Abstract
Purpose
A low serum 25-hydroxyvitamin D [25(OH)D] level in the blood has been correlated with an increased risk of diabetes mellitus; however, the association between serum 25(OH)D level and insulin resistance has not been established in a Korean rural population. The aim of this study was to investigate the independent association between serum 25(OH)D level and insulin resistance in rural Korean adults.
Materials and Methods
This study used data from the Korean Genome Epidemiology Study-Kangwha Study. In the 2011 study, 1200 adults completed health examinations. In an ancillary study, serum 25(OH)D level was measured in a subsample (n=813). After excluding those taking vitamin D supplements, a cross-sectional analysis was carried out on 807 participants (324 men and 483 women) aged 40 to 89 years old. Measured from overnight fasting blood samples, glucose and insulin levels were used to calculate the homeostasis model assessment for insulin resistance (HOMA-IR). Measures of glucose, insulin, and HOMA-IR were log-transformed for parametric tests.
Results
Serum 25(OH)D level was inversely associated with HOMA-IR (β=-0.003, p=0.039) in a univariate analysis. However, the association was not significant after adjustment for sex and age (β=-0.002, p=0.123) or after adjustment for sex, age, body mass index, smoking status, alcohol intake, and regular exercise (β=-0.003, p=0.247).
Conclusion
Our findings suggest that vitamin D is not independently associated with insulin resistance in Korean men and women.
Keywords: Vitamin D, insulin resistance, epidemiology
INTRODUCTION
Vitamin D promotes calcium and phosphate absorption in the intestines, maintains sufficient concentrations of circulating calcium and phosphate levels, and facilities normal mineralization of bone by providing these minerals to bone-forming sites.1,2 Recently, additional functions for vitamin D have also received greater attention, especially concerning its effect on insulin resistance.3,4,5 Nonetheless, while some previous studies reported a significant association between low circulating vitamin D levels and insulin resistance,1,6,7,8,9 others did not.10,11,12,13,14,15,16,17 Associations thereof have proven inconsistent and dependent upon the characteristics of a particular study population, such as ethnicity, sex, and age.10,11 In a prospective cohort study in Denmark, low serum 25-hydroxyvitamin D [25(OH)D] status was significantly associated with unfavorable changes in continuous markers of glucose homeostasis, but not with incident diabetes.17 Therefore, it is still unknown as to whether serum 25(OH)D level has a direct effect on insulin resistance, independent of other risk factors.
The aim of this study was to investigate whether lower serum 25(OH)D level is independently associated with insulin resistance in a rural Korean population.
MATERIALS AND METHODS
Study population
This study used data from the Korean Genome Epidemiology Study (KoGES)-Kangwha study, a rural community-based prospective cohort. The KoGES-Kangwha study enrolled 4906 participants aged 40 years or older between 2006 and 2011. In the 2011 study, 431 new participants completed baseline health examinations and 769 participants completed follow-up health examinations from July to August. An ancillary biomarker study measured serum 25(OH)D levels for a subsample of 813 participants adopted from the 2011 study. We excluded those taking vitamin D supplements (n=4), missing serum insulin measurements (n=1), and body weight measurements (n=1). Finally, a cross-sectional analysis was carried out on 807 participants (324 men and 483 women) aged 40 to 89 years old. All participants provided written informed consent and the study protocol was approved by the Institutional Review Board of Severance Hospital at Yonsei University College of Medicine.
Measurements
Participants were individually interviewed using standardized questionnaires to obtain information about their general characteristics, medical history, medication use, vitamin supplement intake, and lifestyle behaviors, such as smoking, alcohol intake, and regular exercise.18,19,20 Participants were classified as either current smokers or current nonsmokers and current alcohol drinkers or current nondrinkers. Trained interviewers conducted the surveys according to a pre-developed protocol and double-checked whether answers were missing or inappropriate.
All participants wore light clothing for anthropometric measurements. Standing height was measured up to 0.1 cm with a stadiometer (SECA 225, SECA, Hamburg, Germany) and body weight was measured up to 0.1 kg with a digital scale (GL-60000-20, Seoul, Korea) according to protocol. Subsequently, body mass index (BMI) was calculated as body weight in kg divided by standing height in meters squared (kg/m2). Participants were seated for at least 5 minutes before blood pressure measurement, and two measurements were taken within at least 5 minutes using an automatic sphygmomanometer (Dinamap 1846 SX/P; GE Healthcare, Waukesha, WI, USA). If the two measurements differed by 10 mm Hg or more for systolic blood pressure (SBP) or diastolic blood pressure (DBP), a third measurement was taken after 5 minutes, and the last two measurements were averaged for analysis.
Blood samples of all participants were collected from the antecubital vein after at least eight hours of fasting. Enzymatic methods were applied to measure total cholesterol, high-density lipoprotein cholesterol, and triglycerides (ADVIA 1800; Siemens Healthcare Diagnostics Inc., Deerfield, IL, USA). Serum 25(OH)D, currently the best indicator of vitamin D store, was measured by radioimmunoassay (DIASource, Nivelles, Belgium). The intra-assay coefficient of variation was 7.3% at 19.1 ng/mL and 8.8% at 10.4 ng/mL. The inter-assay coefficient of variation was 7.2% at 6.7 ng/mL and 7.3% at 13.1 ng/mL. Fasting blood glucose level was measured using the glucose hexokinase method. Serum insulin level was measured in accordance with the radioimmunometric method, and the intra-assay coefficient of variation was 2.1% at 6.6 uIU/mL and 1.5% at 53.3 uIU/mL. The inter-assay coefficient of variation was 6.5% at 14.4 ng/mL and 6.1% at 100.4 ng/mL. Additionally, insulin resistance of all participants was estimated using the homeostasis model assessment for insulin resistance (HOMA-IR), as the product of fasting blood glucose level (mg/dL) and serum insulin level (uIU/mL) divided by 405.21
Statistical analysis
We investigated differences between general characteristics and concentrations of variables, such as serum 25(OH)D with fasting glucose and insulin resistance for men and women. Continuous variables were assessed using independent t-tests and Wilcoxon rank sum tests, and categorical variables were assessed using chi-squared tests. Fasting glucose, insulin, and HOMA-IR were skewed to the right, thus log-transformed for use in parametric tests. The relationship between serum 25(OH)D level and log-transformed HOMA-IR was evaluated using Pearson's correlation coefficients. Sex was adjusted when analyzing the total population only. Linear regression analyses were used to assess the independent association between serum 25(OH)D and insulin resistance. First, the analyses were performed unadjusted. Then, using multivariate models, we adjusted for 1) sex and age and 2) sex, age, BMI, smoking status, alcohol intake, and regular exercise. Multiple logistic regression models were also used to calculate odds ratios with 95% confidence intervals of increased insulin resistance (defined as HOMA-IR ≥2.5) at an increment of 10 ng/mL 25(OH)D.21 SAS version 9.2 (SAS Institute, Cary, NC, USA) was used for all analyses, and statistical significance was defined as a two-tailed p-value <0.05.
RESULTS
The general characteristics of all participants are presented in Table 1. This study consisted of 807 participants with a mean age of 61.2 years. Mean BMI was 24.5 kg/m2, and mean SBP and DBP were 114.7 mm Hg and 69.4 mm Hg, respectively. Mean serum 25(OH)D level was 25.8 ng/mL and mean HOMA-IR was 1.8. The prevalence of diabetes mellitus was 15.6%, and current cigarette smoking and alcohol drinking were recorded in 9.2% and 39.0%, respectively. Additionally, 43.9% of participants reported exercising regularly.
Table 1.
Characteristics of 324 Men and 483 Women
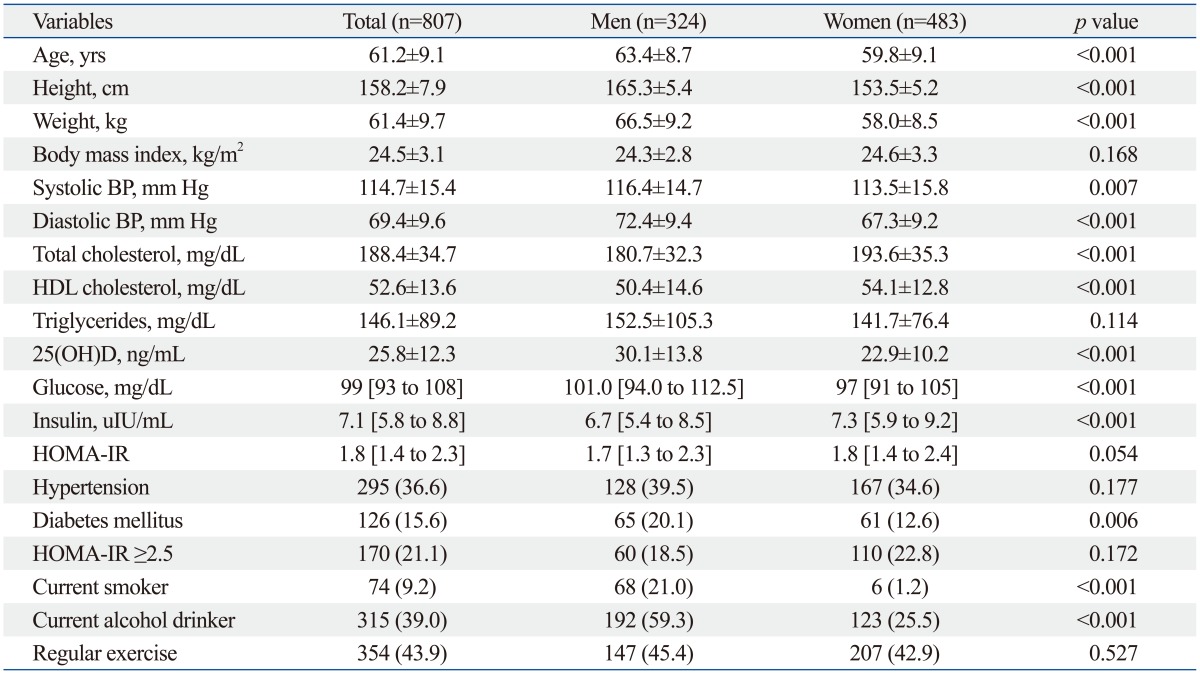
BP, blood pressure; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment for insulin resistance.
Data are expressed as mean±standard deviation, median [inter-quartile range], or number (percent).
Table 2 presents data on the correlation of serum lipids and 25(OH)D and HOMA-IR. Serum 25(OH)D was inversely associated with HOMA-IR in the unadjusted model; however, the association disappeared after adjusting for sex and age. Supplementary Fig. 1 (only online) presents the linear relationship between serum 25(OH)D level and log-transformed HOMA-IR, separately for men and women.
Table 2.
Pearson's Correlation Coefficients of Serum Lipids and 25(OH)D and HOMA-IR (n=807)
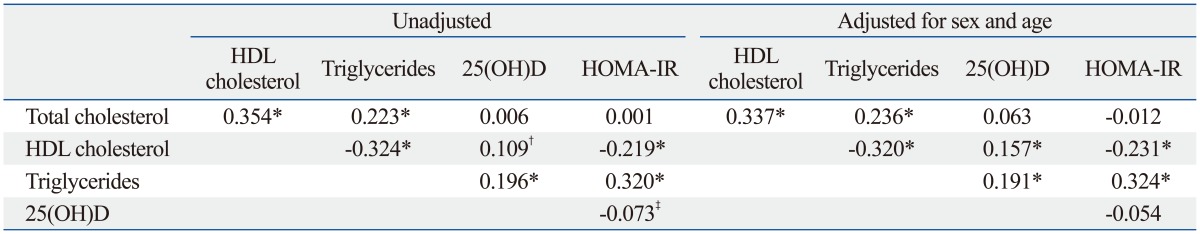
HOMA-IR, homeostasis model assessment for insulin resistance; HDL, high-density lipoprotein.
Triglycerides and HOMA-IR values are log-transformed for analysis.
*p<0.001.
†p<0.01.
‡p<0.05.
Table 3 shows the association between serum 25(OH)D level and log-transformed HOMA-IR. Serum 25(OH)D level was significantly associated with HOMA-IR (β=-0.003, p=0.039) in the unadjusted model. However, the association was no longer present after adjusting for sex and age (β=-0.002, p=0.123). The association remained non-significant after additional adjustment for BMI, smoking status, alcohol intake, and regular exercise (β=-0.002, p=0.247).
Table 3.
Association between Serum 25(OH)D and Log-Transformed HOMA-IR in Total Participants (n=807)
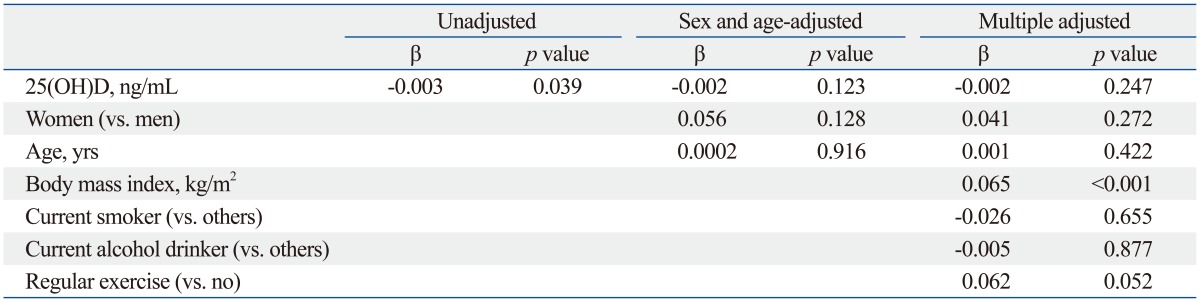
HOMA-IR, homeostasis model assessment for insulin resistance.
The association between serum 25(OH)D level and insulin resistance as a dichotomous measure is shown in Supplementary Table 1 (only online). Increased insulin resistance (defined as HOMA-IR ≥2.5) was not associated with serum 25(OH)D. In the unadjusted analysis, the odds of having insulin resistance was 0.99 (95% CI, 0.86-1.14) at an increment of 10 ng/mL of 25(OH)D. After adjusting for sex, age, BMI, smoking status, alcohol intake, regular exercise, and menopause status, the odds for having insulin resistance was 1.06 (95% CI, 0.91-1.24). When we examined the relation between clinically relevant categories of 25(OH)D (<20, 20-30, and ≥30 ng/mL), we found no association between serum 25(OH)D and insulin resistance (Supplementary Table 2 and 3, only online). The magnitude and direction of these associations were similar to findings in the subgroup analyses categorized by smoking status and alcohol intake (data not shown).
DISCUSSION
The current study investigated the independent association between serum 25(OH)D level and insulin resistance in a rural Korean population. Our findings suggest that vitamin D is not associated with insulin resistance when adjusting for potential confounders in a general population.
The current literature remains inconclusive with regard to whether lower 25(OH)D concentration is independently associated with insulin resistance. Several epidemiologic studies have reported associations between lower circulating vitamin D levels and impaired glucose homeostasis.1,6,7,8,9 Analysis of the U.S. National Health and Nutrition Examination Survey datasets revealed that insufficient serum 25(OH)D concentration is significantly associated with type 2 diabetes and insulin resistance.6 As well, lower circulating 25(OH)D levels were associated with higher fasting insulin and HOMA-IR in a middle-aged to elderly Chinese population9 and a Japanese working population.8 The association between lower 25(OH)D concentration and impaired glucose homeostasis was also reported in the Korean population. In the Fourth Korea National Health and Nutrition Examination Survey (KNHANES), a low serum 25(OH)D concentration was associated with diabetes, fasting insulin, and increased HOMA-IR.1,7 However, in the rural Korean population of the present study, we did not observe an independent association between serum 25(OH)D level and insulin resistance. Our finding is consistent with several previous studies that also did not observe an association between serum 25(OH)D and insulin resistance. In the Third U.S. National Health and Nutrition Examination Survey, an inverse association between serum 25(OH)D level and insulin resistance was found in only non-Hispanic whites and Mexican Americans, but not in non-Hispanic blacks.11 In the Hoorn study, 25(OH)D concentration was not associated with fasting and postprandial glucose levels, and incident diabetes.12 In a healthy Cree community in Quebec, Canada, no association between serum 25(OH)D and insulin homeostasis indices (HOMA-IR and HOMA-beta) was detected.14 Moreover, even in the Asian studies that reported an inverse association between 25(OH)D and insulin resistance, the association was limited to participants who were overweight or obese.8,9 Lastly, a randomized double-blinded trial in women with polycystic ovary syndrome and vitamin D deficiency also did not find vitamin D supplementation to have any effect on insulin resistance.13
Most of the studies that reported inverse associations between serum 25(OH)D level and insulin resistance used nationwide survey data, and participants were from diverse backgrounds. Therein, demonstration of whether the relationships between serum 25(OH)D level and insulin resistance are independent from other risk factors would be difficult, as potential confounders would have unlikely been fully controlled. One of the strengths of our study was that participants were relatively homogeneous in ethnicity and geographical location. These differences in study populations could be one reason for inconsistent findings between our study and the nationwide surveys. Another possible reason for this discrepancy is the difference in the serum 25(OH)D levels. When compared with KNHANES results, our study participants had relatively high serum 25(OH)D levels; mean serum 25(OH)D levels were 25.8 ng/mL in our study and 19.7 ng/mL in the KNHANES. Our study was conducted in a rural community during the summer, while the KNHANES was conducted nationwide throughout the year: serum 25(OH)D concentration is known to be significantly higher in rural areas than in urban areas and typically higher in the summer.1,7,11 Vitamin D level is dependent on sunlight exposure because ultraviolet light is required for the first step of vitamin D synthesis from 7-dehydrocholesterol in the skin.2,22
The current study has several limitations. First, this was a cross-sectional observation study, therefore a causal relationship between serum 25(OH)D and insulin resistance cannot be made. Second, we did not use the glucose clamp method, the gold standard test; instead, we used HOMA-IR as a surrogate measure of insulin resistance. The HOMA-IR equation has been widely used in clinical and epidemiological studies based on a good correlation between estimates of insulin resistance derived from HOMA and from the euglycemic clamp.23,24,25 Furthermore, potential confounding factors such as calcium and parathyroid hormone may be residual confounders. Third, serum 25(OH)D level was not repeatedly measured over four seasons. However, the primary aim of the present study was not to estimate absolute levels of serum 25(OH)D over the year as a descriptive report, but to investigate the association between serum 25(OH)D and insulin resistance in a general population. If 25(OH)D level and insulin resistance are directly related, the relationship should be prominent during the summer season. Thus, our findings are not seriously distorted because 25(OH)D was measured only during the summer. Last, both serum 25(OH)D and insulin resistance were measured only once; thus, we cannot exclude the possibility that random measurement error may have weakened the observed association.
In conclusion, serum 25(OH)D level was significantly associated with insulin resistance in the unadjusted model. However, the association disappeared after adjusting for potential confounders, such as sex, age, BMI, smoking status, alcohol intake, and regular exercise. This study suggests that vitamin D is not independently associated with insulin resistance in a rural-dwelling Korean adult population.
ACKNOWLEDGEMENTS
The Ancillary Biomarker Study was supported by the Global Research Network Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (220-2009-1-E00023). The parent cohort study was supported by grants from the Korea Centers for Disease Control and Prevention (2011-E71002-00). We would like to thank Katherine M. Stefani at the Department of Research Affairs, Yonsei University College of Medicine for her professional editing of our manuscript.
Footnotes
The authors have no financial conflicts of interest.
Supplementary Material
Association between Serum 25(OH)D Level (per 10 ng/mL) and Increased Insulin Resistance
Characteristics of Study Participants by Vitamin D Concentrations
Adjusted OR (95% CI) of Adults Having Insulin Resistance by Vitamin D Concentrations
Association between serum 25(OH)D level and HOMA-IR. HOMA-IR, homeostasis model assessment for insulin resistance.
References
- 1.Choi HS, Kim KA, Lim CY, Rhee SY, Hwang YC, Kim KM, et al. Low serum vitamin D is associated with high risk of diabetes in Korean adults. J Nutr. 2011;141:1524–1528. doi: 10.3945/jn.111.139121. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kabadi SM, Lee BK, Liu L. Joint effects of obesity and vitamin D insufficiency on insulin resistance and type 2 diabetes: results from the NHANES 2001-2006. Diabetes Care. 2012;35:2048–2054. doi: 10.2337/dc12-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee SY, Hwang YC, Chung HY, Woo JT. Vitamin D and diabetes in Koreans: analyses based on the Fourth Korea National Health and Nutrition Examination Survey (KNHANES), 2008-2009. Diabet Med. 2012;29:1003–1010. doi: 10.1111/j.1464-5491.2012.03575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pham NM, Akter S, Kurotani K, Nanri A, Sato M, Hayabuchi H, et al. Serum 25-hydroxyvitamin D and markers of insulin resistance in a Japanese working population. Eur J Clin Nutr. 2012;66:1323–1328. doi: 10.1038/ejcn.2012.169. [DOI] [PubMed] [Google Scholar]
- 9.Lu L, Yu Z, Pan A, Hu FB, Franco OH, Li H, et al. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32:1278–1283. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BK, Park S, Kim Y. Age- and gender-specific associations between low serum 25-hydroxyvitamin D level and type 2 diabetes in the Korean general population: analysis of 2008-2009 Korean National Health and Nutrition Examination Survey data. Asia Pac J Clin Nutr. 2012;21:536–546. [PubMed] [Google Scholar]
- 11.Scragg R, Sowers M, Bell C Third National Health and Nutrition Examination Survey. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 12.Pilz S, van den Hurk K, Nijpels G, Stehouwer CD, Van't Riet E, Kienreich K, et al. Vitamin D status, incident diabetes and prospective changes in glucose metabolism in older subjects: the Hoorn study. Nutr Metab Cardiovasc Dis. 2012;22:883–889. doi: 10.1016/j.numecd.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Ardabili HR, Gargari BP, Farzadi L. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and vitamin D deficiency. Nutr Res. 2012;32:195–201. doi: 10.1016/j.nutres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Del Gobbo LC, Song Y, Dannenbaum DA, Dewailly E, Egeland GM. Serum 25-hydroxyvitamin D is not associated with insulin resistance or beta cell function in Canadian Cree. J Nutr. 2011;141:290–295. doi: 10.3945/jn.110.129619. [DOI] [PubMed] [Google Scholar]
- 15.Gulseth HL, Gjelstad IM, Tierney AC, Lovegrove JA, Defoort C, Blaak EE, et al. Serum vitamin D concentration does not predict insulin action or secretion in European subjects with the metabolic syndrome. Diabetes Care. 2010;33:923–925. doi: 10.2337/dc09-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erdönmez D, Hatun S, Çizmecioğlu FM, Keser A. No relationship between vitamin D status and insulin resistance in a group of high school students. J Clin Res Pediatr Endocrinol. 2011;3:198–201. doi: 10.4274/jcrpe.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husemoen LL, Thuesen BH, Fenger M, Jørgensen T, Glümer C, Svensson J, et al. Serum 25(OH)D and type 2 diabetes association in a general population: a prospective study. Diabetes Care. 2012;35:1695–1700. doi: 10.2337/dc11-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh SM, Kim HC, Ahn SV, Rhee Y, Suh I. Association between depression and bone mineral density in community-dwelling older men and women in Korea. Maturitas. 2012;71:142–146. doi: 10.1016/j.maturitas.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Cho HM, Kim HC, Lee JM, Oh SM, Choi DP, Suh I. The association between serum albumin levels and metabolic syndrome in a rural population of Korea. J Prev Med Public Health. 2012;45:98–104. doi: 10.3961/jpmph.2012.45.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JM, Kim HC, Cho HM, Oh SM, Choi DP, Suh I. Association between serum uric acid level and metabolic syndrome. J Prev Med Public Health. 2012;45:181–187. doi: 10.3961/jpmph.2012.45.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Zivadinov R, Treu CN, Weinstock-Guttman B, Turner C, Bergsland N, O'Connor K, et al. Interdependence and contributions of sun exposure and vitamin D to MRI measures in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2013;84:1075–1081. doi: 10.1136/jnnp-2012-304661. [DOI] [PubMed] [Google Scholar]
- 23.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 24.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 25.Jung CH, Lee WY, Kim BY, Park SE, Rhee EJ, Park CY, et al. The risk of metabolic syndrome according to the white blood cell count in apparently healthy Korean adults. Yonsei Med J. 2013;54:615–620. doi: 10.3349/ymj.2013.54.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association between Serum 25(OH)D Level (per 10 ng/mL) and Increased Insulin Resistance
Characteristics of Study Participants by Vitamin D Concentrations
Adjusted OR (95% CI) of Adults Having Insulin Resistance by Vitamin D Concentrations
Association between serum 25(OH)D level and HOMA-IR. HOMA-IR, homeostasis model assessment for insulin resistance.


