Abstract
Purpose
Cathepsin K is a potent collagenase implicated in human and animal atherosclerosis-based vascular remodeling. This study examined the hypothesis that serum CatK is associated with the prevalence of coronary artery disease (CAD).
Materials and Methods
Between January 2011 and December 2012, 256 consecutive subjects were enrolled from among patients who underwent coronary angiography and percutaneous coronary intervention treatment. A total of 129 age-matched subjects served as controls.
Results
The subjects' serum cathepsin K and high sensitive C-reactive protein (hs-CRP) and high-density lipoprotein cholesterol were measured. The patients with CAD had significantly higher serum cathepsin K levels compared to the controls (130.8±25.5 ng/mL vs. 86.9±25.5 ng/mL, p<0.001), and the patients with acute coronary syndrome had significantly higher serum cathepsin K levels compared to those with stable angina pectoris (137.1±26.9 ng/mL vs. 102.6±12.9 ng/mL, p<0.001). A linear regression analysis showed that overall, the cathepsin K levels were inversely correlated with the high-density lipoprotein levels (r=-0.29, p<0.01) and positively with hs-CRP levels (r=0.32, p<0.01). Multiple logistic regression analyses shows that cathepsin K levels were independent predictors of CAD (odds ratio, 1.76; 95% confidence interval, 1.12 to 1.56; p<0.01).
Conclusion
These data indicated that elevated levels of cathepsin K are closely associated with the presence of CAD and that circulating cathepsin K serves a useful biomarker for CAD.
Keywords: Cathepsin K, coronary artery disease, biomarker, high sensitive C-reactive protein, myocardial ischemia
INTRODUCTION
Cathepsins are a group of lysosomal cysteine proteases belonging to the papain family.1 Recent evidence has implicated the role of cathepsins in the pathogenesis of atherosclerosis-based cardiovascular disease.2,3,4 The protein levels of cathepsins S and K were increased in atherosclerotic plaques and injury-related neointimal lesions in animals and humans.5,6,7,8 Among the same lines of evidence, the genetic and pharmacological inhibition of cathepsins S and K prevented atherosclerotic plaque growth and cardiovascular repair in several animal models.9,10,11,12 Thus, cathepsins represent a viable target to alleviate vascular dysfunction and remodeling in response to multiple pathogenic stresses.
Among the cathepsin family members, cathepsin K has been shown to be one of the most potent mammalian collagenases in vivo and in vitro.11,13,14,15 Data from our group and others have shown that cathepsin K abounds in vascular cells (including smooth muscle cells and endothelial cells) and infiltrated macrophages of human and animal atherogenic lesions.3,16,17,18 The ablation of cathepsin K was shown to ameliorate obesity- and pressure overload-related cardiac dysfunction and remodeling.19,20
We reported that cathepsin K is overexpressed in the intracoronary artery of hypertensive heart failure tissues.21 Several clinical studies have shown that patients with atherosclerosis-related diabetes, aneurysm and chronic kidney disease had increased levels of serum cathepsins S or L.15,22,23 These data suggested that cathepsins levels are associated with atherosclerosis-based cardiovascular disease. However, there is limited information regarding the relationship between circulating cathepsin K and coronary artery disease (CAD). In this study, we tested this relationship in patients with CAD and non-CAD control subjects to explore the relationship between circulating cathepsin K and clinical presentations, and we tried to identify useful blood biomarkers suggestive of CAD with coronary vulnerable plaques.
MATERIALS AND METHODS
Study population and definition
In total, 256 consecutive patients with CAD who underwent percutaneous coronary intervention (PCI) with drug-eluting stent implantation between January 2011 and December 2012 at Yanbian University Hospital (Yanji, China) were considered for inclusion in this study. The patients with CAD were subgrouped into those with stable angina pectoris (SAP; n=50), and those with unstable angina pectoris (UAP) and acute myocardial infarction (AMI) (UAP+AMI, n=206) by symptoms and clinical examinations.
The diagnosis of AMI was based on the elevation of cardiac biomarkers (at least one positive biomarker: creatine kinase-MB or troponin T) and an electrocardiogram indicative of new ischemia (new ST-T change or new left bundle branch block) and a history of prolonged chest pain.24
UAP was diagnosed by typical chest pain at rest in the 24 h prior to the subject's arrival at the hospital, depressed ST ≥0.1 mV, and/or T-wave inversion on an electrocardiogram but a normal creatine kinase-MB level.
SAP was diagnosed as an invariable character of exertional chest pain for 3 months before the subject went to the hospital (with "invariable" meaning the same degree of exertion and excitation provocation and the same location, quality, and 3- to 5-min duration), which was relieved by rest or nitroglycerin.
A total of 129 subjects who showed no evidence of cardiovascular disease-defined as no typical chest pain on exertion, no myocardial infarction (MI) by history or electrocardiogram, negative exercise test, and no significant luminal narrowing of the coronary arteries were recruited as non-CAD controls. Additionally, hypertension was defined as a systolic blood pressure >140 mm Hg, a diastolic blood pressure >90 mm Hg, and/or having received treatment for hypertension. Diabetes mellitus was confirmed when the subject had hemoglobin A1c (HbA1c) levels ≥6.5%, a fasting plasma glucose concentration >126 mg/dL, and/or a history of any anti-hyperglycemic medication or a previous diagnosis of diabetes.
Patients were excluded if they had prior evidence of congenital heart disease, end-stage renal disease with maintenance hemodialysis, primary valvular disease, cardiomyopathy, or secondary cardiac muscle disease caused by any known systemic condition. The study protocol was approved by the ethics committee of Yanbian University Hospital, and written informed consent was obtained from all patients and control subjects.
A blood sample was isolated prior to PCI, and HbA1c, high sensitive C-reactive protein (hs-CRP), and various lipids were measured. The gender, age, body mass index (BMI), systolic and diastolic blood pressures, medication history, and smoking history were recorded for each subject.
Laboratory examination
Human serum cathepsin K levels were determined by using ELISA kits (Biomedica Gruppe, Biomedica Medizinprodukte, Vienna, Austria) in duplicate. Serum levels of creatinine, low-density lipoprotein (LDL), high-density lipoprotein (HDL), hs-CRP, and HbA1c were measured at the clinical laboratory of Yanbian University Hospital (Clinical Laboratory, Yanji, China). Serum cathepsin K values are expressed as ng/mL, and the inter-assay and intra-assay coefficients of variation were <8%.
Quantitative coronary angiogram (QCA)
Coronary angiography was obtained prior to PCI treatment. Angiography showing the maximal degree of stenosis was adapted for the quantitative coronary angiogram (QCA). The QCA analysis was performed using a contour detection minimum cost algorithm (DSA Artis Zee Biplane; Siemens, Erlangen, Germany). All CAD patients showed severe coronary artery stenosis defined as present stenosis ≥50% diameter of at least one major artery. The reference segment dia. was averaged from 5-mm long angiographically normal segments proximal to the lesion; if a normal proximal segment could not be identified, a distal angiographically normal segment was analyzed as described.25
Statistical analysis
Data are presented as means±standard deviation. Comparisons of categorical baseline characteristics were made using the chi-square test. Comparisons of the continuous baseline characteristics were made using an unpaired Student's t-test. The hs-CRP concentrations were logarithmically transformed because the data showed a skewed distribution. If the homogeneity of variance assumption was violated, the nonparametric Kruskal-Wallis test was used instead. The factors that related at the p<0.1 level were selected as independent variable candidates for a multiple logistic regression analysis, which was used to evaluate the independent contributions of clinical parameters to CAD. Correlation coefficients were calculated using a linear regression analysis. StatFlex (version 6.0; Artech, Osaka, Japan) was used for all statistical analyses. p values of less than 0.05 were considered significant.
RESULTS
Baseline clinical characteristics
The baseline characteristics of CAD patients (n=256) and control subjects (n=129) are displayed in Table 1. There were no significant differences in age, gender, or BMI (p>0.05 for all comparisons). The patients with CAD had a significantly higher prevalence of diabetes and hypertension (p<0.01); they were also more likely to have had cerebrovascular disease or myocardial infarction or to have undergone a coronary bypass graft or angioplasty. The frequencies of CAD patients undergoing treatment with antilipid, antidiabetic, antihypertensive, or antiplatelet medications were higher than in the control subjects.
Table 1.
Demographic and Clinical Variables of Control and CAD Patients
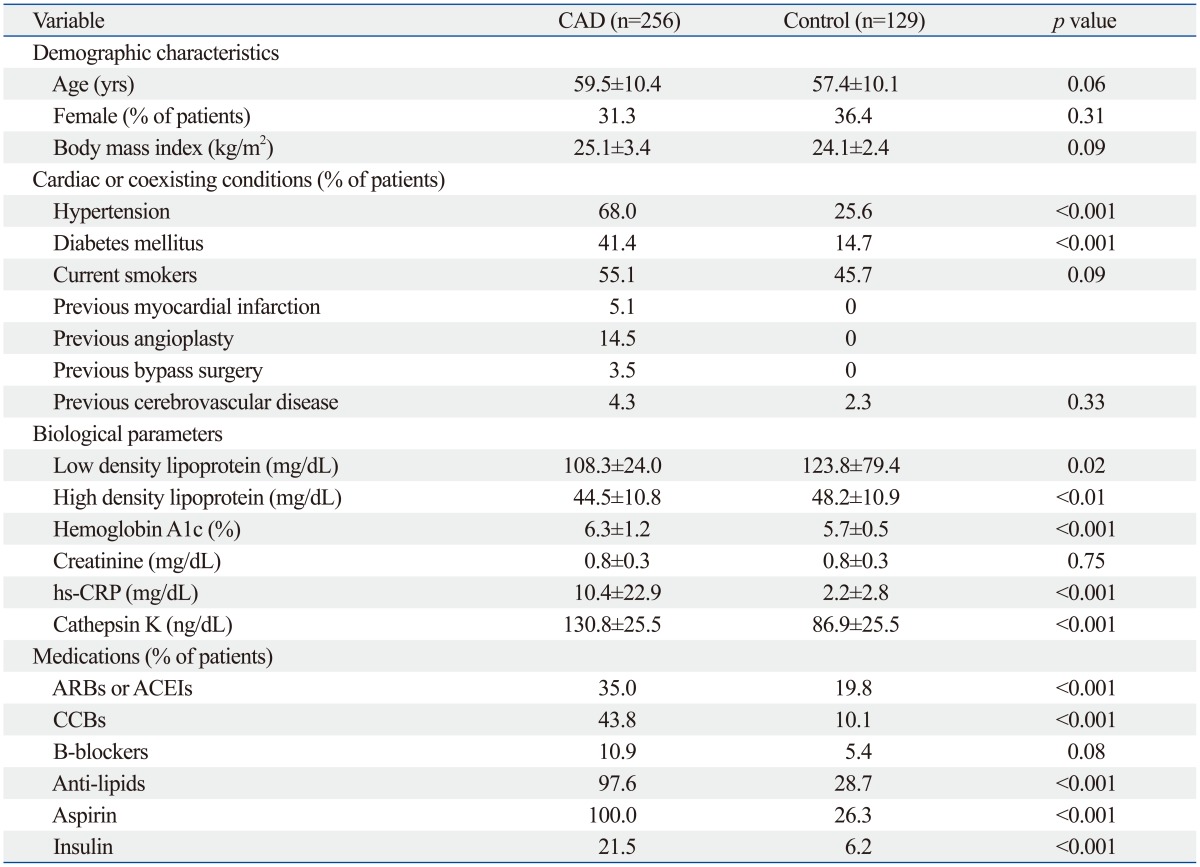
hs-CRP, high sensitive C-reactive protein; ARBs, angiotensin II receptor blockers; ACEI, angiotensin converting enzyme inhibitor; CCBs, calcium channel blockers; CAD, coronary artery disease.
Values are expressed as mean±SD or number (%).
Table 2 shows the clinical characteristics of the UAP+ AMI (called hereafter the "acute coronary syndrome") group and SAP group. There were also no significant differences in age, gender, or BMI (p>0.05 for all comparisons). With the exception of the prevalence of diabetes, there were no significant differences in clinical histories or medications between the two groups (p>0.05 for all comparisons).
Table 2.
Demographic and Clinical Variables of SAP and UAP-AMI
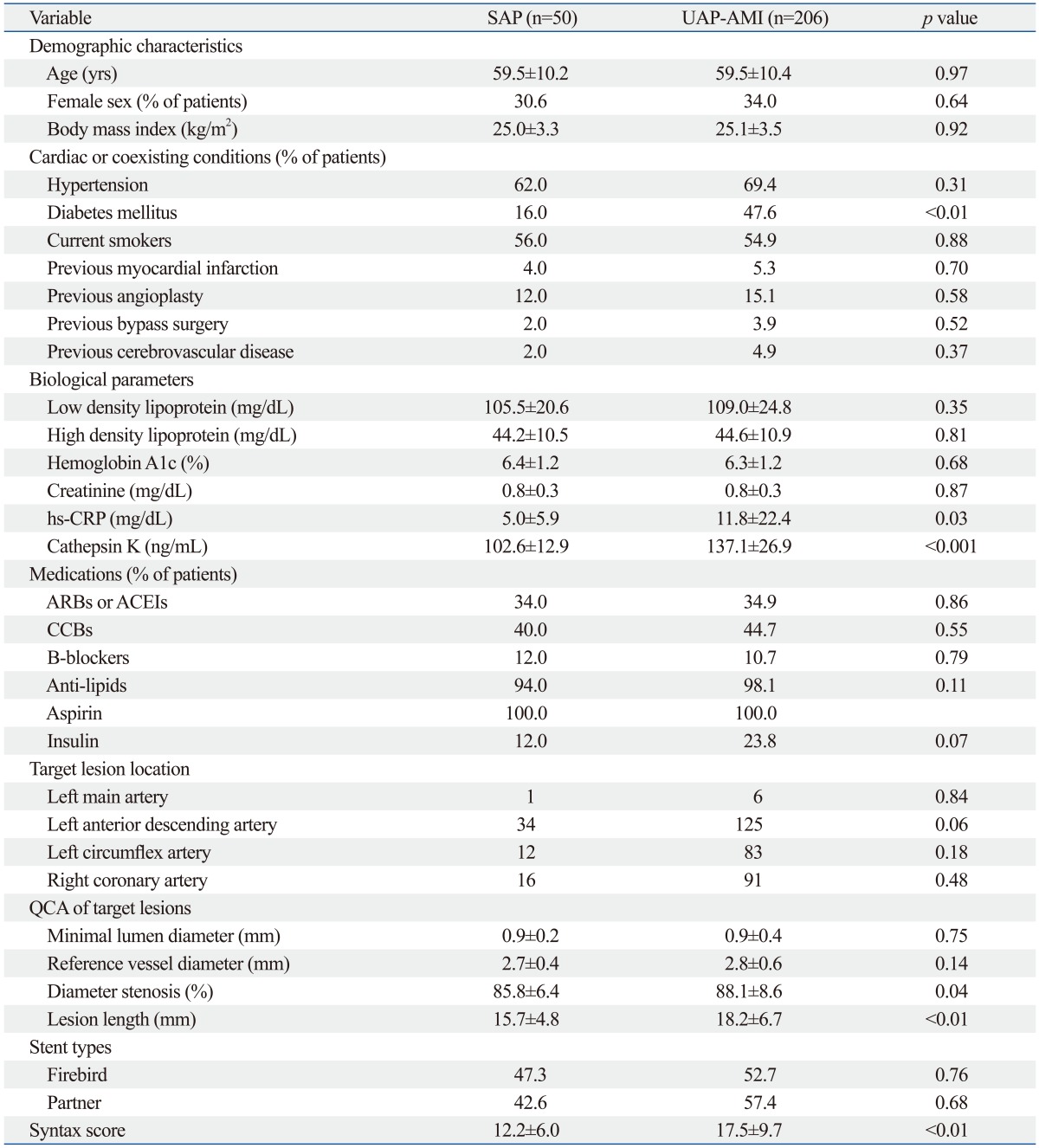
QCA, quantitative coronary angiography; ARBs, angiotensin II receptor blockers; ACEI, angiotensin converting enzyme inhibitor; CCBs, calcium channel blockers; hs-CRP, high sensitive C-reactive protein; SAP, stable angina pectoris; UAP, unstable angina pectoris; AMI, acute myocardial infarction.
Values are expressed as mean±SD or number (%).
Atherosclerotic lesion location and characteristics
As shown in Table 2, with the exception of the diameter stenosis, lesion length, and Syntax score, there were no significant differences in target lesion location or QCA results of the target lesions in the SAP and UAP+AMI groups (p>0.05 for all comparisons).
Circulating biomarkers
As shown in Table 1, compared to the control group, the patients with CAD had significantly higher levels of serum cathepsin K (130.8±25.5 ng/mL vs. 86.9±25.5 ng/mL, p<0.001). The levels of hs-CRP (10.4±22.9 mg/mL vs. 2.2±2.8 mg/mL, p<0.001) and HbA1c (6.3±1.2% vs. 5.7±0.5%, p<0.001) were also significantly higher and the LDL cholesterol (108.3±24.0 mg/mL vs. 123.8±79.4 mg/mL, p=0.02) and HDL cholesterol (44.5±10.8 mg/mL vs. 48.2±10.9 mg/mL, p<0.01) levels were significantly lower in the CAD group than in the control group, but there was no significant difference in the creatinine levels. Moreover, patients with UAP+AMI had significantly higher hs-CRP (11.8±22.4 mg/mL vs. 5.0±5.9 mg/mL, p=0.03) and CatK (137.1±26.9 ng/mL vs. 102.6±12.9 ng/mL, p<0.001) levels than did the SAP patients. However, there were no significant differences in the, LDL, HDL, HbA1c, or creatinine levels between the two CAD subgroups.
The linear regression analysis showed that the cathepsin K levels were positively correlated with the hs-CRP levels (r=0.32, p<0.001), whereas they were negatively correlated with the HDL levels (r=-0.29, p<0.001).
Independence of predictors of CAD
The results of the logistic regression analysis for CAD are shown in Table 3. In the single logistic regression analysis, diabetes mellitus, hypertension, cathepsin K, hs-CRP, LDL cholesterol, and HDL cholesterol were significantly associated with CAD (Table 3). The multiple logistic regression analysis with age, BMI, diabetes mellitus, hypertension, cathepsin K, hs-CRP, LDL cholesterol, and HDL cholesterol revealed that the diabetes mellitus [odds ratio (OR), 7.69; 95% confidence interval (CI), 1.99 to 45.10; p<0.01], hypertension (OR, 9.88; 95% CI, 3.45 to 66.95; p<0.01), hs-CRP (OR, 4.23; 95% CI, 2.99 to 7.68; p<0.01), HDL (OR, 0.89; 95% CI, 0.81 to 0.96; p<0.05) and cathepsin K (OR, 1.76; 95% CI, 1.12 to 1.56; p<0.01) levels were significantly correlated with CAD (Table 3).
Table 3.
Independent Predictors of CAD According to Multivariable Logistic Regression Analysis
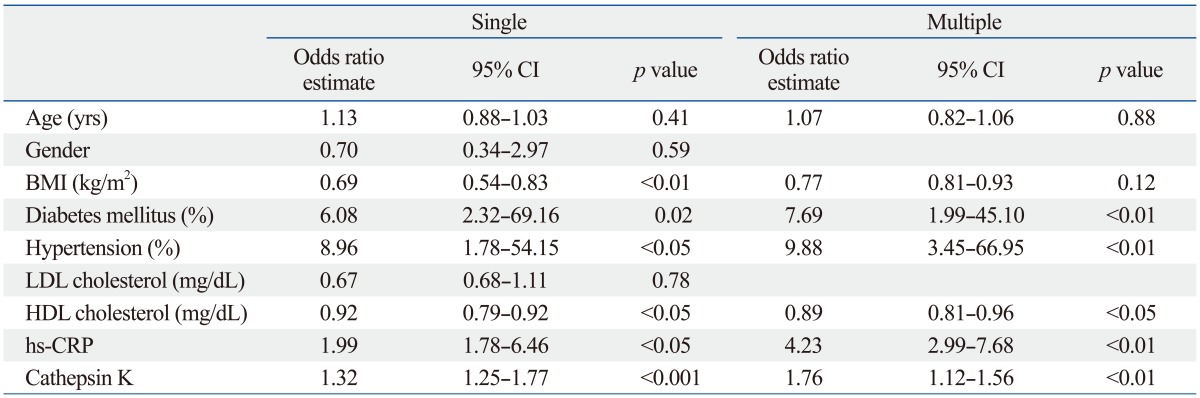
CI, confidence interval; CAD, coronary artery disease; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; hs-CRP, high sensitive C-reactive protein.
Multiple regression model includes all variables at baseline with p<0.05 by univariable analysis.
DISCUSSION
This study provides evidence that elevated levels of circulating cathepsin K are independently associated with the prevalence of CAD after adjusting for conventional CAD risk factors including diabetes-related factors, blood pressure, dyslipidemia and BMI. Excess inflammation is strongly associated with an increased risk of cardiovascular disease.26,27 Cathepsin K mRNA and proteins are highly expressed in human and animal atherosclerotic plaques and injury-induced neointimal lesions,5,7,12 and the expression of the endogenous inhibitor of cysteine proteases, cystatin C, in the plasma and the arterial tissues is decreased in atherosclerosis-based arterial and vein disorders such as aneurysms and varicose veins.6 Thus, these observations indicate that cathepsin K could be a useful marker of CAD associated with inflammation.
Recent studies highlighted that cysteinyl cathepsin K is the most abundant and important protease synthesized by the cardiovascular cells and inflammatory cells, and that it is relevant to atherosclerosis-related cardiovascular disease and its implications.3,28 However, few studies have examined peripheral blood cathepsin K levels in humans with or without CAD. Our present data show that the levels of serum cathepsin K were higher in the patients with CAD than in the control subjects. Our multivariable logistic regression analysis demonstrated that serum cathepsin K levels were independent predictors of CAD. Together with the finding that serum cathepsins S and L were increased in patients with coronary artery extasia or atherosclerotic stenosis,15,29,30 our results suggest that these cysteinyl cathepsins may participate in coronary artery restenosis and aneurysm.
Elevated circulating cathepsin K CAD patients with severe stenosis is consistent with the notion that coronary artery stenosis is a protease-mediated proteolysis involving such as cysteinyl cathepsins K, which is shear stress-sensitive and increased during atherosclerotic lesion and neointimal formation.31,32 Due to the characteristics of the coronary artery anatomy, i.e., plural branches and restriction, oscillatory blood flow during cardiac motion cycle, and collateral blood counter-flow during coronary vessel occlusion, it seems to be that cathepsin K might by secreted by the inflammatory lesions of the coronary artery into the circulation. An increased expression of cathepsin K mRNA and/or protein in in vivo and in vitro cultured vascular endothelium and smooth muscles in response to inflammatory cytokines was reported,5,7,16 suggesting that these vascular cell types may also contribute to increased serum cathepsin K in patients with CAD.
It is well established that atherosclerotic plaque instability and rupture induced by inflammation are the major mechanisms of acute coronary syndrome or an acute clinical event.33,34 Accumulating evidence indicates that elevated levels of CRP, an acute-phase protein widely used as a marker of inflammation, is predictive of the risk of first acute coronary syndrome and acute myocardial infarction.35,36 The severity of the superficial inflammation seen in atherosclerotic lesions has been implicated as a significant correlate of plaque instability and rupture.33,37,38 Here we observed that the patients with UAP and AMI had higher levels of hs-CRP than did the SAP subjects. We detected higher cathepsin K levels in patients with UAP and AMI compared to those with SAP. Moreover, our data revealed a significant positive correlation between cathepsin K and hs-CRP in all subjects. Another study highlighted the cathepsins-mediated metabolism of the major components of the vascular extracellular matrix, including the fibrous cap of atherosclerotic plaques.3
The activation of cathepsins S and K by monocyte/macrophages has been shown to promote plaque instability.18,39 Animal studies demonstrated that the genetic and pharmacological inhibition of cathepsin K alleviates the extracellular matrix metabolism of the atherosclerotic lesion and prevents plaque disruption.18,39,40 Thus, cathepsin K production by activated inflammatory cells and its release into the circulation appear to be strongly linked to the plaque instability and plaque rupture associated with local inflammatory processes within the vascular wall. On the other hand, the most extensively studied molecular candidates for rupture-producing proteases are the matrix metalloproteinases.41 It was shown that a plaque rupture being present in the culprit lesion was closely related to the high levels of metalloproteinase-9 in patients with AMI and UAP.42 Therefore, an increased cathepsin K level together with the evaluation of the matrix metalloproteinase concentration may serve as a noninvasive method of documenting and monitoring coronary inflammatory atherosclerotic plaque vulnerability during acute coronary syndrome.
The involvement of cathepsin K in ApoB-100 proteolytic modification is likely to contribute to the extracellular LDL particle aggregation, lipid droplet formation, and LDL retention of arterial proteoglycans.43 Cathepsin K deficiency resulted in an increase in cholesterol ester storage in macrophages of ApoE-/- bone marrow, which was stored in large lysosomal compartments.18 Our present data revealed a direct negative correlation between cathepsin K and HDL in all subjects. Cathepsin K has been shown to control cholesterol efflux by the degradation of preβ-HDL and apoA-1.44 Thus, cathepsin K-mediated cholesterol uptake and/or efflux could represent a common mechanism in the macrophage-derived foam cell formation and plaque growth.
Study limitations
Here, we have to point out several our study limitations. First, the sample size was too small to restrict the power for proving relationships and differences and to conduct the subgroup analysis of SAP and UAP+MI patients. Second, this study was not designed to determine the relationship of circulating cathepsin K to coronary plaque characteristics (including plaque volumes and fibrous volumes) by intravascular ultrasound. Third, it is well known that serum markers of cathepsin K and collagen turnover are not coronary-specific. It is too difficult to separate cathepsin K and collagen markers from different arteries (carotid artery, peripheral artery, or cerebral artery, etc.) and tissues (myocardium, bone, fat etc.). Additionally, it is unclear how their inclusion or exclusion would influence the present results. Fourth, our recent observations showed that long-term treatment with statins or angiotensin antagonist not only reduced plasma and tissues CatK levels but also prevented cardiovascular and renal injury in animal models.2,12,40 Moreover, the frequencies of patients with CAD under treatment with antihypertensive (angiotensin II receptor blockers or angiotensin converting enzyne inhibitors) and lipid-lowering drugs (statins) were 35.0% or 97.6%, respectively. It is well known that these drugs exert atherosclerotic regression effect in animal and humans.33,34 It might help to explain our unexpected observation. Finally, it is necessary to investigate cardiovascular events as clinical outcomes in future follow-up studies.
A complete elucidation of the pathophysiology of cathepsin K in human atherosclerosis requires further studies. Therefore, the evaluation of circulating cathepsin K may provide a noninvasive method of documenting and monitoring both the extent and mechanism of plaque rupture in CAD patients and of evaluating pharmacologic measures designed to treat this disease. Further investigation and prospective clinical trials are needed to elucidate the exact role of cathepsin K-related proteolysis in CAD and to evaluate the importance and value of monitoring proteolytic activity and extracellular matrix turnover in clinical settings.
ACKNOWLEDGEMENTS
This work was supported in part by the Scientific Research Fund of the Chinese Ministry of Education (nos. 30960128, 82160068) and the Ministry of Education, Science and Technology of Korea (BioR&D program, 2010-0019913).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20:4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng XW, Shi GP, Kuzuya M, Sasaki T, Okumura K, Murohara T. Role for cysteine protease cathepsins in heart disease: focus on biology and mechanisms with clinical implication. Circulation. 2012;125:1551–1562. doi: 10.1161/CIRCULATIONAHA.111.066712. [DOI] [PubMed] [Google Scholar]
- 3.Cheng XW, Huang Z, Kuzuya M, Okumura K, Murohara T. Cysteine protease cathepsins in atherosclerosis-based vascular disease and its complications. Hypertension. 2011;58:978–986. doi: 10.1161/HYPERTENSIONAHA.111.180935. [DOI] [PubMed] [Google Scholar]
- 4.Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120:3421–3431. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi GP, Sukhova GK, Grubb A, Ducharme A, Rhode LH, Lee RT, et al. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104:1191–1197. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng XW, Kuzuya M, Sasaki T, Arakawa K, Kanda S, Sumi D, et al. Increased expression of elastolytic cysteine proteases, cathepsins S and K, in the neointima of balloon-injured rat carotid arteries. Am J Pathol. 2004;164:243–251. doi: 10.1016/S0002-9440(10)63114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns-Kurtis CL, Olzinski AR, Needle S, Fox JH, Capper EA, Kelly FM, et al. Cathepsin S expression is up-regulated following balloon angioplasty in the hypercholesterolemic rabbit. Cardiovasc Res. 2004;62:610–620. doi: 10.1016/j.cardiores.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Sun M, Chen M, Liu Y, Fukuoka M, Zhou K, Li G, et al. Cathepsin-L contributes to cardiac repair and remodelling post-infarction. Cardiovasc Res. 2011;89:374–383. doi: 10.1093/cvr/cvq328. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Sukhova GK, Zhang J, Chen H, Sjöberg S, Libby P, et al. Cathepsin L activity is essential to elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2011;31:2500–2508. doi: 10.1161/ATVBAHA.111.230201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J, Sukhova GK, Zhang J, Chen H, Sjöberg S, Libby P, et al. Cathepsin K deficiency reduces elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2012;32:15–23. doi: 10.1161/ATVBAHA.111.235002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng XW, Murohara T, Kuzuya M, Izawa H, Sasaki T, Obata K, et al. Superoxide-dependent cathepsin activation is associated with hypertensive myocardial remodeling and represents a target for angiotensin II type 1 receptor blocker treatment. Am J Pathol. 2008;173:358–369. doi: 10.2353/ajpath.2008.071126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Hou WS, Brömme D. Collagenolytic activity of cathepsin K is specifically modulated by cartilage-resident chondroitin sulfates. Biochemistry. 2000;39:529–536. doi: 10.1021/bi992251u. [DOI] [PubMed] [Google Scholar]
- 14.Novinec M, Grass RN, Stark WJ, Turk V, Baici A, Lenarcic B. Interaction between human cathepsins K, L, and S and elastins: mechanism of elastinolysis and inhibition by macromolecular inhibitors. J Biol Chem. 2007;282:7893–7902. doi: 10.1074/jbc.M610107200. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Sukhova GK, Yang JT, Sun J, Ma L, Ren A, et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006;184:302–311. doi: 10.1016/j.atherosclerosis.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Cheng XW, Kuzuya M, Nakamura K, Di Q, Liu Z, Sasaki T, et al. Localization of cysteine protease, cathepsin S, to the surface of vascular smooth muscle cells by association with integrin alphanubeta3. Am J Pathol. 2006;168:685–694. doi: 10.2353/ajpath.2006.050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutgens SP, Cleutjens KB, Daemen MJ, Heeneman S. Cathepsin cysteine proteases in cardiovascular disease. FASEB J. 2007;21:3029–3041. doi: 10.1096/fj.06-7924com. [DOI] [PubMed] [Google Scholar]
- 18.Lutgens E, Lutgens SP, Faber BC, Heeneman S, Gijbels MM, de Winther MP, et al. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113:98–107. doi: 10.1161/CIRCULATIONAHA.105.561449. [DOI] [PubMed] [Google Scholar]
- 19.Hua Y, Xu X, Shi GP, Chicco AJ, Ren J, Nair S. Cathepsin K knockout alleviates pressure overload-induced cardiac hypertrophy. Hypertension. 2013;61:1184–1192. doi: 10.1161/HYPERTENSIONAHA.111.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua Y, Zhang Y, Dolence J, Shi GP, Ren J, Nair S. Cathepsin K knockout mitigates high-fat diet-induced cardiac hypertrophy and contractile dysfunction. Diabetes. 2013;62:498–509. doi: 10.2337/db12-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng XW, Obata K, Kuzuya M, Izawa H, Nakamura K, Asai E, et al. Elastolytic cathepsin induction/activation system exists in myocardium and is upregulated in hypertensive heart failure. Hypertension. 2006;48:979–987. doi: 10.1161/01.HYP.0000242331.99369.2f. [DOI] [PubMed] [Google Scholar]
- 22.Smith ER, Tomlinson LA, Ford ML, McMahon LP, Rajkumar C, Holt SG. Elastin degradation is associated with progressive aortic stiffening and all-cause mortality in predialysis chronic kidney disease. Hypertension. 2012;59:973–978. doi: 10.1161/HYPERTENSIONAHA.111.187807. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Ma L, Yang J, Ren A, Sun Z, Yan G, et al. Increased serum cathepsin S in patients with atherosclerosis and diabetes. Atherosclerosis. 2006;186:411–419. doi: 10.1016/j.atherosclerosis.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 25.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 26.Libby P. Mechanisms of acute coronary syndromes. N Engl J Med. 2013;369:883–884. doi: 10.1056/NEJMc1307806. [DOI] [PubMed] [Google Scholar]
- 27.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Liu Z, Cheng Z, Cheng X. Cysteinyl cathepsins: multifunctional enzymes in cardiovascular disease. Chonnam Med J. 2012;48:77–85. doi: 10.4068/cmj.2012.48.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zografos TA, Haliassos A, Korovesis S, Giazitzoglou E, Serelis J, Katritsis DG. Serum cathepsin levels in coronary artery ectasia. Int J Cardiol. 2010;145:606–607. doi: 10.1016/j.ijcard.2010.08.061. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Li X, Peng D, Tan Z, Liu H, Qing Y, et al. Usefulness of serum cathepsin L as an independent biomarker in patients with coronary heart disease. Am J Cardiol. 2009;103:476–481. doi: 10.1016/j.amjcard.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chatzizisis YS, Baker AB, Sukhova GK, Koskinas KC, Papafaklis MI, Beigel R, et al. Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous caps in pigs. Circulation. 2011;123:621–630. doi: 10.1161/CIRCULATIONAHA.110.970038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Platt MO, Ankeny RF, Shi GP, Weiss D, Vega JD, Taylor WR, et al. Expression of cathepsin K is regulated by shear stress in cultured endothelial cells and is increased in endothelium in human atherosclerosis. Am J Physiol Heart Circ Physiol. 2007;292:H1479–H1486. doi: 10.1152/ajpheart.00954.2006. [DOI] [PubMed] [Google Scholar]
- 33.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, Sasaki T, Cheng XW, Iguchi A, Sato K, Kuzuya M. Statin prevents plaque disruption in apoE-knockout mouse model through pleiotropic effect on acute inflammation. Atherosclerosis. 2009;206:355–361. doi: 10.1016/j.atherosclerosis.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Hermus L, Lefrandt JD, Tio RA, Breek JC, Zeebregts CJ. Carotid plaque formation and serum biomarkers. Atherosclerosis. 2010;213:21–29. doi: 10.1016/j.atherosclerosis.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Geluk CA, Post WJ, Hillege HL, Tio RA, Tijssen JG, van Dijk RB, et al. C-reactive protein and angiographic characteristics of stable and unstable coronary artery disease: data from the prospective PREVEND cohort. Atherosclerosis. 2008;196:372–382. doi: 10.1016/j.atherosclerosis.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Liang J, Liu E, Yu Y, Kitajima S, Koike T, Jin Y, et al. Macrophage metalloelastase accelerates the progression of atherosclerosis in transgenic rabbits. Circulation. 2006;113:1993–2001. doi: 10.1161/CIRCULATIONAHA.105.596031. [DOI] [PubMed] [Google Scholar]
- 38.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 39.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki T, Kuzuya M, Nakamura K, Cheng XW, Hayashi T, Song H, et al. AT1 blockade attenuates atherosclerotic plaque destabilization accompanied by the suppression of cathepsin S activity in apoE-deficient mice. Atherosclerosis. 2010;210:430–437. doi: 10.1016/j.atherosclerosis.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz SM, Galis ZS, Rosenfeld ME, Falk E. Plaque rupture in humans and mice. Arterioscler Thromb Vasc Biol. 2007;27:705–713. doi: 10.1161/01.ATV.0000261709.34878.20. [DOI] [PubMed] [Google Scholar]
- 42.Fukuda D, Shimada K, Tanaka A, Kusuyama T, Yamashita H, Ehara S, et al. Comparison of levels of serum matrix metalloproteinase-9 in patients with acute myocardial infarction versus unstable angina pectoris versus stable angina pectoris. Am J Cardiol. 2006;97:175–180. doi: 10.1016/j.amjcard.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Oörni K, Sneck M, Brömme D, Pentikäinen MO, Lindstedt KA, Mäyränpää M, et al. Cysteine protease cathepsin F is expressed in human atherosclerotic lesions, is secreted by cultured macrophages, and modifies low density lipoprotein particles in vitro. J Biol Chem. 2004;279:34776–34784. doi: 10.1074/jbc.M310814200. [DOI] [PubMed] [Google Scholar]
- 44.Lindstedt L, Lee M, Oörni K, Brömme D, Kovanen PT. Cathepsins F and S block HDL3-induced cholesterol efflux from macrophage foam cells. Biochem Biophys Res Commun. 2003;312:1019–1024. doi: 10.1016/j.bbrc.2003.11.020. [DOI] [PubMed] [Google Scholar]


