Abstract
Multiple studies have implicated a role of maternal autoantibodies reactive against fetal brain proteins specific to autism in the etiology of autism spectrum disorders (ASD). In the current study, we examined the impact of brain-reactive maternal autoantibodies of mothers of children with autism (MAU) on offspring behavior in mice compared to offspring exposed to non-reactive IgG of mothers of typically developing children (MTD). Embryonic offspring were exposed to a single intraventricular injection of MAU or MTD IgG on embryonic day 14. Offspring were allowed to mature to adulthood and were subsequently tested for sociability and stereotypic behaviors using a 3-chambered social approach task, marble burying task, and assessment of spontaneous grooming behaviors in response to a novel environment. Results indicate that MAU offspring display autistic-like stereotypic behavior in both marble burying and spontaneous grooming behaviors. Additionally, small alterations in social approach behavior were also observed in MAU offspring compared to MTD offspring. This report demonstrates for the first time the effects of a single, low dose intraventricular exposure of IgG derived from individual MAU samples on offspring behavior.
Keywords: autism spectrum disorders, maternal antibodies, in utero exposure, immune, mouse behavior
1. Introduction
Autism spectrum disorders (ASD) are a group of neurodevelopmental disorders characterized by difficulties in social communication, social interaction, and the presence of restricted and repetitive interests, behaviors, or activities [1]. Currently, it is estimated that 1 in 88 children in the US are diagnosed with ASD [2]. Despite increasing prevalence rates and awareness of the disorder, the etiology of ASD is unknown. While genetic influences are thought to play a large role in its etiology, evidence suggests that susceptibility to ASD has a greater environmental component than previously believed [3]. One potential non-genetic contributing factor for ASD is immune system dysregulation, which has been frequently described in individuals with ASD as well as their family members [4]. Most notably, mothers of children with ASD have been reported to harbor specific antibodies reactive to fetal brain proteins, which are absent in mothers of children who are typically developing or of children with developmental delays [5].
Under normal conditions, the maternal immune system is uniquely regulated during pregnancy to maintain a pathogen-free, yet non-inflammatory, environment for the developing offspring [6, 7]. While the placenta provides a barrier between the mother and developing fetus, nutrition and certain immune factors are allowed to transfer to the fetus. Among these factors, immunoglobulin G (IgG) antibodies transfer at high concentrations beginning around mid-gestation [8], resulting in circulating IgG levels in the newborn that eventually exceed those in maternal circulation owing to neonatal FC receptor-mediated active transport across the placenta. These IgG antibodies, which are maternal in origin, are known to provide the newborn with a passive defense mechanism against different pathogens and persist for up to six months after birth [9]. Under normal conditions, antibodies are unable to cross the blood-brain barrier (BBB) to access the brain. However, the BBB is permissible during early brain development and thus permits maternal antibodies access to the fetal brain [10]. Several deleterious consequences of in utero maternal antibody transfer have been described for autoimmune diseases such as systemic lupus erythematosus, transient neonatal myasthenia gravis, and others [11]. Therefore, brain-reactive maternal antibodies have the potential to exert substantial effects on the developing embryonic brain through their interaction with target antigens.
Several groups have independently identified a strong association between maternal antibody reactivity towards fetal brain proteins and ASD diagnosis in the child [12-14]. Braunschweig et al. was the first to characterize paired reactivity to fetal brain proteins at 37 kDa and 73 kDa in approximately 12% of mothers of children with ASD [12]. Paired reactivity at 37/73 kDa has since been replicated in a larger sample set [15], and the same pattern of reactivity has been observed in prospectively study using collected blood samples collected mid-gestation in mothers who subsequently gave birth to children with autism [16]. Proteins targeted by these maternal antibodies have only recently been identified, each of which is expressed at significant levels in the human fetal brain and has an established role in neurodevelopment [17].
In order to examine whether these maternal antibodies play a direct role in the pathology of ASD, several animal models have been conducted. Data from both rodent [18, 19] and non-human primate [20, 21] experiments suggest that maternal antibodies reactive to fetal brain proteins do indeed play a role in the pathology of a subset of ASD. While informative, these prior animal studies have relied on passive transfer techniques to examine the effect of autism-specific antibodies on offspring behavior. In the present study, mice were directly exposed in utero to purified maternal IgG samples, either from mothers who possess reactivity to both the 37 kDa and 73 kDa fetal brain antigens or from mothers with no history of autism in their families, via embryonic intraventricular injection in order to model the effects of direct exposure of autism-associated IgG in the developing brain. We hypothesized that direct in utero exposure to autism-associated maternal IgG would produce autistic-like behaviors in offspring mice.
2. Material and methods
2.1. IgG preparation
IgG was purified from blood plasma from two mothers of children with autistic disorder (MAU) and from three mothers of children with typical development (MTD). The MAU samples were collected from a group of mothers previously identified to possess IgG antibody reactivity recognizing the 37 kDa and 73 kDa fetal brain proteins, whereas no MTD samples possess fetal brain IgG reactivity 3 years after the birth of the child, immediately following verified diagnosis [22]. IgG samples were purified from plasma under sterile conditions using protein A/G columns and dialyzed against sterile saline. Absence of bacterial contamination was confirmed using the Limulus Amebocyte Lysate Pyrotell test.
2.2. Animals
Five timed pregnant Swiss Webster females obtained from Charles River were used for this study. Mice were individually housed until subsequent offspring were weaned. From these dams, 40 offspring were generated in our laboratory. Both male and female offspring were used in the behavioral assays in this study. All mice were housed in 6 in. × 14 in. × 5.5 in. cages with ad libitum access to food and water. Animals were kept in rooms with a temperature of 70°F and a 12:12 light:dark cycle with lights on at 7:00 AM. All procedures were in accord with protocols approved by the UC Davis Institutional Animal Care and Use Committee.
2.3. Intraventricular injection
Embryonic offspring were randomly assigned to receive injections from purified MAU or MTD IgG, with littermates receiving the same experimental condition. On gestational day 14 (E14), dams were anesthetized prior to embryonic intraventricular injections. An abdominal incision was made through the skin and muscular layers, temporarily exposing the uterine horns. The embryos were then exposed, and purified human IgG at a concentration of 10 μg in a total volume of 1 μl of artificial cerebrospinal fluid (ACSF) from an individual MAU or MTD mother was injected directly into the cerebral ventricle of each offspring by passing a 33-gauge micropipette through the uterine wall and into the cerebral ventricle of each embryonic offspring. The uterine horns were replaced, and the muscular layer and skin were sutured.
2.4. Behavioral Testing
All offspring were weaned on postnatal day (PD) 25, and were housed with sex-matched littermates with up to 4 animals per cage. Behavioral testing began on PD 45 and was conducted during the light phase between 10:00 AM and 2:00 PM. Prior to each behavioral task offspring were allowed to habituate to the testing room for one hour. All testing was conducted blind for treatment group. Offspring behavior was individually recorded for each mouse by Flip video recorders (Cisco Systems, Irvine, CA, USA) and was manually scored by researchers blind to treatment group. Behavioral apparatuses were wiped down with a diluted ethanol alcohol solution between each testing subject.
2.4.1. Open field
On PD 45, behavioral testing began with open field behavior in order to assess both activity and self-grooming behaviors of the offspring in response to a novel environment. The open field apparatus was constructed out of clear Plexiglas and measured 30 cm × 30 cm × 40 cm. During testing, the outer walls of the apparatus were covered to ensure mice could not see outside of the open field. Offspring were individually tested in the open field for a period of 20 minutes. During the first 10 minutes of the task, locomotor activity was scored as the number of line crossings (all four paws crossing a line). During the last 10 minutes of the task, self-grooming behaviors were recorded as a measure of stereotypic behavior [23]. Grooming behaviors recorded included: latency to first grooming event, number of grooming events, total time spent grooming, average time spent grooming per event, fecal boli count, and the number of rearing, jumping, freezing, and digging behaviors.
2.4.2 Social approach
On PD 47, offspring were individually tested using the Crawley 3-chamber social approach task originally described by Nadler et al. [24], which measures social interaction in offspring. The apparatus was constructed from clear Plexiglas and measured 60 cm × 40 cm × 22 cm, consisting of three evenly divided chambers. Retractable doorways within the two dividing walls, measuring 5 × 8 cm, allowed access to the side chambers. During testing, the outer walls of the apparatus were covered to ensure mice could not see outside the apparatus. Non-experimental mice that were never exposed to the experimental mice, referred to as “strangers,” were habituated to small wire cup enclosures (Galaxy Cup, Spectrum Diversified Designs, Inc.) for 5 minutes per day for 5 days prior to social interaction testing.
Experimental offspring were first placed in the central chamber, with the doorways into the two side chambers closed, for 10 minutes in order to habituate to the central chamber. Following habituation to the central chamber, a sex-matched stranger mouse was randomly assigned to a side chamber and placed inside a small wire cup enclosure, while an empty, identical small wire cup was placed in the remaining side chamber, serving as a novel object. After both stimuli were positioned, the doors were simultaneously opened, allowing the experimental subject to freely explore all three chambers for a period of 10 minutes. Measures taken included time spent in each chamber, time spent sniffing each cup, number of entries to each chamber, the total number of grooming events and total time spent grooming. Normal social interaction in this trial can be interpreted as spending significantly more time with the stranger mouse than with the novel object [25].
2.4.3. Marble bury
On PD 50, mice were tested for repetitive behavior using the marble burying task [26]. Cage preparation involved filling clean mouse cages with 5 cm sawdust bedding, followed by gently overlaying 15 glass marbles equidistant in a 3 × 5 arrangement. Mice were individually tested for 30 minutes, after which the number of marbles buried was recorded. Only marbles covered by 66% or more bedding were counted as buried.
2.5. Statistical analyses
Statistical analyses were performed using SPSS 21.0 software (IBM Corp., Armonk, NY, USA). Data from open field, marble burying, and total grooming behaviors in the social approach task were analyzed with a 2×2 univariate ANOVA (IgG treatment group × offspring sex). To assess sociability in the social approach task, paired t-testing was used to examine whether offspring spent significantly more time with the stranger mouse than with the novel object. For all analyses, significance was set at p < 0.05. Values in all figures are reported as mean ± SEM, and all figures were made using GraphPad Prism (La Jolla, CA, USA).
3. Results
3.1. Locomotor Activity
Activity levels were assessed for 36 mice (14 MTD males, 9 MTD females, 7 MAU males, and 6 MAU females). A univariate ANOVA revealed no significant main effects or interactions based on treatment or offspring sex (p < 0.05).
3.2. Spontaneous grooming behavior
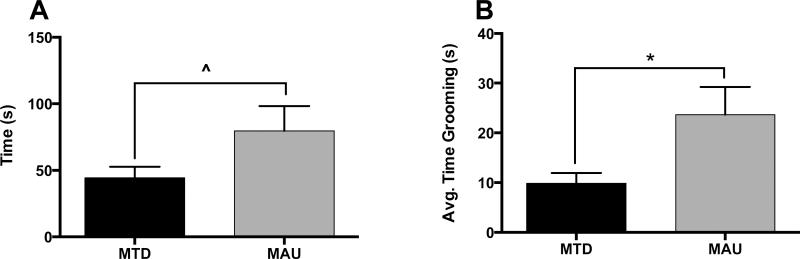
Spontaneous grooming behavior in response to a novel environment was assessed for 37 mice (15 MTD males, 9 MTD females, 7 MAU males, and 6 MAU females). A non-significant trend for treatment was observed for total time spent grooming, with MAU offspring grooming longer than MTD offspring (F1,32 = 3.275, p = 0.0794)(Figure 1A). When analyzing the average time spent grooming per grooming event, MAU offspring were found to groom for significantly longer on average than MTD offspring (F1,32 = 6.835, p = 0.0135)(Figure 1B). No other significant main effects or interactions were observed (p < 0.05).
Figure 1.
Spontaneous grooming behaviors in response to a novel environment in offspring. A) A non-significant trend for treatment was observed for total time spent grooming, with MAU offspring grooming significantly longer than MTD offspring (^p = 0.0794). B) MAU offspring groomed significantly longer on average than MTD offspring (*p = 0.0135).
3.3. Social Approach
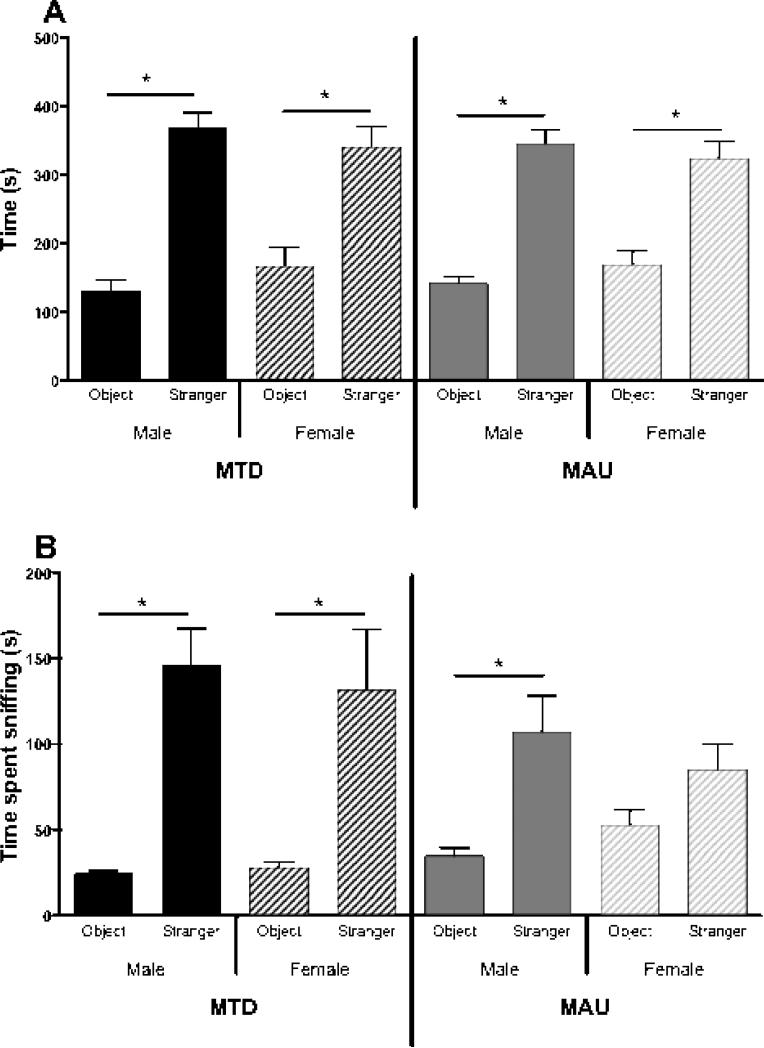
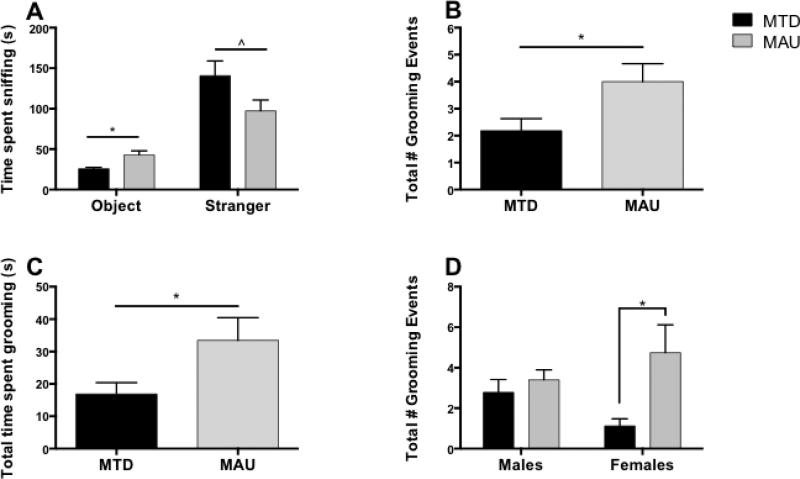
Social interaction levels were assessed for 40 offspring (14 MTD males, 8 MTD females, 10 MAU males, and 8 MAU females). Paired t-testing revealed that all offspring spent significantly more time in the side chamber containing the stranger mouse than in the chamber containing the novel object (p < 0.01), suggesting typical sociability (Figure 2A). However, in analyzing sniff time, female MAU offspring spent a similar amount of time sniffing the novel object and stranger mouse (p = 0.12), whereas all other offspring spent significantly more time sniffing the stranger mouse than the novel object (p < 0.05) (Figure 2B). Furthermore, both male and female MAU offspring spent significantly more time sniffing the novel object than MTD offspring (p = 0.001), and a non-significant trend suggested that MAU offspring spent less time sniffing the stranger mouse than MTD offspring (p = 0.073) (Figure 3A).
Figure 2.
Social interaction behaviors amongst offspring. A) All offspring displayed normal social approach behavior, defined as spending significantly more time in the side chamber containing a stranger mouse than the side chamber containing a novel object (*p < 0.05). B) MAU female offspring sniffed the novel object and stranger for a similar duration (p = 0.12), while all other offspring sniffed the stranger significantly longer (*p < 0.05).
Figure 3.
Extended social interaction behaviors amongst offspring. A) MAU offspring spent more time sniffing the novel object (*p = 0.001) and less time sniffing the stranger mouse (^p = 0.073) than MTD offspring. B) MAU offspring groomed more often than MTD offspring (*p = 0.001). C) MAU offspring spent more time grooming than MTD offspring (*p = 0.026). D) A significant treatment x offspring sex interaction indicated that MAU females groomed more frequently than MTD females (*p = 0.023), but the same effect was not seen in males (*p < 0.05).
Analysis of grooming behavior showed a significant effect of treatment for both total number of grooming events (F1,36 = 7.412, p = 0.010) and total time spent grooming (F1,36 = 5.378, p = 0.026), with MAU offspring grooming significantly more than MTD offspring (Figures 3B and 3C). A non-significant interaction was also seen for total number of grooming events, with MAU offspring grooming more frequently than MTD offspring in females (p = 0.023) but not in males (p = 0.482) (Figure 3D). No other significant main effects or interactions were observed (p < 0.05).
3.4. Marble Bury
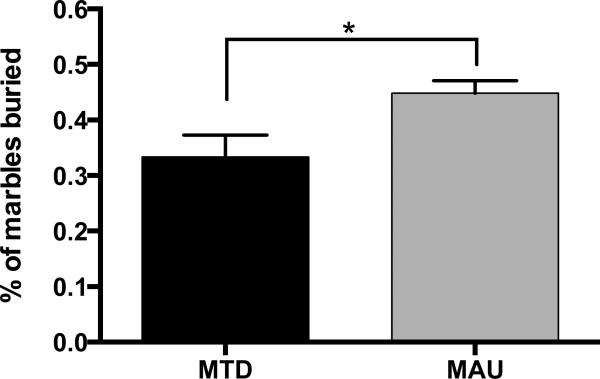
Marble burying behavior was assessed for 25 mice (9 MTD males, 5 MTD females, 6 MAU males, and 5 MAU females). Due to a small sample size, offspring sex was not considered in our analysis. Independent t-testing revealed a significant effect of treatment, with MAU offspring burying significantly more marbles than MTD offspring (t(23) = 2.356, p = 0.027)(Figure 4).
Figure 4.
Marble burying behavior in offspring. MAU offspring buried significantly more marbles than MTD offspring (*p = 0.027).
4. Discussion
The current pilot study describes for the first time the effects of a single intraventricular in utero exposure to purified human IgG of ASD mothers reactive to fetal brain proteins on offspring behavior. Results from both the open field and 3-chamber social approach tasks indicate that offspring exposed to MAU IgG in utero display increased grooming behaviors in response to a novel environment, suggesting autistic-like stereotypical behavior [23, 27]. MAU offspring further displayed autistic-like repetitive behavior in the buried marble task, as MAU offspring buried significantly more marbles than MTD offspring [26, 27]. While MAU offspring did not display autistic-like social interaction deficits in the social approach task, they did display an altered social phenotype in comparison to MTD offspring, as seen in time spent sniffing the novel object and stranger mouse [24, 25, 27]. No significant differences in activity levels were observed in the open field task, supporting the notion that results found in this study were not due to altered locomotor functioning or hyperactivity.
Taken together, these behavioral outcomes support the role of maternal antibodies reactive to fetal brain proteins in the pathology of ASD. Furthermore, the increases in stereotypic behavior observed in the present study recapitulate behaviors of children with maternal autoantibody related (MAR) ASD in the clinical population [17]. Interestingly, of the four animal studies modeling MAR ASD, only one study has been able to produce stereotypic behaviors in MAU offspring [20]. In this study by Martin and colleagues, pregnant rhesus macaques were administered pooled purified IgG from ASD-mothers with reactivity to fetal brain proteins or from mothers of neurotypical children three separate times during their gestation via intravenous injection. Subsequent MAU-exposed offspring displayed only minor alterations in social behavior, but were hyperactive and demonstrated increased whole-body stereotypies compared to controls, similar to findings of the present study [20]. In a separate experiment using rhesus macaques, pregnant females were intravenously injected with purified MAU IgG reactive to 37 and 73 kDa fetal brain proteins or purified MTD IgG six separate times throughout early and mid-gestation [21]. Researchers did not find any alterations in repetitive behaviors, but instead observed that MAU exposed offspring displayed deviant social approach behaviors [21]. Altered social approach behaviors were also seen in adult, but not adolescent, MAU mouse offspring in a 2009 mouse model, in which pregnant female mice were administered six intraperitoneal injections of pooled purified IgG during their gestation [18]. These MAU exposed offspring also demonstrated increased activity and anxiety relative to MTD exposed mice [18]. Finally, a recent mouse model examined the effects of a single exposure to purified IgG, either from ASD-mothers reactive to both 37 and 73 kDa proteins or from MTD-mothers, via tail vein injection on gestational day 12 on offspring behaviors [19]. MAU-exposed offspring did not show any alterations in sociability, but instead had delayed development prior to weaning. Male MAU offspring also demonstrated increases in anxious-like behaviors and a decrease in activity levels compared to MTD male offspring [19]. While MAU exposed offspring did not show stereotypic behaviors in this model, offspring were not tested for these behaviors in response to a novel environment, and instead were only observed in their home cage [19]. Additionally, neither of the mouse models examined the effect of prenatal MAU IgG exposure on marble burying behavior in offspring [18, 19]. The inconsistencies in offspring behaviors amongst the four animal models is most likely results from differences in the route of administration, number of injections administered, gestational timing of injections, animal model used, and most importantly, in the source of IgG used. However, the persistence of ASD-relevant behaviors in MAU-exposed offspring supports the notion that these maternal antibodies play a pathological role in the etiology of MAR ASD.
In the present study, offspring exposed to intraventricular injections of purified human IgG on E14 were subsequently tested for an array of behavioral tasks as adults. Based upon the positive results of this pilot study, expanded future studies utilizing this model will include additional behavioral assays to further assess the behavioral phenotype of exposed offspring. For example, elevated-plus maze or the light-dark box tasks to evaluate potential differences in anxious-like behaviors that have been associated with ASD [27]. Further assessment of social approach and stereotypic behaviors, as well as social communication behaviors would be utilized to further characterize the behavioral phenotype in these offspring. Future studies will utilize a larger number of litters per experimental group to ensure that any potential litter effects on offspring behavior can be assessed. Administration of maternal IgG via embryonic intraventricular injection allows for the creation of a very novel, direct model of MAR ASD. This directed approach would serve as a strong model for observing any physiological or anatomical changes of MAU IgG in the developing offspring brain, as it avoids the issue of differences in neonatal FC receptor functioning between humans and mice that are not addressed in passive transfer studies.
While animal models of autism-specific maternal antibodies suggest a pathological association between these antibodies and ASD, future studies should focus on elucidating the mechanism through which they exert their effects. Future mechanistic-focused studies will allow researchers to determine how the observed behavioral manifestations arise, whether it results as direct interaction between maternal antibodies and their targets in the developing brain, as a consequence of immune activation and inflammation that may be triggered, or a combination of both factors. Additionally, future animal models of maternal antibody-related autism should utilize the recent identification of targeted fetal brain proteins in effort to create a more clinically-relevant model [17]. Furthermore, the ideal animal model of MAR ASD would require the breaking of tolerance to these proteins in females prior to breeding, resulting in dams that produce these circulating antibodies themselves throughout pregnancy. These studies are currently underway. Creation of such a model, as well a better understanding of the underlying mechanism of action of these antibodies, will help researchers develop biological therapeutics for a subset of individuals with ASD in the future.
Highlights.
!! Direct in utero exposure to MAU IgG induced stereotypic behaviors in mice
!! MAU offspring displayed alterations in social interaction behaviors
!! These data support the role of maternal autoantibodies in the pathology of ASD
Acknowledgements
Funding for this project was obtained from the NIMH (R01-MH094681), NIEHS 1 P01 ES11269-01, the U.S. Environmental Protection Agency (U.S.EPA) through the Science to Achieve Results (STAR) program (Grant R829388), NIEHS 1 R01-ES015359, and Shriners Hospitals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.APA . Diagnostic and statistical manual of mental disorders: DSM-V. 5th. American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- 2.Autism, Developmental Disabilities Monitoring Network Surveillance Year Principal I, Centers for Disease C, Prevention: Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1–19. [PubMed] [Google Scholar]
- 3.Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braunschweig D, Van de Water J. Maternal autoantibodies in autism. Arch Neurol. 2012;69(6):693–699. doi: 10.1001/archneurol.2011.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 7.Chaouat G. The Th1/Th2 paradigm: still important in pregnancy? Seminars in immunopathology. 2007;29(2):95–113. doi: 10.1007/s00281-007-0069-0. [DOI] [PubMed] [Google Scholar]
- 8.Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. 1994;1(6):667–669. doi: 10.1128/cdli.1.6.667-669.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heininger U, Desgrandchamps D, Schaad UB. Seroprevalence of Varicella-Zoster virus IgG antibodies in Swiss children during the first 16 months of age. Vaccine. 2006;24(16):3258–3260. doi: 10.1016/j.vaccine.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Frontiers in pharmacology. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox E, Amaral D, Van de Water J. Maternal and fetal antibrain antibodies in development and disease. Dev Neurobiol. 2012;72(10):1327–1334. doi: 10.1002/dneu.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2007;29(2):226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, Pearce DA. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21:351–357. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Brimberg L, Sadiq A, Gregersen PK, Diamond B. Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol Psychiatry. 2013;18(11):1171–1177. doi: 10.1038/mp.2013.101. [DOI] [PubMed] [Google Scholar]
- 15.Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, Hertz-Picciotto I, Van de Water J. Behavioral Correlates of Maternal Antibody Status Among Children with Autism. J Autism Dev Disord. 2011 doi: 10.1007/s10803-011-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, Kharrazi M, Hansen RL, Ashwood P, Van de Water J. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry. 2008;64(7):583–588. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braunschweig D, Krakowiak P, Duncanson P, Boyce R, Hansen RL, Ashwood P, Hertz-Picciotto I, Pessah IN, Van de Water J. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry. 2013;3:e277. doi: 10.1038/tp.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J Neuroimmunol. 2009;211(1-2):39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Braunschweig D, Golub MS, Koenig CM, Qi L, Pessah IN, Van de Water J, Berman RF. Maternal autism-associated IgG antibodies delay development and produce anxiety in a mouse gestational transfer model. J Neuroimmunol. 2012;252(1-2):56–65. doi: 10.1016/j.jneuroim.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauman MD, Iosif AM, Ashwood P, Braunschweig D, Lee A, Schumann CM, Van de Water J, Amaral DG. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Transl Psychiatry. 2013;3:e278. doi: 10.1038/tp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, Van de Water J, Pessah IN. The CHARGE study: An epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7(2):152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 24.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3(5):303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Current protocols in neuroscience / editorial board, Jacqueline N Crawley [et al] 2011 doi: 10.1002/0471142301.ns0826s56. Chapter 8:Unit 8 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204(2):361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11(7):490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]