SUMMARY
Uveal melanoma (UM) is the most common cancer in adult eyes. Approximately eighty percent of UMs harbor somatic activating mutations in GNAQ or GNA11 (encodes Gq or G11 respectively). Herein, we show in both cell culture and human tumors that cancer-associated Gq/11 mutants activate YAP, a major effector of the Hippo tumor suppressor pathway that is also regulated by G-protein coupled receptor (GPCR) signaling. YAP mediates the oncogenic activity of mutant Gq/11 in UM development, and the YAP inhibitor verteporfin blocks tumor growth of UM cells containing Gq/11 mutations. This study reveals an essential role of the Hippo-YAP pathway in Gq/11-induced tumorigenesis and suggests YAP as a potential drug target for UM patients carrying mutations in GNAQ or GNA11.
INTRODUCTION
Uveal melanoma (UM) is the most common intraocular tumor in adults and accounts for ~5% of all melanomas (Singh et al., 2005). UM frequently metastasizes to the liver via a haematogenous route as 90% of the UM metastasis is found in the liver. Once metastasized, there is no effective therapy with an average survival of 2–8 months (Singh et al., 2005). Unlike cutaneous melanoma, UM originates from melanocytes of the choroid, ciliary body, and iris (collectively known as the uvea) derived from the neural crest (Arnesen, 1985). Molecular genetic analyses show that the mutational spectrum of UM is very different from cutaneous melanoma. Instead of the BRAF or NRAS mutations common in cutaneous melanoma, more than 80% of UMs carry activating mutations in either GNAQ or GNA11 (Lamba et al., 2009; Van Raamsdonk et al., 2009; Van Raamsdonk et al., 2010). Only UM derived from the iris, a minor fraction (5%) of total UM cases, harbors BRAF mutations (Henriquez et al., 2007). Notably, the GNAQ mutation is frequent in benign blue naevi, while the GNA11 mutation is frequent in malignant UM (Van Raamsdonk et al., 2010). The Gq and G11 proteins encoded by the GNAQ and GNA11 genes respectively are the alpha subunits of heterotrimeric G-proteins that play an obligatory role in G-protein-coupled receptor (GPCR) signaling. Interestingly, all mutations in Gq or G11 occur at either arginine 183 (R183) or glutamine 209 (Q209) in a mutually exclusive manner, suggesting that these mutations in Gq and G11 have a similar function in tumor promotion (Van Raamsdonk et al., 2010). R183 and Q209 are located in the switch I and switch II domains of Gq/11 proteins, respectively, and these mutations convert the G-proteins into a constitutively active form by decreasing their GTPase activity. Therefore, the cancer-associated mutant Gq/11 would induce constitutive downstream signaling that presumably contributes to tumor development.
Previous works have shown that overexpression of active Gq/11 can induce transformation of normal melanocytes (Van Raamsdonk et al., 2009; Van Raamsdonk et al., 2010). Moreover, down-regulation of mutant Gq/11 in UM cells abolished their ability to form tumors in immunocompromised mice, demonstrating a direct cancer driving function of the active Gq/11 in tumorigenesis (Van Raamsdonk et al., 2009; Van Raamsdonk et al., 2010). Although it has been proposed that Gq/11 activates the MAP kinase, the precise molecular mechanism of these activating Gq/11 mutations in UM development remains to be defined.
The Hippo tumor suppressor pathway normally functions to control tissue homeostasis and limit organ size (Halder and Johnson, 2011; Pan, 2010; Tapon and Harvey, 2012; Yu and Guan, 2013). Core components of the Hippo pathway are represented by a kinase cascade consisting of MST1/2 and Lats1/2. The Lats1/2 kinases phosphorylate and inactivate YAP and TAZ, two homologous transcription co-activators with oncogenic potential. In fact, elevated expression or nuclear enrichment of YAP/TAZ has been observed in multiple types of human cancers (Chan et al., 2008; Steinhardt et al., 2008; Zhao et al., 2007). We recently reported that the Hippo pathway is strongly regulated by GPCR signaling (Miller et al., 2012; Mo et al., 2012; Yu et al., 2012). GPCR signaling can either activate or inhibit YAP activity in a manner dependent on the coupled G-protein. For example, activation of G12/13 stimulates YAP by inducing YAP dephosphorylation, nuclear localization, and transcriptional activity, whereas activation of Gs inhibits YAP by increasing YAP phosphorylation. Interestingly, expression of active Gq/11 (containing the Q209L mutation), but not the wild type, is able to stimulate YAP/TAZ dephosphorylation (Yu et al., 2012), indicating that YAP can be activated by Gq/11. These observations prompted us to investigate if the Hippo-YAP pathway may function as a mediator in active Gq/11-induced tumorigenesis, particularly in UM development.
RESULTS
Activation of YAP by mutant Gq/11 in UM
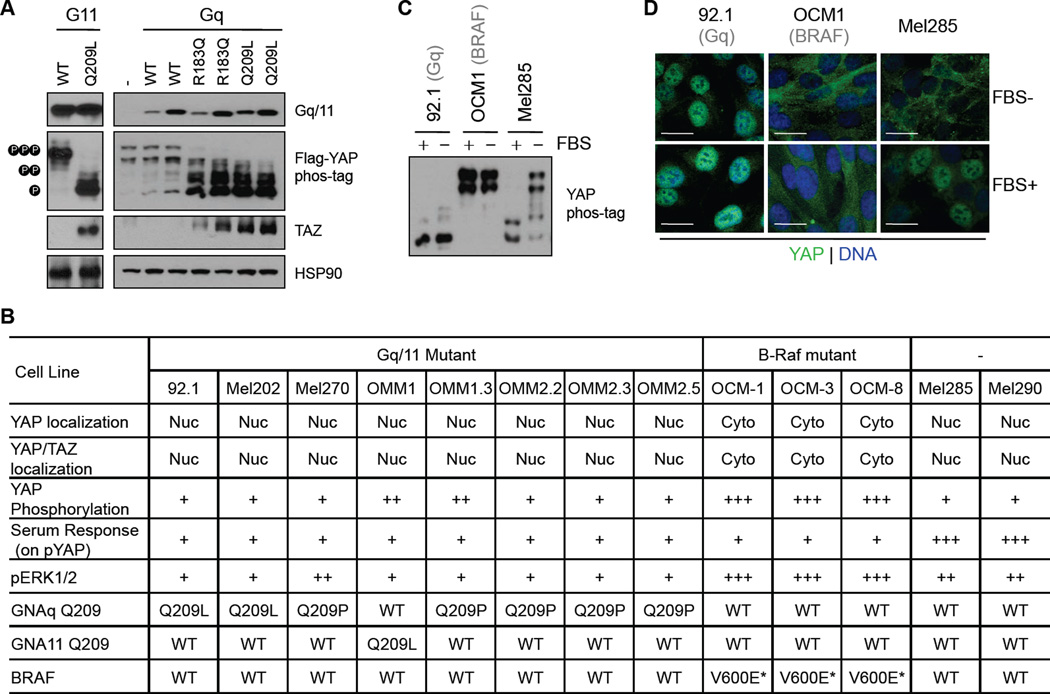
To test whether YAP can be activated by the cancer-associated mutant Gq/11, we firstly determined the effect of GNAQ and GNA11 hotspot mutations found in UM on YAP activity. In HEK293A cells, ectopic expression of mutant Gq/11 (GqR183Q, GqQ209L, or G11Q209L), but not the wild type Gq or G11, caused a dramatic dephosphorylation of co-transfected YAP, as indicated by faster migration of YAP on a phos-tag-containing gel (Figure 1A). Because phosphorylation inhibits YAP, these data suggests that mutant Gq/11 activates YAP. TAZ has two phosphodegrons and Lats-induced phosphorylation promotes TAZ ubiqutination and degradation (Huang et al., 2012; Liu et al., 2010). As expected, the endogenous TAZ protein levels were significantly increased in the presence of mutant Gq/11 (Figure 1A). Lats-induced phosphorylation inhibits YAP/TAZ by promoting YAP/TAZ cytoplasmic sequestration, while dephosphorylated YAP/TAZ translocate to the nucleus and stimulate gene expression. Consistently, over-expression of active Gq/11 mutants, but not wild-type Gq/11, induced nuclear localization of endogenous YAP/TAZ, as assessed by immunofluorescence staining with an antibody that recognizes both YAP and TAZ (Figure S1A–C). These results show that the mutant Gq/11 found in UM potently activates YAP/TAZ, and suggest a model that activation of YAP/TAZ may contribute to mutant Gq/11-induced UM development, given the known oncogenic function of these two transcription co-activators.
Figure 1. Activation of YAP by mutant Gq/11 in UM cell lines.
(A) Effects of UM-associated mutant Gq/11 on YAP/TAZ activity. HEK293A cells were transfected with different Gq/11 plasmids together with FLAG-YAP, and following 12 hr serum starvation, cells were harvested and YAP/TAZ activation status was analyzed by immunoblotting using the indicated antibodies. YAP phosphorylation was assessed using gels containing phos-tag, which slows down migration of phosphorylated YAP during electrophoresis. Endogenous TAZ protein levels were determined with a TAZ specific antibody. For the Gq plasmids, two concentrations of plasmids were used in transfection and the expression levels of transfected Gq were determined by immunoblotting. (B) A table summarizing Gq/11 or BRAF mutation status, YAP phosphorylation, YAP localization, and responses to serum of multiple UM cells. Nuc, Nucleus; Cyto, cytoplasm; wt, wild type; the number of “+” indicate the strength; *, information from Griewank et al., 2012. (C) YAP phosphorylation and response to serum in representative UM cell lines. UM cells were cultured with or without 10% FBS for 16 hr. (D) YAP localization and response to serum in representative UM cell lines. Cells were maintained in the presence or absence of 10% FBS for 16 hr, and after fixation, YAP localization was determined by immunostaining. The green and blue colors represent YAP and DNA staining, respectively. Scale bars represent 5 µm.
See also Figure S1.
We then investigated the YAP/TAZ activation status in a panel of 13 cell lines established from primary or metastatic UM by different laboratories. We sequenced the genes of GNAQ and GNA11. Among these UM cell lines, seven (92.1, Mel202, Mel270, OMM1.3, OMM2.2, OMM2.3, and OMM2.5) contain the GqQ209 mutation, one (OMM1) contains the G11Q209L mutation, and the remaining five tumor lines (OCM1, OCM3, OCM8, Mel285, and Mel290) have no mutation in Gq/11 (Figure 1B). These data are consistent with other recently reported mutation analyses, and among these cell lines, three (OCM1, OCM3, and OCM8) contain BRAFV600E mutation (Griewank et al., 2012). Next, we determined YAP/TAZ phosphorylation and subcellular localization for each of these UM cell lines. Interestingly, all UM cell lines with Gq/11 mutations displayed low or moderate YAP phosphorylation and strong nuclear YAP (or YAP/TAZ) localization (Figure 1B). On the other hand, YAP was highly phosphorylated and exhibited exclusive cytoplasmic localization in BRAF mutant cells (Figure 1B). These observations demonstrate that YAP is activated in Gq/11 mutant UM cells but inactivated in BRAF mutant cells.
YAP/TAZ are known to be activated by serum or lysophosphatidic acid (LPA) (Miller et al., 2012; Yu et al., 2012). Both serum and LPA activate YAP/TAZ by inducing rapid dephosphorylation and nuclear localization. In UM cells with wild-type Gq/11 and BRAF, serum and LPA induced a strong YAP dephosphorylation and concomitantly increased YAP nuclear localization (Figures 1B–D, S1D and S1E). In contrast, in UM cells containing mutated Gq/11, YAP was dephosphorylated and localized in the nucleus regardless of the serum or LPA conditions. Our findings show that YAP/TAZ are indeed more active in UM cell lines containing Gq/11 mutations and are no longer sensitive to serum or LPA. Notably, in UM cells with mutant BRAF, YAP was heavily phosphorylated and the serum and LPA-induced YAP dephosphorylation and nuclear localization were blunted (Figures 1B–D, S1D and S1E). In support, Lats phosphorylation status, an indicator of kinase activity, was higher in BRAF mutant cells compare to that in Gq/11 mutant cells (Figure S1F). These data suggest that YAP activation is not needed for tumor growth of BRAF mutant cells and, moreover, active BRAF might suppress YAP activation.
Previously, Gq/11 mutant-induced activation of the extracellular-signal-regulated kinases (ERK), also known as MAP-kinase (MAPK), was proposed as a potential mechanism for mutant Gq/11 in UM development (Van Raamsdonk et al., 2009; Van Raamsdonk et al., 2010). Indeed, over-expression of Gq/11 mutants moderately induces phosphorylation of ERK (Van Raamsdonk et al., 2009; Van Raamsdonk et al., 2010). Based on Western analyses of the 13 UM cell lines, ERK phosphorylation in Gq/11 mutant UM cell lines was evident, however it was much lower than that of BRAF mutant UM cells (Figure 1B, S1D). Moreover, ERK phosphorylation in the Gq/11 mutant UM cells was no higher than that in UM cells (Mel285 and Mel290) without Gq/11 mutation, suggesting that the ERK pathway is unlikely a major mediator for UM driven by mutant Gq/11. Based on the above data, we speculate that YAP/TAZ activation may play an important role in UM tumorigenesis.
Activation of YAP in uveal melanomas with Gq/11 mutation
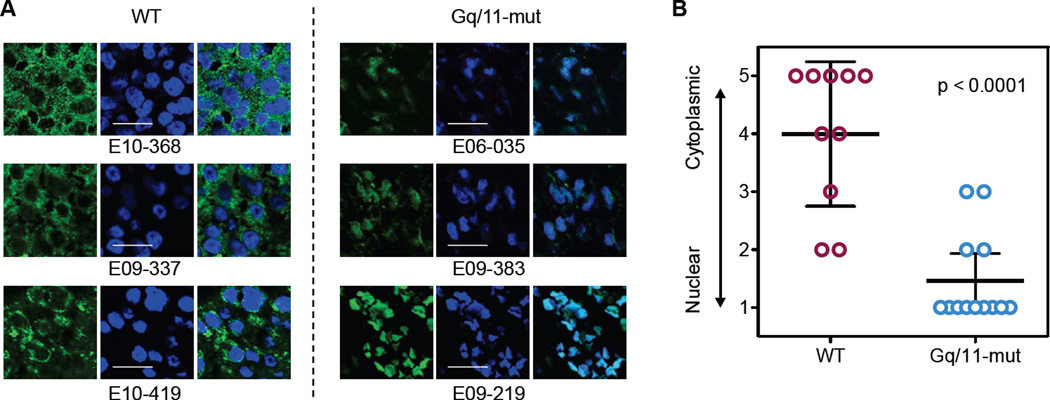
To determine YAP/TAZ activation status in UM, we examined formalin-fixed paraffin-embedded sections of enucleated tumors. We collected 23 UM samples and performed genomic DNA sequencing for exon 4 (R183) and exon 5 (Q209) of Gq/11. Thirteen UM samples have Q209 mutations whereas none has R183 mutation in Gq/11 (Table S1). The same clinical samples were immunostained using a YAP or YAP/TAZ antibody (Figures 2A and Table S1). The subcellular localization of YAP was assessed and scored from 1 to 5, with 1 representing exclusive nuclear localization and 5 representing exclusive cytoplasmic localization (Table S1). When YAP localization data were compared with the mutation status of Gq/11, we observed a strong correlation between mutated Gq/11 and YAP nuclear localization (Figure 2B). Based on these observations, we conclude that mutated Gq/11 is associated with YAP activation in UM, supporting a possible pathological role of YAP in Gq/11 mutation-induced tumorigenesis.
Figure 2. YAP nuclear localization correlates with Gq/11 mutations in UM specimens.
(A) Representative images for YAP localization in UM specimens. Immunofluorescence staining was performed for YAP (Green) and DNA (Blue, DAPI). Shown are three representative samples of wild type (left panels) and Gq/11 mutant (right panels). Scale bars represent 10 µm. (B) Correlation between YAP nuclear localization and Gq/11 mutation in UM specimens. The subcellular localization of YAP were scored from 1 to 5, with “1” representing exclusive nuclear localization and “5” representing for exclusive cytoplasmic localization. Student t test (two-tailed, 95% confidence intervals) was used for statistical analysis, and error bars represent standard deviation (SD).
See also Table S1.
Down-regulation of Gq in UM cells inactivates YAP
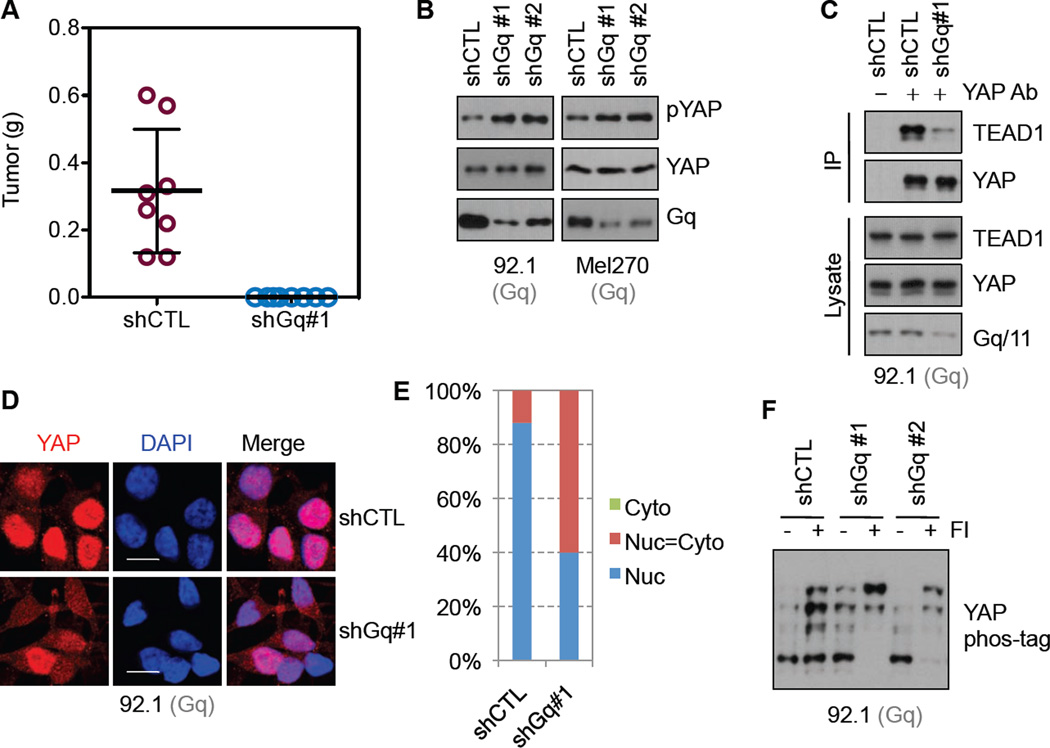
In a subcutaneous xenograft mouse model, 92.1 (GqQ209L) cells transfected with shRNA targeting Gq failed to develop tumor (Figure 3A), confirming an essential role for mutant Gq in tumorigenicity of 92.1 cells. We tested whether the mutant Gq is required for high YAP activity in UM cells. In both 92.1 and Mel270 (GqQ209P) cells, we established stable lines expressing control shRNA or Gq shRNAs (#1 and #2 target different regions). Knockdown of Gq was confirmed by Western blotting (Figure 3B). We observed that YAP phosphorylation (as indicated by the pYAP western blot) was increased in cells expressing Gq shRNA (Figure 3B). When dephosphorylated, YAP localizes in the nucleus and interacts with the TEAD family transcription factors to stimulate gene transcription (Cao et al., 2008; Wu et al., 2008; Zhang et al., 2008; Zhao et al., 2008). We examined the interaction of YAP with TEAD. In Gq knockdown cells, the interaction of YAP and TEAD was decreased while Gq knockdown had no effect on TEAD1 expression (Figure 3C). In addition, YAP nuclear localization was decreased in Gq knockdown cells (Figure3D and 3E), consistent with YAP inactivation. We have recently shown that Gs-PKA signaling stimulates YAP/TAZ phosphorylation, an effect opposite to Gq/11 activation (Kim et al., 2013; Yu et al., 2013; Yu et al., 2012). As expected, YAP phosphorylation was increased in 92.1 cells when treated with Forskolin and IBMX, which increase cellular cAMP and activate PKA (Figure 3F). Notably, Forskolin and IBMX induced a stronger YAP phosphorylation in the Gq knockdown 92.1 cells (Figure 3F), indicating that the active mutant Gq in 92.1 cells functions antagonistically to PKA. The above data support a function for mutant Gq in maintaining YAP in a dephosphorylated and activated status in UM cells.
Figure 3. Down-regulation of mutant Gq in UM cells inactivates YAP.
(A) Tumor formation ability of 92.1 (GqQ209L) cells with or without Gq knockdown. Control or Gq knockdown 92.1 cells were grafted into nude mice subcutaneously, and tumor formation was monitored. Knockdown efficiency is shown in (B). Error bars represent SD. (B) The effect of Gq knockdown on YAP phosphorylation. Stable 92.1 cells expressing control shRNA (shCTL) or two Gq targeting shRNAs (shGq#1 and shGq#2) were established. Gq knockdown efficiency and YAP phosphorylation in these cells were assessed by Western blotting. pYAP indicates Western blotting with an antibody that specially recognizes the S127 phosphorylated YAP. The same experiment was performed in Mel270 (GqQ209P) cells. (C) The effect of Gq knockdown on YAP-TEAD interaction. Immunoprecipitation of YAP from control or Gq knockdown cell lysates was performed, and TEAD1 co-precipitated was determined by immunoblotting. IP denotes immunoprecipitation. (D, E) The effect of Gq knockdown on YAP subcellular localization. Control or Gq knockdown 92.1 cells were serum starved for 16 hr and fixed, and YAP localization was determined. Scale bars represent 5 µm. (F) The effect of Gq knockdown on PKA-induced YAP phosphorylation (inactivation). Control or Gq knockdown cells were treated with Forskorlin (10 µM) and IBMX (100 µM) for 1 hr, and then YAP phosphorylation was determined. Both Forskolin and IBMX increase cAMP and activate PKA, which stimulates YAP phosphorylation.
YAP is required for mutant Gq/11, but not mutant BRAF, driving tumorigenesis
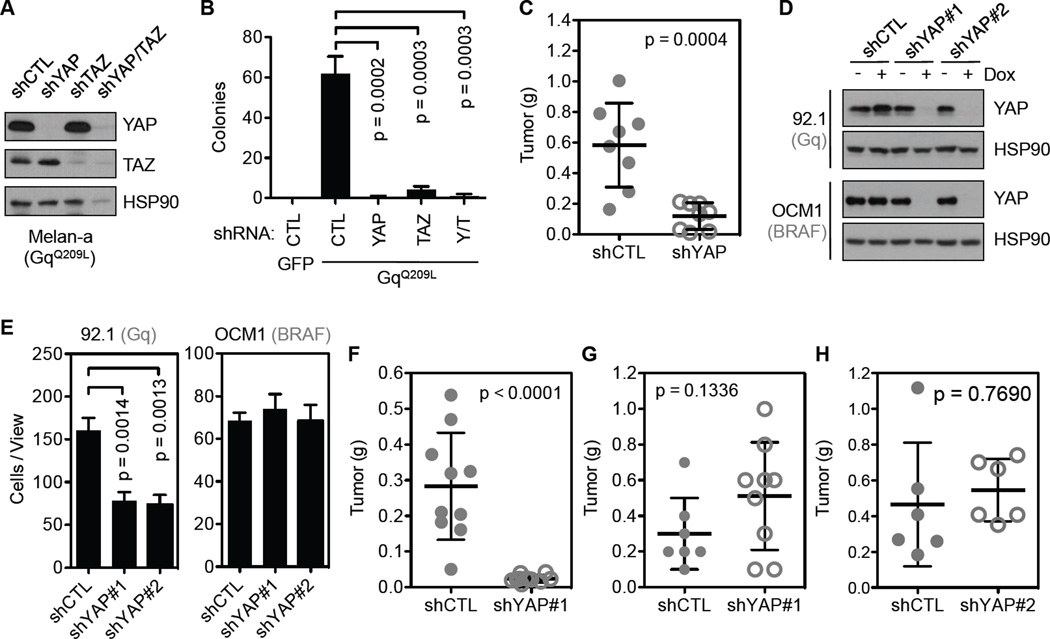
Expression of active Gq/11 (Q209L) in immortalized melanocytes (Melan-a cells) is sufficient to induce cell transformation (Van Raamsdonk et al., 2009; Van Raamsdonk et al., 2010). This offers a well-defined and cleaner system for functional studies than UM-derived cell lines, which certainly contain mutations besides Gq/11. In melan-a cells expressing GqQ209L or G11Q209L, YAP phosphorylation was reduced (Figure S2A and S2B), and the YAP-TEAD interaction was increased (Figure S2C), indicating higher YAP activity. To test the role of YAP/TAZ in GqQ209L-induced cell transformation, we generated GqQ209L-stable melan-a cells expressing control, YAP and/or TAZ shRNAs (Figure 4A). As an indicator of transformation, GqQ209L-stable melan-a cells, but not control melan-a cells expressing green fluorescence protein (GFP), could support anchorage independent growth in soft agar. Importantly, GqQ209L-stable melan-a cells expressing YAP and/or TAZ shRNAs failed to form colonies (Figures 4B and S2D). In addition, when subcutaneously grafted into nude mice, GqQ209L-stable melan-a cells with YAP knockdown exhibited a significant reduction in tumor growth (Figures 4C and S2H). These results indicate that YAP/TAZ are important for Gq-induced neoplastic transformation.
Figure 4. YAP is required for mutant Gq/11-induced tumorigenises.
(A) Knockdown of YAP and/or TAZ in melan-a cells expressing GqQ209L. (B) The effect of YAP and/or TAZ knockdown on anchorage independent growth of melan-a cells expressing GqQ209L. Different melan-a cell lines were cultured in soft agar, and colony formation was assessed. GFP and GqQ209L denote melan-a cells stably expressing GFP control and GqQ209L, respectively. shRNA knockdown of YAP and/or TAZ are indicated, Y/T stand for combination of YAP and TAZ shRNAs. (C) Tumorigenicity of the GqQ209L expressing melan-a cells following YAP knockdown. The same cell lines used in (B) were grafted into nude mice subcutaneously, and tumor formation was monitored. (D) Inducible knockdown of YAP in 92.1 or OCM1 cells. Cells were treated with Dox (2.5 µg/ml) for 3 days, and YAP knockdown was assessed by immunoblotting. (E) The effect of YAP knockdown on cell migration. Migration potential of 92.1 or OCM1 cells following YAP knockdown was assessed using a transwell assay. (F–H) Tumorigenicity of Gq (92.1) mutant and BRAF (OCM1 and OCM8) mutant UM cells following YAP knockdown. Control or YAP knockdown 92.1 (F), OCM1 (BRAF) (G) or OCM8 (H) cells were grafted into nude mice subcutaneously, and tumor formation was monitored. Student t test (two-tailed, 95% confidence intervals) was used for statistical analysis, and error bars represent SD.
See also Figure S2.
To investigate the role of YAP in the tumorigenesis of UM-derived cell lines, we attempted to knockdown YAP in 92.1 cells (GqQ209L), which has high YAP activity, and OCM1 (BRAFV600E) cells, which have low YAP activity. Although it was easy to knockdown YAP in OCM1 cells, we failed to establish an efficient YAP knockdown in 92.1 cells (data not shown). These observations suggest a critical role for YAP in 92.1 cell proliferation, which may be addicted to high YAP activity. We then made an inducible shRNA (pTRIPz system) containing the same YAP targeting sequences used in the conventional vector (pLKO.1), and successfully established both 92.1 and OCM1 stable cells. YAP expression in these cell lines were effectively reduced upon doxycycline (Dox) treatment that induced expression of the shRNAs (Figure 4D). In vitro, the proliferation of 92.1 cells was slightly reduced upon doxycycline treatment, whereas knockdown of YAP in OCM1 cells showed no significant effect on cell proliferation (Figure S2E and S2F). We also assessed the cell migratory potential of these cells. Again knockdown of YAP in 92.1 but not OCM1 cells reduced cell migration in a trans-well assay (Figures 4E and S2G). Furthermore, knockdown of YAP greatly impaired tumor formation of 92.1 cells (Figure 4F) but not OCM1 (Figure 4G) or OCM8 cells (Figure 4H) in nude mice (Figure S2H). Together, these observations suggest that YAP plays a pivotal role in tumorigenesis of Gq/11Q209L-induced, but not BRAFV600E-induced, UM.
YAP inhibitory drug verteporfin selectively suppresses Gq/11 mutant UM tumorigenesis
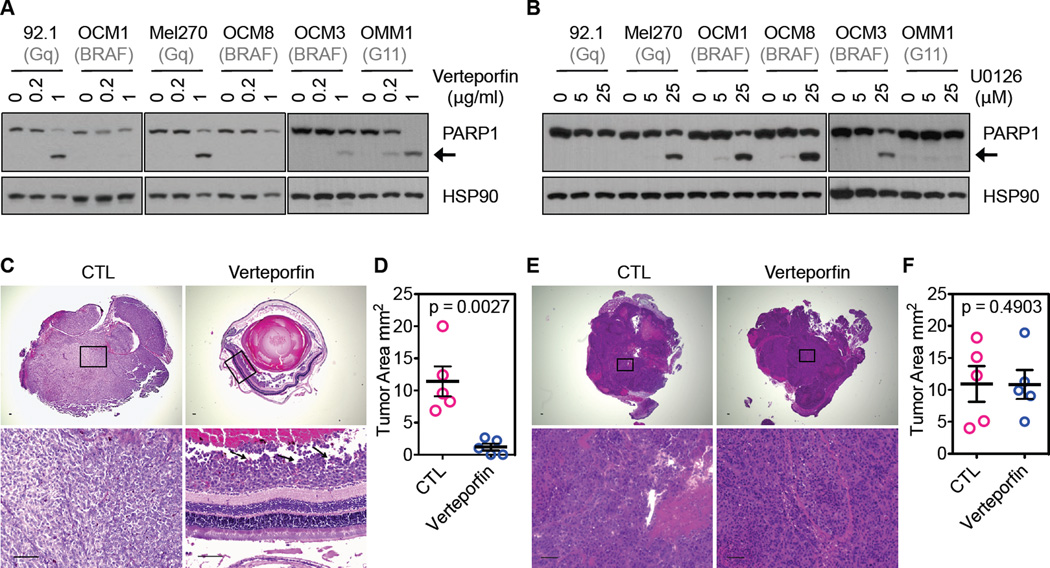
The strong correlation between the Gq/11 mutation and YAP activation in UM specimens and UM cell lines and the effectiveness of YAP knockdown in preventing tumor growth of Gq/11 mutated UM cells in a mouse xenograft model promoted us to test the effect of pharmacological inhibition of YAP on the tumorigenesis of UM cells. It has recently been reported that the porphyrin family compounds, such as verteporfin, disrupts the YAP-TEAD interaction and therefore inhibits the function of YAP in liver size control (Liu-Chittenden et al., 2012). Verteporfin is an FDA approved drug for photodynamic therapy to eliminate abnormal blood vessels in the eye (Bressler and Bressler, 2000). Interestingly, when treated with verteporfin, the UM cells with Gq/11 mutations were effectively killed, as indicated by the cleavage of poly (ADP-ribose) polymerase-1 (PARP-1, an apoptosis marker; Figures 5A and S3A). Similarly, the Gq/11 mutant UM cells were sensitive to growth inhibition by verteporfin (Figure S3B). In comparison, the BRAF mutant cells were more resistant to both growth inhibition and apoptosis in response to verteporfin treatment (Figures 5A, S3A, and S3B). On the other hand, the BRAF mutant UM cells were more easily killed by U0126 (an inhibitor for MEK, the ERK activating kinase), while the Gq mutant cells were resistant to U0126 (Figure 5B). These results suggest a model that YAP activation is more important for Gq/11 mutant tumor cells, whereas ERK activation is more important for BRAF mutant tumor cells. Our data indicate that verteporfin may be used to selectively kill tumor cells with elevated YAP activity, such as UM containing mutations in Gq/11.
Figure 5. YAP inhibitor suppresses tumor growth of Gq/11 mutated UM cells.
(A) Sensitivity of Gq/11 mutant and BRAF mutant UM cells to Verteporfin, a YAP inhibitor. Gq mutant cells or BRAF mutant cells were treated with 0.2 µg/ml or 1 µg/ml of verteporfin for 48 hr, and cell lysates were assessed for PARP1 cleavage (the black arrow indicate the position of cleaved PARP1, an indicator of cell death. (B) Sensitivity of Gq/11 mutant and BRAF mutant UM cells to U0126, a MEK inhibitor. UM cells were treated with 5 µM and 25 µM of U0126 for 36 hr, and then cell apoptosis was assessed by PARP1 cleavage. (C–D) Effects of Verteporfin treatment on tumor growth of 92.1 cells in an orthotopic UM mouse model. Prior to injection into the suprachoroidal space of the eye, 92.1 cells were mixed with nanoparticles containing verteporfin or buffer (control, CTL). After injection, verteporfin was delivered systematically to mice (treated) via intraperitoneal injection. Tumor formation was monitored by OCT, and tumors were harvested and sectioned for histological analysis. Representative sections of the eye showed the presence of large pigmented melanoma xenografts filling the eyes of all vehicle-treated animals (left panels in C), whereas tumors in the vertepofin treatment group (right panels in C) were smaller as indicated by black arrows. The tumor areas from 5 injected eyes were quantified and are shown in (D). (E, F) Effect of Verteporfin treatment on tumor growth of OCM1 cells. Similar experiments were performed as (C, D) using OCM1 cells. Student t test (two-tailed, 95% confidence intervals) was used for statistical analysis, and error bars represent SD. Scale bars represent 100 µm.
See also Figure S3.
To assess the role of verteporfin in inhibiting tumorigenesis of UM cells, we used an orthotopic mouse model. Tumor cells were injected into the suprachoroidal space of the eye of the severe combined immunodeficiency (SCID) mice. Tumor formation was monitored by non-invasive fundus examinations and optical coherent tomography (OCT) in live animals and histological analyses after mice were euthanized (Figure S3C and S3D). 92.1 cells showed aggressive endophytic growth, resulting in filling of inside of the eye with tumor cells (Figure S3C and S3D). We investigated effect of verteporfin on inhibition of tumor growth in vivo. Clinical grade verteporfin (40 µg/injection) was packaged into nanoparticles and mixed with 92.1, Mel270, or OCM1 cells prior to injection into the eye. As negative controls, UM cells were co-injected with empty nanoparticles. For the verteporfin treated group, mice were also administered 100 mg/kg verteporfin via an intraperitoneal route every other day over a period of 14 days, whereas control mice were injected with PBS. After 6 weeks, when compared to the control group, vertepofin treatment significantly reduced tumor growth of the Gq mutant 92.1 and Mel270 cells (Figures 5C, 5D, S3E and S3F). In contrast, vertepofin treatment had little effect on tumor growth of the BRAF mutant OCM1 cells (Figure5E and 5F). Therefore, these mouse model studies demonstrate that verteporfin is effective in inhibiting tumor cell growth and might be considered for targeted treatment of UM with Gq/11 mutations and elevated YAP activity.
DISCUSSION
UM is the most common intraocular tumor in adults and frequently metastasizes to the liver. Early stage UM can be treated by radiation or enucleation (removal of the eye), but there is no effective treatment for metastatic UM which is the most feared complication and the main cause of death (Singh et al., 2005). Enucleation has been the last resort to prevent metastasis and has a long lasting adverse and psychological impact on patients, even though enucleation does not significantly improve the outcome of survival (COMS, 1998). Therefore, a systemic treatment of metastasis is urgently needed. The high penetrance of Gq/11-activating mutations and the essential role of mutant Gq/11 in UM oncogenisis warrants the need for an in-depth mechanistic understanding of Gq/11 in tumorigenesis. Moreover, the establishment of mutant Gq/11 as a cancer driver suggests a potential of developing targeted therapies for UM treatment. Unfortunately, a drug that targets constitutively active Gq/11 is currently not available. It is therefore important to identify downstream effectors essential for Gq/11-induced tumorigenesis, and these effectors may provide opportunities to develop molecular-targeted drugs for UM management.
In this report, we reveal a strong correlation between Gq/11 mutations and YAP activation in UM. We have established a causal relationship between Gq/11 mutation and YAP activation, and show that YAP is essential in transducing the oncogenic activity of mutant Gq/11 to induce UM. Thus, YAP may serve as a drug target for pharmaceutical intervention of UM. Indeed, this concept is strongly supported by our data that down-regulation of YAP selectively inhibits tumor growth of UM cells containing mutated Gq/11. Furthermore, verteporfin inhibits proliferation of UM cells with Gq/11 mutations in vitro and is effective in suppressing their growth in a mouse model. Verteporfin is already an FDA approved drug for eye disease indications such as macular degeneration, therefore it would be relatively easy to adapt this drug for UM treatment. Hence, the result of UM inhibition by verteporfin offers exciting possibilities not only for treatment of local intraocular UM, but also for deadly metastasis. Notably, the FDA approved application of verteporfin is based on photodynamic therapy to eliminate neovascularization of blood vessels. Light activation of the drug is required for verteporfin to inhibit neovascular angiogenesis but is not required to disrupt the interaction between YAP and TEAD (Liu-Chittenden et al., 2012). Therefore, verteporfin could have a dual function, inhibiting angiogenesis and YAP activity, both of which can positively contribute to inhibiting UM. Our data indicate that verteporfin should be considered for UM treatment. It is equally important to note that verteporfin is ineffective towards the BRAF mutant UM cells, which are sensitive to MEK inhibitors. These observations suggest that the therapeutic effects of verteporfin observed on the Gq/11 mutant UM cells are not due to general toxicity, but rather target specific and mechanism based inhibition of the Gq/11 mutant UM cells.
It has been previously shown that the active Gq/11 mutant also stimulates the ERK pathway (Van Raamsdonk et al., 2009; Van Raamsdonk et al., 2010). This is not surprising because G-proteins are known to initiate multiple downstream signaling events. However, activation of the ERK pathway by active Gq/11 is less potent compared to that of the BRAFV600E mutation, which is frequently found in cutaneous melanoma (Figures 1B and S1D). BRAF inhibition is an effective target based therapy commonly used to treat BRAFV600E-mutant melanoma. Our study indicates that YAP activation is more important than ERK activation in UM. Consistent with this notion, down-regulation of YAP or verteporfin treatment induces more cell death and tumor inhibition in Gq/11 mutant UM cells than BRAF mutant UM cells (Figures 4F–H and 5). Further studies are needed to determine the effect of a combined treatment inhibiting both MEK and YAP in treating UM with the Gq/11 mutation.
YAP/TAZ are frequently activated in human cancers (Harvey et al., 2013). However, the underlying mechanisms leading to YAP/TAZ activation in cancers are largely unknown. Mutations in the Hippo pathway components are rare in human cancers. One notable example is the neurofibromin 2 (NF2) tumor suppressor. Mutation in NF2 activates YAP, and YAP activation is required for NF2-induced tumorigenesis (Zhang et al., 2010). Our report not only reveals a mechanism of YAP activation in Gq/11-induced tumorigenesis, but also suggests that YAP activation plays a critical role in cancer development with altered GPCR signaling. In addition to UM, other types of neoplastic lesions, such as blue naevi (Van Raamsdonk et al., 2009) and leptomeningeal melanocytic lesions (Kusters-Vandevelde et al., 2010) also contain prevalent Gq/11 mutations. More recently, the GqR183Q mutation has been identified in the 80–90% of Sturge-Weber Syndrome and Port-Wine Stains patients (Shirley et al., 2013). We also showed that GqR183Q also potently activated YAP (Figures 1A and S1A–C). Interestingly, most of these tumors or overgrowths, including those caused by NF2 loss-of-function mutations, are derived from the neuroectoderm. Activation of YAP/TAZ by Gq/11 or NF2 mutations in these tumors suggests that the Hippo pathway plays an important role in the development and differentiation of the neuroectoderm, consistent with a role of the Hippo pathway in regulating neural progenitor cells (Cao et al., 2008).
GPCRs constitute the largest family of cell surface receptors encoded by the human genome. Although cancer-associated mutations in GPCR signaling is less frequent than receptor tyrosine kinases, extensive cancer genome sequencing has revealed that approximately 20% of all human cancers may have altered GPCR signaling (O'Hayre et al., 2013). For example, mutation of metabotropic glutamate receptor occurs at an appreciable frequency in cutaneous melanoma (Prickett et al., 2011). Besides genetic alterations (mutation or amplification), GPCRs are stimulated by their cognate ligands, and therefore altered ligand levels can also lead to abnormal GPCR signaling. For example, LPA is a potent mitogen and strongly activates YAP/TAZ (Miller et al., 2012; Yu et al., 2012). In fact, LPA is elevated and defined as a biomarker for ovarian cancer (Mills and Moolenaar, 2003). We have recently shown that YAP is broadly regulated by a large number of GPCRs (Yu et al., 2012). Collectively, these observations suggest a potential paradigm that YAP activation plays a broad role in cancers driven by altered GPCR signaling, and thus YAP inhibitors, such as verteporfin, represent a potential therapeutic for cancers with altered GPCR signaling.
EXPERIMENTAL PROCEDURES
Extended Experimental Procedures are shown in Supplemental Information.
Cell culture
UM cell lines, provided by Dr. Martine Jager (Leiden University), were maintained in RPMI 1640 medium with 10% FBS. Melan-a cells, a gift from Dr. Dorothy Bennett (St George University), were cultured in RPMI medium with 10% FBS and 200 nM TPA. HEK293A, HEK293T, and HEK293P cells were cultured in DMEM medium with 10% FBS. All medium were supplemented with 50 µg/mL penicillin/streptomycin. Cells were maintained at 37°C with 5% CO2.
Animal work
All animal procedures were carried out according to protocols approved by the Institutional Animal Care and Use Committee of University of California, San Diego (UCSD). For subcutaneous xenograft experiments, 12-week-old male nude mice were used. Cells (Melan-a, 92.1, OCM1, or OCM8) with manipulations of YAP or Gq expression were grafted subcutaneously into both flanks of mice, and tumor growth was monitored three times a week. Mice were euthanized after 10 weeks of cell injection or until the tumor size reached 1 cm3 to document the formation of primary tumors. For the orthotopic UM mouse model, male 4-week-old SCID mice were used. Mice were anesthetized and 50,000 cells of 92.1, Mel270, or OCM1 were injected into the suprachoroidal space in the right eye using a 33-gauge needle. Tumor formation was monitored every week by fundus examinations and OCT (Spectralis, Heiderberg Engineering). Mice were euthanized after 6 weeks because of the development of very large masses in the eyes of several mice in the control group. Eyes were enucleated and fixed in 4% PFA, and subjected to histological analysis.
Human clinical samples
The patients were diagnosed with uveal melanoma by clinical history, complete ophthalmic examination, ultrasound, and ancillary studies. Twenty three enucleated eyes due to large uveal melanoma lesions were collected with patients’ consent and approval of the Institutional Review Board of West China Hospital. UM specimens were paraffin-embedded and sectioned for histology and immunofluorescence staining. The use of human tissue samples were conducted in accordance with the protocols approved by the Institutional Review Boards at West China Hospital and University of California, San Diego.
Other methods are described in the Supplemental Experimental Procedures.
Supplementary Material
Highlights.
-
-
Uveal Melanoma-associated mutant Gq/11 activates YAP
-
-
YAP activation correlates with mutations of Gq/11 in uveal melanomas
-
-
YAP is essential for mutant Gq/11-induced uveal melanoma growth
-
-
YAP inhibitor suppresses mutant Gq/11-induced uveal melanoma development
Significance.
Uveal melanoma (UM) is the most common type of adult eye cancer, currently there is no effective treatment especially for metastatic UM. The majority of UMs has activating mutations in two homologous G-proteins Gq or G11 (encoded by GNAQ or GNA11 respectively). We found that the Hippo pathway effector YAP is activated in UM containing mutant Gq/11, and inhibition of YAP by either genetic or pharmacological approaches blocks tumor growth of Gq/11-mutanted UM cells in mouse models, suggesting a strategy for UM intervention by inhibiting YAP. This mechanism that YAP activation mediates mutant Gq/11 signaling in tumorigenesis may serve as a paradigm for general pathogenesis of human cancers with aberrant expression or mutations of G-protein-coupled receptor (GPCR) or G-proteins.
ACKNOWLEDGMENTS
We would like to thank Drs. Martine Jager, Jerry Niederkorn, and Dorothy Bennett for providing cell lines. We also thank Steven Plouffe for critical reading of the manuscript. This study was supported by grants from NIH (EY022611 and CA132809) and CIRM (RB2-01547) to K.L.G. and NIH (EY022611 and EY024134) and Research to Prevent Blindness to K.Z. and 973 program (2014CB964900 and 2013CB967500, China) to J.L. and W.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPRELEMTAL INFORMATION
Supplemental information includes Supplemental Experimental Procedures and Supplemental Data (4 figures) can be found with this article online at: http://
REFERENCES
- Arnesen K. The neural crest origin of uveal melanomas. Int Ophthalmol. 1985;7:143–147. doi: 10.1007/BF00128360. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Bressler SB. Photodynamic therapy with verteporfin (Visudyne): impact on ophthalmology and visual sciences. Invest Ophthalmol Vis Sci. 2000;41:624–628. [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes & development. 2008;22:3320–3334. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Guo K, Ng CP, Lee I, Hunziker W, Zeng Q, Hong W. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- COMS. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma II: initial mortality findings. COMS report no. 10. Am J Ophthalmol. 1998;125:779–796. doi: 10.1016/s0002-9394(98)00039-7. [DOI] [PubMed] [Google Scholar]
- Griewank KG, Yu X, Khalili J, Sozen MM, Stempke-Hale K, Bernatchez C, Wardell S, Bastian BC, Woodman SE. Genetic and molecular characterization of uveal melanoma cell lines. Pigment Cell Melanoma Res. 2012;25:182–187. doi: 10.1111/j.1755-148X.2012.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Henriquez F, Janssen C, Kemp EG, Roberts F. The T1799A BRAF mutation is present in iris melanoma. Invest Ophthalmol Vis Sci. 2007;48:4897–4900. doi: 10.1167/iovs.07-0440. [DOI] [PubMed] [Google Scholar]
- Huang W, Lv X, Liu C, Zha Z, Zhang H, Jiang Y, Xiong Y, Lei QY, Guan KL. The N-terminal phosphodegron targets TAZ/WWTR1 for SCFbeta-TrCP dependent degradation in response to PI3K inhibition. The Journal of biological chemistry. 2012 doi: 10.1074/jbc.M112.382036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Lee S, Kuninaka S, Saya H, Lee H, Lim DS. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013;32:1543–1555. doi: 10.1038/emboj.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusters-Vandevelde HV, van Engen-van Grunsven IA, Kusters B, van Dijk MR, Groenen PJ, Wesseling P, Blokx WA. Improved discrimination of melanotic schwannoma from melanocytic lesions by combined morphological and GNAQ mutational analysis. Acta Neuropathol. 2010;120:755–764. doi: 10.1007/s00401-010-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba S, Felicioni L, Buttitta F, Bleeker FE, Malatesta S, Corbo V, Scarpa A, Rodolfo M, Knowles M, Frattini M, et al. Mutational profile of GNAQQ209 in human tumors. PLoS One. 2009;4:e6833. doi: 10.1371/journal.pone.0006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. The Journal of biological chemistry. 2010;285:37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes & development. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Yang J, Deran M, Wu C, Su AI, Bonamy GM, Liu J, Peters EC, Wu X. Identification of Serum-Derived Sphingosine-1-Phosphate as a Small Molecule Regulator of YAP. Chem Biol. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease activated receptor PAR. Genes & development. 2012;29 doi: 10.1101/gad.197582.112. doi:10.1101/gad.197582.197112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, Gutkind JS. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer. 2013;13:412–424. doi: 10.1038/nrc3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickett TD, Wei X, Cardenas-Navia I, Teer JK, Lin JC, Walia V, Gartner J, Jiang J, Cherukuri PF, Molinolo A, et al. Exon capture analysis of G protein-coupled receptors identifies activating mutations in GRM3 in melanoma. Nat Genet. 2011;43:1119–1126. doi: 10.1038/ng.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, North PE, Marchuk DA, Comi AM, Pevsner J. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971–1979. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AD, Bergman L, Seregard S. Uveal melanoma: epidemiologic aspects. Ophthalmol Clin North Am. 2005;18:75–84. viii. doi: 10.1016/j.ohc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA. Expression of Yes-associated protein in common solid tumors. Human pathology. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N, Harvey KF. The Hippo pathway--from top to bottom and everything in between. Semin Cell Dev Biol. 2012;23:768–769. doi: 10.1016/j.semcdb.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, Simpson EM, Barsh GS, Bastian BC. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes & development. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes & development. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP Pathway by G-Protein-Coupled Receptor Signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & development. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes & development. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.