Abstract
A brain-computer interface (BCI) is a communication system that takes recorded brain signals and translates them into real-time actions, in this case movement of a cursor on a computer screen. This work applied Fitts’ law to the evaluation of performance on a target acquisition task during sensorimotor rhythm-based BCI training. Fitts’ law, which has been used as a predictor of movement time in studies of human movement, was used here to determine the information transfer rate, which was based on target acquisition time and target difficulty. The information transfer rate was used to make comparisons between control modalities and subject groups on the same task. Data were analyzed from eight able-bodied and five motor disabled participants who wore an electrode cap that recorded and translated their electroencephalogram (EEG) signals into computer cursor movements. Direct comparisons were made between able-bodied and disabled subjects and between EEG and joystick cursor control in able-bodied subjects. Fitts’ law aptly described the relationship between movement time and index of difficulty for each task movement direction when evaluated separately and averaged together. This study showed that Fitts’ law can be successfully applied to computer cursor movement controlled by neural signals.
1. Introduction
Brain-computer interfaces (BCIs), which link neural signal-based commands to computer inputs, have advanced significantly over the past decade. A BCI is a communication system that does not depend on the brain’s normal input/output pathways of peripheral nerves and muscles [33]. Instead, communication between a computer and the brain is made by reading and processing neural signals for input control. This has the ultimate promise of providing functionality and independence to individuals with severe motor disabilities by enabling self-modulation of neural signals to control assistive devices such as computers, wheelchairs, or prosthetic limbs, rather than depending on residual muscle control.
Previous studies have demonstrated that humans can operate a computer cursor using neural signals from the scalp (i.e. electroencephalogram or EEG) [35, 33, 15], the surface of the brain (i.e. electrocorticogram or ECoG) [16, 9], or within the brain (i.e. local field potentials and action potentials) [12, 14]. Although the methods vary, cursor control is often accomplished by training the participant to use motor imagery to modulate neural signals [35, 16, 34, 9].
The majority of BCI studies involving the use of motor imagery for computer cursor control have been conducted in the able-bodied population [35, 19, 18]. Some studies have included a disabled participants [15, 18, 19, 27, 35], but more are needed. If the intended users of BCI technology are individuals with motor disabilities, then the ready adaptation of BCIs for this population is uncertain. Although testing BCI technology on able-bodied subjects provides a good starting point, the user-BCI interaction is unlikely to be exactly the same for disabled individuals. Further, even among individuals with disabilities, it is anticipated that the ability to effectively use a BCI will differ based on the duration and type of disability. For example, it is unknown if individuals with different types of motor disabilities engage the traditional neurocognitive networks involved in motor imagery. It is also unknown if plasticity occurs over the course of BCI training which may allow the networks to rewire or strengthen. Other factors such as mental effort, cognitive ability, and attention span will also play a role in whether a BCI implementation is successful for a specific BCI user. Beyond changes in the brain, external factors such as noise from power wheelchairs, artificial ventilators, and other life support equipment may interfere with neural signal recordings and limit the communication bandwidth. This study evaluated a computer-based target acquisition task executed with a BCI system that measured EEG signals from the scalp in both able-bodied and disabled participants. The tasks were based on the Fitts’ law paradigm and subjects used motor imagery to control the computer cursor movement. Subject performance on the cursor movement tasks was evaluated using Fitts’ law to quantify information processing rates.
1.1 Fitts’ Law
General background information on Fitts’ law is presented here as it forms for the basis for the remainder of this article. Fitts’ law, first proposed in 1954 as a model of human psychomotor behavior, has become one of the most widely cited and adopted models from experimental psychology [10]. Fitts’ law is derived from Shannon’s Theorem 17, which was developed to describe information transfer in electronic communication systems [25, 10]. The Fitts’ index of difficulty (ID) describes the relative difficulty of a particular movement used in a task, and is based on the distance (A) from the starting point to the target, and the width (W) of the target (equation 1) such that movement time (MT) is a function of ID (equation 2). The information transfer rate (bits/sec) is calculated by taking the reciprocal of the slope, b, in the regression equation.
| (1) |
| (2) |
Fitts is best known for the reciprocal tapping task, which involved moving a stylus back and forth between two targets and is often referred to as the “Fitts’ task paradigm.” Tasks in the original tapping experiment were carried out mainly with lower arm movements and subjects performed the tasks as quickly and accurately as possible. The average information processing rate for all tasks was around 10–12 bits/sec, which is considered to be the performance capacity of the human motor system [10]. Although Fitts’ law was originally used to describe human limb movement, with particular focus on the hand and arm, it has proved to be a reliable predictor of MT in psychomotor studies involving a variety of limb and muscle groups. There is a consistent high correlation between Fitts’ measure of task difficulty and time required to complete a movement task.
Fitts’ law has previously been used for a variety of computer input devices, including the hand-held mouse, joystick, touchpad, and trackball [2, 8]. Several alternative computer input devices for people with disabilities have also been evaluated, including a head-controlled pointer [23, 13], foot pedal [7], chin stylus [1], and eye tracker [32]. All of these devices rely on substitution of residual muscle groups possessing better control than those which are functionally impaired.
The intent of EEG-based BCI use is to enable control using only motor imagery, without muscle involvement. We hypothesize that Fitts’ law may be an effective tool for comparing BCI subjects, modalities, and tasks. A major difference between Fitts’ law and other tools that have been used for BCI comparison is the emphasis of time over accuracy. In order to perform the Fitts’ analysis, there is an assumption that the subjects are performing at a consistent high level of performance. This will eliminate some subjects from analysis, but data from the ones that remain can be used to focus on time as a measure of performance. In the current study, Fitts’ law was applied to BCI tasks to determine the relationship between target acquisition time and index of difficulty. The Fitts’ law regression line provided information about the task information transfer rate and reaction time for each task, which was then used to make comparisons of subject groups and cursor control modalities.
2. Methods
2.1 Participants
Participants included disabled and able-bodied volunteers. Twelve disabled subjects (7 men and 5 women) aged 27–65 (mean age = 50.4 years) were originally enrolled in this study (table 1). Inclusion criteria included subject participation in more than five one-hour sessions and consistent target acquisition task accuracy exceeding 80%. The data from five subjects was ultimately used for the analysis based on satisfying these criteria. Eight able-bodied participants (5 men and 3 women) aged 19–29 (mean age = 25.7 years), who also participated in another study on EEG-based BCIs, were recruited for this experiment based on participation in more than five one-hour sessions and consistent task accuracy exceeding 90%. Consistent task accuracy is a requirement for applying Fitts law to the data.
Table 1.
Disabled subject demographics.
| # | Description of Disability | Gender | Age | Duration of disability | Speech affected (Y/N)a | > 5 BCI sessions (Y/N) | Fitts’ analysis (Y/N) |
|---|---|---|---|---|---|---|---|
| 1 | Spinal muscular atrophy (type 2) | F | 27 | Since birth | Y | Y | N |
| 2 | Spinal cord injury (level T-10) | M | 51 | 8 years | N | N | N |
| 3 | Locked-in syndrome | M | 59 | 20 years | Y | Y | N |
| 4 | Amyotrophic lateral sclerosis | M | 50 | 18 years | Y | Y | Y |
| 5 | Amyotrophic lateral sclerosis | F | 46 | 19 years | Y | Y | Y |
| 6 | Spinal cord injury (level C4-5) | M | 50 | 9 years | N | Y | N |
| 7 | Post-polio | F | 65 | 51 years | N | N | N |
| 8 | Post-polio | M | 60 | N | Y | Y | |
| 9 | Muscular dystrophy (MD) and post-polio | M | 54 | MD -Since birth Polio - 51 years | Y | Y | Y |
| 10 | Cerebral palsy | M | 47 | Since birth | Y | N | N |
| 11 | Spinal muscular atrophy (type 2/3) | F | 49 | Since birth | N | Y | Y |
| 12 | Spinal muscular atrophy (type 3) | F | 47 | Since birth | N | Y | N |
The degree of speech impairment varied from difficult to understand to no speech at all
All subjects were naïve to the BCI task upon enrollment in the main study, with the exception of one able-bodied subject who participated in electrocorticogram (ECoG) based BCI experiments 1.5 years prior to commencing participation in the EEG experiments presented here. The tasks in the prior experiments were different than those used here, and her performance did not differ significantly from the subjects who were naïve to BCI tasks. All participated with informed consent and this study was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board.
2.2 Experimental Hardware and Software
Participants donned a 16-channel electrode cap (Electro-Cap International Inc., Eaton, OH), which measured and transmitted their EEG signals to a computer via a g.USBamp amplifier (Guger Technologies, Graz, Austria) or a Pentusa amplifier and signal processing system (Tucker-Davis Technologies, Alachua, FL). There was no difference in the data recorded between the two amplifiers. The pure tin electrodes were 9 mm in diameter and the cap configuration followed the standard 10–20 system for electrode placement (see [26] for details).
BCI2000, a general-purpose BCI software package (Wadsworth Center in Albany, New York), was used for these experiments [24]. BCI2000 uses a standardized data format for offline analysis. The code for BCI2000 is open-source, and the program was modified with in-house software routines for the tasks used in this experiment [24].
2.3 Experimental Procedure: Screening and Parameter Selection
A screening procedure was performed during the first experimental session to determine the EEG spectral frequency components of specific electrodes that subjects could self-modulate by using motor imagery. Subjects imagined different types of movements (e.g. clenching their hands, tapping their feet, sticking out their tongue) in response to visual cues presented on a computer screen. These data were processed in the frequency domain using autoregressive spectral analysis and if a high correlation (r2 > 0.3) was observed between the power change and an active response time segment, it was concluded that the subject could use that imagery to self-modulate the specific signal component.
Based on the analysis, 3–5 Hz bandwidths (e.g. 12–15 Hz) for specific electrodes were assigned a preferred direction for cursor movement (e.g. left or right). A specific electrode, frequency range, and weighting factor were used to control each direction of cursor movement (up, down, left, right). During each trial the EEG power spectral content of the chosen signals was measured continuously, and the signal magnitude was the independent variable in a linear equation that controlled cursor motion in real time. For example, the vertical direction of cursor movement is described by equation 3 where: MV = vertical cursor movement in pixels, UV = amplitude for up direction, DV = amplitude for down direction, w = weight, a = gain (slope), and b = mean control signal for the user’s previous performance (intercept) [35].
| (3) |
Once the parameters selected from the screening tasks were established, participants were trained to use motor imagery to control an onscreen target acquisition task with a computer cursor. Subjects were initially instructed to use the imagery that produced the best response during the screening tasks, but they were allowed to experiment to find the imagery that worked best for them.
Based on subject performance and screening results, initial BCI2000 parameters were sometimes changed during the session to achieve stable task performance. Although small changes were made as needed, these were minimized in an attempt to keep parameters relatively constant throughout the sessions.
2.4 Experimental Procedure: Target Acquisition Task
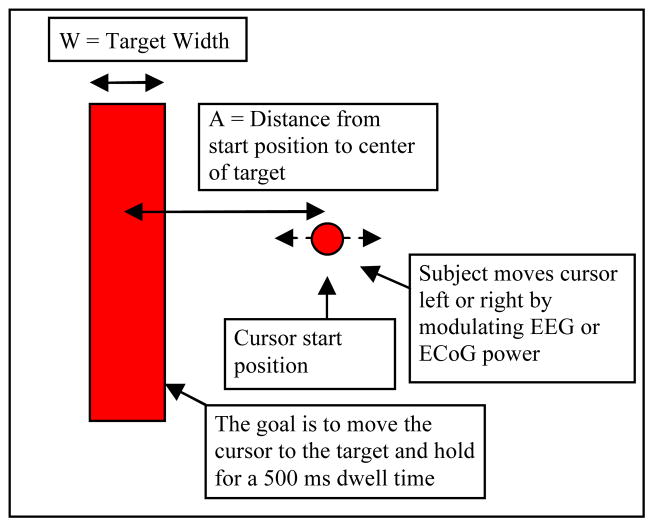
At the start of each trial, a rectangular target appeared on the screen for a particular ID (figure 1). After two seconds, a circular “cursor” appeared at the center of the screen. The subject was instructed to use specific types of imagery to control the cursor movement in each direction. For example, cursor movement in the horizontal direction was typically accomplished by instructing subjects to imagine clenching their left or right hand to make the cursor move left or right, respectively. The instructions were to move the cursor into the target as quickly and accurately as possible and dwell within the target for at least 500 ms. When the center of the cursor moved into the target, the target changed color to indicate the cursor was inside the target area and the subject needed to hold it there. After 500 ms, the target flashed and disappeared to indicate a successful acquisition. The MT for a successful acquisition was the elapsed time from the cursor’s first appearance onscreen until the cursor’s center entered the perimeter of the target. The 500 ms dwell time was not included in the MT, but the dwell was necessary for successful target acquisition. This task varies from traditional BCI target movement tasks where the cursor touches the target, but does not have to dwell, for an acquisition.
Figure 1.
Target acquisition task based on Fitts’ law. The task was to move the circular cursor to the rectangular target. One target is shown, but targets of varying indices of difficulty were presented one at a time on each side of the screen during the task.
If the cursor did not come in contact with the target after 15 s, the trial ended. However, if the cursor came in contact with the target, but did not dwell 500 ms, the timer kept going until the target was acquired or 15 s elapsed without contacting the target. There was a two second rest period between trials, and subjects completed as many trials as possible in sets lasting three minutes.
There were seven IDs for the one-dimension (1-D) tasks: 0.58, 0.74, 1.14, 1.22, 2.0, 2.59, and 3.70 bits. This meant there were 28 possible target positions (i.e. seven each on the top, bottom, left, and right sides of the screen), although the horizontal and vertical dimensions were evaluated separately because cursor movement for each dimension required different types of imagery. For example, in a 1-D horizontal task set, targets would appear only on the left or right side of the screen and cursor movement was constrained to the horizontal (left-right) direction.
After learning how to control both horizontal (left-right) and vertical (up-down) cursor movements, these were put together into a combined dimension task where six of the same seven indices of difficulty (0.58 – 2.59 bits) were evaluated, for a total of 24 possible target positions that appeared randomly in a test set. In this combined dimension task targets were constrained to horizontal and vertical locations and did not involve a true 2-D movement where diagonal targets were introduced. However, unless the subject was able to bring the cursor directly from the start position to the target (by moving it up, down, left, or right), they did have to combine the motor imagery as described below.
Finally, subjects who were successful with the combined tasks (>90% accuracy) advanced to a true two-dimension (2-D) task where the targets could be located diagonally from the cursor start position. Subjects could acquire targets by simultaneously or sequentially performing the imagery attributed to each direction of cursor movement. For example, if a target was located to the bottom-left corner of the screen, and the subject used foot movement imagery for downward cursor movement and left hand clenching imagery for leftward cursor movement, they could either think about the foot and hand movement together or sequentially to acquire the target. The 2-D task had eight targets, but only 2 levels of ID: 1.59 and 1.92 bits.
In addition to the BCI tasks, able-bodied subjects also performed the task with a hand-held joystick (Logitech Attack 3, Logitech Inc.) in order to make a direct task comparison between manual and brain-controlled movement.
2.5 Data Analysis
The mean MT for each ID was calculated separately for each 1-D movement direction (up, down, left, or right) and then averaged across directions. Comparisons were made between disabled and able-bodied subjects who performed the same tasks with both brain and joystick control. Group MT means were calculated and regressed against ID for each condition. The average predicted MT from the regression analysis was used as the dependent variable in repeated measures analysis of variance (ANOVA).
Data from the first session and trials for which parameters had to be adjusted on subsequent days were excluded from analysis. Exclusion of these adjustment trials was necessary because they were typically related to minor changes in the electrode cap position or signal quality, not subject performance. MT greater than three standard deviations from the mean were identified for individual subjects for each ID of each task. These were considered outliers and excluded from the analysis.
3. Results
Five motor disabled participants performed the 1-D task at a level sufficient for evaluation with Fitts law. Eight able-bodied participants performed the 1-D task, four performed the combined dimension task, and two performed the 2-D task. The relationship between ID and MT for 1-D cursor movement is shown in figures 2 and 3 for disabled and able-bodied subjects, respectively. The Fitts’ law regression model averaged across subjects for each 1-D task direction is shown next to each graph. Note that left and right targets were tested separately from up and down targets to constrain cursor movement to one dimension. However, each movement direction was assigned a unique combination of electrode(s), frequency band(s), and weighting factor(s) to control cursor movement. Subjects were instructed to use specific types of imagery for each movement direction.
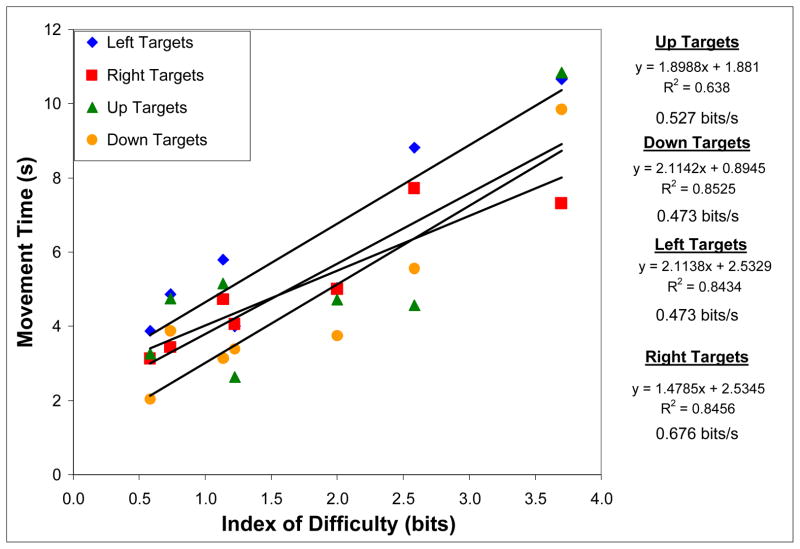
Figure 2.
Relationship between index of difficulty and movement time at four target locations (left, right, top, or bottom of screen) for five motor disabled subjects performing a one-dimensional BCI cursor movement task at >90% accuracy. A linear trend line was fit to each dataset, with the equation, r2, and bit rate shown to the right of the graph.
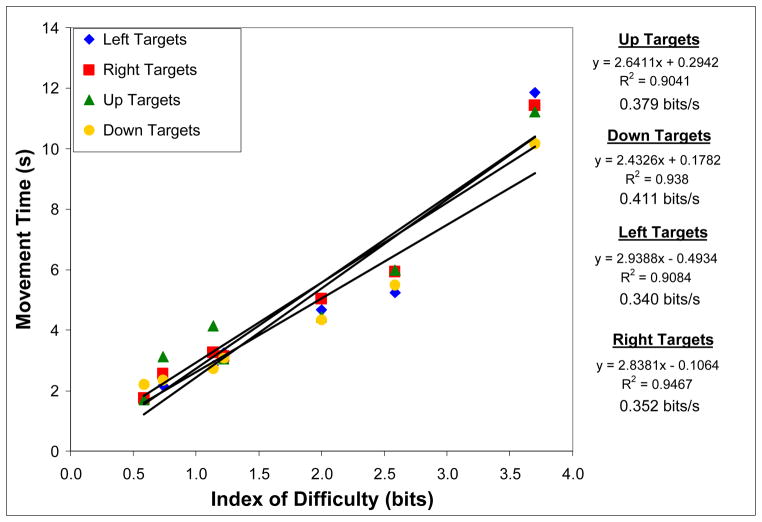
Figure 3.
Relationship between index of difficulty and movement time at four target locations (left, right, top, or bottom of screen) for eight able-bodied subjects performing a one-dimensional BCI cursor movement task at >90% accuracy. A linear regression line was fit to each dataset, with the equation, r2, and bit rate shown to the right of the graph.
In order to test if the Fitts’ law regression lines were the same for each movement direction, a repeated measures ANOVA was performed to compare the predicted means averaged across ID from the least squares regression analysis for each movement direction. There were no significant main effects for 1-D task movement direction in disabled [F(3,1) = 2.705; p=0.414] or able [F(3,5) = 0.635; p=0.624] subjects, or for combined dimension task movement direction in able subjects [F(3,1) = 99.31; p=0.074].
Since no significant differences were found between the Fitts’ law regression lines for each movement direction, the MT at the first six levels of ID was averaged across movement direction and a pooled regression was plotted in figure 5. The first six ID levels were used because the 3.70 bit ID was most commonly missed by subjects in 1-D tasks and furthermore multi-dimensional tasks were carried out only with the first six levels of ID.
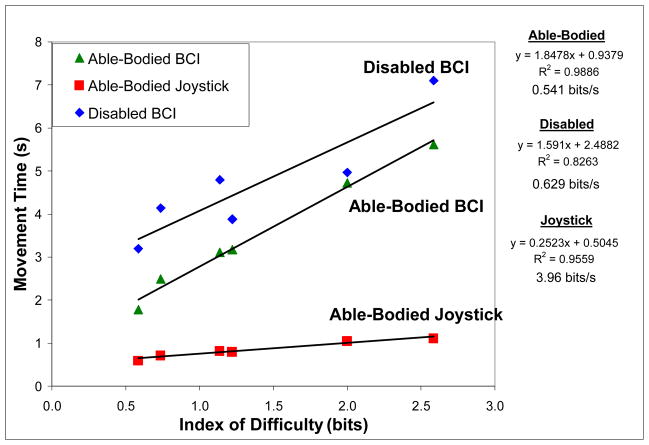
Figure 5.
Relationship between index of difficulty and movement time averaged across target locations for one-dimensional BCI cursor movement tasks in disabled and able-bodied subjects. A manual joystick task performed by able-bodied subjects is also included. A linear trend line was fit to each dataset, with the equation, r2, and bit rate shown to the right of the graph.
After averaging across disabled subjects, the 1-D MT ranged from 3.20 s (SD = 0.84 s) to 7.01 s (SD = 1.14 s) at the lowest and highest IDs, 0.58 and 2.59, respectively. The ID did not significantly affect average MT (for the first 5 levels of ID since there were not enough subjects to do the analysis with 6 levels) [F(4,1) = 2.04, p>0.05] and the linear regression of average MT against ID resulted in r2 = 0.83.
After averaging across able-bodied subjects, 1-D brain-controlled MT ranged from 1.78 s (SD=0.10 s) to 11.21 s (SD=1.54 s) at the lowest and highest IDs, respectively. The ID significantly affected average MT [F(5,3) = 236.76, p<0.001] and the linear regression of average MT against ID resulted in r2 = 0.99.
The joystick MT ranged from 0.60 s (SD=0.11 s) to 1.12 s (SD=0.13 s) at the lowest and highest IDs, respectively. The ID significantly affected average MT [F(5,3) = 54.16, p<0.01] and the linear regression of average MT against ID resulted in r2 = 0.96.
A between groups ANOVA was performed after averaging MT across movement direction. There were statistically significant main effects for group (able-bodied or disabled) [F(1,11) = 21.75; p<0.01] and modality (disabled brain-control and able-bodied joystick control) [F(1,11) = 214.54; p<0.001].
The 2-D BCI task consisting of eight unique targets was performed at >80% accuracy by two subjects. There were only two levels of ID, so a regression line was not fit to the data. Table 2 shows the mean MT to each of the eight target locations averaged across subjects. The mean MT averaged across the up, down, left, and right targets was 4.84 s (SD=1.70 s), while the average MT for the diagonal targets was 7.99 s (SD=2.71 s).
Table 2.
Average movement time for the 2-D brain-controlled task.
| Movement direction | ID (bits) | Na | Average MT (s) (SD) |
|---|---|---|---|
| Up | 1.59 | 2 | 6.42 (2.3) |
| Down | 1.59 | 2 | 4.98 (2.14) |
| Left | 1.59 | 2 | 4.23 (1.29) |
| Right | 1.59 | 2 | 3.73 (0.61) |
| Up/Left | 1.92 | 2 | 8.25 (6.46) |
| Up/Right | 1.92 | 2 | 8.61 (0.01) |
| Down/Left | 1.92 | 2 | 8.43 (0.94) |
| Down/Right | 1.92 | 2 | 6.7 (2.07) |
N = Number of subjects
4. Discussion
One of the important goals of BCI is to benefit individuals with motor disabilities. It is not definitively known, however, if the brains of motor disabled individuals have undergone reorganization precluding the ability to generate appropriate neural signals. This is of particular concern for BCI strategies focusing on motor imagery, as in the current study. Imaging studies have produced conflicting reports of whether individuals with spinal cord injury have normal somatotopic organization of the motor cortex. Shoham et al. (2001) used functional magnetic resonance imaging (fMRI) to study tetraplegic patients (1–5 years post injury) and healthy controls while they executed or attempted limb movement [28]. They found that motor-cortical activation in the SCI patients was similar to normal somatotopic organization in primary and non-primary sensorimotor areas. The authors concluded that even if reorganization does occur following SCI, attempt-related activation is not affected, so sensorimotor signals can be used for BCI control in this population [28]. Turner et al. (2001) also used fMRI to study somatotopy in SCI patients, but came to different conclusions than Shoham et al. [31]. Turner’s study was set up similarly, with SCI, amputee, and able-bodied subjects executing or attempting limb movement while undergoing fMRI. The overall pattern of activity (determined by the blood-oxygen-level dependent response), including pre-motor area activity, was weaker in SCI subjects than the other subjects during limb movement attempts. However, SCI subjects had more activity in medial wall (supplementary motor area) and posterior parietal areas. The posterior parietal activation may be related to the role those areas play in sensorimotor integration and the intention to move. The authors concluded that the medial pre-motor area is a location to consider for BCI use in SCI populations [31].
BCI participants are typically instructed to use cognitive tasks, such as motor imagery, to modulate neural signals. However, there are many ways these motor imagery instructions can be interpreted by subjects. The instruction, “imagine moving your hands” can be understood in any of the following ways: remember or bring to mind feeling of hand moving, visualize own hand moving, visualize another’s hand or abstract hand moving, combine remembered feeling of hand moving with visualization of hand moving, or form intention to move hand (while ensuring that it does not move) [3]. There is also a distinction between first and third person perspective for motor imagery tasks. A first person perspective involves motor-kinesthetic information processing while a third person perspective uses visuo-spatial information processing. Curran and Stokes (2003) discuss evidence, both from sports psychology and anecdotes, that first person imagery is more effective [3]. A study comparing classification accuracy for BCI tasks also found that kinesthetic imagery was better than visual-motor imagery [21]. However, neither of these studies evaluated tasks in disabled subjects. Even if individuals with motor disabilities can generate motor imagery, they may not do it in the same way as able-bodied subjects.
Several studies unrelated to BCI have evaluated motor imagery tasks in individuals with different types of disabilities. Motor imagery has been defined as “a dynamic state during which a subject mentally simulates a given action” [4]. Imagery of movement is thought to involve the same neural processes involved in programming and preparing for real movement. Evidence from neuroimaging, behavioral, and lesion studies has contributed to this theory [22, 5].
Multiple investigators have shown similar changes (i.e. deficits or slowing) in real movement and motor imagery in individuals with different types of brain lesions that cause motor impairment. However, the type and extent of impairment differs based on the lesion type and location. Two related investigations of subjects with motor and parietal cortex lesions found differences in the ability to execute motor imagery. Sirigu et al. (1995) evaluated real and imagined hand movements in a subject with a right motor cortex lesion [29]. Contralesional (i.e. left side) slowing was observed during real and imagined hand movements, and bilateral movements were slowed to the extent of the contralesional hand. A visually guided pointing task based on Fitts’ law was also performed; a linear relationship was found between ID and MT for actual and imagined movement conditions in the left and right hands. However, significant differences were found between left and right hand movement times for both conditions. The authors concluded that while the motor cortex plays a role in the conscious representation of movement, it is not critical for the activation and monitoring of motor images [29].
In a separate study, Sirigu et al. (1996) evaluated the speed of mentally simulated and real hand movements in individuals with and without parietal cortex lesions [30]. Subjects performed or imagined a continuous thumb-fingers opposition sequence by keeping up with a metronome beat, which progressively increased in speed. Subjects reported when the metronome beat was too fast for them to keep up with. This “breaking point” was similar for both real and imagined movements in able-bodied subjects. However, subjects with parietal cortex lesions were either too fast, too slow, or inconsistent from trial to trial during the imagery tasks [30]. Therefore, unlike motor cortex lesions, parietal cortex lesions appear to interfere with the generation of motor imagery [29, 30].
There also may be differences in the ability to produce imagery in individuals who have had their disability since birth, as in cerebral palsy (CP). Spastic CP is generally considered a motor execution disorder. However, studies have shown that individuals with right-sided spastic hemiparetic CP (i.e. left brain damage) also have deficits in anticipatory planning, possibly due to left-hemisphere dominance for motor planning. Based on these prior findings, Mutsaarts et al. (2007) hypothesized that individuals with right hemiparetic CP will have greater impairments in motor imagery than individuals with left hemiparetic CP or able-bodied control subjects [20]. The experimental task involved making laterality judgments based on pictures of hands in different orientations. Reaction time increased linearly as a function of angular rotation of the stimuli for control and left hemiparetic CP subjects. However, right hemiparetic CP subjects did not have an increased reaction time, suggesting a disorder in motor imagery. The authors proposed that this may be related to left-hemisphere dominance for motor planning [20]. Similar findings have been found for other brain lesions, such as stroke [11] and hemi-Parkinson’s disease [6], which may affect only one side of the brain. These findings need to be considered in BCI studies, because if all of the participants with a certain disability have a left sided lesion, that disability type may wrongly be considered ineligible for BCI use.
In the current study, five of the nine disabled subjects participating in at least five sessions successfully performed the BCI tasks by modulating neural signals generated with motor imagery. Fitts’ law aptly described the relationship between movement time and index of difficulty for each 1-D movement direction when evaluated separately and after averaging together. There were no significant differences between the regression lines for the four movement directions even though different combinations of electrodes and frequency bands were used corresponding to different types of motor imagery for each direction. Therefore, not only could these disabled subjects perform motor imagery, but four unique signals could be discerned and used for BCI cursor movement.
The difference between the Fitts’ law regression lines for 1-D brain-controlled cursor movement was statistically significant (p<0.05); the disabled subjects had a similar slope (AB – 1.59 s/bit, D – 1.85 s/bit), but larger y-intercept (AB – 0.94 s, D – 2.49 s). If the y-intercept describes the reaction time to initiate a task [17], based on previous studies demonstrating slowing in motor imagery corresponding to the degree of real movement slowing, it is logical that disabled subjects in this study had an increased y-intercept. The difference in regression lines for joystick control by able-bodied subjects and BCI carried out by both subject groups was statistically significant (p<0.05); the bit rate (inverse slope) for joystick control was also higher than that for brain-controlled tasks (3.96 bits/s compared with 0.54 and 0.63 bits/s for AB and D subjects, respectively). However, for individuals unable to use a mouse, joystick, or other alternative communication device, bit rates on the order of 0.6 bits/s may be acceptable. The fact that EEG-based BCI target acquisition tasks can be carried out by at least some disabled subjects reinforces the notion that even with non-invasive recording methods, appropriate neural signals can be generated to represent four movement directions.
The disabled subjects in this study were not all able to perform the BCI tasks equally well. One contributing factor may be the type and duration of disability, which can impact the ability to effectively execute motor imagery. Ideally, comparisons would be made between sub-groups of disabled subjects, but the small sample in this study had a wide range of disabilities. Even preliminary conclusions cannot be drawn because the results from this study are mixed. Two low performing subjects were affected with their disability since birth and two were affected for a long duration (9+ years). However, two high performing subjects were also affected since birth and the other three were affected for a long duration (18+ years). Further investigation with larger numbers of subjects in each disability category will be necessary to determine disability related factors that affect performance and strategies that can be used to improve performance in each group.
It is also possible that motor disability is unrelated to whether or not an individual can perform BCI tasks. The able-bodied group had a similar percentage of subjects (~50%) who were considered low performers. Many intrinsic factors can be considered, including motivation, cognitive ability, past experience with motor imagery (i.e. related to sports or music), experience with computers and video games, and day-today variation in mood, fatigue, and stress. Age may also play a role. The mean age of the disabled subjects was 50.4 years, compared with 25.7 years for the able-bodied subjects. Factors such as attention span and the ability to learn and adapt to new tasks may decline with age and affect the ability of a user to perform brain-controlled tasks.
This study also has implications for enhancing understanding of brain plasticity and neurocognitive networks involved when motor imagery is used to control external devices. Yue and Cole (1992) demonstrated that motor imagery can lead to increases in strength and improvements in real performance [36]. If there are changes that occur in the brain with BCI training, there may be implications for rehabilitation in people with motor disabilities. For example, if BCI training were to commence shortly after a spinal cord injury or stroke, the continual use of motor imagery may function to strengthen and maintain the neurocognitive networks involved in movement planning and execution. Therefore, it is possible that BCI training can be used as a tool for rehabilitation while at the same time allowing the individual to use their neural signals to substitute for the impaired motor system. Fitts’ law could be used to evaluate short and long-term changes in BCI performance, motor imagery (similar to the Sirigu study [30]), and real movement in individuals with motor disabilities.
This study showed that Fitts’ law can be successfully applied to computer cursor movement tasks using neural signals, which subjects modulated using motor imagery. Fitts’ law aptly described the relationship between movement time and index of difficulty for each 1-D and combined dimension task movement direction when evaluated separately and averaged together. It is notable that even though the imagined movements involved different actions using different parts of the body (e.g. hand clenching and foot tapping), Fitts’ law still aptly described the imagined movements and they were similar across movement directions. Learning to use a motor imagery to perform BCI tasks may not be much different than learning to use motor actions to control handheld devices, such as a joystick or mouse. This observation can be incorporated into the design and evaluation of future BCI studies, including the comparison between non-invasive and invasive modalities.
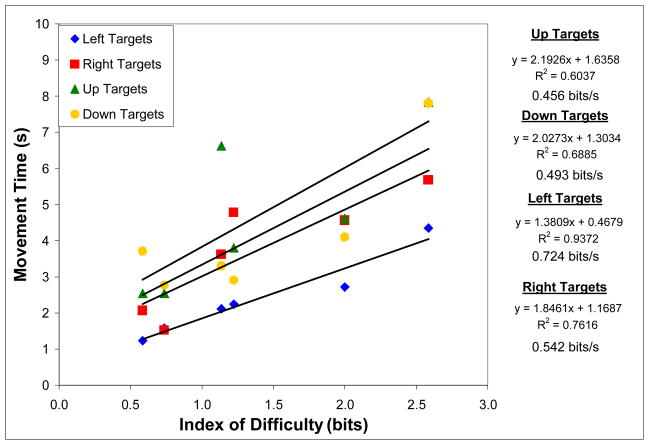
Figure 4.
Relationship between index of difficulty and movement time at four target locations (left, right, top, or bottom of screen) for four able-bodied subjects performing a combined dimension BCI cursor movement task at >90% accuracy. A linear regression line was fit to each dataset, with the equation, r2, and bit rate shown to the right of the graph.
Acknowledgments
This work was supported in part by: National Institute on Disability and Rehabilitation Research (NIDRR), U.S. Department of Education (H133E030012); Clinical Neuroengineering Training Program (NIH T90 DK070079); Training and Education to Advance Multidisciplinary Clinical Research Program (NIH K12 HD049077); and Medical Scientist Training Program. The authors wish to thank Gerwin Schalk from the Wadsworth Center for technical and software assistance; and the study participants for their time, effort, and helpful feedback.
References
- 1.Andres RO, Hartung KJ. Prediction of Head Movement Time Using Fitts Law . Human Factors. 1989;31:703–13. [Google Scholar]
- 2.Card SK, English WK, Burr BJ. Evaluation of Mouse, Rate-Controlled Isometric Joystick, Step Keys, and Text Keys for Text Selection on a CRT . Ergonomics. 1978;21:601–13. [Google Scholar]
- 3.Curran EA, Stokes MJ. Learning to control brain activity: a review of the production and control of EEG components for driving brain-computer interface (BCI) systems . Brain & Cognition. 2003;51:326–36. doi: 10.1016/s0278-2626(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 4.Decety J. The neurophysiological basis of motor imagery . Behavioural Brain Research. 1996;77:45–52. doi: 10.1016/0166-4328(95)00225-1. [DOI] [PubMed] [Google Scholar]
- 5.Decety J, Jeannerod M. Mentally simulated movements in virtual reality: Does Fitts’s law hold in motor imagery? Behavioural Brain Research. 1995;72:127–34. doi: 10.1016/0166-4328(96)00141-6. [DOI] [PubMed] [Google Scholar]
- 6.Dominey P, Decety J, Broussolle E, Chazot G, Jeannerod M. Motor imagery of a lateralized sequential task is asymmetrically slowed in hemi-Parkinson’s patients . Neuropsychologia. 1995;33:727–41. doi: 10.1016/0028-3932(95)00008-q. [DOI] [PubMed] [Google Scholar]
- 7.Drury CG. Application of Fitts Law to Foot-Pedal Design . Human Factors. 1975;17:368–73. [Google Scholar]
- 8.Epps BW. Proceedings of the Human Factors Society - 30th Annual Meeting. Santa Monica, CA: Human Factors Society; 1986. Comparison of Six Cursor Control Devices Based on Fitts’ Law Models; pp. 327–31. [Google Scholar]
- 9.Felton EA, Wilson JA, Williams JC, Garell PC. Electrocorticographically Controlled Brain-Computer Interfaces Using Motor and Sensory Imagery in Patients with Temporary Subdural Electrode Implants: Report of Four Cases . Journal of Neurosurgery. 2007;106:495–500. doi: 10.3171/jns.2007.106.3.495. [DOI] [PubMed] [Google Scholar]
- 10.Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement . Journal of Experimental Psychology. 1954;47:381–91. [PubMed] [Google Scholar]
- 11.Giuliani CA, Purser JL, Light KE, Genova PA. Impairments in arm control in subjects with left and right hemisphere stroke . Neurorehabilitation. 1997;9:71–87. doi: 10.3233/NRE-1997-9107. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia . Nature. 2006;442:164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 13.Jagacinski RJ, Monk DL. Fitts Law in Two Dimensions with Hand and Head Movements . Journal of Motor Behavior. 1985;17:77–95. doi: 10.1080/00222895.1985.10735338. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy PR, Bakay RA, Moore MM, Adams K, Goldwaithe J. Direct control of a computer from the human central nervous system . IEEE Transactions on Rehabilitation Engineering. 2000;8:198–202. doi: 10.1109/86.847815. [DOI] [PubMed] [Google Scholar]
- 15.Kubler A, Nijboer F, Mellinger J, Vaughan TM, Pawelzik H, Schalk G, McFarland DJ, Birbaumer N, Wolpaw JR. Patients with ALS can use sensorimotor rhythms to operate a brain-computer interface . Neurology. 2005;64:1775–7. doi: 10.1212/01.WNL.0000158616.43002.6D. [DOI] [PubMed] [Google Scholar]
- 16.Leuthardt EC, Schalk G, Wolpaw JR, Ojemann JG, Moran DW. A brain-computer interface using electrocorticographic signals in humans . Journal of Neural Engineering. 2004;1:63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- 17.MacKenzie IS. Fitts’ Law as a Research and Design Tool in Human-Computer Interaction . Human-Computer Interaction. 1992;7:91–139. [Google Scholar]
- 18.McFarland DJ, McCane LM, Wolpaw JR. EEG-based communication and control: short-term role of feedback . IEEE Transactions on Rehabilitation Engineering. 1998;6:7–11. doi: 10.1109/86.662615. [DOI] [PubMed] [Google Scholar]
- 19.McFarland DJ, Miner LA, Vaughan TM, Wolpaw JR. Mu and beta rhythm topographies during motor imagery and actual movements . Brain Topography. 2000;12:177–86. doi: 10.1023/a:1023437823106. [DOI] [PubMed] [Google Scholar]
- 20.Mutsaarts M, Steenbergen B, Bekkering H. Impaired motor imagery in right hemiparetic cerebral palsy . Neuropsychologia. 2007;45:853–9. doi: 10.1016/j.neuropsychologia.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Neuper C, Scherer R, Reiner M, Pfurtscheller G. Imagery of motor actions: Differential effects of kinesthetic and visual-motor mode of imagery in single-trial EEG . Cognitive Brain Research. 2005;25:668–77. doi: 10.1016/j.cogbrainres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, Bazzocchi M, diPrampero PE. Primary motor and sensory cortex activation during motor performance and motor imagery: A functional magnetic resonance imaging study . Journal of Neuroscience. 1996;16:7688–98. doi: 10.1523/JNEUROSCI.16-23-07688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radwin RG, Vanderheiden GC, Lin ML. A method for evaluating head-controlled computer input devices using Fitts’ law . Hum Factors. 1990;32:423–38. doi: 10.1177/001872089003200405. [DOI] [PubMed] [Google Scholar]
- 24.Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system . IEEE Transactions on Biomedical Engineering. 2004;51:1034–43. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 25.Shannon CE, Weaver W. The Mathematical Theory of Communication. Urbana: University of Illinois Press; 1964. [Google Scholar]
- 26.Sharbrough F, Chatrian G, Lesser R, Luders H, Nuwer M, Picton T. American Electroencephalographic Society Guidelines for Standard Electrode Position Nomenclature . Journal of Clinical Neurophysiology. 1991;8:200–02. [PubMed] [Google Scholar]
- 27.Sheikh H, McFarland DJ, Sarnacki WA, Wolpaw JR. Electroencephalographic(EEG)-based communication: EEG control versus system performance in humans . Neuroscience Letters. 2003;345:89–92. doi: 10.1016/s0304-3940(03)00470-1. [DOI] [PubMed] [Google Scholar]
- 28.Shoham S, Halgren E, Maynard EM, Normann RA. Motor-cortical activity in tetraplegics . Nature. 2001;413:793. doi: 10.1038/35101651. [DOI] [PubMed] [Google Scholar]
- 29.Sirigu A, Cohen L, Duhamel JR, Pillon B, Dubois B, Agid Y, Pierrotdeseilligny C. Congruent Unilateral Impairments for Real and Imagined Hand Movements . Neuroreport. 1995;6:997–1001. doi: 10.1097/00001756-199505090-00012. [DOI] [PubMed] [Google Scholar]
- 30.Sirigu A, Duhamel J-R. The mental representation of hand movements after parietal cortex damage . Science. 1996;273:1564. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- 31.Turner JA, Lee JS, Martinez O, Medlin AL, Schandler SL, Cohen MJ. Somatotopy of the motor cortex after long-term spinal cord injury or amputation . IEEE Trans Neural Syst Rehabil Eng. 2001;9:154–60. doi: 10.1109/7333.928575. [DOI] [PubMed] [Google Scholar]
- 32.Ware C, Mikaelian HH. Proceedings of the SIGCHI/GI conference on Human factors in computing systems and graphics interface. Toronto, Ontario: Canada ACM Press; 1987. An evaluation of an eye tracker as a device for computer input; pp. 183–8. [Google Scholar]
- 33.Wolpaw JR, Birbaumer N, Heetderks WJ, McFarland DJ, Peckham PH, Schalk G, Donchin E, Quatrano LA, Robinson CJ, Vaughan TM. Brain-computer interface technology: a review of the first international meeting . IEEE Transactions on Rehabilitation Engineering. 2000;8:164–73. doi: 10.1109/tre.2000.847807. [DOI] [PubMed] [Google Scholar]
- 34.Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control . Clinical Neurophysiology. 2002;113:767–91. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 35.Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans . PNAS. 2004:0403504101. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yue G, Cole KJ. Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions . J Neurophysiol. 1992;67:1114–23. doi: 10.1152/jn.1992.67.5.1114. [DOI] [PubMed] [Google Scholar]