Abstract
The emergence of avian satellite cells during development has been studied using markers that distinguish adult from fetal cells. Previous studies by us have shown that myogenic cultures from fetal (Embryonic Day 10) and adult (12–16 weeks) chicken pectoralis muscle (PM) each regulate expression of the embryonic isoform of fast myosin heavy chain (MHC) differently. In fetal cultures, embryonic MHC is coexpressed with a ventricular MHC in both myocytes (differentiated myoblasts) and myotubes. In contrast, myocytes and newly formed myotubes in adult cultures express ventricular but not embryonic MHC. In the current study, the appearance of myocytes and myotubes which express ventricular but not embryonic MHC was used to determine when adult myoblasts first emerge during avian development. By examining patterns of MHC expression in mass and clonal cultures prepared from embryonic and posthatch chicken skeletal muscle using double-label immunofluorescence with isoform-specific monoclonal antibodies, we show that a significant number of myocytes and myotubes which stain for ventricular but not embryonic MHC are first seen in cultures derived from PM during fetal development (Embryonic Day 18) and comprise the majority, if not all, of the myoblasts present at hatching and beyond. These results suggest that adult type myoblasts become dominant in late embryogenesis. We also show that satellite cell cultures derived from adult slow muscle give results similar to those of cultures derived from adult fast muscle. Cultures derived from Embryonic Day 10 hindlimb form myocytes and myotubes that coexpress ventricular and embryonic MHCs in a manner similar to cells of the Embryonic Day 10 PM. Thus, adult and fetal expression patterns of ventricular and embryonic MHCs are correlated with developmental age but not muscle fiber type.
INTRODUCTION
Satellite cells, which are cells located underneath the basement membrane of the muscle fiber, are considered to be the myogenic precursors of postnatal and adult skeletal muscle (Mauro, 1979; Campion, 1984). In the neonate, these precursor cells are proliferative, adding nuclei to the fibers (Moss and LeBlond, 1971). In the adult, satellite cells are mitotically quiescent but divide in response to injury (Mauro, 1979; Campion, 1984), as well as in response to more subtle stresses such as stretch, exercise, denervation, or mild compression (see White and Esser, 1989 for a review). The resulting progeny (adult myoblasts) either fuse into preexisting fibers or form new myofibers (Snow, 1977a,b; Mauro, 1979). Identification of satellite cells in the intact muscle is ultimately dependent on the existence of the basement membrane which surrounds myofibers in later stages of embryogenesis (Kelly and Zacks, 1969; Mayne et al., 1989; reviewed in Grounds and Yablonka-Reuveni, 1992). Using this approach, satellite cells were detected in late stages of development (Kelly and Zacks, 1969; Mauro, 1979). Identification of myogenic precursor cells by location is ambiguous, since other cell types could be in a satellite position (Mauro, 1979), and recent studies raised the possibility that adult myogenic precursors may not be limited to this location (Kennedy et al., 1989).
Although the localization of myogenic precursor cells underneath the basement membrane defines a specific site for these precursors in late development and onward, it is not clear whether satellite cells (or adult myoblasts) are different from myoblasts present at previous stages of development. Studies on mammalian myoblasts (reviewed by Cossu and Molinaro, 1987) have shown differences between fetal and adult myoblasts in culture, including differential sensitivity to phorbol ester inhibition of fusion (Cossu et al., 1983, 1987; Senni et al., 1987). This difference was used to trace the appearance of phorbol-ester-resistant myogenic cells during human and mouse muscle histogenesis (Cossu et al., 1985, 1988).
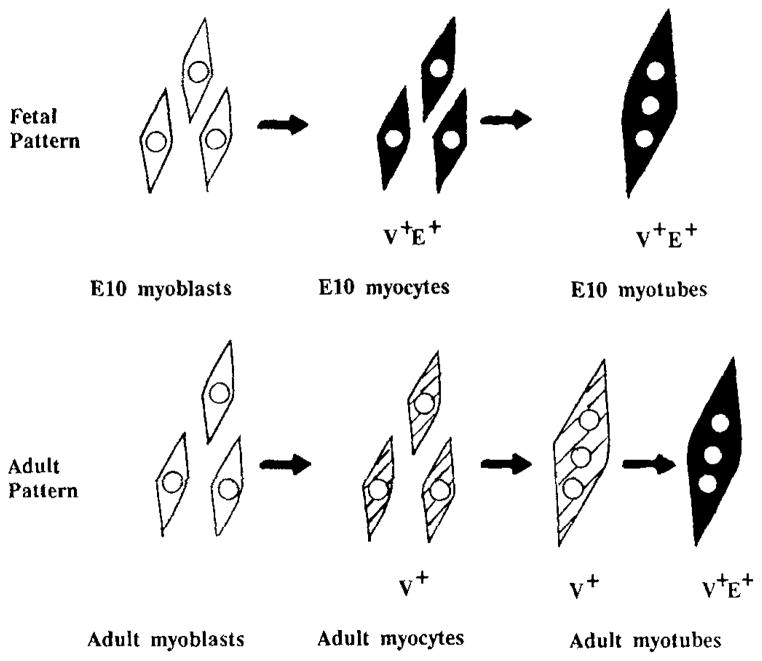
Studies of avian myogenic cells have established the existence of early and late myoblasts, based on their media requirements and the morphology of the fibers they formed in culture (White et al., 1975; Seed and Hauschka, 1984). Later studies by Stockdale and coworkers described the appearance of “embryonic” and “fetal” myoblasts which are present during the embryonic and fetal stages of embryogenesis. These correspond to early and late myoblasts and have distinct myosin heavy chain (MHC)1 expression patterns (Crow and Stockdale, 1986; Miller and Stockdale, 1986; Stockdale and Miller, 1987). The aforementioned work did not address the question of whether avian adult myoblasts represent a separate lineage or population of myoblasts or whether they are identical to fetal myoblasts (see Stockdale and Miller, 1987 for a review; Feldman and Stockdale, 1991). Distinctions between avian fetal and adult myoblasts have been described (Yablonka-Reuveni et al., 1987; Yablonka-Reuveni and Nameroff, 1990; Yablonka-Reuveni and Seifert, 1992; Nameroff and Rhodes, 1989; Feldman and Stockdale, 1992). Recently, we reported a difference in the regulation of embryonic fast MHC expression between fetal myoblasts from Day 10 chicken embryos (E10 myoblasts) and adult chicken myoblasts in culture (Hartley et al., 1991). In the latter study, double-immunofluorescence labeling with specific monoclonal antibodies showed that both fetal (E10) myocytes (differentiated myoblasts) and myotubes coexpress embryonic fast and ventricular MHCs. In contrast, adult myocytes express ventricular MHC alone while adult myotubes first express ventricular MHC and then, after a lag, coexpress embryonic MHC. We termed this initial expression of ventricular but not embryonic MHC in adult myogenic cultures the adult pattern of MHC expression. This study identified a distinct difference in fast MHC expression between adult and fetal cultures; Figure 1 is a summary of these results in a pictorial form. The adult pattern was unchanged in passaged adult myoblasts and in myoblasts isolated from regenerating adult muscle, and blocking fusion with EGTA inhibited the expression of the embryonic isoform in adult but not in fetal cultures. Clonal cultures also clearly demonstrated the existence of adult and fetal patterns. Thus, the expression of ventricular but not embryonic MHC in myocytes and newly formed myotubes is a stable marker for adult myoblasts.
Fig. 1.
Summary of ventricular and embryonic MHC expression patterns for fetal (E10) and adult myogenic cultures, based on previously published results (Hartley et al., 1991). This summary is limited to the expression of these two isoforms and does not address possible heterogeneity in expression of other MHC isoforms by fibers within fetal and adult cultures as was shown by Stockdale and co-workers (Stockdale and Miller, 1987; Miller and Stockdale, 1989; Feldman and Stockdale, 1991). V+E+, coexpression of ventricular and embryonic MHC isoforms as detected by isoform specific mAbs. V+, expression of ventricular MHC only.
In the current study we use this adult pattern of MHC expression as a marker to trace the first appearance of adult myoblasts (or satellite cells) during the fetal stage of chicken development. We show that a significant number of myoblasts with the adult pattern are first seen during late embryogenesis (E18) and comprise the majority, if not all, of the myoblasts present at hatch. Prior to E18 we can identify far smaller numbers of cells with the adult pattern. We also show that the adult pattern is similar for myoblasts from both fast and slow adult skeletal muscles, whereas myoblasts from different fetal muscles show the fetal pattern of coexpression of ventricular and embryonic MHC in both myocytes and myotubes. Thus, patterns of ventricular and embryonic MHC expression are correlated with developmental age but not muscle fiber type.
METHODS
Animals
Embryonated chicken eggs (White Leghorn) and adult chickens (12- to 16-week-old White Leghorns) were purchased from H & N International (Redmond, WA). Eggs were maintained in a forced air incubator at 37.5°C. Newly hatched and 3-week-old chickens were from the same stock of eggs and were kindly provided by the laboratory of Dr. E. Rubel, Department of Otolaryngology, University of Washington.
Isolation and Culture of Posthatch and Adult Myoblasts
Cells from newly hatched, 3-week posthatch, and 12- to 16-week (adult) chickens were isolated from the pectoralis muscle as well as from adult anterior and posterior latissimus dorsi muscles (ALD and PLD, respectively) as previously described (Hartley et al., 1991; Yablonka-Reuveni et al., 1987; Yablonka-Reuveni, 1989). Briefly, 2 to 5 g of muscle was finely minced and treated with collagenase 3 (Worthington, Freehold, NJ) followed by trypsin to release cells. The cell suspension was then subjected to Percoll density centrifugation to separate myoblasts from contaminating myofibril debris and nonmyogenic cells. Cells were plated in complete medium (85% Eagle’s minimal essential medium (MEM), 10% horse serum, 5% chicken embryo extract, and penicillin and streptomycin at 105 units/liter each) at 1–2 × 105 per 35-mm dish for mass cultures and 100 cells per 60-mm dish for clonal cultures. Horse serum was from Sigma (St. Louis, MO) and was preselected for maximal clonal growth. Dishes were precoated with 2% gelatin (Sigma). Medium was replaced 24–30 hr after plating and every other day thereafter for mass cultures and every third day for clonal cultures.
Isolation and Culture of Fetal Myoblasts
Primary cultures were prepared from the pectoralis muscles of 10-, 14-, and 18-day embryonic chicks and the hindlimb muscles of E10 chicks essentially as previously described (Hartley et al., 1991; Yablonka-Reuveni et al., 1988). The muscles were excised, finely minced, and dissociated into single cells by trypsin digestion for E10 tissue, and with both collagenase and trypsin digestions for E14 and E18 tissue as described above for posthatch myoblasts. The crude cell suspensions were enriched for myogenic cells by Percoll density centrifugation and cells were plated as mass and clonal cultures as described above for adult myoblasts. Collagenase was not included in routine preparations of E10 myoblasts, but was included as a control in some experiments. No significant differences were observed in results obtained with E10 myoblasts isolated with or without collagenase digestion.
Myosin Heavy Chain Isoform Detection
Double-immunofluorescent labeling
Embryonic and ventricular MHCs were colocalized in myogenic cultures using double-immunofluorescent labeling as previously described (Hartley et al., 1991). Embryonic fast MHC was detected using mAb EB165. EB165 reacts with all known fast embryonic isoforms of MHC in the chicken and also with the adult fast isoform (Cerny and Bandman, 1987; Bandman et al., 1990). As determined with a mAb against adult fast MHC, the adult isoform is not expressed in 1- to 7-day-old myogenic cultures from E10 (Yablonka-Reuveni et al., 1990; Hartley and Yablonka-Reuveni, 1990) and adult pectroalis muscle (data not shown). Therefore, reactivity with EB165 in the current study is due to the presence of the embryonic isoform. Ventricular MHC was detected with fluorescein-conjugated mAb HV11 which is specific for a chicken ventricular myosin (Bandman et al., 1990; Bourke et al., 1991). In the adult, mAb HV11 reacts exclusively with the ventricular myocardium. It also reacts with differentiating muscle cells in the somites, limb buds, developing heart, and regenerating fast and slow skeletal muscle in vivo (Bourke et al., 1991). In addition, immunoblots of MHC peptide maps demonstrated that the same MHC isoform is expressed in the adult ventricle and in fetal myogenic cultures (Bourke et al., 1991). While mAb HV11 detects a major ventricular isoform, other minor MHC isoforms are also present in the chicken ventricle (Evans et al., 1988). Fluoresceinated HV11 was prepared as described (Goding, 1976) with the following modifications: Fluorescein isothiocyanate was dissolved in acetone at −20°C (1 mg/ml) and unbound fluorochrome was removed with extensive dialysis in PBS for at least 4 days.
Cultures were fixed for 30–60 sec in a cold solution of 70% ethanol:formalin:acetic acid (AFA, 20:2:1) and incubated for at least 1 hr in 0.05 M Tris, 0.15 M NaCl, and 1% normal goat serum to block nonspecific binding. Double-immunofluorescent staining was performed as described (Hartley et al., 1991). Briefly, for double-labeling of mass and clonal cultures, cultures were incubated first with mAb EB165 followed by rhodamine-conjugated goat anti-mouse IgG (Organon-Technika Cappel, West Chester, PA). Cultures were then blocked with 1% normal mouse serum and reacted with fluorescein-conjugated HV11. Controls were performed as previously described, including double-labeling of chicken smooth muscle cultures with an indirectly labeled mAb reactive with smooth muscle cells followed by directly labeled HV11 (which does not react with smooth muscle cells) to ensure that HV11 reacts only with its antigen and not with the GAM IgG nonspecifically (Hartley et al., 1991). Cultures were viewed with a Zeiss photomicroscope equipped with phase and epifluorescence optics.
Quantitation of myosin-positive cells
The number of myosin-positive cells in mass cultures was determined by quantitating the nuclei within mononucleated and multinucleated cells reacting with the MHC mAbs. For quantitation, cultures were viewed with either a 40X or a 100X objective, depending on cell density. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI, 1 μg/ml) and visualized using Hoescht filters. At least 500 nuclei in 10 or more fields were counted using duplicate plates, unless otherwise stated. The number of mononucleated and multinucleated MHC-positive cells was determined as well as the total number of MHC-positive cells. For an example of how raw data were collected and analyzed for mass cultures see Table 1.
TABLE 1.
Distribution of Myogenic Cells Reacting with Anti-ventricular and/or Anti-embryonic MHC mAbs
| Source of cells | Culture age (days) | Total nuclei | Number of fields | Nuclei in E+V+ cells
|
Nuclei in V+ cells
|
Total+ cells
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Myotube | Myocyte | Myotube | Myocyte | Myotube | Myocyte | ||||
| E14 | 1 | 500 | 47 | 4 | 30 | 2 | 4 | 6 | 34 |
| 510 | 45 | 4 | 24 | 0 | 0 | 4 | 24 | ||
| 2 | 516 | 12 | 12 | 34 | 0 | 13 | 12 | 47 | |
| 530 | 15 | 9 | 24 | 4 | 6 | 13 | 30 | ||
| 3 | 967 | 10 | 499 | 39 | 0 | 18 | 499 | 57 | |
| 752 | 10 | 426 | 39 | 3 | 6 | 429 | 45 | ||
| 4 | 981 | 10 | 466 | 6 | 10 | 4 | 476 | 10 | |
| 1359 | 10 | 628 | 3 | 3 | 7 | 631 | 10 | ||
| 5 | 1836 | 10 | 824 | 8 | 19 | 22 | 843 | 30 | |
| 2206 | 10 | 1061 | 6 | 24 | 20 | 1085 | 26 | ||
| E18 | 1 | 501 | 42 | 0 | 0 | 0 | 0 | 0 | 0 |
| 504 | 42 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 2 | 506 | 31 | 0 | 0 | 0 | 2 | 0 | 2 | |
| 511 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 505 | 14 | 3 | 0 | 0 | 3 | 3 | 3 | |
| 501 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 1097 | 10 | 0 | 0 | 11 | 28 | 11 | 28 | |
| 942 | 10 | 0 | 2 | 4 | 29 | 4 | 31 | ||
| 4.5 | 1518 | 10 | 30 | 1 | 184 | 75 | 214 | 76 | |
| 1445 | 10 | 37 | 0 | 160 | 45 | 197 | 45 | ||
| 5 | 1635 | 10 | 475 | 12 | 382 | 59 | 857 | 71 | |
| 2009 | 10 | 694 | 5 | 349 | 53 | 1043 | 58 | ||
| 6* | 552 | 10 | 183 | 0 | 3 | 4 | 186 | 4 | |
| 572 | 10 | 207 | 1 | 0 | 1 | 207 | 2 | ||
| 1-day | 1 | 500 | 104 | 0 | 0 | 0 | 0 | 0 | 0 |
| 501 | 95 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 2 | 504 | 60 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 506 | 69 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 503 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 519 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 4 | 507 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 525 | 15 | 0 | 0 | 4 | 3 | 4 | 3 | ||
| 5 | 1002 | 10 | 0 | 0 | 0 | 35 | 0 | 35 | |
| 814 | 10 | 0 | 0 | 0 | 22 | 0 | 22 | ||
| 6 | 3202 | 10 | 17 | 1 | 314 | 87 | 331 | 88 | |
| 2097 | 10 | 0 | 0 | 151 | 87 | 151 | 87 | ||
| 7 | 3637 | 10 | 219 | 0 | 1249 | 59 | 1468 | 59 | |
| 3632 | 10 | 381 | 1 | 1073 | 35 | 1454 | 36 | ||
| 8* | 688 | 10 | 195 | 1 | 37 | 3 | 232 | 4 | |
| 897 | 10 | 234 | 1 | 11 | 0 | 245 | 1 | ||
| 3-week | 1 | 524 | 37 | 0 | 0 | 0 | 8 | 0 | 8 |
| 500 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 2 | 505 | 22 | 0 | 0 | 2 | 9 | 2 | 9 | |
| 513 | 19 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| 3 | 926 | 10 | 0 | 0 | 0 | 12 | 0 | 12 | |
| 875 | 10 | 0 | 0 | 2 | 11 | 2 | 11 | ||
| 4 | 2296 | 10 | 0 | 0 | 383 | 81 | 383 | 81 | |
| 2258 | 10 | 0 | 0 | 330 | 79 | 330 | 79 | ||
| 5 | 3072 | 10 | 870 | 0 | 954 | 22 | 1824 | 22 | |
| 2726 | 10 | 437 | 0 | 1244 | 20 | 1681 | 20 | ||
| 6* | 893 | 10 | 195 | 0 | 4 | 10 | 109 | 10 | |
| 867 | 10 | 260 | 0 | 3 | 7 | 260 | 7 | ||
Note. Duplicate cultures were fixed and double-labeled at each time point with mAbs against embryonic fast MHC (EB165 indirectly labeled with GAM-TRITC) and ventricular MHC (HV11 directly labeled with fluorescein). Nuclei were counterstained with DAPI and the number of nuclei within MHC-positive cells versus total nuclei was determined using a 40X objective, except where indicated (*), when counts were determined using a 100X objective.
For clonal analysis, clones were classified as myogenic if they contained myocytes and/or myotubes reacting with either or both of the mAbs. Typically, at the first time of analysis, clones contained either myocytes only (1-day and 3-week posthatch cultures) or myocytes and small myotubes (myotubes containing 10 nuclei or less; E14 and E18 cultures). At later time points, clones in all culture types contained myocytes and myotubes of varying sizes. In addition to myocytes and myotubes, clones also contained myosin-negative cells. Micrographs of clones containing myocytes and myotubes which are representative of clones in the current study can be seen in previous publications (Yablonka-Reuveni et al., 1987; Yablonka-Reuveni and Nameroff, 1990).
RESULTS
Myoblasts Which Express Ventricular but not Embryonic MHC upon Differentiation Become Dominant during Late Chicken Embryogenesis
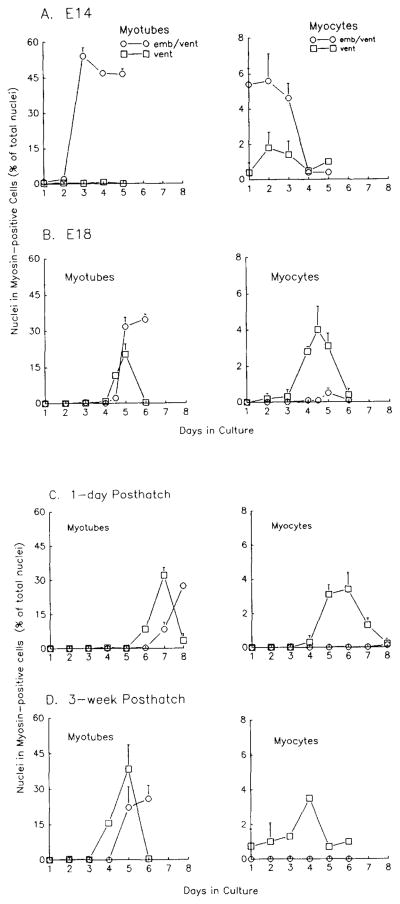
Our previous studies established that adult- and fetal-derived (E10) myogenic cultures from chicken pectoralis muscle regulate the expression of embryonic fast MHC differently. The fetal pattern is the coexpression of both embryonic fast and ventricular MHCs in myocytes and myotubes, while the adult pattern is the expression of ventricular MHC only in myocytes and initially in myotubes, which subsequently coexpress embryonic MHC (Fig. 1). To investigate when myoblasts with the adult pattern first appear, we examined the expression of embryonic and ventricular MHCs in myogenic cultures from chick pectoralis muscle at progressively later stages of development, using double-label immunofluorescence with isoform-specific mAbs as described under Methods (mAb EB165 for embryonic fast MHC and mAb HV11 for ventricular MHC). Table 1 shows the number of nuclei in either myotubes or myocytes reacting with the anti-ventricular and anti-embryonic MHC mAbs from the four developmental ages studies, E14, E18, 1-day, and 3-week posthatch cultures. Figure 2 graphically depicts the data in Table 1 as the percentages of total nuclei which are in myotubes or myocytes which reacted with mAb HV11 or both mAbs HV11 and EB165. The left panels in Fig. 2 show the percentages of total nuclei which are in myotubes reactive with the different mAbs, while the right panels depict myocytes only. In cultures derived from E14 pectoralis muscle (Table 1 and Fig. 2A), a majority of myocytes and myotubes stain with both HV11 and EB165 mAbs, similar to E10 cultures (Hartley et al., 1991). Myocytes reacting with HV11 alone were present in low but detectable numbers in E14 cultures (Fig. 2A, right panel and Table 1), as were those seen previously in E10 cultures (Hartley et al., 1991). E18 myogenic cultures, however, contain primarily myocytes reacting only with mAb HV11 and very few myocytes reacting with both mAbs (Fig. 2B, right panel, Table 1), reminiscent of adult cultures (as shown in Hartley et al., 1991). In E18 cultures, mAb EB165 coreactivity is not seen until after fusion which begins on Day 4 (Fig. 2B, left panel and Table 1). The 4.5-day time point shows that a majority of initial myotubes are ventricular-positive (Fig. 2B, left panel and Table 1), but by Day 5 more than half of the myotubes coreact with both HV11 and EB165 mAbs.
Fig. 2.
Frequency of MHC-positive myocytes and myotubes in double-labeled cultures from the PM of E14, E18, 1-day posthatched, and 3-week posthatched chickens. (A) E14, (B) E18, (C) 1-day posthatch, and (D) 3-week posthatch cultures. Duplicate cultures were double-labeled at each time point with mAbs against embryonic fast MHC (EB165 indirectly labeled with GAM-TRITC) and ventricular MHC (HV11 directly labeled with fluorescein). Nuclei were counterstained with DAPI and the number of nuclei within MHC-positive cells versus total nuclei was determined. At least 500 nuclei in 10 or more fields were counted per plate. Error bars represent standard deviation and are smaller than the symbols if not seen. Values decrease due to continued proliferation of nonmyogenic cells. emb/vent, coexpression of embryonic and ventricular MHC; vent, expression of ventricular MHC.
We also studied MHC expression in myogenic cultures prepared from newly hatched and young chicks. Table 1 and Figs. 2C and 2D show that myogenic cultures from both 1-day (2C) and 3-week (2D) posthatch chicks have similar MHC expression patterns, and these patterns are identical to the adult pattern (Hartley et al., 1991). Myocytes in 1-day and 3-week posthatch-derived cultures react exclusively with mAb HV11. Myotubes reacting with mAb HV11 but not mAb EB165 are present on Days 6 and 7 in cultures from 1-day posthatched chicks (Fig. 2C, left panel and Table 1) and on Days 4 and 5 in myogenic cultures from 3-week posthatch chicks (Fig. 2D, left panel and Table 1), after which double-labeled myotubes predominate. A very low number of double-labeled myocytes was detected in cultures from newly hatched chicks, similar to the frequency seen previously in adult cultures (Table 1 in Hartley et al., 1991). As discussed in the latter paper, these may be cells that undergo the transition in MHC expression which is associated with maturation without fusion. Differentiation is delayed in 1-day posthatch compared to 3-week posthatch cultures. This delay was also observed in 1-day posthatch clonal cultures (Table 2 and data not shown).
TABLE 2.
Reactivity of Clones with Anti-ventricular and/or Anti-embryonic MHC mAbs
| Source of cells | Culture age (days) | Number of clones
|
Total MHC+ clones | |
|---|---|---|---|---|
| E+V+ | V+ | |||
| E14 | 5 | 43 | 9 | 52 |
| 10 | 57 | 0 | 57 | |
| 15 | 50 | 0 | 50 | |
| E18 | 7 | 22 | 11 | 33 |
| 10 | 37 | 5 | 42 | |
| 15 | 50 | 0 | 50 | |
| 1-day | 8 | 0 | 5 | 5 |
| 11 | 8 | 19 | 27 | |
| 15 | 15 | 12 | 27 | |
| 3-week | 7 | 0 | 14 | 14 |
| 11 | 0 | 16 | 16 | |
| 15 | 3 | 19 | 22 | |
Note. Cells were plated at a clonal density and myogenic clones were marked prior to fixation and staining. Three (E18, 1-day, and 3-week) or four (E14) cultures were fixed for each time point and double-labeled as under Methods, except that nuclei were not counterstained. For cultures from E18, 1-day, and 3-week chicks the total numbers of clones screened for the first time point were 45, 26, and 22, respectively, but only myogenic clones (with MHC+ cells or myotubes) are shown. E+V+, clones with myocytes and myotubes reactive with both HV11 and EB165 mAbs. V+, clones with myocytes and myotubes reactive with HV11 mAb.
The decrease in the percentage of MHC-positive cells which occurs with time in all cultures is due to continued proliferation of nonmyogenic cells, which are present in primary cultures. This population may also contain nondifferentiating myogenic cells.
Clonal Analysis of Double-Labeled Fetal, Posthatch, and Adult Myoblasts
We had previously demonstrated that adult clones differentiate later than E10 clones and that the myocytes in adult clones express only ventricular MHC while the myotubes begin to undergo a transition from expressing ventricular MHC to coexpressing both ventricular and embryonic MHCs after an extended time in culture (Hartley et al., 1991). In comparison, a majority of E10 myogenic clones contain myocytes and myotubes which coexpress both MHC isoforms, although at the earliest time examined (i.e., Day 5 of culture) a small percentage of clones (6%) were shown to contain exclusively myocytes and myotubes which express only ventricular MHC. This may indicate that a small number of clonable adult myoblasts are present by Embryonic Day 10.
In the current study, we pursued this clonal analysis on myogenic cells derived from pectoralis muscles of different age chickens. This type of analysis, although limited to clonable cells, offers a means to study progeny of individual myogenic progenitors and could indicate not only when myogenic clones expressing ventricular MHC alone become dominant, but also whether the transition from the fetal to the adult phenotype could occur within single clones associated with a specific developmental age. For clonal analysis, freshly isolated myoblasts were plated at a low density (100 cells/60-mm dish) to ensure that each clone derived from one precursor cell. Clones were marked prior to fixation and then double-labeled as under Methods. If clones contained myocytes and/or myotubes which reacted only with the mAb HV11, they were classified as ventricular-positive (V+), and if they contained myocytes and/or myotubes which reacted with both mAbs (HV11 and EB165), they were classified as embryonic-positive, ventricular-positive (E+V+, or double-labeled); E+V− clones (those containing only embryonic-positive myocytes/myotubes) were never seen. These results are summarized in Table 2. In clonal cultures from 1-day and 3-week posthatch chicks, myotubes were not present on Days 7 and 8 of culture, respectively, but ventricular-positive myocytes were. At both posthatch stages, ventricular-positive clones containing myocytes only (Days 7 and 8) and both myocytes and myotubes (Days 11 and 15) were present at all time points examined and comprised all of the myogenic clones at the earliest day examined (Table 2). In 11- and 15-day clonal cultures from newly hatched chicks, some clones also contained myotubes positive for both ventricular and embryonic MHC and thus were classified as E+V+ (30% of all clones by Day 11 and 50% of all clones by Day 15). In clonal cultures from 3-week chicks, clones were V+ only until 15 days of culture, at which time double-labeled myotubes were present in some of the clones (14% of all clones), similar to previously reported results from adult cultures (Hartley et al., 1991). Myocytes in 3-week posthatch clonal cultures were exclusively ventricular-positive.
In contrast to the postnatal stages, a majority of E14 clones are E+V+, with 9 V+ (17% of total myogenic clones) and 43 E+V+ clones on Day 5 of culture, at which time there are numerous small myotubes and many myocytes. All E14 clones were double-labeled thereafter. In E18 clones, fusion was first seen on Day 7, at which time there were a few small myotubes and many aligned, bipolar cells. On this day, 11 E18 clones were V+ (33% of total clones) while 22 were E+V+. On Day 10, E18 clonal cultures still contained V+ clones (12% of total clones), unlike E14 clonal cultures in which V+ clones were present only on Day 5. By Day 15 in culture, all E18 clones contained myotubes which were double-labeled and thus were classified as E+V+.
E14 and E18 E+V+ clones are heterogeneous, ranging from clones which contain E+V+ myotubes but not myocytes, E+V+ myocytes but not myotubes, and clones which contain both E+V+ myotubes and myocytes. In addition, the majority of these clones contain V+ myocytes and myotubes. The number of E+V+ clones containing a particular type(s) of myocytes was quantitated for Day 5 in E14 and Day 7 in E18 clones, which were the first time points examined. Of the 43 E14 double-labeled clones, 1 had no myocytes, 8 contained only E+V+ myocytes, 11 contained only V+ myocytes, and 23 contained both E+V+ and V+ myocytes (within each of these 23 clones the proportion of V+ myocytes varied from about 30 to 90% of all the myocytes). Of 22 double-labeled E18 clones, 3 contained E+V+ myocytes, 5 contained V+ myocytes, and 14 contained both E+V+ and V+ myocytes (again, within each of these 14 clones the proportion of V+ myocytes varied from about 30 to 90% of the total myocytes). The 11 E14 clones and the 5 E18 which contain V+ myocytes only are similar to adult or posthatch clones in that they are double-labeled because they contain E+V+ myotubes. Although preliminary, the finding of clones containing both V+ and E+V+ myocytes suggests that at E14 and E18 there are progenitor cells capable of giving rise to both V+ and E+V+ myocytes.
The Adult and Fetal Patterns of MHC Expression Are Correlated with Developmental Age but not Muscle Fiber Type
To determine whether the adult pattern of ventricular/embryonic MHC expression is peculiar to adult myoblasts from the pectoralis muscle or if it is a common trait of myoblasts from other adult skeletal muscles, we examined expression of embryonic and ventricular MHCs in cultures of adult chicken ALD, a slow tonic, and PLD, a fast twitch muscle. We also examined E10 hindlimb muscles, which consist of fast and fast/slow fibers, to determine if the fetal pattern of ventricular/embryonic MHC coexpression is present in other fetal muscles.
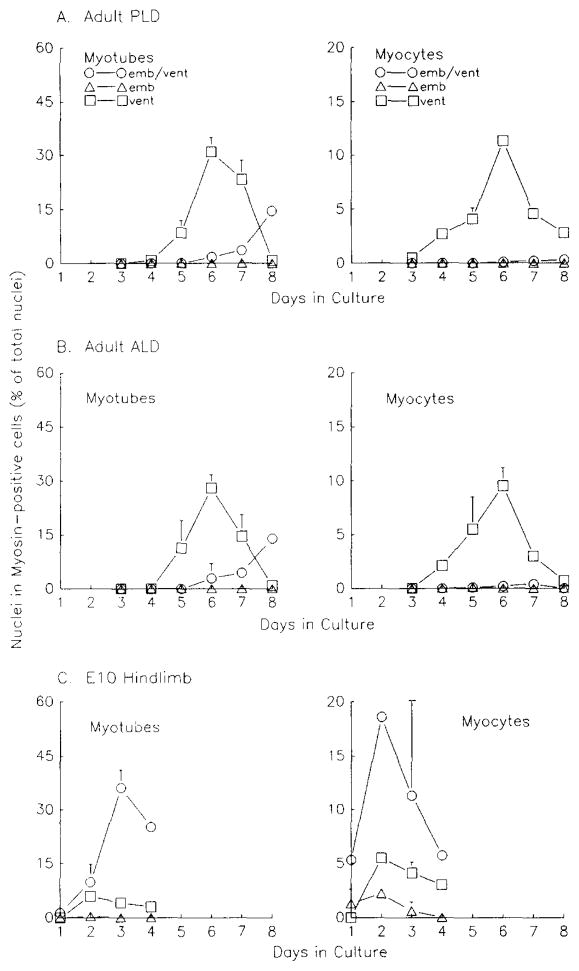
We found that the adult pattern is present in both fast and slow adult muscles, as is the fetal pattern in mixed fast/slow fetal muscles. Figure 3A shows results from adult PLD cultures (a fast muscle) and Fig. 3B shows adult ALD cultures (a slow muscle). The left panels show the percentages of myotubes reactive with the different mAbs, while the right panels depict myocytes only. In both ALD and PLD cultures, a majority of myocytes are reactive with the ventricular mAb and are present by the fourth day in culture (Figs. 3A and 3B, right panels). Fusion begins by Day 5, and the ventricular-positive myotubes begin to coreact with the anti-embryonic mAb by Day 6 in culture. By Day 8, a majority of remaining myotubes in both ALD and PLD cultures are double-labeled with both mAbs (approximately 83 and 94% of MHC-positive nuclei in myotubes for PLD and ALD, respectively) (Figs. 3A and 3B, left panels).
Fig. 3.
Frequency of MHC-positive myocytes and myotubes in double-labeled adult and fetal primary myogenic cultures from various muscles. (A) Adult posterior latissimus dorsi (PLD)-, (B) Adult anterior latissimus dorsi (ALD)-, and (C) E10 hindlimb-derived cultures. Duplicate cultures were labeled and data obtained as in Fig. 2. Error bars show standard deviation; where there are no error bars, the symbols are bigger than the standard deviation. Values decrease due to continued proliferation of nonmyogenic cells. emb/vent, coexpression of embryonic and ventricular MHCs; emb, expression of embryonic MHC; vent, expression of ventricular MHC.
MHC reactivity in E10-derived hindlimb cultures (Fig. 3C) parallels the fetal pattern seen in E10 PM cultures (Hartley et al., 1991). Myocytes and myotubes in E10 hindlimb cultures react with both embryonic and ventricular mAbs upon differentiation in culture. Double-labeled myocytes are already present on Day 1 of culture (Fig. 3C, right panel), and the percentage of nuclei in double-labeled myotubes increases until Day 3 (left panel), at which time most fusion has occurred. There is a low number of single-labeled myocytes which react with either mAb in E10 hindlimb cultures, in a manner similar to E10 pectoralis muscle-derived cultures Hartley et al., 1991).
DISCUSSION
In this study, we were concerned with two issues. First, using the expression of ventricular but not embryonic MHC as a marker for adult myoblasts, we were interested in defining the time during development when adult myoblasts emerge. Second, we wanted to determine if the specific adult and fetal MHC phenotypes could be used as common markers for all adult and fetal myoblasts, regardless of the muscle type from which they were isolated.
The Developmental Appearance of Adult Myoblasts
Double-immunolabeling with MHC isoform-specific mAbs of primary myogenic cultures from progressively later stages of chicken development allowed us to identify when myoblasts with the adult phenotype of MHC expression first appear during development. The results indicate that all myoblasts in 3-week chickens and newly hatched chicks give rise to myogenic cultures with the adult pattern of MHC expression, supporting the theory that all myoblasts at these stages are of the adult (satellite cell) type. Adult type myoblasts (those giving rise to myocytes expressing ventricular but not embryonic MHCs) are also present during fetal development, their frequency increasing by E18. For example, in mass cultures the percentages of myocytes expressing only ventricular MHC are 16% for E10 (Hartley et al., 1991), 24% for E14, and 86% for E18 on the days when the highest numbers of myocytes are present (Day 2 for E10 and E14 and Day 5 for E18). Also like adult cultures, myotubes in E18 mass cultures initially express ventricular MHC alone and eventually coexpress embryonic MHC, but this coexpression occurs sooner in E18 cultures (Fig. 2B) than in 1-day and 3-week posthatch cultures (Figs. 2C and 2D). This quicker expression of embryonic MHC (which was also observed in E18 clones) may reflect a more synchronous transition, perhaps due to the physiological state of the cells in the animal.
Clonal analysis of myoblasts from the different ages revealed a dramatic difference between the number of ventricular only expressing clones following hatching and that in E18 (Table 2), but the difference in number of these clones in E18 versus E14 is more subtle (33 vs 17% of total clones at the first time point) than that seen in mass cultures. One explanation for this discrepancy between mass and clonal cultures is that clonal analysis is limited to clonable cells, and as it was previously reported by Quinn et al. (1984, 1989), myogenic cells from the breast muscle of chicken embryos form both large clones (equivalent to those studied in the present results) and small clones (which result from one to four cell divisions). The majority of myoblasts from E17 to E18 form large clones while only about 25% of E14 clones and about 5% of E10 clones are of this type (Quinn et al., 1984). Hence it is possible that many of the progenitors which give rise to E+V+ cells exclusively may differentiate quickly (i.e., form small clones) and thus would not be included in our clonal analysis. Data from E14 mass cultures support this possibility, as E+V+ myocytes are present by the first day of culture.
The two extremes of ventricular and embryonic MHC expression, coexpression during fetal development and expression of ventricular MHC alone during posthatch stages, may result from the presence of two separate lineages: myoblasts which give rise to E+V+ myocytes and myotubes (fetal myoblasts) and myoblasts which give rise to V+ myocytes and myotubes which are initially V+ but become E+V+ (adult myoblasts). Chick-quail chimera studies suggest that, like myoblasts found during development, satellite cells originate in the somites (Armand et al., 1983). Hence, it is possible that adult myoblasts are predetermined at very early developmental stages, but are not dominant until late embryogenesis. If one assumes that adult and fetal myoblasts represent two predetermined populations or lineages, then the transition from fetal to adult dominance may occur as fetal myoblasts differentiate and fuse into myotubes, whereas adult myoblasts continue to proliferate, as suggested for the replacement of embryonic with fetal myoblasts (reviewed in Stockdale and Miller, 1987), or adult myoblasts may be quiescent in the embryo with their numbers remaining stable, while fetal myoblasts continue to terminally differentiate. The presence of E14 and E18 clones containing both E+V+ and V+ myocytes does not support the existence of two separate lineages that are determined early in development, but rather suggests that a progenitor cell capable of giving rise to both types of myoblasts (fetal and adult) exists during development. This bipotential precursor cell would become restricted in potential as development progresses, perhaps in response to the changing environment in the developing muscle, and would give rise to predominantly adult myoblasts posthatch. Since MHC expression in clonal cultures cannot be followed continuously, the finding of E14 and E18 clones which contain both V+ and E+V+ myocytes could also be explained by a progression of MHC expression from V+ only to E+V+ within individual myocytes in a clone. The existence of clones (from posthatch stages) which contain exclusively V+ myocytes even after an extended time in culture does not support this hypothesis. Definitively determining the lineal relationship between adult and fetal myoblasts was beyond the scope of this study, and a more thorough analysis is needed to further address these lineage relationships.
Evidence for a Common Adult and Fetal Pattern of Embryonic Fast MHC Expression in Different Muscle Types
Diversity in MHC expression patterns has been shown by Stockdale and co-workers (Stockdale and Miller, 1987; Miller and Stockdale, 1989; Feldman and Stockdale, 1991). Their studies established the existence of different subpopulations within embryonic and adult myoblast populations based on the MHC(s) expressed by myotubes formed in culture. For example, adult myoblasts can be divided into at least two subpopulations, fast and fast/slow, based on the differential expression of fast and slow MHCs in myotubes (Feldman and Stockdale, 1991). We now show that regardless of this diversity, myoblasts from adult fast and slow muscles share a common adult pattern of ventricular and embryonic MHC expression, as do fetal myoblasts from different fetal muscles. The adult pattern of ventricular but not embryonic fast MHC expression in myocytes and newly formed myotubes that is seen in cultures derived from the adult PM is also present in cultures from an adult slow muscle (ALD) and from a fast muscle other than the PM (PLD). Cultures derived from E10 hindlimb muscles (these muscles are fast, fast/slow, or mixed fast and fast/slow) contained myocytes and myotubes that coexpressed ventricular and embryonic MHCs in a manner similar to cultures of the E10 PM (which is a fast muscle).
Other investigators have shown that in vivo, embryonic and regenerating adult muscles (fast and slow) initially express both a ventricular and a fast isoform of MHC (Sweeney et al., 1989; Kennedy et al., 1989; Stewart et al., 1989). The fast isoform was later shown to be embryonic fast MHC (Stewart et al., 1989; Bourke et al., 1991). Our results show that fetal myogenic cultures also coexpress ventricular and embryonic MHC(s), while adult myogenic cultures at first express ventricular MHC only and then after a lag coexpress embryonic MHC, but only in myotubes. This lag was not observed during in vivo experiments on regenerating muscle (Sweeney et al., 1989; Kennedy et al., 1989; Bourke et al., 1991), perhaps because the earliest time point examined following cold injury was 3 days, and at this time the majority of myotubes expressed both MHC isoforms (Bourke et al., 1991). In addition, it is not clear whether myocytes exist as such in regenerating muscle, as they may fuse into myotubes immediately upon differentiation. Also, unambiguously distinguishing between myocytes and early myotubes in tissue sections may not be possible (Kennedy et al., 1989).
In conclusion, our results suggest that adult myoblasts begin to predominate in late chicken embryogenesis and represent virtually all myoblasts present at hatch. Similar conclusions on the appearance of adult myoblasts are reported in a recent study by Feldman and Stockdale (1992). Furthermore, our results suggest that adult and fetal patterns of MHC expression are consistent for both fast and slow muscles. Preliminary observations by us also indicate that fetal and adult myoblasts may arise from a common precursor, but further studies are needed to clarify this issue. The mechanisms which regulate fetal and adult patterns of MHC expression remain to be elucidated, as does the reason for the existence of multiple myogenic populations or lineages during development. In an attempt to understand the mechanism(s) underlying these patterns of MHC expression, we are currently comparing the expression of ventricular and embryonic MHC genes to that of myogenic determination genes. Myogenic determination genes are putative muscle-specific transcriptional regulators (Weintraub et al., 1991) and are expressed in different patterns during muscle development (Ott et al., 1991; Lyons et al., 1991; Hinterberger et al., 1991; Sassoon et al., 1989). In view of this, differential expression of these genes may result in the specific patterns of MHC expression observed for fetal and adult myoblasts.
Acknowledgments
This work was supported by grants from the NIH (AR39677 to Z.Y.-R. and AG08573 to E.B.), the Muscular Dystrophy Association (to Z.Y.-R. and to E.B.), and the USDA (91-337206-6715 to E.B.). During the course of this study Z.Y.-R. was also supported by a grant-in-aid from the American Heart Association. R.S.H. was supported by Predoctoral Developmental Biology Training Grant HD07183 from the NIH.
Footnotes
Abbreviations used: PM, pectoralis muscle; ALD, anterior latissimus dorsi; PLD, posterior latissimus dorsi; MHC, myosin heavy chain; E10, Embryonic Day 10; E14, Embryonic Day 14; E18, Embryonic Day 18; EGTA, ethylene glycol bis(β-aminoethyl ether) N,N,N′,N′,-tetra-acetic acid; DAPI, 4,6-diamidino-2-phenylindole.
References
- Armand O, Boutineau A-M, Mauger A, Pautou M-P, Kieny M. Origin of satellite cells in avian skeletal muscles. Arch Anat Microsc. 1983;72:163–181. [PubMed] [Google Scholar]
- Bandman E, Bourke DL, Wick M. Regulation of myosin heavy chain expression during development, maturation, and regeneration in avian muscle: The role of myogenic and non-myogenic factors. In: Pette D, editor. The Dynamic State of Muscle Fibers. de Gruyter; Berlin: 1990. pp. 127–138. [Google Scholar]
- Bandman E, Matsuda R, Strohman RC. Developmental appearance of myosin heavy and light chain isoforms in vivo and in vitro in chicken skeletal muscle. Dev Biol. 1982;93:508–518. doi: 10.1016/0012-1606(82)90138-5. [DOI] [PubMed] [Google Scholar]
- Bourke DL, Wilie SR, Wick M, Bandman E. Differentiating skeletal muscles initially express a ventricular myosin heavy chain. Basic Appl Myol. 1991;1:13–21. [Google Scholar]
- Campion DR. The muscle satellite cell: A review. Int Rev Cytol. 1984;87:225–247. doi: 10.1016/s0074-7696(08)62444-4. [DOI] [PubMed] [Google Scholar]
- Cerny LC, Bandman E. Expression of myosin heavy-chain isoforms in regenerating myotubes of innervated and denervated chicken pectoral muscle. Dev Biol. 1987;119:350–362. doi: 10.1016/0012-1606(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Cossu G, Cicinelli P, Fieri C, Coletta M, Molinaro M. Emergence of TPA-resistent “satellite” cells during muscle histogenesis of human limb. Exp Cell Res. 1985;160:403–411. doi: 10.1016/0014-4827(85)90187-9. [DOI] [PubMed] [Google Scholar]
- Cossu G, Eusebi F, Grassi F, Wanke E. Acetylcholine receptors are present in undifferentiated satellite cells but not in embryonic myoblasts in culture. Dev Biol. 1987;123:43–50. doi: 10.1016/0012-1606(87)90425-8. [DOI] [PubMed] [Google Scholar]
- Cossu G, Molinaro M. Cell Heterogeneity in the myogenic lineage. Curr Top Dev Biol. 1987;23:185–208. doi: 10.1016/s0070-2153(08)60625-0. [DOI] [PubMed] [Google Scholar]
- Cossu G, Molinaro M, Pacifici M. Differential response of satellite cells and embryonic myoblasts to a tumor promoter. Dev Biol. 1983;98:520–524. doi: 10.1016/0012-1606(83)90382-2. [DOI] [PubMed] [Google Scholar]
- Cossu G, Ranaldi G, Senni MI, Molinaro M, Vivarelli E. “Early” mammalian myoblasts are resistant to phorbol ester-induced block of differentiation. Development. 1988;102:65–69. doi: 10.1242/dev.102.1.65. [DOI] [PubMed] [Google Scholar]
- Crow MT, Stockdale FE. Myosin expression and specialization among the earliest muscle fibers of the developing avian limb. Dev Biol. 1986;113:238–254. doi: 10.1016/0012-1606(86)90126-0. [DOI] [PubMed] [Google Scholar]
- Evans D, Miller JB, Stockdale FE. Developmental patterns of expression and coexpression of myosin heavy chains in atria and ventricles of the avian heart. Dev Biol. 1988;127:376–383. doi: 10.1016/0012-1606(88)90324-7. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Stockdale FE. Skeletal muscle satellite cell diversity: Satellite cells form fibers of different types in cell culture. Dev Biol. 1991;143:320–334. doi: 10.1016/0012-1606(91)90083-f. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Stockdale FE. Temporal appearance of satellite cells during myogenesis. Dev Biol. 1992 doi: 10.1016/0012-1606(92)90107-r. in press. [DOI] [PubMed] [Google Scholar]
- Goding JW. Conjugation of antibodies with fluorochromes: Modifications to the standard methods. J Immunol Methods. 1976;13:215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Yablonka-Reuveni Z. Molecular and cell biology of skeletal muscle regeneration. In: Partridge T, editor. Molecular and Cell Biology of Muscular Dystrophy. Chapman & Hall; London: 1992. in press. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Bandman E, Yablonka-Reuveni Z. Myoblasts from fetal and adult skeletal muscle regulate myosin expression differently. Dev Biol. 1991;148:249–260. doi: 10.1016/0012-1606(91)90334-y. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Yablonka-Reuveni Z. Long-term maintenance of primary myogenic cultures on a reconstituted basement membrane. In Vitro Cell Dev Biol. 1990;26:955–961. doi: 10.1007/BF02624469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterberger TJ, Sassoon DA, Rhodes SJ, Konieczny SF. Expression of the muscle regulatory factor MRF4 during somite and skeletal myofiber development. Dev Biol. 1991;147:144–156. doi: 10.1016/s0012-1606(05)80014-4. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Zacks SI. The histogenesis of rat intercostal muscle. J Cell Biol. 1969;42:135–169. doi: 10.1083/jcb.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy JM, Sweeney LJ, Gao L. Ventricular myosin expression in developing and regenerating muscle, cultured myotubes, and nascent myofibers of overloaded muscle in the chicken. Med Sci Sports Exercise. 1989;21:S187–S197. [PubMed] [Google Scholar]
- Lyons GE, Muhlebach S, Moser A, Masood R, Paterson BM, Buckingham ME, Perriard JC. Developmental regulation of creatine kinase gene expression by myogenic factors in embryonic mouse and chick skeletal muscle. Development. 1991;113:1017–1029. doi: 10.1242/dev.113.3.1017. [DOI] [PubMed] [Google Scholar]
- Mauro A. Muscle Regeneration. Raven Press; New York: 1979. [Google Scholar]
- Mayne R, Swasdison S, Sanderson RD, Irwin MH. Extracellular matrix, fibroblasts, and the development of skeletal muscle. In: Stockdale F, Kedes L, editors. Cellular and Molecular Biology of Muscle Development. A. R. Liss; New York: 1989. pp. 107–116. [Google Scholar]
- Miller JB, Stockdale FE. Developmental regulation of the multiple myogenic cell lineages of the avian embryo. J Cell Biol. 1986;103:2197–2208. doi: 10.1083/jcb.103.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JB, Stockdale FE. Multiple cellular processes regulate expression of slow myosin heavy chain isoforms during avian myogenesis in vitro. Dev Biol. 1989;136:393–404. doi: 10.1016/0012-1606(89)90265-0. [DOI] [PubMed] [Google Scholar]
- Moss FP, LeBlond CP. Satellite cells as a source of nuclei in muscles of growing rats. Anat Rec. 1971;170:421–436. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Nameroff M, Rhodes LD. Differential response among cells in the chick embryo myogenic lineage to photosensitization by Merocyanine 540. J Cell Physiol. 1989;141:475–482. doi: 10.1002/jcp.1041410305. [DOI] [PubMed] [Google Scholar]
- Ott MO, Bober E, Lyons G, Arnold H, Buckingham M. Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development. 1991;111:1097–1107. doi: 10.1242/dev.111.4.1097. [DOI] [PubMed] [Google Scholar]
- Quinn LS, Nameroff M, Holtzer H. Age dependent changes in myogenic precursor cell compartment sizes: Evidence for the existence of a stem cell. Exp Cell Res. 1984;154:65–82. doi: 10.1016/0014-4827(84)90668-2. [DOI] [PubMed] [Google Scholar]
- Quinn LS, Rhodes LD, Nameroff M. Characterization of sequential myogenic cell lineage compartments. In: Kedes LH, Stockdale FE, editors. Cellular and Molecular Biology of Muscle Development. A. R. Liss; New York: 1989. pp. 37–46. [Google Scholar]
- Sassoon D, Lyons G, Wright WE, Lin V, Lassar A, Weintraub H, Buckingham M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989;341:303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- Seed J, Hauschka SD. Temporal separation of the migration of distinct myogenic precursor populations into the developing chick wing bud. Dev Biol. 1984;106:389–393. doi: 10.1016/0012-1606(84)90237-9. [DOI] [PubMed] [Google Scholar]
- Senni MI, Castrignano F, Poiana G, Cossu G, Scarcella G, Biagioni S. Expression of adult fast pattern of acetylcholinesterase molecular forms by mouse satellite cells in culture. Differentiation. 1987;36:194–198. doi: 10.1111/j.1432-0436.1987.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Snow MH. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. I. Fine structural analysis. Anat Rec. 1977a;188:182–200. doi: 10.1002/ar.1091880205. [DOI] [PubMed] [Google Scholar]
- Snow MH. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. II. An autoradiographic study. Anat Rec. 1977b;188:201–218. doi: 10.1002/ar.1091880206. [DOI] [PubMed] [Google Scholar]
- Stewart AFR, Kennedy JM, Bandman E, Zak R. A myosin isoform repressed in hypertrophied ALD muscle of the chicken reappears during regeneration following cold injury. Dev Biol. 1989;135:367–375. doi: 10.1016/0012-1606(89)90186-3. [DOI] [PubMed] [Google Scholar]
- Stockdale FE, Miller JB. The cellular basis of myosin heavy chain isoform expression during development of avian skeletal muscles. Dev Biol. 1987;123:1–9. doi: 10.1016/0012-1606(87)90420-9. [DOI] [PubMed] [Google Scholar]
- Sweeney LJ, Kennedy JM, Zak R, Kokjohn K, Kelley SW. Evidence for expression of a common myosin heavy chain phenotype in future fast and slow skeletal muscle during initial stages of avian myogenesis. Dev Biol. 1989;133:361–374. doi: 10.1016/0012-1606(89)90040-7. [DOI] [PubMed] [Google Scholar]
- Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S, Zhuang Y, Lassar A. The myoD gene family: Nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- White NK, Bonner PH, Nelson DR, Hauschka SD. Clonal analysis of vertebrate myogenesis. IV. Medium-dependent classification of colony-forming cells. Dev Biol. 1975;44:346–361. doi: 10.1016/0012-1606(75)90405-4. [DOI] [PubMed] [Google Scholar]
- White TP, Esser KA. Satellite cell growth and growth factor involvement in skeletal muscle growth. Med Sci Sports Exercise. 1989;21:S158–S163. [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. Application of density centrifugation and flow cytometry for the isolation and characterization of myogenic and fibroblast-like cells from skeletal muscle. In: Kedes LH, Stockdale FE, editors. Cellular and Molecular Biology of Muscle Development. A. R. Liss; New York: 1989. pp. 869–879. [Google Scholar]
- Yablonka-Reuveni Z, Anderson SK, Bowen-Pope DF, Nameroff M. Biochemical and morphological differences between fibroblasts and myoblasts from embryonic chicken skeletal muscle. Cell Tissue Res. 1988;252:339–348. doi: 10.1007/BF00214376. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Bowen-Pope DF, Hartley RS. Proliferation and differentiation of myoblasts: The role of platelet-derived growth factor and the basement membrane. In: Pette D, editor. The Dynamic State of Muscle Fibers. de Gruyter; Berlin: 1990. pp. 693–706. [Google Scholar]
- Yablonka-Reuveni Z, Quinn LS, Nameroff M. Isolation and clonal analysis of satellite cells from chicken pectoral muscle. Dev Biol. 1987;119:252–259. doi: 10.1016/0012-1606(87)90226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Nameroff M. Temporal differences in desmin expression between myoblasts from embryonic and adult chicken skeletal muscle. Differentiation. 1990;45:21–28. doi: 10.1111/j.1432-0436.1990.tb00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Seifert RA. Proliferation of chicken myoblasts is regulated by specific isoforms of platelet-derived growth factor: Evidence for differences between myoblasts from mid and late stages of development. 1992. Submitted for publication. [DOI] [PubMed] [Google Scholar]