Abstract
Purpose
To determine whether renal injury induced by ischemia-reperfusion (I/R) could be further improved by mesenchymal stem cells (MSCs) modified with survivin.
Materials and Methods
Lentiviral vectors were used to introduce the survivin gene into MSCs and the MSCs modified with survivin were transplanted into established mice models of renal I/R injury. Seven days later, serum creatinine (Scr) and blood urea nitrogen (BUN) were measured and the survival of MSCs was determined. Hematoxylin and eosin staining was used to assess renal pathological change. The expressions of hepatocyte growth factor (HGF) and basic fibroblast growth factor (bFGF) in kidney tissue were detected by western blot.
Results
Mice transplanted with survivin-modified MSCs demonstrated good renal function recovery with Scr and BUN decline close to normal levels and improvement of renal I/R injury repair. Additionally, the survival of transplanted MSCs modified with survivin was enhanced and the expression of HGF and bFGF in kidney tissue was increased.
Conclusion
Our results demonstrated that MSCs engineered to over-express survivin could enhance their therapeutic effect on renal I/R injury in mice, probably via the improved survival ability of MSCs and increased production of protective cytokines in ischemic tissue.
Keywords: Marrow-derived mesenchymal stem cells, ischemia-reperfusion, survivin, transplantation
INTRODUCTION
Ischemia-reperfusion (I/R) injury frequently occurs in clinical practice and is the most common reason for delayed graft function. Renal I/R can occur in various important clinical conditions, such as kidney transplantation, kidney vascular surgery, cardiac failure and shock resuscitation,1,2,3,4 and renal injury induced by I/R is a serious burden on both society and patients. Over the past several years, studies have identified a variety of approaches to treating renal I/R injury, which includes improving hypothermic organ preservation in kidney transplantation and applying a variety of pharmaceuticals.5,6 However, the clinical treatment for I/R renal injury is still limited and few specific pharmaceutical interventions are able to modulate renal I/R injury. Therefore, there is an urgent need to develop effective strategies to treat I/R-induced renal injury.
Mesenchymal stem cells (MSCs) are multipotent stem cells located within the stroma of the bone marrow and other organs, and have been discovered to transdifferentiate into cells of different germ layers.7 One of the important characteristics of MSCs is their tissue repair potential, due to their migration and differentiation abilities and capacity to secrete various growth factors.8,9 In recent years, MSCs have been applied in the treatment of kidney injury.10,11 However, study has shown that the survival ability of simple transplantation of MSCs in ischemic tissue is very limited.12 Recent studies have demonstrated that combination of apoptosis inhibitors with MSCs or anti-apoptosis gene-modified MSCs for transplantation improves the recovery of tissue ischemia,13,14 suggesting that anti-apoptosis strategies might advance the use of MSCs in treatment of renal I/R injury.
Survivin, the smallest member of the inhibitor of apoptosis protein family, exhibits a strong anti-apoptotic function in regulation of cell proliferation. Researchers have demonstrated that transplantation with survivin-modified MSCs can improve the cardiac performance of rats after myocardial infarction and better recovery of neurological function after a experimental stroke.15,16 However, it is unclear whether such MSCs could lead to a better therapeutic effect in renal I/R injury. In this study, we investigated the effects of transplantation with survivin-modified MSCs on renal I/R injury in mice.
MATERIALS AND METHODS
Animals
High-specified-pathogens free (SPF) level healthy, male, C57BL/6 mice were obtained from the experimental animal research center of Chongqing Medical University. The committee for experimental animals of Chongqing Medical University approved all of the experimental procedures, and the procedures complied with the Guidelines for the Care and Use of Laboratory Animals.
MSCs preparation
Mouse bone marrow mesenchymal stem cells were cultured as previously described.17 Briefly, the femur and tibia of 6-week-old male C57BL/6 mice were taken under sterile conditions, and the marrow cavity was repeatedly flushed with 0.9% saline. After centrifuging the bone marrow at 1000 rpm for 5 min, the supernatant was discarded, and 5 mL F12-dulbecco's modified eagle's medium (DMEM) containing 10% phosphate-buffered saline (PBS) was added to the precipitate. Next, the mixture was resuspended, seeded in cell culture flasks, and then incubated at 37℃ in a 5% CO2 incubator. At day 3, the medium was replaced to remove the suspended cells, and consequent replacement of culture medium was performed once every three days. When the adherent cells were grown to 90% confluence, the cells were digested with 0.25% trypsin and then subcultured at a ratio of 1:2.
The cultured bone marrow mesenchymal stem cells of the third passage were collected and digested with 0.25% trypsin, washed with 0.01 mol/L PBS, and made into single cell suspensions, to which the monoclonal antibodies of CD90 fluorescein isothiocyanate (FITC), CD34 phycoerythrin (PE), CD45 PE, CD29-PEs, and CD44 PE were added. After incubation at room temperature for 30 min away from light, the cells were washed with 0.01 mol/L PBS and detected by flow cytometry.
Lentiviral vectors construction
Full-length mouse survivin cDNA without termination codon was amplified by polymerase chain reaction from pUC18-survivin and then used for construction of recombinant lentiviral expression vector pLV.Ex3d.p-neo-EF1A-survivin-internal ribosome entry site-enhanced green fluorescent protein (IRES-EGFP) using Gateway Tech-nology. The identity of survivin cDNA was confirmed by sequencing. The primer sequence was 5'-GGGGAAAGTTTGTACAA AAAAGCAGGCTGCCACCATGGGAGCTCCGGCG CT-3' and 5'-GGGGACCACTTTGTACAAGAAAGCTG GGTTTAGGCAGCCAGCTGCTCA-3'. The recombinant plasmids were transfected into 293FT cells for producing lenti-survivin vectors. The pLV.Ex3d.p/neo-EF1A-IRES-EGFP was designed as the control lentivirus and named the Lenti-mock.
Lentiviral transfection
MSCs (5×105) were infected by lentivirus with a multiplicity of infection of 8.15 MSCs infected with survivin recombinant lentivirus were named survivin-MSCs, and MSCs infected with mock lentivirus were defined as mock-MSCs. All MSCs were expanded to three passes and then used for transplantation.
Western blot
MSCs were lysed and subjected to sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE) and electrotransferred to a nitrocellulose membrane (Amersham, Piscataway, NJ, USA). The blot was then treated with anti-survivin1 polyclonal antibody (Santa Cruz, CA, USA) and incubated at 4℃. The appropriate horseradish peroxidase (HRP) conjugated IgG was used as a secondary antibody. Antibody on membrane was visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA). Western blot for β-actin was used as an internal sample.
Animal model
Mice were anesthetized with pentobarbital (0.3 mg/g) and subjected to an ischemic post-condition as described previously.18 In brief, a midline laparotomy was performed and bilateral kidneys were subjected to 40 minutes of ischemia, performed via bilateral renal pedicle clamping with no vascular damage, followed by reperfusion. During the whole procedure, core body temperatures were monitored with a rectal probe and maintained at 37℃ on a homoeothermic table.
Animals were randomly divided into four groups (5 mice per group): 1) sham group, in which the kidneys were exposed for 40 minutes without clamping bilateral pedicles, 24 hours after which intravenous injection of 0.2 mL PBS was performed; 2) I/R group, in which intravenous injection of 0.2 mL PBS was performed after 24-hour reperfusion; 3) mock-MSCs group, in which 0.2 mL PBS containing 1×106 EGFP-MSCs were intravenously injected after 24-hour reperfusion; and 4) surviving-MSCs group, in which 0.2 mL PBS containing 1×106 survivin-MSCs were intravenously injected after 24 hours of reperfusion.
Mice from each group were sacrificed at 7 d after transplantation. Blood was obtained via cardiac puncture, and centrifuged with 2000×g to obtain serum, which was then stored at -80℃ for further studies. The renal tissues were harvested and fixed in 4% PBS-buffered formaldehyde or immediately frozen and stored at -80℃ for different procedures.
Renal function analysis
Serum creatinine (Scr) and blood urea nitrogen (BUN) were measured by an Auto Analyzer (Olympus, Optical Co. Ltd., Tokyo, Japan) with BUN and Scr test kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), following the manufacturer's instructions.
Histologic examination
Kidney tissues in each group were selected to undergo hematoxylin and eosin (H&E) staining. For histological evaluation, three kidney sections per rat were examined under a light microscope (Olympus, Tokyo, Japan). The kidney damage characteristics included tubular cell swelling, congestion, tubular dilatation, brush border loss, cytoplasmic vacuole, and nuclear loss. The renal injury was semi-quantitatively scored according to the criteria described previously [6]: 0=normal kidney; 1=minimal damage (less than 5% area of the cortex or outer medulla); 2=mild damage (5-25% area of the cortex or outer medulla); 3=moderate damage (25-75% involvement of the cortex or outer medulla); and 4=severe damage (more than 75% involvement of the cortex or outer medulla). Mean scores were presented per group by two examiners.
MSCs survival detection
The kidney tissues were preserved in 10% neutral-buffered formalin, paraffin embedded, and then sectioned into 4 µm-thick slices according to the standard procedure. EGFP expression was examined using a fluorescence microscope (Olympus, Optical Co, Ltd., Tokyo, Japan). GFP-positive MSCs were counted in five randomly selected non-overlapping fields (original magnification ×200) in each section to calculate the number of MSCs.
Western blot for HGF and bFGF in kidney tissue
Frozen tissue was thawed in ice-cold lysis buffer. Tissues were homogenized and lysed on ice for 1 h. Then, tissue lysates were subjected to a centrifugation at 10000 rpm for 10 min at 4℃. Total cellular proteins (50 µg) were subjected to SDS-PAGE, and transferred to nitrocellulose membranes (Amersham, Piscataway, NJ, USA). Specific polyclonal antibody against hepatocyte growth factor (HGF) and basic fibroblast growth factor (bFGF) (Santa Cruz, CA, USA) diluted in TBS-T containing 5% nonfat milk was used to detect indicated proteins. The appropriate HRP conjugated IgG was used as a secondary antibody. Antibody on membranes was visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA). Western blot for β-actin was used as an internal sample.
Statistical analysis
Data are presented as mean±standard deviation. The means of the different groups were compared using one-way analysis of variance and the Student-Newman-Keuls test. The statistical analysis was conducted with SPSS software, version 13.0 (SPSS Inc., Chicago, IL, USA). All p-values less than 0.05 were considered statistically significant.
RESULTS
Phenotypic characterization of MSCs
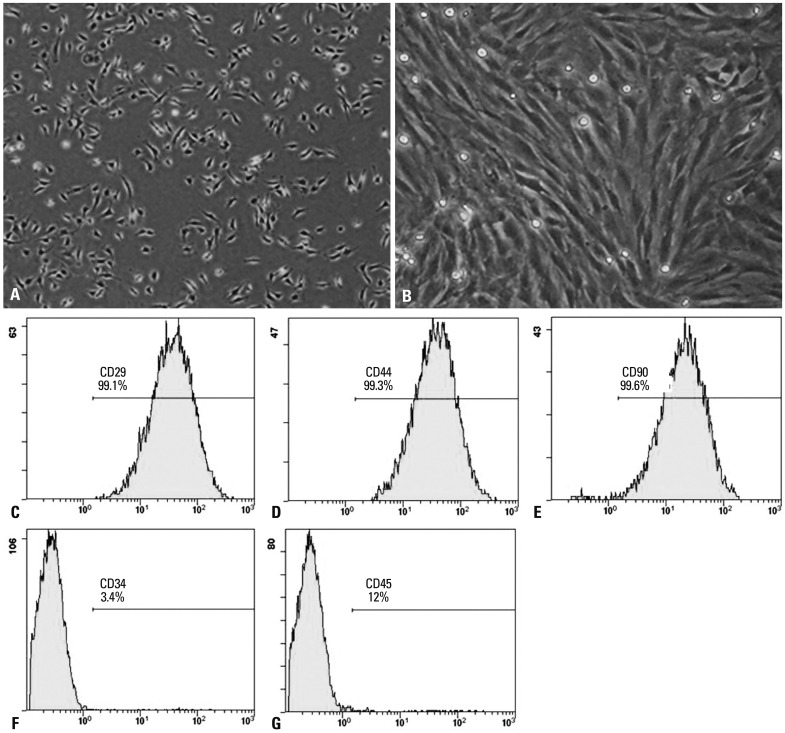
Freshly isolated and inoculated bone marrow mesenchymal stem cells were round and suspended as single cells in culture (Fig. 1A). At day 8, long-spindle cells (Fig. 1B). Passaged cells (mostly spindle cells) were uniformly distributed, and covered the bottom of the plates every 4 to 5 days. Third passage MSCs highly expressed the surface marker molecules CD29 (99.1%), CD44 (99.3%), and CD90 (99.6%), and lowly expressed CD34 (3.4%) and CD45 (1.2%) (Fig. 1C-G). These results were consistent with the expression characteristics of mouse MSCs.
Fig. 1.
Phenotypic characterization of MSCs. (A) The initial passage MSCs grew as a morphologically homogeneous population of fibroblast-like cells. (B) Third passage MSCs grew as whorls of densely packed spindle-shaped. (C-G) Flow cytometry analyzed the surface markers CD29, CD44, CD90, CD34, and CD45 in MSCs. MSC, mesenchymal stem cell.
Efficiency of gene transduction and survivin expression
After infection with survivin recombinant lentivirus and mock lentivirus, MSCs were stimulated to overexpress EGFP (Fig. 2A and B). Western blot revealed the expression of survivin in survivin-MSCs, but not in mock-MSCs (Fig. 2C).
Fig. 2.
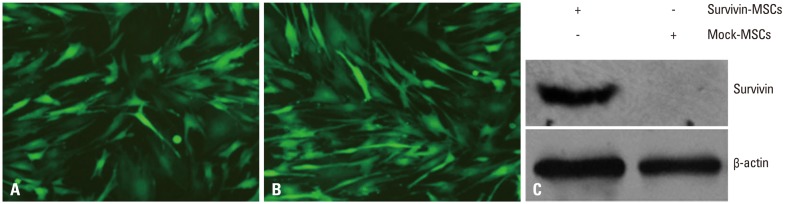
Efficiency of gene transduction and survivin expression. (A) Expression of EGFP in mock lentivirus-transduced MSCs. (B) Expression of EGFP in MSCs modified with survivin recombinant lentivirus. (C) Survivin expression in MSCs was detected by western blot. MSCs were infected with survivin recombinant lentivirus and mock lentivirus. Total cell lysates were harvested and the presence of survivin protein was detected by specific antibody. β-actin was used as a loading control. MSC, mesenchymal stem cell; EGFP, enhanced green fluorescent protein.
Survivin enhances the protective effect of MSCs on renal function
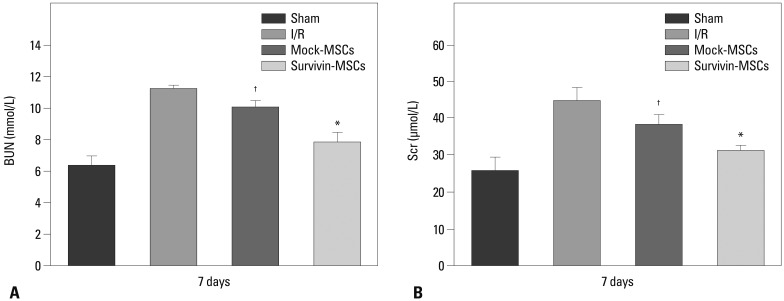
At 7 days after MSCs transplantation, BUN and Scr increased significantly in the I/R group but not the mock-MSCs group, in which BUN and Scr rose moderately. However, the survivin-MSCs group showed no obvious increase in BUN and Scr, which were close to the sham group, and both were lower than the mock-MSCs group (p<0.05) (Fig. 3).
Fig. 3.
Protective effect of survivin-MSCs on renal function. At 7 days after MSCs transplantation, BUN (A) and Scr (B) measured by Auto Analyzer with BUN and Scr test kits. Scr, serum creatinine; BUN, blood urea nitrogen; MSC, mesenchymal stem cell; I/R, ischemia-reperfusion. *,†p<0.05 versus control group.
Survivin-MSCs attenuated renal injury after renal ischemia-reperfusion in mice
Kidney tissue specimens were collected to exam renal damage by H&E staining (Fig. 4A). The score of renal damage was significantly increased in the I/R group, compared with the sham group, and higher than the mock-MSCs group (p<0.05) (Fig. 4B). However, the survivin-MSCs group showed lower renal damage score than the mock-MSCs group (p<0.05) (Fig. 4B).
Fig. 4.
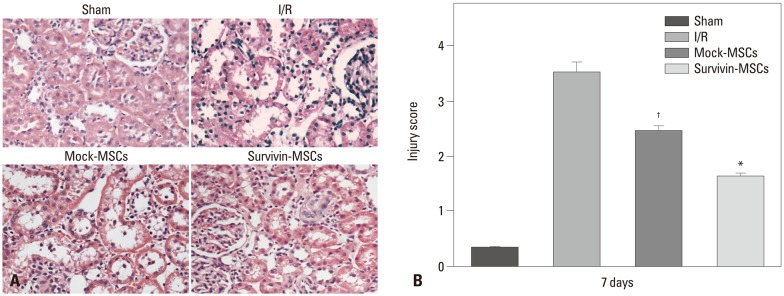
Survivin-MSCs attenuated renal injury. (A) Kidney tissue specimens were collected for H&E staining ×200. (B) The renal injury was semi-quantitatively scored. Data are presented as the mean±SD. *p<0.05, compared with control group. †p<0.05, compared with control group. H&E, hematoxylin and eosin, MSC, mesenchymal stem cell; I/R, ischemia-reperfusion.
Survivin enhanced the survival of transplanted MSCs
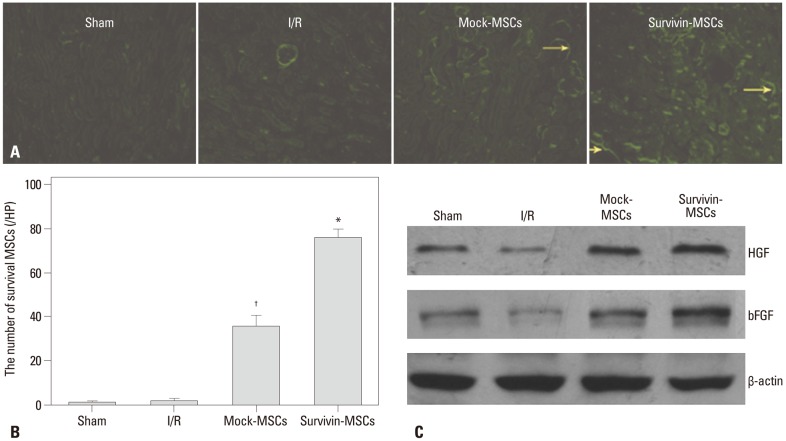
To observe the survival of MSCs in the kidneys of mice transplanted with MSCs, EGFP expression in renal slices was examined using a fluorescence microscope. Doing so showed a significantly greater number of MSCs in the survivin-MSCs group than the mock-MSCs group (Fig. 5A and B); however, there was nearly no EGFP expression in the sham group and I/R group (Fig. 5A and B).
Fig. 5.
Survival of transplanted MSCs and HGF and bFGF expression in kidney tissue. (A) Kidney tissue specimens were collected to observe EGFP expression in renal slices by fluorescence microscope. EGFP-positive cells (yellow arrows). (B) Quantitative analysis of the number of survival MSCs at 7 days after transplantation. Data are presented as the mean±SD. *p<0.05, compared with control group. †p<0.05, compared with control group. (C) Kidney tissues were collected and lysed. Tissue lysates were prepared and subjected to western blot. Specific antibodies were used to detect HGF and bFGF. β-actin was used as a loading control. MSC, mesenchymal stem cell; HGF, hepatocyte growth factor; bFGF, basic fibroblast growth factor; EGFP, enhanced green fluorescent protein; I/R, ischemia-reperfusion.
Survivin-MSCs improved expression of HGF and bFGF in kidney tissue
HGF and bFGF in kidney tissue were measured by western blot. The expression of HGF and bFGF in the I/R group was deregulated, compared to the sham group, and lower than that in the mock-MSCs group. Nevertheless, the survivin-MSCs group showed enhanced expression of HGF and bFGF, compared to mock-MSCs (Fig. 5C).
DISCUSSION
Renal I/R injury is an inevitable clinical challenge after renal transplantation or kidney vascular surgery. Such injury involves pro-inflammatory mediator release, which is positively associated with organ damage and dysfunction. MSCs with their broad immunomodulatory and tissue protective properties make them promising candidates for conditioning the I/R environment to combat I/R injury. Recently, MSCs-based therapies have been applied in a large number of diseases, including stroke, myocardial infarction, and even, I/R-induced acute renal failure.9,19,20 However, studies reported limited migration of transplanted MSCs to I/R tissue and organs, as well as low survival,21 which seriously constrained the function of MSCs. In this study, therefore, we used recombinant lentivirus vector carrying survivin to modify MSCs, and observed their effects on renal I/R injury in mice.
In the present study, we demonstrated that MSCs modified with survivin up-regulated the expression of survivin protein, and promoted the better recovery of renal function at 7 days after MSCs transplantation in I/R injured mice. Moreover, we observed that overexpression of survivin improved the protective effect of MSCs against renal tubular cell injury on H&E staining. However, we did not detect any tubular-like cells derived from the transplanted MSCs via morphous change, which was not in accordance with the results reported previously by Huls, et al.,22 who reported that approximately 10% of the integral tubular segments originated from transplanted MSCs after renal ischemic injury. The reason for this may originate from differences in experimental models, and further research should be conducted. Additionally, we found that the survival number of transplanted mock-MSCs in the kidney was quite few, in which several factors may be involved, including strong inflammatory and oxidative stress reactions, variety of pro-apoptosis factors and chemokines, and the lethal influences on the transplanted cells induced by I/R injury.16 However, the amount of survival MSCs modified with survivin was significantly more than mock-MSCs. Our results demonstrated that survivin can improve the post-transplantation survival of MSCs, due to its powerful anti-apoptosis effect.23
In order to elucidate the renoprotective effect of modification for MSCs with survivin, we further measured several renotropic factors, such as HGF and bFGF, which are secreted by MSC and are known to both decrease the apoptosis of endothelial and tubular cells, as well as to stimulate the proliferation of surviving cells.24 Our results indicated that MSCs modified with survivin could enhance secretion of HGF and bFGF uniformly, demonstrating that the paracrine effect may be a major factor in renal recovery after renal I/R in mice. Furthermore, we found there was an analog trend between increase of these renotropic factors and the survival of transplanted MSCs in I/R injured renal tissue. This suggests that enhancing the paracrine effect of these renoprotective factors by MSCs may indirectly result from improving the survival of transplanted MSCs due to modification with survivin. Nevertheless, whether survivin affects the differentiation of MSCs into tubular cells is still not certain, and how survivin upregulates the expression of HGF and bFGF was not investigated. In any case, our study may be helpful to strengthen our comprehension of MSC transplantation in renal I/R injury.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Grant No. 81370706).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Snoeijs MG, Vink H, Voesten N, Christiaans MH, Daemen JW, Peppelenbosch AG, et al. Acute ischemic injury to the renal microvasculature in human kidney transplantation. Am J Physiol Renal Physiol. 2010;299:F1134–F1140. doi: 10.1152/ajprenal.00158.2010. [DOI] [PubMed] [Google Scholar]
- 2.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 3.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 4.Chua HR, Glassford N, Bellomo R. Acute kidney injury after cardiac arrest. Resuscitation. 2012;83:721–727. doi: 10.1016/j.resuscitation.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Henry SD, Guarrera JV. Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev (Orlando) 2012;26:163–175. doi: 10.1016/j.trre.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Hu L, Yang C, Zhao T, Xu M, Tang Q, Yang B, et al. Erythropoietin ameliorates renal ischemia and reperfusion injury via inhibiting tubulointerstitial inflammation. J Surg Res. 2012;176:260–266. doi: 10.1016/j.jss.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 7.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 8.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 9.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 10.Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, et al. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 11.Duffield JS, Bonventre JV. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int. 2005;68:1956–1961. doi: 10.1111/j.1523-1755.2005.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mylotte LA, Duffy AM, Murphy M, O'Brien T, Samali A, Barry F, et al. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells. 2008;26:1325–1336. doi: 10.1634/stemcells.2007-1072. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Li Y, Wang L, Lu M, Chopp M. Caspase inhibition by Z-VAD increases the survival of grafted bone marrow cells and improves functional outcome after MCAo in rats. J Neurol Sci. 2002;199:17–24. doi: 10.1016/s0022-510x(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 14.Wei L, Cui L, Snider BJ, Rivkin M, Yu SS, Lee CS, et al. Transplantation of embryonic stem cells overexpressing Bcl-2 promotes functional recovery after transient cerebral ischemia. Neurobiol Dis. 2005;19:183–193. doi: 10.1016/j.nbd.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Fan L, Lin C, Zhuo S, Chen L, Liu N, Luo Y, et al. Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur J Heart Fail. 2009;11:1023–1030. doi: 10.1093/eurjhf/hfp135. [DOI] [PubMed] [Google Scholar]
- 16.Liu N, Zhang Y, Fan L, Yuan M, Du H, Cheng R, et al. Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by Survivin on experimental stroke in rats. J Transl Med. 2011;9:105. doi: 10.1186/1479-5876-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng W, Bivalacqua TJ, Chattergoon NN, Hyman AL, Jeter JR, Jr, Kadowitz PJ. Adenoviral gene transfer of eNOS: high-level expression in ex vivo expanded marrow stromal cells. Am J Physiol Cell Physiol. 2003;285:C1322–C1329. doi: 10.1152/ajpcell.00141.2003. [DOI] [PubMed] [Google Scholar]
- 18.Villanueva S, Suazo C, Santapau D, Pérez F, Quiroz M, Carreño JE, et al. NFAT5 is activated by hypoxia: role in ischemia and reperfusion in the rat kidney. PLoS One. 2012;7:e39665. doi: 10.1371/journal.pone.0039665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362:200–203. doi: 10.1016/j.neulet.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Li T, Wei X, Bianchi G, Hu J, Sanchez PG, et al. Mesenchymal stem cell transplantation improves regional cardiac remodeling following ovine infarction. Stem Cells Transl Med. 2012;1:685–695. doi: 10.5966/sctm.2012-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 22.Huls M, Russel FG, Masereeuw R. Insights into the role of bone marrow-derived stem cells in renal repair. Kidney Blood Press Res. 2008;31:104–110. doi: 10.1159/000121387. [DOI] [PubMed] [Google Scholar]
- 23.Chiou SK, Jones MK, Tarnawski AS. Survivin - an anti-apoptosis protein: its biological roles and implications for cancer and beyond. Med Sci Monit. 2003;9:PI25–PI29. [PubMed] [Google Scholar]
- 24.Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]