein-1 [25]. The original sequencing of murine Rgnef contained a frame shift error that altered the coding sequence for the last 36 amino acids [20] (Protein: NP_036156, Nucleotide: NM_012026). This region is homologous to human Rgnef (GeneID 64283, NM_001080479) and as noted in a prior review [26], Rgnef contains a consensus PDZ-binding motif (IVYL) at the C-terminus, a feature shared by a subset of other GEFs [27]. One unique feature of Rgnef is that it can bind directly to focal adhesion kinase (FAK) and this interaction is dependent upon a short Rgnef peptide region (1292–1301) near the coiled-coil domain [28, 29].
Fig. (1). Rgnef protein domains and structure.
(A) Mouse Rgnef protein schematic. Shown are the leucine-rich domain (Leu), zinc-finger motif (Zn), tandem Dbl-homology (DH) and pleckstrin-homology (PH) domains, FAK-binding domain (1292–1301), coiled-coil domain (coil), and PDZ-binding motif. Also shown are the locations of the GEF-inactivating mutation (Y1003A), lipid-binding mutation (R1098A/K1100A), and RhoA-GTP binding residues (A1151/A1153). (B) PH domain alignment of Lbc RhoGEF subfamily members. Highlighted in gray are putative locations of beta-strands (b1–b7), asterisks indicate identical residues. In red is the location of residues necessary for efficient PI lipid binding in Rgnef. In blue are residues necessary for binding to activated RhoA across all Lbc subfamily GEFs. (C) Left, theoretical structure of the Rgnef DH-PH domain at the plasma membrane. Rgnef binds to PI lipids (red) at the plasma membrane through conserved residues in the PH domain (residues in red, PH domain in blue). Rgnef also potentially binds to RhoA-GTP (green) at the plasma membrane through conserved hydrophobic residues (yellow) in the PH domain. These factors potentially localize and orient Rgnef for its GEF activity towards RhoA-GDP (purple) through the DH domain (cyan). Right, top down view of Rgnef in complex with RhoA-GTP and RhoA-GDP. Theoretical Rgnef DH-PH model created in Swiss-Model. RhoA-GDP crystal structure from PDB 1X86. RhoA-GTP crystal structure from PDB 3KZ1. Theoretical Rgnef DH-PH model created in Swiss-Model based on PDB 3KZ1 [97] (D) Simplified model of Rgnef function downsteam of integrin signaling. Cell binding ECM leads to integrin clustering and activation at the membrane, generating increased phoshatidylinositol lipids at adhesion sites. Rgnef PH domain associates with concentrated membrane lipids and facilitates FAK localization at nascent adhesions. FAK activation promotes FA maturation and Rgnef RhoA-GEF catalytic activity promotes actomyosin contractility, both required for proper cell motility.
FAK is a cytoplasmic protein-tyrosine kinase that is recruited to and activated at cell adhesion sites termed focal adhesions [30]. FAK acts downstream of various growth factor and integrin receptors in the control of cell shape and cell-cell adhesion changes needed for efficient cell movement [31]. Although a variety of FAK-associated signaling pathways have been characterized through analysis of FAK knockout mice/cells [32], FAK kinase-dead knockin mice/cells [33–35], and pharmacological FAK inhibition [36], the mechanisms associated with FAK recruitment and activation at receptor sites remains unclear. The tightly controlled process of cell migration involves many precise spatiotemporally regulated molecules. Since both FAK and the Rgnef effector RhoA have been shown to play significant roles in migration, the direct interaction of these two proteins likely confers an additional layer of regulation. Thus, the interaction between Rgnef and FAK is important as this provides a point of integration for the generation of contractile forces and activation of signaling cascades regulating cell movement [29]. Moreover, emerging evidence supports the importance of Rgnef-FAK interactions in promoting tumor progression [37]. In this review, we will expand upon a novel concept that Rgnef also functions as a scaffold in a GEF-independent manner to enhance FAK activation downstream of integrins [38] and how this may impact tumor biology.
DEVELOPMENT: POTENTIAL COMPENSATION BETWEEN GEFS FROM KNOCKOUT STUDIES
Regulated cell movement is a fundamental process during multicellular animal development. From C. elegans to primates, tissue formation results from the orchestrated migration of various cells during gastrulation, organogenesis, vasculogenesis, and neuronal pathfinding [39, 40]. Rho GTPases are key regulators of cell motility and therefore, it is not surprising that inactivation results in developmental abnormalities. RhoA, RhoB, and RhoC are related and RhoA knockout in mice leads to embryonic lethality whereas loss of RhoB or RhoC result in milder phenotypes [41–43]. These results suggest a fundamental role for RhoA whereas RhoB and RhoC may have overlapping and tissue- or disease-specific roles apart from activating common RhoA targets. Since there are ~3 times as many GEFs that activate Rho-family GTPases [10], a major challenge in the field is to understand how temporal and spatial activation of GEFs relates to RhoA activation and cell function. A standard approach is to analyze the effect of loss of expression in a transgenic mouse model. However, few developmental defects have been observed in mice lacking RhoGEFs [44, 45]. This may be attributable to either redundancy during development or tissue-specific RhoGEF expression.
Analyses of heterozygous crosses of transgenic Rgnef knockout mice showed that Rgnef−/− mice were present at normal Mendelian ratios on embryonic day 13.5 [44]. However, Rgnef−/− mice were born at a significantly lower Mendelian frequency. At birth, Rgnef−/− mice exhibit an overall smaller size than Rgnef+/− or Rgnef+/+ littermates. Analyses of Rgnef−/− offspring did not reveal apparent tissue abnormalities and this size difference was negligible by 6 to 8 weeks of age. It is likely that there is an important role for Rgnef in mouse growth or development, but that some type of partial redundancy or compensation may be occurring to lessen or bypass the potential restriction point between embryonic day 13.5 and birth. Highest Rgnef expression was found in the brain, ovary, and spleen of 10 week old mice [44]. Although roles for Rgnef have been proposed in neuronal [23, 46, 47] and immune cell [48, 49] function, Rgnef−/− mice are fertile and do not exhibit obvious defects. Moreover, partial embryonic lethal phenotypes are uncommon in other RhoGEF transgenic mouse models (Table I). Except for AKAP13 (ARHGEF13) [50], Sos1 [51], Ect2 (ARHGEF31) [52], β-Pix (ARHGEF7), and Trio (ARHGEF23) knockouts which result in embryonic lethality [53], other RhoGEF knockouts have non-lethal phenotypes (Table 1).
Interestingly, as observed with loss of Rgnef, knockout of the RhoA effector proteins ROCK1 or ROCK2 (Rho-associated protein kinases) also result in partial embryo lethality and birth of small pups [54, 55]. ROCK2 loss was associated with late placental dysfunction and ROCK1 loss with cellular actomyosin bundling defects. Future studies of Rgnef knockout embryos in utero will be focused on identifying potential phenotypes as a means to link Rgnef to RhoA signaling in vivo. Many of the restricted hematopoietic or neural defects associated with RhoGEF loss are linked to potential alterations in cell movement (Table 1). For instance, Lsc/p115 (ARHGEF1) loss is associated with marginal zone B-cell and neutrophil migration defects [56, 57]. In culture, Rgnef−/− fibroblasts exhibit defects in adhesion formation and cell movement when stimulated by extracellular matrix proteins such as fibronectin [44]. This has been associated with decreased integrin-mediated signaling to RhoA as well as FAK activation as discussed below.
INTEGRIN-RHOA SIGNALING AXIS
Integrin receptors are heterodimeric transmembrane proteins comprised of alpha and beta subunits that cluster upon binding to extracellular matrix proteins and signal across the membrane in both directions [58]. Integrins generate signals within cells with respect to external surroundings and establish a physical linkage to the actin cytoskeleton to facilitate cell adhesion, shape change, and tension. Cell adhesion complexes (also called focal adhesions, FAs) consist of integrins and various cytoplasmic proteins such as talin, vinculin, paxillin, and alpha-actinin. FA formation is associated with the activation of kinases, including FAK and c-Src, that phosphorylate substrates such as p130Cas or cortactin promoting the binding of adaptor proteins like Crk or Nck and the establishment of large multi-protein signaling complexes at FAs. Linkages of Crk and Nck to actin nucleating protein complexes such as N-WASP or Arp2/3 alter actin branching with effects on cell protrusion activity. These early signaling events are associated with cell spreading, cycles of GTPase activation and inactivation, which occur concurrent with the formation, maturation, and eventual turnover of FAs [59]. All of these events must be precisely coordinated to enable efficient directional cell movement.
Canonical cell migration models postulate that Rac promotes membrane protrusion at the leading edge and Rho regulates contractility in the cell body [7]. However, studies with FRET-based probes for Rho GTPases revealed high levels of RhoA activity at both the leading and trailing edges of cells [60]. The occurrence of high Rac and Rho activity at leading edge is likely cyclical and/or may occur at distinct sites. At the leading edge, Rac activation can provide the necessary “push” (decrease in cell contractility) needed for lamellipodial growth and Rho activation then facilitates the “pull” (increase in cell contractility) to stabilize growing lamellipodia in part through FA maturation [61].
Biochemically, cell adhesion to fibronectin (FN) initially triggers an overall transient decrease in RhoA activity levels (at 15 to 30 min), followed by an extended phase of RhoA activation associated with FA maturation [62, 63]. It is the coordination of GAP and GEF activity that promotes RhoA cyclic regulation upon FN binding. Interestingly, FAK is linked to FN-mediated cyclic RhoA regulation through associations with both p190RhoGAP [64] and Rgnef [29]. FAK expression and activity promoted FA localization and tyrosine phosphorylation of p190RhoGAP [34, 64] and this is associated with increased GAP activity, cell protrusion, and establishment of polarity [65]. The FAK-p190RhoGAP interaction is indirect and dependent upon the binding of p120RasGAP to both FAK and p190RhoGAP [64]. In the absence of FAK expression or activity, RhoA activity is high and deregulated [62]. In addition to the loss of p190RhoGAP regulation, FAK−/− fibroblasts exhibit high levels of Rgnef expression due in part to compensatory signaling from the FAK-related Pyk2 kinase [29]. Elevated Rgnef expression contributes to aberrant FAK−/− fibroblast morphology, RhoA activity, and increased FA formation. However, in normal fibroblasts, Rgnef knockdown prevents FN-stimulated RhoA regulation, FA formation, and cell motility [29]. Despite published putative roles for LARG (ARHGEF12), Lsc/p115 (ARHGEF1), and GEFH1 (ARHGEF2) in FN-stimulated RhoA regulation [66, 67], Rgnef knockout fibroblasts exhibit defects in FN-stimulated RhoA regulation that are rescued by Rgnef re-expression [44]. Taken together, these studies establish the importance of Rgnef in RhoA regulation downstream of integrins. Simplistically, too much or not enough Rgnef expression in cells inhibits cell movement, as the formation of overabundance or too few FAs limits cell motility.
COMPLEX INTERACTIONS BETWEEN RGNEF AND FAK
In this integrin-Rho signaling axis, it remains undetermined how Rgnef becomes activated to facilitate RhoA GTP binding. Using a binding assay with a nucleotide-free mutant of RhoA [66], Rgnef became activated 60 min after replating cells on FN [44]. Rgnef tyrosine phosphorylation after FN replating occurs at 60 min and this was disrupted by deletion of the FAK binding site (1292–1301) on Rgnef [29]. Rgnef tyrosine phosphorylation is associated with the localization of Rgnef to FAs and this is correlated with the ability of Rgnef to activate RhoA. However, the molecular mechanisms linking integrin signaling to Rgnef and RhoA activation is undetermined. In particular, it is not known how phosphorylation and the activity of different Rgnef domains act to control Rgnef function.
Despite over twenty years of research on FAK [68], the mechanisms through which FAK associates with integrin signaling complexes at FAs also remains unclear. Although FAK and paxillin co-localize to the earliest adhesions formed upon cell attachment to FN [69], other mutational and knockout studies have concluded that paxillin is important but not essential for FAK recruitment to nascent adhesions [70, 71]. Additionally, direct binding between FAK and talin may contribute to but is not essential for adhesion localization of FAK [72, 73]. It is the C-terminal region of FAK termed the focal adhesion targeting (FAT) domain that binds to paxillin and talin and facilitates FAK localization to integrin adhesion sites. The FAK FAT domain also binds to Rgnef residues 1292–1301 [28].
Interestingly, Rgnef Δ1292–1301 over-expression results in a similar phenotype to neurons that lack FAK [74]. This result was originally interpreted as Rgnef being downstream of FAK and that Δ1292–1301 Rgnef would block signaling leading to RhoA activation. However, an alternative possibility is that if Rgnef also functions upstream of FAK, expression of Rgnef Δ1292–1301 would not bind FAK and may inhibit FAK. To this end, recent studies in Rgnef−/− fibroblasts found that FAK activation (FAK Y397 phosphorylation) and paxillin tyrosine phosphorylation were inhibited at early time points (5 to 30 min) after cell adhesion to FN [38]. This was associated with decreased FAK co-localization at FAs. Rgnef mutagenesis and re-expression studies found that the Rgnef PH domain or FAK binding region were required as part of a mechanism promoting FAK FA localization, FAK activation, and paxillin tyrosine phosphorylation. Interestingly, Rgnef PH domain mutation (R1098A, K1100A) prevented phosphatidylinositol 4-P and phosphatidylinositol 4,5P2 binding and these residues are conserved within related GEFs (Fig. 1B). Modeling of the Rgnef DH-PH domain structure reveals that R1098 and K1100 may be located within a surface exposed pocket that could potentially form a phosphatidylinositol headgroup binding site (Fig. 1C). In this way, it is likely that Rgnef lipid binding and scaffolding play an unexpected but important role in promoting FAK recruitment and activation at FAs.
Moreover, re-expression of a GEF-inactivating Rgnef point mutation (Y1003A) [21] in Rgnef−/− fibroblasts was sufficient to promote FAK FA localization and activation upon cell adhesion to FN [38]. However, Rgnef Y1003A did not promote paxillin tyrosine phosphorylation. This separates FAK and paxillin tyrosine phosphorylation downstream of integrins. Interestingly, myosin II activity and the generation of cell tension promote FAK-mediated paxillin tyrosine phosphorylation leading to adhesion maturation and cytoskeletal-matrix linkage reinforcement [75]. Thus, since Rgnef−/− fibroblasts do not efficiently activate RhoA upon cell adhesion to FN [44], and RhoA activation of ROCK can stimulate cell tensional forces through myosin-mediated contractility [76], it may be that Rgnef-mediated RhoA activation allows for FAK-mediated paxillin tyrosine phosphorylation at FAs in response to contractility signals or FA maturation.
As summarized in a simplistic model (Fig. 1D), cell binding to matrix leads to integrin receptor clustering and activation. Signals are generated to increase phosphatidylinositol lipids within the plasma membrane near adhesion sites, and this facilitates Rgnef membrane association via the Rgnef PH domain. FAK binding to Rgnef is not regulated by cell adhesion, but the translocation of Rgnef to the membrane brings FAK to nascent adhesion sites and likely facilitates the formation of a complex between FAK and paxillin within FAs. Through processes that remain unclear, but may involve release of intramolecular inhibitory constraints [77] and intermolecular FAK transphosphorylation at Y397 [78], FAK becomes catalytically active. Rgnef-mediated RhoA activation and increased contractility facilitate FAK-mediated paxillin tyrosine phosphorylation important for FA maturation and the further recruitment of proteins such as vinculin to FAs. Inhibition of any of these steps prevents efficient cell movement.
RGNEF AND RHO - MORE THAN ONE CONNECTION
The recombinant DH-PH domain of Rgnef possesses exchange activity for RhoA and this is blocked by a point mutation (Y1003A) within the DH domain [21]. It is the DH domain that provides the canonical interface for Rho GTPase binding. PH domains bind to lipids and other protein targets [17]. Mutagenesis and in vitro binding assays have confirmed that the Rgnef PH domain binds phosphatidylinositol lipids and this is mediated in part by Rgnef residues R1098A and K1100A [38]. The PH domain of Rgnef also bound directly to activated RhoA and this was dependent on hydrophobic residues F1154 and I1156 [79]. In three-dimensional models of the Rgnef PH domain, this hydrophobic patch does not overlap with the R1098A and K1100A residues involved in phosphatidylinositol lipid binding (Fig. 1C). Interestingly, mutation of Rgnef F1154 and I1156 in the full-length protein also attenuated RhoA activation, as assayed by a serum-response element gene reporter, when compared to wild type Rgnef [79]. This RhoGEF-activated RhoA binding interaction is conserved within the Lbc-family of RhoGEFs. It is proposed that this interaction could serve as a positive feedback loop, perhaps working in tandem with PH domain lipid-binding residues to correctly orient RhoGEFs at the plasma membrane or relieving auto-inhibition. In fact, several unrelated proteins including RhoGEFs have been shown to bind to activated GTPases through their PH domain, suggesting that this could be a common regulatory mechanism [80–82]. It will be of interest to test whether this Rgnef hydrophobic patch regulates its subcellular localization and whether the Rgnef PH domain also binds efficiently to other GTPases such as RhoC. This adds another layer to the possible mechanisms by which RhoA and RhoC are spatiotemporally regulated in normal and transformed cells.
RGNEF AND FAK IN CANCER
Studies of the molecular mechanisms controlling FAK activation are of potential clinical importance due to the fact that FAK controls various aspects of tumor progression [83]. Small molecules that act as ATP-competitive inhibitors of FAK activity are in various stages of development and human clinical trials testing [84–88]. What remains unclear are the molecular mechanisms driving elevated FAK activation in tumor cells. Notably, Rgnef mRNA and protein expression are significantly increased during colorectal tumor progression and dominant-negative expression of the Rgnef C-terminal domain resulted in smaller, less invasive tumors with reduced paxillin tyrosine phosphorylation as analyzed in an orthotopic model [37]. This tumor inhibitory activity of Rgnef-C required the presence of the FAK binding site and we speculate it may be associated with the prevention of FAK or Rho GTPase activation. Early studies identified Dbl (ARHGEF21) in a cell transformation-based screen [89], various RhoGEFs are over-expressed in tumors [90], and small molecule inhibitors of RhoGEFs that disrupt binding to RhoGTPases are being developed [91]. Thus, targeted inhibition of RhoGEFs like Rgnef may result in dual inhibition of FAK and Rho GTPase signaling pathways.
Mechanistic screens for RhoGEF inhibitors include in vitro invasion assays, as RhoA and RhoC GTPases have been linked to an invasive cell phenotype [76]. In fact, recent studies point to the importance of a RhoA-FAK signaling axis in KRAS-driven non-small cell lung cancer (NSCLC) [92]. This study concluded that since RhoA silencing and FAK pharmaceutical inhibition yielded similar anti-tumor effects on NSCLC tumor bearing KRAS and INK4A/Arf mutations, that activation of a RhoA-FAK signaling axis is a genotype-specific vulnerability of high grade tumors. FAK activity is also an important factor promoting breast cancer tumor growth and metastasis [87, 93, 94]. Structures termed invadopodia on carcinoma cells degrade surrounding matrix and allow for enhanced tumor cell invasion [95]. In breast carcinoma cells, Rgnef was shown to activate RhoC to facilitate invadopodia formation [22]. Although functional connections between FAK and Rgnef have not been established in breast cancer, invasive matrix degradation is dependent upon FAK signaling [93, 96]. Understanding the mechanisms of Rgnef spatiotemporal regulation and interactions with FAK and RhoA or RhoC GTPases in vivo will provide new insights on the molecular pathways involved in cancer progression.
CONCLUDING REMARKS
In this review we have emphasized the dual function of Rgnef, which acts as a GEF for RhoA and RhoC, and plays a novel scaffolding role in FAK recruitment and activation. The Rgnef-FAK interaction is critical for both normal cell migration and tumorigenesis, as FAK contributes to several hallmarks of cancer, including survival, proliferation, angiogenesis, and invasion. Future studies will be aimed at understanding the molecular mechanisms behind Rgnef-FAK signaling in tumor progression to better understand how these pathways can be targeted in the future for more effective treatments.
Further, the recent discovery of novel RhoA-GTP binding patch on the PH domain provides a new opportunity to understand how Rgnef spatiotemporally regulates Rho GTPases, and vice versa. Due to recent evidence that a lipid-binding mutation in the PH domain prevents FAK membrane localization, further investigation of the role of the Rgnef PH domain with regard to lipid binding, necessity in promoting FAK activation, and interactions with RhoA/C in the context of tumor progression are warranted.
The use of Rgnef-null mouse and cell lines has provided a powerful system to dissect signaling pathways downstream of integrins at focal adhesions. Already, the use of these cells has revealed a novel method of FAK recruitment and allowed us to separate FAK and paxillin phosphorylation downstream of integrins for the first time. These knockout systems will be a valuable tool in examining the role of Rgnef and its binding partners in cellular signaling, development, and cancer.
ACKNOWLEDGEMENTS
This work was supported by NIH CA180769 to D.D.S. N.L.G.M. was supported by NIH 1F32CA159558.
ABBREVIATIONS
- ATP
adenosine triphosphate
- C-terminus
carboxy terminus
- Dbl
diffuse B-cell lymphoma
- DH
dbl-homology
- ECM
extracellular matrix
- FA
focal adhesion
- FAK
focal adhesion kinase
- FAT
focal adhesion kinase
- FN
fibronectin
- FRET
fluorescence resonance energy transfer
- GAP
GTPase activating protein
- GDP
guanosine diphosphate
- GTP
guanosine triphosphate
- NSCLC
non-small cell lung cancer
- PDZ
post synaptic density protein, disc large tumor suppressor, zona occludens-1
- PH
pleckstrin homology
- ROCK
Rho-associated protein kinase
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Moissoglu K, Schwartz MA. Integrin signalling in directed cell migration. Biol Cell. 2006;98:547–555. doi: 10.1042/BC20060025. [DOI] [PubMed] [Google Scholar]
- 2.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Revs Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 3.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 4.Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 2010;26:315–333. doi: 10.1146/annurev.cellbio.011209.122036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 9.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 11.Ligeti E, Welti S, Scheffzek K. Inhibition and termination of physiological responses by GTPase activating proteins. Physiological reviews. 2012;92:237–272. doi: 10.1152/physrev.00045.2010. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem. 2012;81:637–659. doi: 10.1146/annurev-biochem-052810-093700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rittinger K. Snapshots form a big picture of guanine nucleotide exchange. Sci Signal. 2009;2:pe63. doi: 10.1126/scisignal.291pe63. [DOI] [PubMed] [Google Scholar]
- 14.Pakes NK, Veltman DM, Williams RS. Zizimin and Dock guanine nucleotide exchange factors in cell function and disease. Small GTPases. 2013;4:22–27. doi: 10.4161/sgtp.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaiswal M, Dvorsky R, Ahmadian MR. Deciphering the molecular and functional basis of Dbl family proteins: a novel systematic approach toward classification of selective activation of the Rho family proteins. J Biol Chem. 2013;288:4486–4500. doi: 10.1074/jbc.M112.429746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soisson SM, Nimnual AS, Uy M, Bar-Sagi D, Kuriyan J. Crystal structure of the Dbl and pleckstrin homology domains from the human Son of sevenless protein. Cell. 1998;95:259–268. doi: 10.1016/s0092-8674(00)81756-0. [DOI] [PubMed] [Google Scholar]
- 17.Lemmon MA. Pleckstrin homology domains: not just for phosphoinositides. Biochem Soc Trans. 2004;32:707–711. doi: 10.1042/BST0320707. [DOI] [PubMed] [Google Scholar]
- 18.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 19.Viaud J, Gaits-Iacovoni F, Payrastre B. Regulation of the DH-PH tandem of guanine nucleotide exchange factor for Rho GTPases by phosphoinositides. Adv Biol Regul. 2012;52:303–314. doi: 10.1016/j.jbior.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Gebbink MF, Kranenburg O, Poland M, van Horck FP, Houssa B, Moolenaar WH. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J Cell Biol. 1997;137:1603–1613. doi: 10.1083/jcb.137.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Horck FP, Ahmadian MR, Haeusler LC, Moolenaar WH, Kranenburg O. Characterization of p190RhoGEF, a RhoA-specific guanine nucleotide exchange factor that interacts with microtubules. J Biol Chem. 2001;276:4948–4956. doi: 10.1074/jbc.M003839200. [DOI] [PubMed] [Google Scholar]
- 22.Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A Novel Spatiotemporal RhoC Activation Pathway Locally Regulates Cofilin Activity at Invadopodia. Curr Biol. 2011;21:635–644. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canete-Soler R, Wu J, Zhai J, Shamim M, Schlaepfer WW. p190RhoGEF Binds to a destabilizing element in the 3' untranslated region of light neurofilament subunit mRNA and alters the stability of the transcript. J Biol Chem. 2001;276:32046–32050. doi: 10.1074/jbc.M104104200. [DOI] [PubMed] [Google Scholar]
- 24.Zhai J, Lin H, Shamim M, Schlaepfer WW, Canete-Soler R. Identification of a novel interaction of 14-3-3 with p190RhoGEF. J Biol Chem. 2001;276:41318–41324. doi: 10.1074/jbc.M107709200. [DOI] [PubMed] [Google Scholar]
- 25.Meyer D, Liu A, Margolis B. Interaction of c-Jun amino-terminal kinase interacting protein-1 with p190RhoGEF and its localization in differentiated neurons. J Biol Chem. 1999;274:35113–35118. doi: 10.1074/jbc.274.49.35113. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Mata R, Burridge K. Catching a GEF by its tail. Trends Cell Biol. 2007;17:36–43. doi: 10.1016/j.tcb.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Chi CN, Bach A, Stromgaard K, Gianni S, Jemth P. Ligand binding by PDZ domains. Biofactors. 2012;38:338–348. doi: 10.1002/biof.1031. [DOI] [PubMed] [Google Scholar]
- 28.Zhai J, Lin H, Nie Z, Wu J, Canete-Soler R, Schlaepfer WW, et al. Direct interaction of focal adhesion kinase with p190RhoGEF. J Biol Chem. 2003;278:24865–24873. doi: 10.1074/jbc.M302381200. [DOI] [PubMed] [Google Scholar]
- 29.Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, et al. PyK2 and FAK connections to p190RhoGEF regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123:1007–1013. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 31.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 32.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Progress in Biophysics & Molecular Biology. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 33.Zhao X, Peng X, Sun S, Park AY, Guan JL. Role of kinase-independent and -dependent functions of FAK in endothelial cell survival and barrier function during embryonic development. J Cell Biol. 2010;189:955–965. doi: 10.1083/jcb.200912094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim ST, Chen XL, Tomar A, Miller NL, Yoo J, Schlaepfer DD. Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation. J Biol Chem. 2010;285:21526–21536. doi: 10.1074/jbc.M110.129999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, et al. VEGF-induced vascular permeability is mediated by FAK. Dev Cell. 2012;22:146–157. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons JT, Slack-Davis J, Tilghman R, Roberts WG. Focal adhesion kinase: targeting adhesion signaling pathways for therapeutic intervention. Clin Cancer Res. 2008;14:627–632. doi: 10.1158/1078-0432.CCR-07-2220. [DOI] [PubMed] [Google Scholar]
- 37.Yu HG, Nam JO, Miller NL, Tanjoni I, Walsh C, Shi L, et al. p190RhoGEF (Rgnef) promotes colon carcinoma tumor progression via interaction with focal adhesion kinase. Cancer Res. 2011;71:360–370. doi: 10.1158/0008-5472.CAN-10-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller NL. A non-canonical role for Rgnef (190RhoGEF) in promoting integrin-stimulated focal adhesion kinase activation. 2013 doi: 10.1242/jcs.135509. (in revision). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perrimon N, Pitsouli C, Shilo BZ. Signaling mechanisms controlling cell fate and embryonic patterning. CSH Perspect Biol. 2012;4:a005975. doi: 10.1101/cshperspect.a005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139:3471–3486. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- 41.Liu AX, Rane N, Liu JP, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol Cell Biol. 2001;21:6906–6912. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen E, Brakebusch C. Rho GTPase function in development: how in vivo models change our view. Exp Cell Res. 2012;318:1779–1787. doi: 10.1016/j.yexcr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Hakem A, Sanchez-Sweatman O, You-Ten A, Duncan G, Wakeham A, Khokha R, et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes & development. 2005;19:1974–1979. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller NL, Lawson C, Chen XL, Lim ST, Schlaepfer DD. Rgnef (p190RhoGEF) knockout inhibits RhoA activity, focal adhesion establishment, and cell motility downstream of integrins. PLoS One. 2012;7:e37830. doi: 10.1371/journal.pone.0037830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samson T, van Buul JD, Kroon J, Welch C, Bakker EN, Matlung HL, et al. The guanine-nucleotide exchange factor SGEF plays a crucial role in the formation of atherosclerosis. PLoS One. 2013;8:e55202. doi: 10.1371/journal.pone.0055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H, Han JR, Park J, Oh M, James SE, Chang S, et al. Delta-catenin-induced dendritic morphogenesis. An essential role of p190RhoGEF interaction through Akt1-mediated phosphorylation. J Biol Chem. 2008;283:977–987. doi: 10.1074/jbc.M707158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Droppelmann CA, Keller BA, Campos-Melo D, Volkening K, Strong MJ. Rho guanine nucleotide exchange factor is an NFL mRNA destabilizing factor that forms cytoplasmic inclusions in amyotrophic lateral sclerosis. Neurobiol Aging. 2013;34:248–262. doi: 10.1016/j.neurobiolaging.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 48.Lee JR, Ha YJ, Kim HJ. Cutting edge: induced expression of a RhoA-specific guanine nucleotide exchange factor, p190RhoGEF, following CD40 stimulation and WEHI 231 B cell activation. J Immunol. 2003;170:19–23. doi: 10.4049/jimmunol.170.1.19. [DOI] [PubMed] [Google Scholar]
- 49.Ha YJ, Jeong JH, Park Y, Lee JR. Increased p190RhoGEF expression in activated B cells correlates with the induction of the plasma cell differentiation. Exp Mol Med. 2012;44:138–148. doi: 10.3858/emm.2012.44.2.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayers CM, Wadell J, McLean K, Venere M, Malik M, Shibata T, et al. The Rho guanine nucleotide exchange factor AKAP13 (BRX) is essential for cardiac development in mice. J Biol Chem. 2010;285:12344–12354. doi: 10.1074/jbc.M110.106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qian X, Esteban L, Vass WC, Upadhyaya C, Papageorge AG, Yienger K, et al. The Sos1 and Sos2 Ras-specific exchange factors: differences in placental expression and signaling properties. EMBO J. 2000;19:642–654. doi: 10.1093/emboj/19.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cook DR, Solski PA, Bultman SJ, Kauselmann G, Schoor M, Kuehn R, et al. The ect2 rho Guanine nucleotide exchange factor is essential for early mouse development and normal cell cytokinesis and migration. Genes & cancer. 2011;2:932–942. doi: 10.1177/1947601912437035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Brien SP, Seipel K, Medley QG, Bronson R, Segal R, Streuli M. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc Natl Acad Sci USA. 2000;97:12074–12078. doi: 10.1073/pnas.97.22.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, et al. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, et al. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubtsov A, Strauch P, Digiacomo A, Hu J, Pelanda R, Torres RM. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity. 2005;23:527–538. doi: 10.1016/j.immuni.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Francis SA, Shen X, Young JB, Kaul P, Lerner DJ. Rho GEF Lsc is required for normal polarization, migration, and adhesion of formyl-peptide-stimulated neutrophils. Blood. 2006;107:1627–1635. doi: 10.1182/blood-2005-03-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 59.Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 60.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 61.Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol. 2009;21:676–683. doi: 10.1016/j.ceb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Sci. 2005;118:4917–4919. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- 64.Tomar A, Lim ST, Lim Y, Schlaepfer DD. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J Cell Sci. 2009;122:1852–1862. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell. 2001;12:2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dubash AD, Wennerberg K, Garcia-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. J Cell Sci. 2007;120:3989–3998. doi: 10.1242/jcs.003806. [DOI] [PubMed] [Google Scholar]
- 67.Nalbant P, Chang YC, Birkenfeld J, Chang ZF, Bokoch GM. Guanine nucleotide exchange factor-H1 regulates cell migration via localized activation of RhoA at the leading edge. Molecular Biology of the Cell. 2009;20:4070–4082. doi: 10.1091/mbc.E09-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 69.Choi CK, Zareno J, Digman MA, Gratton E, Horwitz AR. Cross-correlated fluctuation analysis reveals phosphorylation-regulated paxillin-FAK complexes in nascent adhesions. Biophys J. 2011;100:583–592. doi: 10.1016/j.bpj.2010.12.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, et al. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901–915. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scheswohl DM, Harrell JR, Rajfur Z, Gao G, Campbell SL, Schaller MD. Multiple paxillin binding sites regulate FAK function. J Mol Signal. 2008;3:1. doi: 10.1186/1750-2187-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lawson C, Lim ST, Uryu S, Chen XL, Calderwood DA, Schlaepfer DD. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J Cell Biol. 2012;196:223–232. doi: 10.1083/jcb.201108078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawson C, Schlaepfer DD. Integrin adhesions: who's on first? What's on second? Connections between FAK and talin. Cell Adh Migr. 2012;6:302–306. doi: 10.4161/cam.20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nature neuroscience. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188:877–890. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 77.Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nature Rev Mol Cell Biol. 2010;11:802–814. doi: 10.1038/nrm2996. [DOI] [PubMed] [Google Scholar]
- 78.Toutant M, Costa A, Studler JM, Kadare G, Carnaud M, Girault JA. Alternative splicing controls the mechanisms of FAK autophosphorylation. Mol Cell Biol. 2002;22:7731–7743. doi: 10.1128/MCB.22.22.7731-7743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Medina F, Carter AM, Dada O, Gutowski S, Hadas J, Chen Z, et al. Activated RhoA is a Positive Feedback Regulator of the Lbc family of RhoGEFs. J Biol Chem. 2013 doi: 10.1074/jbc.M113.450056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jaffe AB, Aspenstrom P, Hall A. Human CNK1 acts as a scaffold protein, linking Rho and Ras signal transduction pathways. Mol Cell Biol. 2004;24:1736–1746. doi: 10.1128/MCB.24.4.1736-1746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jezyk MR, Snyder JT, Gershberg S, Worthylake DK, Harden TK, Sondek J. Crystal structure of Rac1 bound to its effector phospholipase C-beta2. Nat Struct Mol Biol. 2006;13:1135–1140. doi: 10.1038/nsmb1175. [DOI] [PubMed] [Google Scholar]
- 82.Chen Z, Medina F, Liu MY, Thomas C, Sprang SR, Sternweis PC. Activated RhoA binds to the pleckstrin homology (PH) domain of PDZ-RhoGEF, a potential site for autoregulation. The Journal of biological chemistry. 2010;285:21070–21081. doi: 10.1074/jbc.M110.122549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Met Rev. 2009;28:35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
- 84.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68:1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 85.Halder J, Lin YG, Merritt WM, Spannuth WA, Nick AM, Honda T, et al. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67:10976–10983. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]
- 86.Tanjoni I, Walsh C, Uryu S, Tomar A, Nam JO, Mielgo A, et al. PND-1186 FAK inhibitor selectively promotes tumor cell apoptosis in three-dimensional environments. Cancer Biol Ther. 2010;9:764–777. doi: 10.4161/cbt.9.10.11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walsh C, Tanjoni I, Uryu S, Tomar A, Nam JO, Luo H, et al. Oral delivery of PND-1186 FAK inhibitor decreases tumor growth and spontaneous breast to lung metastasis in pre-clinical models. Cancer Biol Ther. 2010;9:778–790. doi: 10.4161/cbt.9.10.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ward KK, Tancioni I, Lawson C, Miller NL, Jean C, Chen XL, et al. Inhibition of focal adhesion kinase (FAK) activity prevents anchorage-independent ovarian carcinoma cell growth and tumor progression. Clinical & experimental metastasis. 2012 doi: 10.1007/s10585-012-9562-5. PMID: 23275034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eva A, Vecchio G, Rao CD, Tronick SR, Aaronson SA. The predicted DBL oncogene product defines a distinct class of transforming proteins. Proc Natl Acad Sci USA. 1988;85:2061–2065. doi: 10.1073/pnas.85.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barrio-Real L, Kazanietz MG. Rho GEFs and cancer: linking gene expression and metastatic dissemination. Sci Signal. 2012;5:pe43. doi: 10.1126/scisignal.2003543. [DOI] [PubMed] [Google Scholar]
- 91.Shang X, Marchioni F, Evelyn CR, Sipes N, Zhou X, Seibel W, et al. Small-molecule inhibitors targeting Gprotein- coupled Rho guanine nucleotide exchange factors. Proc Natl Acad Sci U S A. 2013;110:3155–3160. doi: 10.1073/pnas.1212324110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Konstantinidou G, Ramadori G, Torti F, Kangasniemi K, Ramirez RE, Cai Y, et al. RHOA-FAK Is a Required Signaling Axis for the Maintenance of KRAS-Driven Lung Adenocarcinomas. Cancer Discov. 2013;3:444–457. doi: 10.1158/2159-8290.CD-12-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mitra SK, Lim ST, Chi A, Schlaepfer DD. Intrinsic focal adhesion kinase activity controls orthotopic breast carcinoma metastasis via the regulation of urokinase plasminogen activator expression in a syngeneic tumor model. Oncogene. 2006;25:4429–4440. doi: 10.1038/sj.onc.1209482. [DOI] [PubMed] [Google Scholar]
- 94.Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest. 2009;119:252–266. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clinical & experimental metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAKp130Cas complex. J Cell Biol. 2012;196:375–385. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 98.Missy K, Hu B, Schilling K, Harenberg A, Sakk V, Kuchenbecker K, et al. AlphaPIX Rho GTPase guanine nucleotide exchange factor regulates lymphocyte functions and antigen receptor signaling. Mol Cell Biol. 2008;28:3776–3789. doi: 10.1128/MCB.00507-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramakers GJ, Wolfer D, Rosenberger G, Kuchenbecker K, Kreienkamp HJ, Prange-Kiel J, et al. Dysregulation of Rho GTPases in the alphaPix/Arhgef6 mouse model of X-linked intellectual disability is paralleled by impaired structural and synaptic plasticity and cognitive deficits. Hum Mol Genet. 2012;21:268–286. doi: 10.1093/hmg/ddr457. [DOI] [PubMed] [Google Scholar]
- 100.Taymans JM. The GTPase function of LRRK2. Biochem Soc Trans. 2012;40:1063–1069. doi: 10.1042/BST20120133. [DOI] [PubMed] [Google Scholar]
- 101.Kaartinen V, Gonzalez-Gomez I, Voncken JW, Haataja L, Faure E, Nagy A, et al. Abnormal function of astroglia lacking Abr and Bcr RacGAPs. Development. 2001;128:4217–4227. doi: 10.1242/dev.128.21.4217. [DOI] [PubMed] [Google Scholar]
- 102.Kaartinen V, Nagy A, Gonzalez-Gomez I, Groffen J, Heisterkamp N. Vestibular dysgenesis in mice lacking Abr and Bcr Cdc42/RacGAPs. Dev Dyn. 2002;223:517–525. doi: 10.1002/dvdy.10071. [DOI] [PubMed] [Google Scholar]
- 103.Devon RS, Orban PC, Gerrow K, Barbieri MA, Schwab C, Cao LP, et al. Als2-deficient mice exhibit disturbances in endosome trafficking associated with motor behavioral abnormalities. Proc Natl Acad Sci U S A. 2006;103:9595–9600. doi: 10.1073/pnas.0510197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hadano S, Yoshii Y, Otomo A, Kunita R, Suzuki-Utsunomiya K, Pan L, et al. Genetic background and gender effects on gross phenotypes in congenic lines of ALS2/alsin-deficient mice. Neurosci Res. 2010;68:131–136. doi: 10.1016/j.neures.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 105.Arthur WT, Ellerbroek SM, Der CJ, Burridge K, Wennerberg K. XPLN, a guanine nucleotide exchange factor for RhoA and RhoB, but not RhoC. J Biol Chem. 2002;277:42964–42972. doi: 10.1074/jbc.M207401200. [DOI] [PubMed] [Google Scholar]
- 106.Serbanovic-Canic J, Cvejic A, Soranzo N, Stemple DL, Ouwehand WH, Freson K. Silencing of RhoA nucleotide exchange factor, ARHGEF3, reveals its unexpected role in iron uptake. Blood. 2011;118:4967–4976. doi: 10.1182/blood-2011-02-337295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aoki T, Ueda S, Kataoka T, Satoh T. Regulation of mitotic spindle formation by the RhoA guanine nucleotide exchange factor ARHGEF10. BMC cell biology. 2009;10:56. doi: 10.1186/1471-2121-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Winkler S, Mohl M, Wieland T, Lutz S. GrinchGEF--a novel Rho-specific guanine nucleotide exchange factor. Biochem Biophys Res Commun. 2005;335:1280–1286. doi: 10.1016/j.bbrc.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 109.Oliver AW, He X, Borthwick K, Donne AJ, Hampson L, Hampson IN. The HPV16 E6 binding protein Tip-1 interacts with ARHGEF16, which activates Cdc42. Br J Cancer. 2011;104:324–331. doi: 10.1038/sj.bjc.6606026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harada K, Hiramoto-Yamaki N, Negishi M, Katoh H. Ephexin4 and EphA2 mediate resistance to anoikis through RhoG and phosphatidylinositol 3-kinase. Exp Cell Res. 2011;317:1701–1713. doi: 10.1016/j.yexcr.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 111.Kawasaki Y, Jigami T, Furukawa S, Sagara M, Echizen K, Shibata Y, et al. The Adenomatous Polyposis Coli-associated Guanine Nucleotide Exchange Factor Asef Is Involved in Angiogenesis. Journal of Biological Chemistry. 2010;285:1199–1207. doi: 10.1074/jbc.M109.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kawasaki Y, Sagara M, Shibata Y, Shirouzu M, Yokoyama S, Akiyama T. Identification and characterization of Asef2, a guanine-nucleotide exchange factor specific for Rac1 and Cdc42. Oncogene. 2007;26:7620–7267. doi: 10.1038/sj.onc.1210574. [DOI] [PubMed] [Google Scholar]
- 113.Kawasaki Y, Tsuji S, Muroya K, Furukawa S, Shibata Y, Okuno M, et al. The adenomatous polyposis coli-associated exchange factors Asef and Asef2 are required for adenoma formation in Apc(Min/+) mice. Embo Reports. 2009;10:1355–1362. doi: 10.1038/embor.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cho YJ, Cunnick JM, Yi SJ, Kaartinen V, Groffen J, Heisterkamp N. Abr and Bcr, two homologous Rac GTPase-activating proteins, control multiple cellular functions of murine macrophages. Mol Cell Biol. 2007;27:899–911. doi: 10.1128/MCB.00756-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang H, Li Y, Wang Y, Han ZG, Cai B. C9orf100, a new member of the Dbl-family guanine nucleotide exchange factors, promotes cell proliferation and migration in hepatocellular carcinoma. Mol Med Rep. 2012;5:1169–1174. doi: 10.3892/mmr.2012.783. [DOI] [PubMed] [Google Scholar]
- 116.Hirsch E, Pozzato M, Vercelli A, Barberis L, Azzolino O, Russo C, et al. Defective dendrite elongation but normal fertility in mice lacking the Rho-like GTPase activator Dbl. Mol Cell Biol. 2002;22:3140–3148. doi: 10.1128/MCB.22.9.3140-3148.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Whitehead LP, Lambert QT, Glaven JA, Abe K, Rossman KL, Mahon GM, et al. Dependence of Dbl and Dbs transformation on MEK and NF-kappa B activation. Mol Cell Biol. 1999;19:7759–7770. doi: 10.1128/mcb.19.11.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Z, Adams HC, 3rd, Whitehead IP. The rho-specific guanine nucleotide exchange factor Dbs regulates breast cancer cell migration. J Biol Chem. 2009;284:15771–15780. doi: 10.1074/jbc.M901853200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salazar MA, Kwiatkowski AV, Pellegrini L, Cestra G, Butler MH, Rossman KL, et al. Tuba, a novel protein containing bin/amphiphysin/Rvs and Dbl homology domains, links dynamin to regulation of the actin cytoskeleton. J Biol Chem. 2003;278:49031–49043. doi: 10.1074/jbc.M308104200. [DOI] [PubMed] [Google Scholar]
- 120.Srougi MC, Burridge K. The Nuclear Guanine Nucleotide Exchange Factors Ect2 and Net1 Regulate RhoB-Mediated Cell Death after DNA Damage. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shi L, Butt B, Ip FCF, Dai Y, Jiang LW, Yung WH, et al. Ephexin1 Is Required for Structural Maturation and Neurotransmission at the Neuromuscular Junction. Neuron. 2010;65:204–216. doi: 10.1016/j.neuron.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koyano Y, Kawamoto T, Shen M, Yan W, Noshiro M, Fujii K, et al. Molecular cloning and characterization of CDEP, a novel human protein containing the ezrin-like domain of the band 4.1 superfamily and the Dbl homology domain of Rho guanine nucleotide exchange factors. Biochem Biophys Res Commun. 1997;241:369–375. doi: 10.1006/bbrc.1997.7826. [DOI] [PubMed] [Google Scholar]
- 123.Cheadle L, Biederer T. The novel synaptogenic protein Farp1 links postsynaptic cytoskeletal dynamics and transsynaptic organization. J Cell Biol. 2012;199:985–1001. doi: 10.1083/jcb.201205041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kubo T, Yamashita T, Yamaguchi A, Sumimoto H, Hosokawa K, Tohyama M. A novel FERM domain including guanine nucleotide exchange factor is involved in Rac signaling and regulates neurite remodeling. Journal of Neuroscience. 2002;22:8504–8513. doi: 10.1523/JNEUROSCI.22-19-08504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takegahara N, Kang S, Nojima S, Takamatsu H, Okuno T, Kikutani H, et al. Integral roles of a guanine nucleotide exchange factor, FARP2, in osteoclast podosome rearrangements. Faseb Journal. 2010;24:4782–4792. doi: 10.1096/fj.10-158212. [DOI] [PubMed] [Google Scholar]
- 126.He X, Kuo YC, Rosche TJ, Zhang X. Structural Basis for Autoinhibition of the Guanine Nucleotide Exchange Factor FARP2. Structure. 2013;21:355–364. doi: 10.1016/j.str.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Olson MF, Pasteris NG, Gorski JL, Hall A. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr Biol. 1996;6:1628–1633. doi: 10.1016/s0960-9822(02)70786-0. [DOI] [PubMed] [Google Scholar]
- 128.Huber C, Martensson A, Bokoch GM, Nemazee D, Gavin AL. FGD2, a CDC42-specific exchange factor expressed by antigen-presenting cells, localizes to early endosomes and active membrane ruffles. J Biol Chem. 2008;283:34002–34012. doi: 10.1074/jbc.M803957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hayakawa M, Matsushima M, Hagiwara H, Oshima T, Fujino T, Ando K, et al. Novel insights into FGD3, a putative GEF for Cdc42, that undergoes SCFFWD1/beta-TrCP-mediated proteasomal degradation analogous to that of its homologue FGD1 but regulates cell morphology and motility differently from FGD1. Genes to Cells. 2008;13:329–342. doi: 10.1111/j.1365-2443.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 130.Horn M, Baumann R, Pereira JA, Sidiropoulos PNM, Somandin C, Welzl H, et al. Myelin is dependent on the Charcot-Marie-Tooth Type 4H disease culprit protein FRABIN/FGD4 in Schwann cells. Brain. 2012;135:3567–3583. doi: 10.1093/brain/aws275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheng C, Haasdijk R, Tempel D, van de Kamp EHM, Herpers R, Bos F, et al. Endothelial Cell-Specific FGD5 Involvement in Vascular Pruning Defines Neovessel Fate in Mice. Circulation. 2012;125 doi: 10.1161/CIRCULATIONAHA.111.064030. 3142-+. [DOI] [PubMed] [Google Scholar]
- 132.Aijaz S, D'Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell. 2005;8:777–786. doi: 10.1016/j.devcel.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 133.Li H, Oliver T, Jia W, He YW. Efficient dendritic cell priming of T lymphocytes depends on the extracellular matrix protein mindin. EMBO J. 2006;25:4097–4107. doi: 10.1038/sj.emboj.7601289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Reid T, Bathoorn A, Ahmadian MR, Collard JG. Identification and characterization of hPEM-2, a guanine nucleotide exchange factor specific for Cdc42. J Biol Chem. 1999;274:33587–33593. doi: 10.1074/jbc.274.47.33587. [DOI] [PubMed] [Google Scholar]
- 135.Shimojima K, Sugawara M, Shichiji M, Mukaida S, Takayama R, Imai K, et al. Loss-of-function mutation of collybistin is responsible for X-linked mental retardation associated with epilepsy. J Hum Genet. 2011;56:561–565. doi: 10.1038/jhg.2011.58. [DOI] [PubMed] [Google Scholar]
- 136.Hussain NK, Jenna S, Glogauer M, Quinn CC, Wasiak S, Guipponi M, et al. Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol. 2001;3:927–932. doi: 10.1038/ncb1001-927. [DOI] [PubMed] [Google Scholar]
- 137.Yu Y, Chu PY, Bowser DN, Keating DJ, Dubach D, Harper I, et al. Mice deficient for the chromosome 21 ortholog Itsn1 exhibit vesicle-trafficking abnormalities. Human Molecular Genetics. 2008;17:3281–3290. doi: 10.1093/hmg/ddn224. [DOI] [PubMed] [Google Scholar]
- 138.McGavin MK, Badour K, Hardy LA, Kubiseski TJ, Zhang J, Siminovitch KA. The intersectin 2 adaptor links Wiskott Aldrich Syndrome protein (WASp)-mediated actin polymerization to T cell antigen receptor endocytosis. J Exp Med. 2001;194:1777–1787. doi: 10.1084/jem.194.12.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cahill ME, Xie Z, Day M, Photowala H, Barbolina MV, Miller CA, et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A. 2009;106:13058–13063. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nature Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 141.D'Angelo R, Aresta S, Blangy A, Del Maestro L, Louvard D, Arpin M. Interaction of ezrin with the novel guanine nucleotide exchange factor PLEKHG6 promotes RhoG-dependent apical cytoskeleton rearrangements in epithelial cells. Mol Biol Cell. 2007;18:4780–4793. doi: 10.1091/mbc.E06-12-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu D, Asiedu M, Wei Q. Myosin-interacting guanine exchange factor (MyoGEF) regulates the invasion activity of MDA-MB-231 breast cancer cells through activation of RhoA and RhoC. Oncogene. 2009;28:2219–2230. doi: 10.1038/onc.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Alberts AS, Treisman R. Activation of RhoA and SAPK/JNK signalling pathways by the RhoA-specific exchange factor mNET1. EMBO J. 1998;17:4075–4085. doi: 10.1093/emboj/17.14.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kawata H, Shimada N, Kamiakito T, Komatsu K, Morita T, Ota T, et al. RhoC and guanine nucleotide exchange factor Net1 in androgen-unresponsive mouse mammary carcinoma SC-4 cells and human prostate cancer after short-term endocrine therapy. Prostate. 2012;72:1071–1079. doi: 10.1002/pros.21511. [DOI] [PubMed] [Google Scholar]
- 145.Coisy-Quivy M, Touzet O, Bourret A, Hipskind RA, Mercier J, Fort P, et al. TC10 controls human myofibril organization and is activated by the sarcomeric RhoGEF obscurin. J Cell Sci. 2009;122:947–956. doi: 10.1242/jcs.040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ford-Speelman DL, Roche JA, Bowman AL, Bloch RJ. The rho-guanine nucleotide exchange factor domain of obscurin activates rhoA signaling in skeletal muscle. Mol Biol Cell. 2009;20:3905–3917. doi: 10.1091/mbc.E08-10-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lange S, Ouyang K, Meyer G, Cui L, Cheng H, Lieber RL, et al. Obscurin determines the architecture of the longitudinal sarcoplasmic reticulum. J Cell Sci. 2009;122:2640–2650. doi: 10.1242/jcs.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Randazzo D, Giacomello E, Lorenzini S, Rossi D, Pierantozzi E, Blaauw B, et al. Obscurin is required for ankyrinB-dependent dystrophin localization and sarcolemma integrity. J Cell Biol. 2013;200:523–536. doi: 10.1083/jcb.201205118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Welch HCE, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, et al. P-Rex1, a PtdIns(3,4,5)P-3- and G beta gamma-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108:809–821. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 150.Dong X, Mo Z, Bokoch G, Guo C, Li Z, Wu D. P-Rex1 is a primary Rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr Biol. 2005;15:1874–1879. doi: 10.1016/j.cub.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 151.Naikawadi RP, Cheng N, Vogel SM, Qian F, Wu D, Malik AB, et al. A critical role for phosphatidylinositol (3,4,5)-trisphosphate-dependent Rac exchanger 1 in endothelial junction disruption and vascular hyperpermeability. Circ Res. 2012;111:1517–1527. doi: 10.1161/CIRCRESAHA.112.273078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Qian F, Le Breton GC, Chen J, Deng J, Christman JW, Wu D, et al. Role for the guanine nucleotide exchange factor phosphatidylinositol-3,4,5-trisphosphate-dependent rac exchanger 1 in platelet secretion and aggregation. Arterioscler Thromb Vasc Biol. 2012;32:768–777. doi: 10.1161/ATVBAHA.111.243675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rosenfeldt H, Vazquez-Prado J, Gutkind JS. P-REX2, a novel PI-3-kinase sensitive Rac exchange factor. FEBS Lett. 2004;572:167–171. doi: 10.1016/j.febslet.2004.06.097. [DOI] [PubMed] [Google Scholar]
- 154.Donald S, Humby T, Fyfe I, Segonds-Pichon A, Walker SA, Andrews SR, et al. P-Rex2 regulates Purkinje cell dendrite morphology and motor coordination. Proc Natl Acad Sci U S A. 2008;105:4483–4488. doi: 10.1073/pnas.0712324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Niu J, Profirovic J, Pan H, Vaiskunaite R, Voyno-Yasenetskaya T. G Protein betagamma subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: regulation of cell shape and reactive oxygen species production. Circ Res. 2003;93:848–856. doi: 10.1161/01.RES.0000097607.14733.0C. [DOI] [PubMed] [Google Scholar]
- 156.Mikelis CM, Palmby TR, Simaan M, Li W, Szabo R, Lyons R, et al. PDZ-RhoGEF and LARG are essential for embryo development. J Biol Chem. 2013 doi: 10.1074/jbc.M112.428599. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rumenapp U, Freichel-Blomquist A, Wittinghofer B, Jakobs KH, Wieland T. A mammalian Rho-specific guanine-nucleotide exchange factor (p164-RhoGEF) without a pleckstrin homology domain. Biochem J. 2002;366:721–728. doi: 10.1042/BJ20020654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mitin N, Rossman KL, Der CJ. Identification of a Novel Actin-Binding Domain within the Rho Guanine Nucleotide Exchange Factor TEM4. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Souchet M, Portales-Casamar E, Mazurais D, Schmidt S, Leger I, Javre JL, et al. Human p63RhoGEF, a novel RhoA-specific guanine nucleotide exchange factor, is localized in cardiac sarcomere. J Cell Sci. 2002;115:629–640. doi: 10.1242/jcs.115.3.629. [DOI] [PubMed] [Google Scholar]
- 160.Rumenapp U, Blomquist A, Schworer G, Schablowski H, Psoma A, Jakobs KH. Rho-specific binding and guanine nucleotide exchange catalysis by KIAA0380, a Db1 family member. FEBS Lett. 1999;459:313–318. doi: 10.1016/s0014-5793(99)01270-3. [DOI] [PubMed] [Google Scholar]
- 161.Ueda H, Nagae R, Kozawa M, Morishita R, Kimura S, Nagase T, et al. Heterotrimeric G protein betagamma subunits stimulate FLJ00018, a guanine nucleotide exchange factor for Rac1 and Cdc42. J Biol Chem. 2008;283:1946–1953. doi: 10.1074/jbc.M707037200. [DOI] [PubMed] [Google Scholar]
- 162.Lybaek H, Oyen N, Fauske L, Houge G. A 2.1 Mb deletion adjacent but distal to a 14q21q23 paracentric inversion in a family with spherocytosis and severe learning difficulties. Clinical Genetics. 2008;74:553–559. doi: 10.1111/j.1399-0004.2008.01072.x. [DOI] [PubMed] [Google Scholar]
- 163.Griswold AJ, Ma DQ, Sacharow SJ, Robinson JL, Jaworski JM, Wright HH, et al. A De Novo 1.5 Mb Microdeletion on Chromosome 14q23.2–23.3 in a Patient With Autism and Spherocytosis. Autism Research. 2011;4:221–227. doi: 10.1002/aur.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ishikawa K, Toru S, Tsunemi T, Li MS, Kobayashi K, Yokota T, et al. An autosomal dominant cerebellar ataxia linked to chromosome 16q22.1 is associated with a single-nucleotide substitution in the 5 '-untranslated region of the gene encoding a protein with spectrin repeat and Rho guanine-nucleotide exchange-factor domains. American Journal of Human Genetics. 2005;77:280–296. doi: 10.1086/432518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.De Toledo M, Coulon V, Schmidt S, Fort P, Blangy A. The gene for a new brain specific RhoA exchange factor maps to the highly unstable chromosomal region 1p36.2–1p36.3. Oncogene. 2001;20:7307–7317. doi: 10.1038/sj.onc.1204921. [DOI] [PubMed] [Google Scholar]
- 166.Maystadt I, Rezsohazy R, Barkats M, Duque S, Vannuffel P, Remacle S, et al. The nuclear factor kappaB-activator gene PLEKHG5 is mutated in a form of autosomal recessive lower motor neuron disease with childhood onset. Am J Hum Genet. 2007;81:67–76. doi: 10.1086/518900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fam NP, Fan WT, Wang Z, Zhang LJ, Chen H, Moran MF. Cloning and characterization of Ras-GRF2, a novel guanine nucleotide exchange factor for Ras. Mol Cell Biol. 1997;17:1396–1406. doi: 10.1128/mcb.17.3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brambilla R, Gnesutta N, Minichiello L, White G, Roylance AJ, Herron CE, et al. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- 169.Itier JM, Tremp GL, Leonard JF, Multon MC, Ret G, Schweighoffer F, et al. Imprinted gene in postnatal growth role. Nature. 1998;393:125–126. doi: 10.1038/30120. [DOI] [PubMed] [Google Scholar]
- 170.de Mora JF, Esteban LM, Burks DJ, Nunez A, Garces C, Garcia-Barrado MJ, et al. Ras-GRF1 signaling is required for normal beta-cell development and glucose homeostasis. EMBO J. 2003;22:3039–3049. doi: 10.1093/emboj/cdg280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fernandez-Medarde A, Barhoum R, Riquelme R, Porteros A, Nunez A, de Luis A, et al. RasGRF1 disruption causes retinal photoreception defects and associated transcriptomic alterations. Journal of Neurochemistry. 2009;110:641–652. doi: 10.1111/j.1471-4159.2009.06162.x. [DOI] [PubMed] [Google Scholar]
- 172.Borras C, Monleon D, Lopez-Grueso R, Gambini J, Orlando L, Pallardo FV, et al. RasGrf1 deficiency delays dging in mice. Aging-Us. 2011;3:262–276. doi: 10.18632/aging.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fan WT, Koch CA, de Hoog CL, Fam NP, Moran MF. The exchange factor Ras-GRF2 activates Ras-dependent and Rac-dependent mitogen-activated protein kinase pathways. Curr Biol. 1998;8:935–938. doi: 10.1016/s0960-9822(07)00376-4. [DOI] [PubMed] [Google Scholar]
- 174.Ruiz S, Santos E, Bustelo XR. RasGRF2, a guanosine nucleotide exchange factor for Ras GTPases, participates in T-cell signaling responses. Mol Cell Biol. 2007;27:8127–8142. doi: 10.1128/MCB.00912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ellerbroek SM, Wennerberg K, Arthur WT, Dunty JM, Bowman DR, DeMali KA, et al. SGEF, a RhoG guanine nucleotide exchange factor that stimulates macropinocytosis. Mol Biol Cell. 2004;15:3309–3319. doi: 10.1091/mbc.E04-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Samson T, van Buul JD, Kroon J, Welch C, Bakker EN, Matlung HL, et al. The Guanine-Nucleotide Exchange Factor SGEF Plays a Crucial Role in the Formation of Atherosclerosis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Curtis C, Hemmeryckx B, Haataja L, Senadheera D, Groffen J, Heisterkamp N. Scambio, a novel guanine nucleotide exchange factor for Rho. Mol Cancer. 2004;3:10. doi: 10.1186/1476-4598-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AW, Weinberg RA. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993;363:45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- 179.Nimnual AS, Yatsula BA, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 180.Esteban LM, Fernandez-Medarde A, Lopez E, Yienger K, Guerrero C, Ward JM, et al. Ras-guanine nucleotide exchange factor Sos2 is dispensable for mouse growth and development. Mol Cell Biol. 2000;20:6410–6413. doi: 10.1128/mcb.20.17.6410-6413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- 182.Yoo S, Kim Y, Lee H, Park S, Park S. A Gene Trap Knockout of the Tiam-1 Protein Results in Malformation of the Early Embryonic Brain. Mol Cells. 2012;34:103–108. doi: 10.1007/s10059-012-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hoshinoso M, Sone M, Fukata M, Kurodad S, Kaibuchi K, Nabeshima Y, et al. Identification of the stef gene that encodes a novel guanine nucleotide exchange factor specific for Rac1. J Biol Chem. 1999;274:17837–17844. doi: 10.1074/jbc.274.25.17837. [DOI] [PubMed] [Google Scholar]
- 184.Wang Z, Kumamoto Y, Wang P, Gan X, Lehmann D, Smrcka AV, et al. Regulation of Immature Dendritic Cell Migration by RhoA Guanine Nucleotide Exchange Factor Arhgef5. Journal of Biological Chemistry. 2009;284:28599–28606. doi: 10.1074/jbc.M109.047282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fischer KD, Zmuldzinas A, Gardner S, Barbacid M, Bernstein A, Guidos C. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+ CD8+ thymocytes. Nature. 1995;374:474–477. doi: 10.1038/374474a0. [DOI] [PubMed] [Google Scholar]
- 186.Tarakhovsky A, Turner M, Schaal S, Mee PJ, Duddy LP, Rajewsky K, et al. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature. 1995;374:467–470. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- 187.Doody GM, Bell SE, Vigorito E, Clayton E, McAdam S, Tooze R, et al. Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nature Immunol. 2001;2:542–547. doi: 10.1038/88748. [DOI] [PubMed] [Google Scholar]
- 188.Sauzeau V, Jerkic M, Lopez-Novoa JM, Bustelo XR. Loss of Vav2 proto-oncogene causes tachycardia and cardiovascular disease in mice. Mol Biol Cell. 2007;18:943–952. doi: 10.1091/mbc.E06-09-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fujikawa K, Miletic AV, Alt FW, Faccio R, Brown T, Hoog J, et al. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J Exp Med. 2003;198:1595–1608. doi: 10.1084/jem.20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Quevedo C, Sauzeau V, Menacho-Marquez M, Castro-Castro A, Bustelo XR. Vav3-deficient mice exhibit a transient delay in cerebellar development. Mol Biol Cell. 2010;21:1125–1139. doi: 10.1091/mbc.E09-04-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kusuhara S, Fukushima Y, Fukuhara S, Jakt LM, Okada M, Shimizu Y, et al. Arhgef15 Promotes Retinal Angiogenesis by Mediating VEGF-Induced Cdc42 Activation and Potentiating RhoJ Inactivation in Endothelial Cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang Y, Suzuki H, Yokoo T, Tada-Iida K, Kihara R, Miura M, et al. WGEF is a novel RhoGEF expressed in intestine, liver, heart, and kidney. Biochem Biophys Res Commun. 2004;324:1053–1058. doi: 10.1016/j.bbrc.2004.09.153. [DOI] [PubMed] [Google Scholar]