Abstract
A method to directly measure the intracellular pressure of adherent, migrating cells is described in the Basic Protocol. This approach is based on the servo-null method where a microelectrode is introduced into the cell to directly measure the physical pressure of the cytoplasm. We also describe the initial calibration of the microelectrode as well as the application of the method to cells migrating inside three-dimensional (3D) extracellular matrix (ECM).
Keywords: Intracellular pressure, fibroblasts, motility, extracellular matrix
INTRODUCTION
Cells are able to maintain positive intracellular pressure compared to their environment. For example, indirect estimates of intracellular pressure conclude that red blood cells, migrating keratocytes, and non-adherent blebbing cells have intracellular pressures ranging from 20 – 100 Pa (Charras et al., 2008; Dai and Sheetz, 1999; Keren et al., 2009; Rand and Burton, 1964), while pressure in cells undergoing cytokinesis ranges slightly higher from 100 – 300 Pa (Charras et al., 2008; Stewart et al., 2011). The direct measurement of intracellular pressures by the servo-null method (see Basic Protocol) shows these estimated pressures are relatively low compared to other cell types and processes; large cells such as Xenopus oocytes, Amoeba proteus (Yanai et al., 1996), and skeletal muscle cells of the giant barnacle (Balanus nubilis, (Rabbany et al., 1994)) range in pressure from 30 – 6000 Pa. Despite these demonstrations that cells are capable of significantly elevating their intracellular pressure, the mechanisms generating intracellular pressure as well as its potential roles in cell function have been relatively unclear.
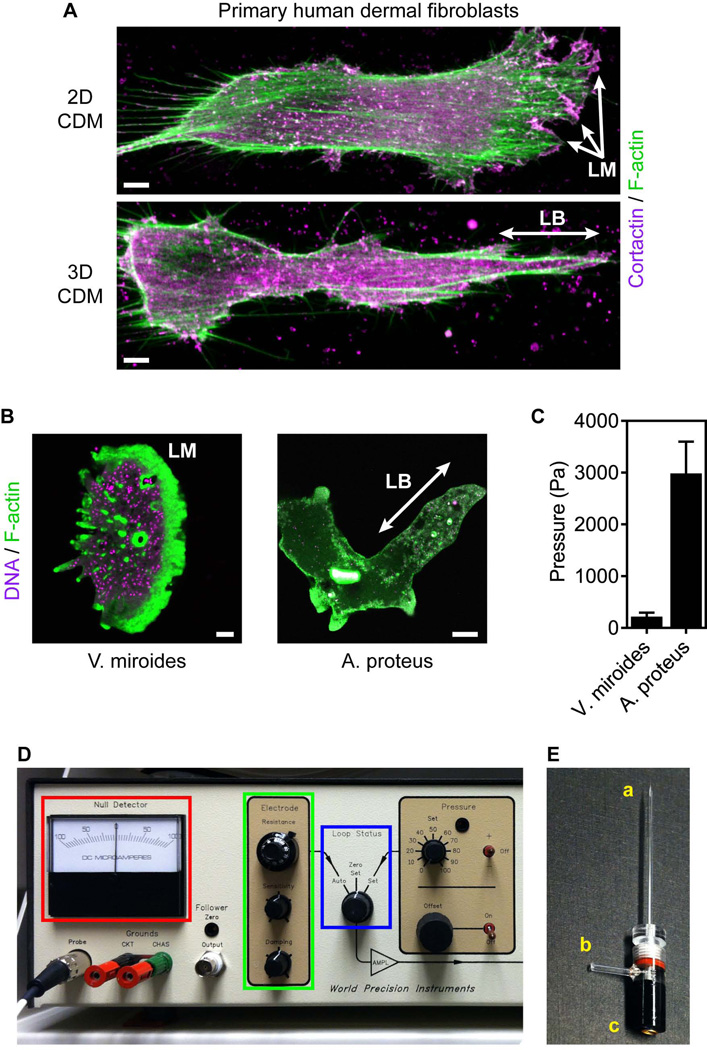
We have adapted the servo-null method (Fein, 1972; Fox and Wiederhielm, 1973; Kelly and Macklem, 1991) to directly measure the intracellular pressure of primary human fibroblasts migrating on two-dimensional (2D) surfaces and in 3D extracellular matrix (ECM) in order to investigate the potential role of pressure in specifying the type of protrusion used by migrating cells (reviewed in (Petrie and Yamada, 2012)). Fibroblasts can migrate either with low intracellular pressure (~300 Pa) using flat, actin-rich lamellipodia, or elevate their intracellular pressure (to ~ 2200 Pa) and form blunt, cylindrical lobopodia (see Fig. 1A, (Petrie et al., 2012; Petrie et al., 2014)). Interestingly, this relationship between pressure and protrusion identity is maintained in certain single-cell protozoa (see Fig. 1B and C), demonstrating the potential broad applicability of the method to deciphering the cellular role and regulation of intracellular pressure across a range of cell types.
Figure 1.
Direct intracellular pressure measurements in migrating cells. (A) Maximally projected confocal stacks of primary human dermal fibroblasts migrating on (upper panel) 2D and in 3D (lower panel) cell-derived matrix (CDM) stained for filamentous actin (F-actin) with rhodamine-phalloidin (green) and the lamellipodia marker cortactin (magenta). Fibroblasts can migrate on 2D surfaces using low-pressure lamellipodia (LM) and in 3D matrix using high-pressure lobopodia (LB). Scale bars, 5 µm. (B) F-actin structures in the protists Vanella miroides (lamellipodia) and Amoeba proteus (lobopodia). Cells were stained with SYBR green (DNA, magenta) and rhodamine-phalloidin (F-actin, green). Scale bars 10 µm in (A) and 50 µm in (B). (C) The intracellular pressure of lamellipodial V. miroides and lobopodial A. proteus. (D) The control panel of the 900A micropressure system (WPI). (E) Micropipette and microelectrode holder labeled for the microelectrode tip (a), air connection (b) and the microelectrode connection (c).
This protocol will describe how to apply the servo-null method to adherent fibroblasts migrating on 2D surfaces and inside 3D ECM. This method requires a small electrolyte-filled microelectrode (0.5 – 1 µm in diameter, see Fig. 1E) to penetrate through the plasma membrane and cell cortex and come in direct contact with the cytoplasm (see Fig. 2A). The microelectrode is connected to the 900A micropressure system (WPI) which simultaneously monitors and maintains the resistance of the microelectrode when it is in the cytoplasm. Positive intracellular pressure pushes the electrolyte farther into the micropipette, increasing the resistance of the circuit. The micropressure system then inputs pressure to compensate, pushing the electrolyte back to its original position to restore the resistance of the circuit to its original null or zero condition. The compensation pressure is equal to the pressure inside the cell. The physical penetration of the cell by the microelectrode is similar to microinjection, a technique where a micropipette penetrates into the cytoplasm or nucleus to directly deliver small molecules such as mRNA or protein, without permanently damaging the cell (Mendoza et al., 2012; Ridley and Hall, 1992). Thus, any cell type that can tolerate microinjection is a promising candidate on which to use the servo-null method to directly measure intracellular pressure.
Figure 2.
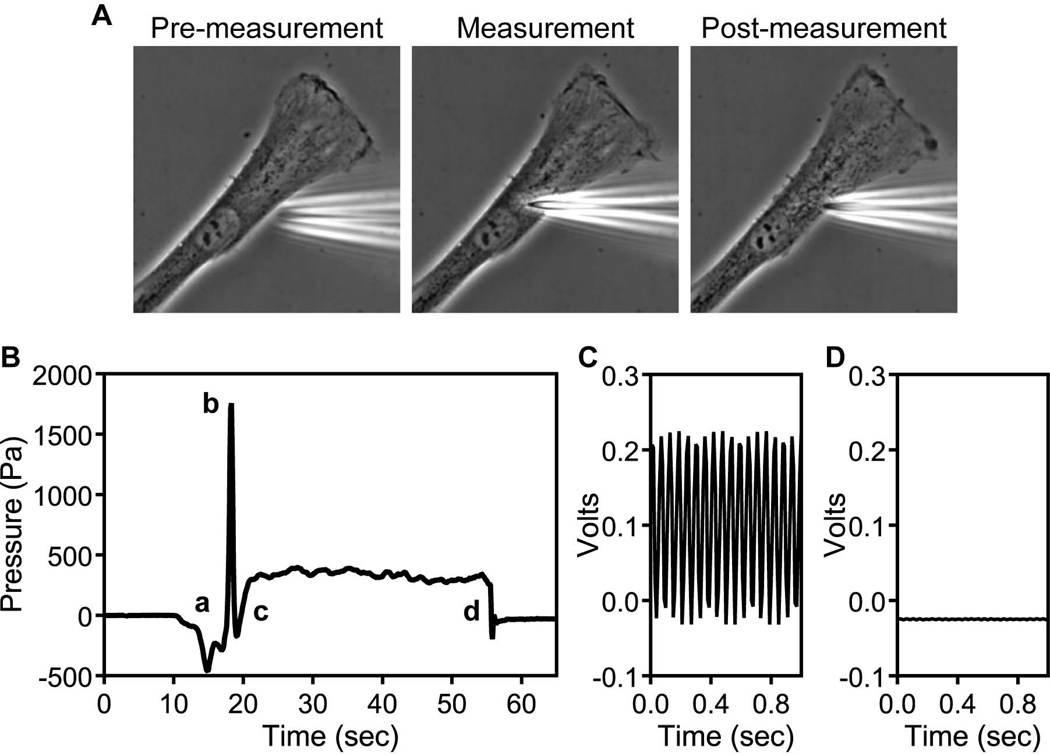
Characteristics of a successful intracellular pressure measurement. (A) A primary human dermal fibroblasts before, during, and after the intracellular pressure measurement. Note overall cell morphology is unaffected. (B) A characteristic pressure measurement of a human fibroblast migrating on a 2D glass surface. The baseline reading becomes unstable as the micropipette comes in close proximity to the cell surface (a), there is a transient pressure spike as the tip of the microelectrode penetrates the plasma membrane and cell cortex (b). The pressure rises slightly as the microelectrode is withdrawn slightly without leaving the cytoplasm (c). There is a stable intracellular reading for ≥ 10 sec before the microelectrode is fully withdrawn and the reading returns to the initial stable baseline reading (d). (C) The initial oscillation when the microelectrode is first connected to the system and the loop status is set to auto. (D) The fully stabilized oscillation of a calibrated microelectrode.
Important measurement parameters of the servo-null method have been determined. It is compatible with a variety of micropipette sizes and can measure the pressure in solutions of physiological salt concentration, where there is a large enough difference between the conductivity of the cytoplasm and the electrolyte in the microelectrode (Fein, 1972; Fox and Wiederhielm, 1973). The micropressure system can detect pressure changes in a variety of environments, including culture media, egg white, egg yolk, 1.5% agar gels, and 3D ECM (Petrie et al., 2014; Yanai et al., 1996). The microelectrode is sufficiently small (with a 0.5 µm tip opening) to measure the pressure of structurally distinct cellular compartments (Petrie et al., 2014). In summary, this technique should be generally applicable to the measurement of intracellular pressure in a variety of cells and environments, and will complement other biophysical approaches such as measuring membrane tension (Dai and Sheetz, 1999) to better understand the role of intracellular pressure in cell function.
BASIC PROTOCOL 1
DIRECT MEASUREMENT OF INTRACELLULAR PRESSURE
In this protocol, we describe how to directly measure the intracellular pressure of adherent fibroblasts migrating across a two-dimensional (2D) surface and in 3D ECM using the servo-null method (Kelly and Macklem, 1991). In vitro models of 3D extracellular matrix can provide a more physiological environment to study cellular mechanisms than a 2D glass surface (Petrie et al., 2012). Intracellular pressure may play a more important role in 3D migration compared to 2D (Petrie et al., 2014), so it will be important to be able to successfully make pressure measurements in this environment. We have used cell-derived matrix and collagen gels as 3D ECM models and successfully performed intracellular pressure measurements on cells embedded in these environments. Negative controls are essential when performing pressure measurements in 3D matrix because physical occlusion of the microelectrode tip by the matrix fibers can increase the intracellular pressure reading (see Critical Parameters). For in-depth protocols on how to make these matrix models and culture cells in them see (Artym and Matsumoto, 2010; Beacham et al., 2007). A support protocol describes how to calibrate each microelectrode.
Materials
Human primary dermal fibroblasts
High-glucose Dulbecco’s modified eagle medium (DME) supplemented with 10% fetal bovine serum (FBS)
Cell-derived matrix
1.7 mg/ml collagen gel
Sigmacote (Sigma, cat. no. SL2)
900A Micropressure System (WPI, cat. no. SYS-900A)
4-axis motorized micromanipulator (Sutter Instrument, cat. no. MPC-325)
1.0 mm outer diameter micropipette with filament, 0.5 µm opening (WPI, cat. no. TIP05TW1F)
Microelectrode holder (1.0 mm) with Ag/AgCl half-cell and air column connections (WPI, cat. no. MEH6SF10)
Reference electrode (WPI, cat. no. DRIREF-2)
Low-walled glass bottom culture dish 50/40 mm (Warner Instruments, cat. no. 64-0760)
Inverted microscope with environmental controls to maintain 37 °C, 10% CO2, and humidity
Phase contrast microscope objective
Data acquisition system (WPI, cat. no. LAB-TRAX4-24T)
BNC-to-BNC Cable, M-M (WPI, cat. no. 2851)
Computer with LabScribe 2 software (WPI)
50-ml disposable conical tubes
2-ml disposable plastic serological pipets
Plate cells in the low-walled culture dish at a density of approximately 10 cells/mm2 in 2 ml of media and culture overnight at 37 °C to have polarized, motile single cells to measure the next day.
The next day, aliquot additional media into a 50-ml disposable conical tube (~4 ml / culture dish) and maintain at 37 °C.
Gently wash each dish of cells with media to remove any debris which could clog the micropipette.
Place the dish of cells on the microscope in the environmental chamber and remove the lid of the dish. Look through the oculars and bring the cells into focus.
Secure the reference electrode so its tip is immersed at the side of the media-filled dish and not exerting pressure on the dish itself. This will help prevent the reference electrode from coming into contact with the microelectrode during data acquisition or interfering with the x-y movement of the dish on the stage.
Connect the reference electrode to the ground connection on the 900A system (see Fig. 1D).
With the Loop Status control of the 900A system set to Zero Set (see Fig. 1D, blue box), position the calibrated microelectrode (see Support Protocol 1) on the micromanipulator at a 45° angle with respect to the horizontal. After the tip has been filled, but prior to the calibration procedure (see Support Protocol), the tip of the microelectrode can be siliconized by dipping it in Sigmacote and allowing it to be air-dried. This can help prevent the glass micropipette from sticking to matrix components.
Now using the micromanipulator to control the position of the microelectrode, move the microelectrode so its tip is centered above the objective and slowly lower it toward the surface of the media while watching the Null Detector on the 900A system (see Fig. 1D, red box). While the microelectrode is in the air, the Null Detector needle will be in the far left position indicating an open circuit. Once the tip of the microelectrode contacts the media containing the reference electrode, the Null Detector needle will return to the zero position indicating the circuit is now closed.
Once both the reference electrode and the microelectrode are immersed in the media, set the Loop Status control to Auto. This will cause the 900A system to maintain the electrolyte level within the microelectrode and help prevent debris from clogging the tip.
Looking through the oculars, raise the focus of the objective into the media. Slowly move the tip of the microelectrode back and forth over the objective so the movement of its shadow will help you identify its position and bring the tip into focus.
Using the serological pipet, gently add 4 ml of pre-warmed media to the dish. The meniscus on the glass micropipette will move away from the field of view and improve the quality of the phase contrast image.
Allow at least 15 min for the apparatus to equilibrate to 37°C. This will prevent thermal-induced movement of the micropipette and its holder from interfering with the subsequent pressure measurements.
Re-position the micropipette in the field of view and lower it until the tip is approximately 10 µm above the layer of cells.
With the pressure output signal connected to the data acquisition system via the BNC-to-BNC cable and the data acquisition system connected to a computer via the USB connection, launch the LabScribe 2 software and begin recording.
Looking through the oculars, slowly lower the microelectrode towards the desired target region (see Fig. 2A). When the tip of the microelectrode comes into contact with the outer surface of the cell, the character of the pressure reading will change from the stable baseline of the medium, to unstable (see Fig. 2B).
Switch the micromanipulator to the diagonal mode of movement.
Penetrate the cell with a rapid (≤ 0.3 sec) 1 µm diagonal movement to penetrate the plasma membrane and cell cortex. The pressure trace will record a spike as the tip of the microelectrode penetrates this cellular barrier (see Fig. 2B). Putting the micromanipulator into the diagonal, pulsatile mode will move the tip downward at a 45 angle in short, quick bursts and will help the tip reach the embedded cell without the tip getting stuck in the surrounding matrix. If the embedded cell is close enough to the surface of the matrix, the tip can be inserted into the matrix just above the cell and then gently moved side to side to clear a portion of the matrix away from the plasma membrane and allow easier penetration.
Withdraw the micropipette approximately 0.5 µm to avoid compressing the cytoplasm (see Critical Parameters), but keeping the tip of the microelectrode inside the cell.
Record the intracellular pressure for at least 10 s and then smoothly withdraw the tip of the microelectrode out of the cell and back into the medium. Continue recording for 5 s to confirm the baseline reading in the media is stable and unchanged. If it is not, this could indicate the tip has become damaged or clogged during the intracellular pressure reading and both the tip and the reading should be discarded. Continuous intracellular pressure recordings can be made over seconds to hours, depending on the experimental parameters.
Looking through the oculars make sure the morphology of the cell has not been affected by the measurement (see Fig. 2A). Damaged cells will tend to immediately burst, lose polarity, or round up. If the morphology has been noticeably affected by the measurement, the pressure reading should be discarded.
Review each pressure trace. The characteristics of a successful measurement are equivalent stable baselines before and after each measurement, a pressure spike as the tip penetrates the cell, a slight decrease and subsequent increase in pressure after the needle is partially withdrawn, and a stable intracellular reading. The intracellular pressure is the average of the stable intracellular pressure reading minus the baseline pressure in the media (see Fig. 2B).
As long as the tip of the microelectrode does not become obstructed or damaged allowing the baseline pressure readings remain stable, it can be used to make additional measurements.
SUPPORT PROTOCOL 1
CALIBRATING THE MICROELECTRODE
Prior to making a pressure reading, each new microelectrode must be calibrated so that the resistance detected by the 900A system is set to zero (or null). It is the ability of the 900A system to maintain this zero resistance by controlling the pressure in the air column behind the electrolyte solution in the microelectrode which is the fundamental basis of each pressure measurement (Kelley and Macklem, 1991). External pressure differences increase or decrease the electrolyte fluid level in the tip, which changes the resistance, which is restored by pressure inputs by the machine, and the compensation pressure is directly equal to the pressure outside the electrode.
Additional Materials (also see Basic Protocol)
1M KCl
0.1 M KCl
MicroFil flexible needle (WPI, cat. no. MF34G-5)
Disposable 10 ml syringe
Pressure calibration chamber (WPI, cat. no. CAL900A)
Set-up the 900A micropressure system according the manufacturer’s instructions. Make sure you have access to clean, dry pressure and vacuum sources that can be regulated to 46000 and −20000 Pa, respectively.
Fill the 10 ml syringe with 1M KCl and attach the MicroFil flexible needle.
Completely backfill the micropipette with 1M KCl and remove any air bubbles.
Fill the microelectrode holder with 1M KCl and secure the microelectrode inside. Visually inspect to confirm there are no air bubbles between the tip of the microelectrode and the Ag/AgCl half-cell that could prevent completion of the circuit (see Fig. 1E).
Fill the pressure calibration chamber with 0.1 M KCl and insert the microelectrode and holder. Be careful not to damage the tip on the sides of the chamber or on the electrode within the chamber.
Connect the microelectrode holder to the 900A system using the electrical and air column connections (see Fig. 1E). The Null Detector needle will deflect to the far left position (see Fig. 1D, red box).
Turn the Electrode Resistance dial clockwise (see Fig. 1D, green box) to move the needle back to the zero position. When the needle is at zero, the number on the dial indicates the actual resistance of the microelectrode tip (approximately 1250k Ohms for a 0.5 µm tip). Abnormally low resistance could indicate a broken tip, while abnormally high resistance may result from an air bubble blocking the circuit.
Continue turning the Electrode Resistance dial clockwise until the Null Detector needle reads 75 µA.
Begin recording the pressure signal and switch the Loop Status to Auto. The needle will move back to approximately zero and the signal will begin oscillating (see Fig. 2C).
Using the damping and sensitivity controls on the 900A system (see Fig. 1D, green box), reduce the oscillation to the lowest level possible (see Fig. 2D). Turn the sensitivity knob counterclockwise slowly while monitoring the oscillating signal. If the sensitivity is reduced too much, the signal will go off the scale. If this occurs, return the sensitivity knob to the start position (see Fig. 1D, green box), turn the damping knob 1/8th of a turn clockwise and re-adjust the sensitivity control while monitoring the oscillation of the signal. With practice you should be able to achieve the stable signal shown in Fig. 2D.
Once the pressure signal is stabilized, switch the Loop Status to Zero Set and proceed with the steps outlined in the Basic Protocol.
COMMENTARY
Background Information
A limited number of alternative methods have been developed to indirectly estimate pressure in different contexts. If the internal and external forces on a cell are at equilibrium, intracellular pressure can be calculated based on external pressure, membrane tension, and/or curvature of the plasma membrane using the general law of Laplace (Charras et al., 2008; Dai and Sheetz, 1999; Mitchison and Swann, 1954; Rand and Burton, 1964). Alternatively, measuring the extent of deformation of round cells in response to applied force can also be used to estimate intracellular pressure (Cole, 1932; Stewart et al., 2011). Finally, intracellular pressure has also been estimated based on the flow of cytoplasmic fluid within migrating keratocytes (Keren et al., 2009). With the exception of monitoring cytoplasmic flow, each of these alternative methods require either round or blebbing cells and cannot be applied to adherent cells migrating on 2D surfaces or in 3D ECM.
The servo-null method is the only approach we are aware of that is capable of directly measuring intracellular pressure. This method does not rely on the simplifying assumptions necessary in order to apply the Law of Laplace and does not require establishing the tension of the plasma membrane, a quantity that depends on both the degree of membrane-cytoskeleton attachment (pressure-independent), as well as in-plane membrane tension (pressure-dependent). During the servo-null method, the micropipette penetrates the plasma membrane and cortex to directly read the physical pressure exerted by the cytoplasm, preventing the tension of the plasma membrane from confounding the pressure measurement. Also, the servo-null method does not require round, detached, or blebbing cells and can therefore be applied to cells in situ, such as fibroblasts migrating in 3D ECM (Petrie et al., 2014). Despite these fundamental differences in how these approaches measure intracellular pressure, it is important to note that they each have independently concluded that resting cells maintain a positive intracellular pressure of approximately 20 – 300 Pa.
Critical Parameters
Physical blockage of the microelectrode tip can mimic a genuine increase in intracellular pressure. Thus, it is essential, especially when measuring the pressure of cells in 3D ECM, that appropriate controls are incorporated to eliminate artificially elevated pressure readings due to occlusion of the microelectrode tip. For primary human fibroblasts migrating in cell-derived matrix or collagen, we routinely include a control where actomyosin contractility is inhibited to confirm we could measure the expected reduction in pressure, despite the presence of the physical matrix structure.
Troubleshooting
This is a technique that requires skill, patience, and lots of practice. It seems unlikely that this approach can be applied with immediate success. We found two separate tasks had to be mastered before we could routinely perform intracellular pressure measurements. The first was successfully calibrating the microelectrode without breaking its tip and avoiding air bubbles within the micropipette and micropipette holder. We recommend starting with a large-bore micropipette (5 µm opening) to help avoid small bubbles at the tip. Also the pressure calibration chamber can be sealed and pressure applied via a side port to elevate the pressure outside the microelectrode and confirm its sensitivity. Once microelectrode calibration has been accomplished, the next step is to position the microelectrode on the microscope and successfully measure intracellular pressure. We recommend plating cells on a thick, soft substrate initially, such as collagen (Artym and Matsumoto, 2010). This will allow you to miss the cells in practice runs, without breaking the tip of the microelectrode on the hard glass surface and necessitating a potentially time consuming calibration of a new microelectrode. Occasionally, a particular microelectrode will seem to burst every cell, or the baseline reading in the media will be unstable. Calibrating a new microelectrode often solves these issues. Finally, the opening of the microelectrode can become blocked by debris floating in the media even before an intracellular pressure measurement is attempted. Switching the loop status to auto mode (see Fig. 1D) as soon as possible once the tip of the microelectrode is submerged in the media will help prevent this from occurring. If debris does get stuck on the microelectrode, repeatedly inserting the micropipette through the air-media interface can remove debris. Additionally, the micropressure system can be used to temporarily apply positive pressure inside the microelectrode to push debris back out of the tip and into the media.
Anticipated Results
The number and variability of the pressure measurements obtained using this protocol will depend on the cell type under study. In the case of migrating human primary fibroblasts 10 – 20 measurements should be sufficient to establish statistically meaningful measurements to compare to the appropriate positive and negative controls.
Time Considerations
This technique requires a substantial investment of time to become proficient at both filling and calibrating each microelectrode and then successfully making an intracellular pressure measurement without destroying either the particular cell of interest or the microelectrode. When we initially started, one successful pressure measurement was considered a very good day. Encouragingly, after two weeks of constant practice we were able to successfully measure approximately 10 cells per day. An expert should expect to be able to measure the intracellular pressure of 50–100 cells per day.
Literature Cited
- Artym VV, Matsumoto K. Imaging cells in three-dimensional collagen matrix. Curr. Protoc. Cell Biol. 2010;Chapter 10(Unit 10.18):11–20. doi: 10.1002/0471143030.cb1018s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham DA, Amatangelo MD, Cukierman E. Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr. Protoc. Cell Biol. 2007;Chapter 10(Unit 10):19. doi: 10.1002/0471143030.cb1009s33. [DOI] [PubMed] [Google Scholar]
- Charras GT, Coughlin M, Mitchison TJ, Mahadevan L. Life and times of a cellular bleb. Biophys. J. 2008;94:1836–1853. doi: 10.1529/biophysj.107.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole KS. Surface forces of the Arbacia egg. J. Cell. Comp. Physiol. 1932;1:1–9. [Google Scholar]
- Dai J, Sheetz MP. Membrane tether formation from blebbing cells. Biophys. J. 1999;77:3363–3370. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein H. Microdimensional pressure measurements in electrolytes. J. App. Physiol. 1972;32:560–564. doi: 10.1152/jappl.1972.32.4.560. [DOI] [PubMed] [Google Scholar]
- Fox JR, Wiederhielm CA. Characteristics of the servo-controlled micropipet pressure system. Microvascular Res. 1973;5:324–335. doi: 10.1016/0026-2862(73)90046-0. [DOI] [PubMed] [Google Scholar]
- Kelly SM, Macklem PT. Direct measurement of intracellular pressure. Am. J. Physiol. 1991;260:C652–C657. doi: 10.1152/ajpcell.1991.260.3.C652. [DOI] [PubMed] [Google Scholar]
- Keren K, Yam PT, Kinkhabwala A, Mogilner A, Theriot JA. Intracellular fluid flow in rapidly moving cells. Nature Cell Biol. 2009;11:1219–1224. doi: 10.1038/ncb1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza MC, Besson S, Danuser G. Quantitative fluorescent speckle microscopy (QFSM) to measure actin dynamics. Curr. Protoc. Cytometry. 2012;Chapter 2(Unit 2–18) doi: 10.1002/0471142956.cy0218s62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison JM, Swann MM. The Mechanical Properties of the Cell Surface: I. The Cell Elastimeter. J. Exp. Biol. 1954;31:443–460. [Google Scholar]
- Petrie RJ, Gavara N, Chadwick RS, Yamada KM. Nonpolarized signaling reveals two distinct modes of 3D cell migration. J. Cell Biol. 2012;197:439–455. doi: 10.1083/jcb.201201124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie RJ, Koo H, Yamada KM. Generation of compartmentalized pressure by a nuclear piston drives 3D cell motility. 2014 doi: 10.1126/science.1256965. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie RJ, Yamada KM. At the leading edge of three-dimensional cell migration. J. Cell Sci. 2012;125:5917–5926. doi: 10.1242/jcs.093732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbany SY, Funai JT, Noordergraaf A. Pressure generation in a contracting myocyte. Heart Vessels. 1994;9:169–174. doi: 10.1007/BF01746060. [DOI] [PubMed] [Google Scholar]
- Rand RP, Burton AC. Mechanical Properties of the Red Cell Membrane. I. Membrane Stiffness and Intracellular Pressure. Biophys. J. 1964;4:115–135. doi: 10.1016/s0006-3495(64)86773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Stewart MP, Helenius J, Toyoda Y, Ramanathan SP, Muller DJ, Hyman AA. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature. 2011;469:226–230. doi: 10.1038/nature09642. [DOI] [PubMed] [Google Scholar]
- Yanai M, Kenyon CM, Butler JP, Macklem PT, Kelly SM. Intracellular pressure is a motive force for cell motion in Amoeba proteus. Cell Motil. Cytoskeleton. 1996;33:22–29. doi: 10.1002/(SICI)1097-0169(1996)33:1<22::AID-CM3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]