Abstract
Cerebral aneurysms treated by traditional endovascular methods using platinum coils have a tendency to be unstable, either due to chronic inflammation, compaction of coils, or growth of the aneurysm. We propose to use alternate filling methods for the treatment of intracranial aneurysms using polyurethane based shape memory polymer (SMP) foams. SMP polyurethane foams were surgically implanted in a porcine aneurysm model to determine biocompatibility, localized thrombogenicity, and their ability to serve as a stable filler material within an aneurysm. The degree of healing was evaluated via gross observation, histopathology and low vacuum scanning electron microscopy (LV-SEM) imaging after zero, thirty and ninety days. Clotting was initiated within the SMP foam at time zero (less than one hour exposure to blood prior to euthanization), partial healing was observed at thirty days, and almost complete healing had occurred at ninety days in vivo, with minimal inflammatory response.
Keywords: aneurysm, endothelialization, embolization, pathology, shape memory polymer
1.0 Introduction
1.1 Cerebral aneurysms and subarachnoid hemorrhage
Aneurysms are geometric structural abnormalities of the vasculature manifested as a bulging or out pouching of the vessel wall. These can occur almost anywhere in the body but are particularly worrisome when they are present in the intracranial circulation. Due to the thinned, weakened wall, aneurysms are at risk of rupture, and in the United States, approximately one in fifty adults has an unruptured aneurysm1. Upon rupture, a subarachnoid hemorrhage (SAH) is said to have occurred, and this can be a severely debilitating or fatal event2. Approximately thirty thousand people per year in the United States suffer SAH from a ruptured cerebral aneurysm1.
1.2 Current treatments and limitations
Current treatment of these arterial abnormalities involves isolation from the normal vasculature, and subsequent stabilization of the vulnerable portion of the artery. Isolation methods involve either surgical clipping or filling of the aneurysm with platinum coils, thereby preventing subsequent rupture. Surgical clipping, although highly effective for treating aneurysms3, involves invasive surgery in the form of a craniotomy. Additionally, for patients who are not viable candidates for surgery or whose aneurysm is in an area of the brain in which surgical access involves increased risk, surgery is either not an option or is not the preferred option1,4,5. For these patients and many treatment centers filling methods are the preferred treatment of aneurysms 6,7.
Filling methods involve endovascular navigation to the site of the aneurysm with the aid of fluoroscopy and intra-arterial injection of iodinated contrast for vessel and aneurysm opacification. The aneurysm is then entered with a microcatheter and filled, reducing the pressure on the aneurysm wall and promoting clotting and healing within the aneurysm. For optimal treatment, the filling material would become incorporated into the aneurysm sac and isolated from the parent vessel by the formation of an endothelial cell layer and neointima proliferation across the neck of the aneurysm. This encasing of the filler and restoration of the parent vessel essentially isolates the aneurysm sac from blood flow, permanently stabilizing the weakened portion of the artery and preventing subsequent rupture.
Various materials are currently used for endovascular treatment of aneurysms. The most common is a soft, wound platinum coil. There are variations of this, including hydrogel-coated coils and poly-glycolic poly-lactic acid coated or impregnated coils. These devices have proven to be clinically successful at filling small aneurysms (< 4 mm in diameter)8. However, these devices could be improved with respect to their biological activity9 and safety of delivery10. Previously it has been demonstrated by Murayama et al. and Szikora et al. that despite the relatively inert nature of platinum, these coils can be an ongoing source of inflammation within the aneurysm after implantation11,12. The latter group reported that after multiple years, bare platinum coils are still not completely endothelialized at the aneurysm and parent artery interface, and therefore, the aneurysm is not optimally stabilized12. Coils have a tendency to have low filling volume as compared to total aneurysm volume8,9,13 and may compact over time14, allowing aneurysm recanalization15–19. This results in re-exposure of the aneurysm wall and subsequent rupture or re-rupture or can potentially result in formation of aneurysms adjacent to the original aneurysm14. Recanalization may require additional treatment, thus adding to the overall cost of treatment and increasing risk to the patient. Other efficacy issues surrounding these coils involve migration of coils into the parent vessel20,21, difficulty in treating wide necked aneurysms4, and potential rupture of the aneurysm during coil delivery10, often with devastating results.
Another material used for endovascular treatment of aneurysms is a liquid ethylene-vinyl copolymer (Onyx HD-500, ev3, Irvine, California). This entails placing a balloon catheter in the parent artery over the neck of the aneurysm, temporarily halting blood flow, while the polymer is injected into the aneurysm via a second microcatheter. The material polymerizes in situ. This technique does not guarantee a seal at the balloon-artery interface and can potentially lead to the occlusion of the parent artery or of distal arteries. Also, complications can arise if the material migrates into the parent artery22,23.
1.3 Proposed treatment of intracranial aneurysms by SMP foams
Previously, polymer coils and foams have been proposed as an alternative filling material for treatment of cerebral aneurysms11,24–27. Polymer coated platinum coils have also been used for aneurysm treatment and have been shown to have favorable results in vivo. However, despite encouraging animal studies9,12, the benefits have not been borne out clinically, likely due to the relatively rapid biodegradability28,29. It has also been shown that polyurethane foams have favorable biocompatibility in vivo for larger aortic aneurysm animal models30. In this research we focus on the biocompatibility of polyurethane based SMP foams as an aneurysm filling material. We sought to observe the tissue response after implantation of a SMP foam device in a swine aneurysm model. The swine vein pouch aneurysm model, reported by Guglielmi et al. in 1994, was chosen due to its relatively low cost, large arteries and veins within the neck and similar fibrinolytic and coagulation system to humans31,32. This model is also significant due to the similarity in the achievable dimensions of aneurysm sacs that can be made during the process of attaching the vein pouch to the artery. Similarities in dimension and blood interactions between the device and the in vivo environment within the swine model, facilitate extrapolation of the pathological data acquired from this study to the potential reaction to the implant within humans 31.
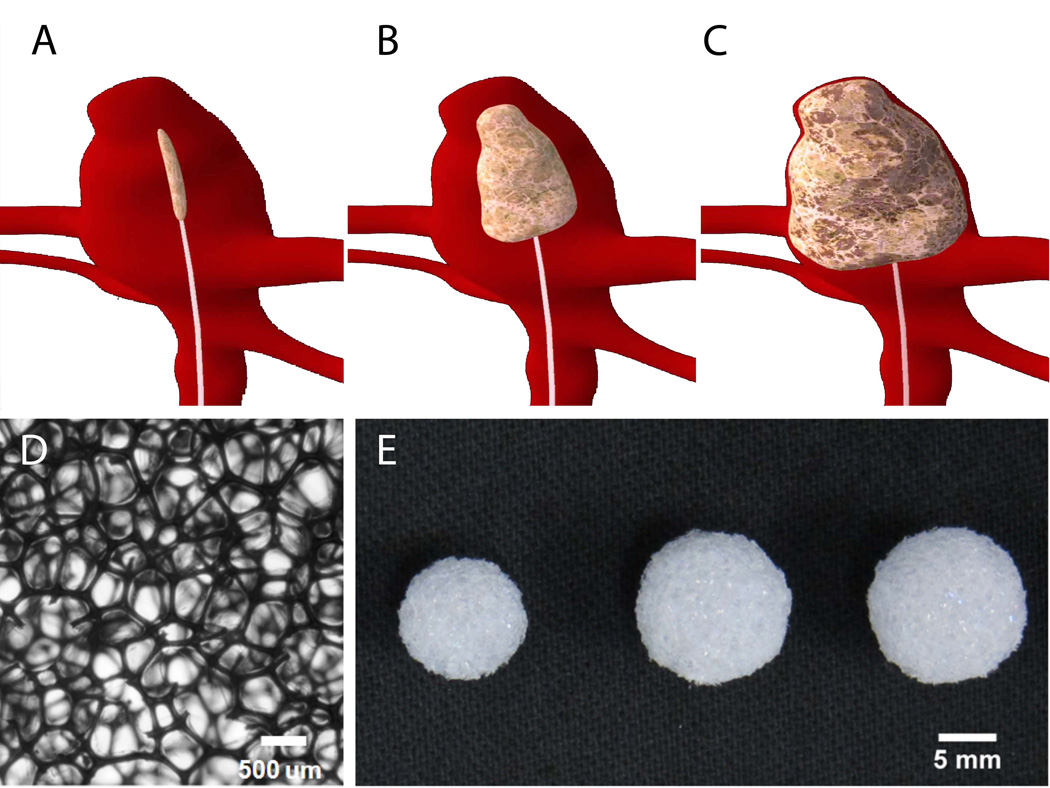
SMPs are materials that have the ability to be made into a primary shape, and upon an increase in the bulk temperature of the material above its transition temperature, can be deformed and programmed into a temporary shape. Programming of these materials occurs when the temperature of the material is brought below its transition temperature while maintaining the deformation, for our device a crimped temporary shape would be desirable (figure 1A). A device made from these materials would remain in this programmed shape until it encounters an additional stimulus that raises the material’s temperature above its transition temperature, at which point it would recover to its primary shape, for our device an expanded shape to fill the aneurysm sac would be desirable (figure 1B-C). This ability to change from one shape to another via the application of a stimulus has made these materials attractive for medical device development25. These polymers possess characteristics similar to other polymeric materials, including the ability to be molded or foamed into an open celled architecture.
Figure 1.
Proposed SMP foam devices for aneurysm filling: A) Crimped (temporary shape) SMP foam device delivered to the aneurysm via catheter, B) Intermediate image of the foam expanding within the aneurysm, as it recovers to its permanent shape via stimuli, C) Treated aneurysm with fully expanded foam, D) A detail of the foam, scale bar is 500 µm, E) Foam devices (scale bar = 5 mm).
Due to these favorable characteristics, we have developed polyurethane based SMP foam for the treatment of intracranial aneurysms33. These low density foams have an expansion force less than that which would pose risk of aneurysm rupture34. Both the foams and neat materials have demonstrated biocompatibility in vitro31,33. Biocompatibility of the foams also has been demonstrated in vivo26. To further verify biocompatibility in vivo, we have implanted these foams in a porcine vein pouch aneurysm model31 for zero (< 1 hour contact with the circulation prior to sacrifice), thirty and ninety days. Gross evaluation, low vacuum scanning electron microscopy (LV-SEM), and histology were performed to assess the tissue response induced by the implanted foams. Inflammation induced by the U.S. Food and Drug Administration (FDA) approved silk and polypropylene sutures (7–0 Prolene™ (Ethicon, Inc., San Angelo, TX)) used to create the experimental aneurysms was also quantified for comparison.
2.0 Materials and Methods
2.1 SMP fabrication
Filling devices were fabricated out of polyurethane SMP foams based on the H60 chemistry reported by Singhal et al.33. An isocyanate pre-polymer was prepared by mixing 38% of the total equivalents of hexamethylene diisocyanate (HDI) (TCI America, Portland, Oregon) with the hydroxyl monomers N,N,N’,N’- tetrakis(hydroxypropyl)ethylenediamine (HPED) (Sigma-Aldrich, St. Louis, Missouri) and triethanolamine (TEA) (Sigma-Aldrich, St. Louis, Missouri) at which a single phase was formed. This pre-polymer was allowed to cure at room temperature for two days prior to foaming. At the point of foam fabrication, the balance of hydroxyl monomers to isocyanate monomers, had a net isocyanate index of 105. These monomers were then added to the pre-polymer. Subsequent addition of catalysts, surfactants, water (chemical blowing agent), and Enovate (Honeywell Inc., Morristown, New Jersey) (Physical blowing agent), were added and mixed to achieve a homogeneous solution33. This solution was then put in the oven at 90 °C where it rose. The foam was then heat cured for twenty minutes in the oven, followed by a room temperature cure for at least two days.
2.2 Device fabrication
2.2.1 Fabrication
Spherical SMP foam devices were fabricated using a scalpel into dimensions ranging from 8 to 12 mm in diameter (Figure 1E). Varying sizes of SMP foam devices were fabricated to allow the vascular surgeon to select the appropriate size to fill the aneurysm.
2.2.2 Cleaning
Removal of surfactants and catalysts from the devices were achieved by cleaning via 0.1 M hydrochloric acid (BDH Chemicals, West Chester, Pennsylvania) and detergent, Contrad® 70 solution (Decon Laboratories, Inc., King of Prussia, Pennsylvania). The devices were initially submerged in 0.1N hydrochloric acid in glass vials, and placed in a sonication bath for two hours. This step was followed by rinsing the samples in deionized water, and changing the acidic solution in the vial to an 80:20 volume %, deionized water- Contrad® 70 solution. They were then placed into the sonication bath for fifteen minutes. The samples were washed multiple times in deionized water to remove residual detergent (removal of detergent was determined by an absence of bubble generation upon shaking). After the samples were free of detergent, they submerged in a vial containing deionized water and placed back into the sonication bath for another fifteen minutes. These steps were repeated once more to ensure thorough cleaning and complete removal of residual mobile species. The cleaned samples were then dried in an oven over night (~twelve hours) at 50°C under vacuum. The dried samples were visually examined under magnification for any deformation or presence of loose struts on the surface, and a scalpel was used to manually trim loose struts and to restore dimensions and spherical shape.
2.2.3 Sterilization
Each device was individually sealed in a sterilization pouch and sterilized by ethylene oxide (EtO), and allowed to de-gas for forty-eight hours prior to implantation.
2.3 Porcine Animal Model
All animal experiments were conducted in accordance with policies set by the Texas A&M University Institutional Animal Care and Use Committee (IACUC), and met all federal requirements, as defined in the Animal Welfare Act (AWA), the Public Health Service Policy (PHS), and the Humane Care and Use of Laboratory Animals. Additionally, NIH guidelines (or for non-U.S. residents similar national regulations) for the care and use of laboratory animals (NIH Publication #85-23 Rev. 1985) were observed. A porcine vein pouch saccular sidewall aneurysm model was utilized in this study31.
Saccular sidewall aneurysms were created in 3–4 month old swine weighing approximately 30–40 kg. Anesthesia was induced via intramuscular injection of ketamine, xylazine, and acepromazine (20 mg/kg, 2 mg/kg, and 0.2 mg/kg, respectively) with intubation and use of isoflurane for maintenance of anesthesia. Using sterile technique a 10 cm incision was made in the midline of neck. After reflecting the sternocleidomastoid muscle medially, a 4 cm long segment of the external jugular vein was isolated and excised after a ligature is placed at each end of the segment. This segment of the vein was then divided transversely, yielding two 2 cm open ended pouches. The carotid arteries were then subsequently exposed and cleaned of adventitia. Vascular clamps were placed at each end of the area of interest on the artery to provide temporary vessel occlusion. A 7 mm arteriotomy was then made, and end-to-side anastomosis of the venous pouch to the carotid artery will be performed using 7–0 Prolene™ (Ethicon, Inc., San Angelo, TX) sutures. An aneurysm between 8–10 mm in diameter was then created on each carotid artery, which resulted in two aneurysms per animal. After confirmation of hemostasis, the subcutaneous tissues were closed.
Angiography was performed to visualize the integrity of the aneurysm and to assess for any premature thrombosis. An SMP device was soaked in 37°C saline and then placed in each aneurysm by opening the top suture and reclosing after proper placement had been achieved. Aneurysm filling and integrity were assessed via angiography after implantation of the SMP devices.
The implanted devices remained in place for zero (less than one hour exposure to blood prior to euthanization), thirty or ninety days. At the end of each implantation time point the animals were sacrificed. Each of the aneurysms and their parent vessels were isolated, harvested, and preserved with formalin for gross and histological evaluation.
2.4 Gross evaluation of healing
The parent vessels were bisected parallel to the direction of blood flow to allow visualization of the aneurysm neck. Each aneurysm was observed at the main artery and graft interface for endothelialization of the lesion en face.
2.5 Microscopic evaluation of healing
LV-SEM imaging was used to determine the degree of endothelialization at the parent vessel and device interface (i.e., across the neck of the aneurysm). Histology was used to determine the amount of healing and inflammation at the dome of the aneurysm. Multiple stains including Haematoxylin & eosin (H&E), Masson’s trichrome and Mallory’s phosphotunsic acid hematoxylin (PTAH) were used.
2.5.1 LV-SEM: Endothelialization
A major component of aneurysm stabilization and healing is endothelialization at the device and parent vessel interface and restoration of the intima of the parent artery across the aneurysm neck. Presence of neointima and progression of the covering were assessed via LV-SEM at each time point.
2.5.2 Histopathology
Multiple stains were used to characterize the healing in and around the aneurysms and to evaluate the degree of inflammation present. The pathology slides were scanned by an Aperio® ScanScope® slide scanner, and the resulting images were evaluated by ImageScope® (Aperio Technologies, Inc., Vista, California), a pathology slide viewing software. Magnifications reported in histology figures are based on these scanned images.
Haematoxylin & Eosin (H&E) is a micro-atomic stain that provides information about the general pathology of a tissue and was used as a general stain to evaluate inflammation and neovascularization. The nuclei of cells are stained blue/black by haematoxylin35. Eosin is an acid dye that stains cytoplasm various shades of red, pink and orange35.
Masson’s trichrome is one of two connective tissue stains that were used to evaluate elastin and fibrin. With this stain, collagen and reticular fibers appear blue/green, cytoplasm and red blood cells red, and the nuclei black/gray35,36.
Mallory’s phosphotunsic acid hematoxylin (PTAH) is the second connective tissue stain used. This stain was used to determine the amount of residual fibrin at the aneurysm dome. PTAH is also used to determine the amount of collagen and elastin that has been deposited. This stain colors nuclei of cells a deep blue, fibrin fibers a lighter blue, collagen reddish, and coarse elastin fibers bluish35.
3.0 Results
3.1 Aneurysm embolization
Based on fluoroscopic imaging performed during implantation and LV-SEM and histological evaluation at zero, thirty and ninety days after implantation, all aneurysms showed complete filling with SMP foam at all time points.
3.2 Gross evaluation of healing
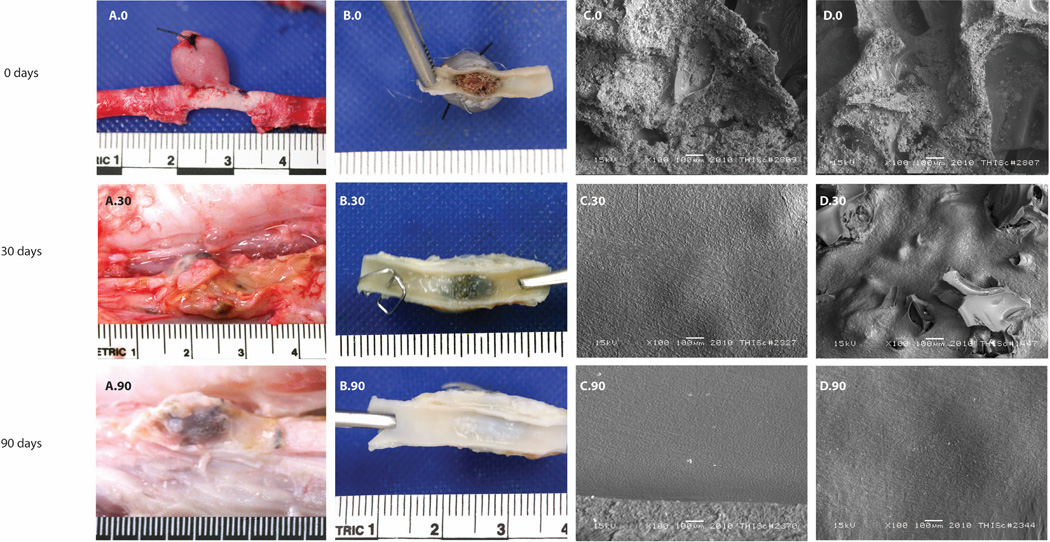
Gross evaluation of aneurysm healing is summarized in column A and B of Figure 2. For zero days, the surgical site was clean as expected, and the polymer had visible thrombus throughout the matrix where it had been in contact with blood flow. For the thirty day implants, the outer surface was composed of a white to slightly opaque dense connective tissue, with tan to golden brown patches. In Figure 2(B.30), the carotid artery was bisected to expose the en face view of the ostium (neck) of the aneurysm sac. The surface of the ostium was white, glistening and appeared to cover the aneurysm neck. However, there appeared to be focal areas where polymer was exposed. For implants that had remained in vivo for ninety days, the external surface of the aneurysms showed multifocal adhesions of dense white connective tissue with tan to golden brown coloration. The carotid artery was bisected to visualize the ostium and it appeared to be white, glistening, intact and with no evidence of exposed polymer.
Figure 2.
Gross and microscopic evaluation of the healing response of the implanted SMP foams: A.0) thirty minute implant of SMP foam in vein pouch model, B.0) en face view of the aneurysm artery interface after thirty minutes in vivo, C.0) LV-SEM shows partial protrusion of foam material into the lumen, D.0) The porous surface in contact with the vessel lumen has patchy aggregates of fibrin-enmeshed erythrocytes attached to the struts of the foam, A.30) Dissected aneurysm in situ after thirty days of implantation. The outer surface of the aneurysm was composed of dense white to slightly opaque connective tissue with patchy tan to golden brown discoloration,B.30) Excised aneurysm with two clips that mark the cranial end. In patchy areas this outer capsule was adherent to adjacent skeletal muscle. The left carotid artery was bisected parallel with the long-axis to visualize the ostium of the left aneurysm sac. The cranial end was labeled with two clips, C.30) Most of the ostium shows an imprint of polymer foam beneath the endothelial covering, D.30) After thirty days of implantation, there is a focal area of disruption and polymer exposed to the lumen, A.90) The right carotid in situ showed an outer surface of artery and aneurysm with multifocal adhesions of dense white connective tissue, B.90) The right carotid artery was bisected parallel with the long-axis to visualize the ostium of the left aneurysm sac. The surface covering the ostium was white, glistening and appeared intact, C.90) The bisected section of artery and en face ostium of aneurysm sac shows an endothelial cell covered surface, D.90) Evidence of proliferating mural thrombosis is not present. The endothelial cell morphology across the ostium is mostly spindle-shaped and parallel with the long axis.
3.3 Microscopic evaluation of healing
3.3.1 LV-SEM: Endothelialization
Figure 2, columns C and D correspond to the middle lower edge of and middle of the en face regions respectively, and summarize the endothelialization and topography at the foam/parent artery interface with subsequent rows indicating the results of the three time points: zero, thirty and ninety days. At zero days the LV-SEM micrographs indicate patchy aggregates of fibrin-enmeshed red blood cells on the surface of the polymer implant. After thirty days there was presence of a discontinuous endothelial layer over the polymer struts. En face images of the ostium at ninety days show an endothelial cell layer comprised of cobblestone patterned and spindle-shaped endothelial cells. These endothelial cells aligned parallel with the direction of blood flow in the parent vessel. The first row in Table 1 summarizes the topographical LV-SEM evaluation of all aneurysms. After ninety days, all of the aneurysms were completely covered by endothelial cells.
Table 1. Four pathological metrics of healing: 1. condition of endothelium covering, 2. residual thrombus, 3. connective tissue, and 4. lumen narrowing.
1. For characterization of endothelium covering, the ideal condition is defined as 100% endothelium coverage of the aneurysm ostium (score = 0). The following conditions are the intermediate gradations between ideal and less than ideal listed in descending order: greater than 95% endothelium coverage (score = 1), 90–95% endothelium coverage (score = 2), 80–-89% endothelium coverage (score = 3). The less than ideal condition is defined as <80% coverage (score = 4) determined by LV-SEM. 2. For characterization of thrombus the ideal condition is defined as 0% of SMP foam surface covered by thrombus (score = 0). The following conditions are the intermediate gradations between Ideal and less than ideal listed in descending order: <1% covered by thrombus (score = 1), 1–4% covered by thrombus (score = 2), 5–10% covered by thrombus (score = 3). Less than ideal was defined as >10% covered by thrombus (score = 4) determined by histology. 3. For characterization of Connective Tissue the ideal condition is defined as the aneurysm being 100% composed of connective tissue (score = 0). The following conditions are the intermediate gradations between ideal and less than ideal listed in descending order: 75% composed of connective tissue (score = 1), 50% composed of connective tissue (score = 2), 25% composed of connective tissue (score = 3). Less than ideal was defined as 0% (score = 4) composed of connective tissue determined by histology. 4. For characterization of lumen narrowing the ideal was defined as a lack of lumen narrowing (score = 0). Intermediate gradations between ideal and less than ideal listed in descending order: <5% lumen narrowing (score = 1), 5–20% lumen narrowing (score = 2), 20–35% lumen narrowing (score = 3). Less than ideal was defined as >35% lumen narrowing (score = 4) determined by LV-SEM and histology.
| 30 days, n = 7 | 90 days, n = 8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1. endothelium covering | 0% | 25% | 50% | 25% | 0% | 0% | 0% | 0% | 0% | 100% |
| 2. residual thrombus | 0% | 0% | 0% | 43% | 57% | 0% | 0% | 0% | 0% | 100% |
| 3. connective tissue | 0% | 57% | 14% | 29% | 0% | 0% | 0% | 0% | 0% | 100% |
| 4. lumen narrowing | 0% | 0% | 29% | 71% | 0% | 0% | 0% | 0% | 100% | 0% |
Row two of Table 1 summarizes the data regarding mural thrombus at the base of the aneurysms evaluated by LV-SEM imaging. There was less than 1% of the surface area of the base of the aneurysm covered with thrombus after thirty days, and no thrombus remaining after ninety days.
3.3.2 Histopathology
3.3.2.1 Connective tissue: Aneurysm composition
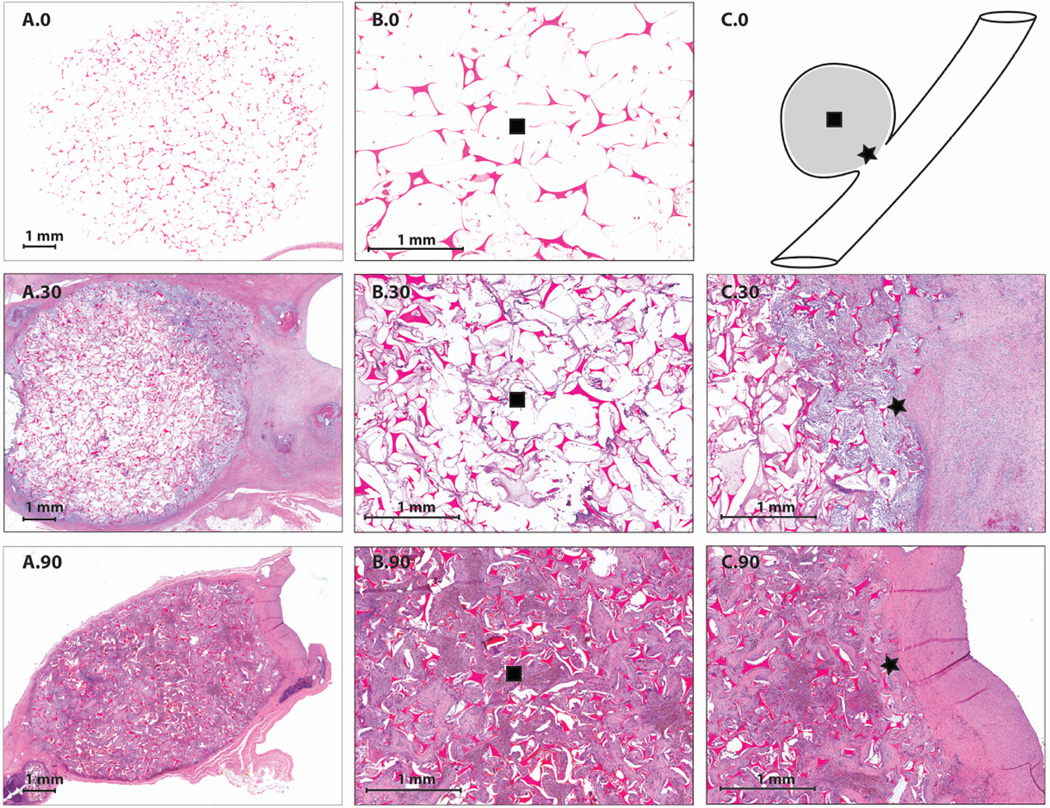
Row three of Table 1 summarizes the composition of the connective tissue of each aneurysm at the three time points, which includes multinucleated giant cells, macrophages, lymphocytes, and focal aggregates of neutrophils. The amount of connective tissues was determined by pathology, in which a cross section of the dome was evaluated. It was shown that at zero days there were no connective tissue present (Figure 3 (A.0 and B.0)). At thirty days there were connective tissues present within the aneurysm dome between 25–75% observed throughout all aneurysms (Figure 3 (A.30, B.30 and C.30)). At ninety days there was 75% connective tissue present throughout the aneurysms (Figure 3 (A.90, B.90 and C.90)).
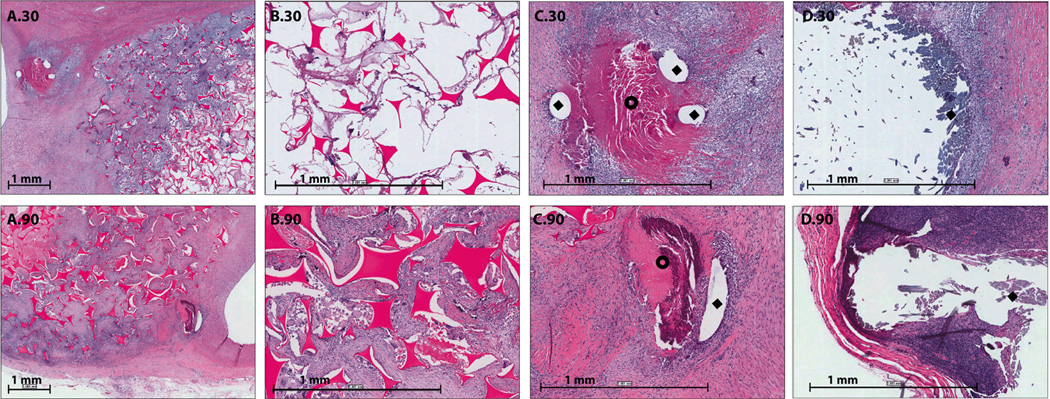
Figure 3.
H&E Staining (1X and 4X magnification): A.0) 1X H&E staining of a bisected aneurysm after thirty minutes of implantation, B.0) 4X H&E staining of the central core of the bisected aneurysm after thirty minutes of implantation, C.0) orientation of SMP foam implant within the aneurysm dome and parent artery, A.30) 1X H&E staining of a bisected aneurysm after thirty days of implantation, B.30) 4X H&E staining of the central core of the bisected aneurysm after thirty days of implantation, C.30) 4X H&E staining of foam and parent artery interface after thirty days of implantation, A.90) 1X H&E staining of a bisected aneurysm after thirty days of implantation, B.90) 4X H&E staining of the central core of the bisected aneurysm after ninety days of implantation, C.90) 4X H&E staining of foam and parent artery interface after ninety days of implantation.
3.3.2.2 Remnant fibrin present in aneurysm
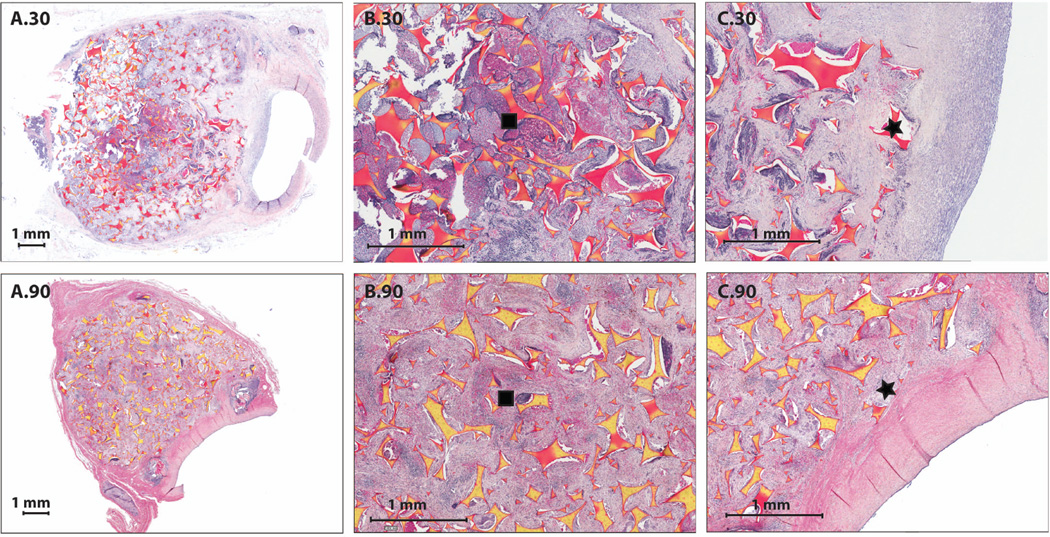
Table S1 summarizes the amount of remnant fibrin, or the amount of fibrin that had not been resorbed or fully degraded, throughout the aneurysm domes at each time point observed via pathology. At the zero day time point (less than one hour exposure to blood prior to euthanization), there was between 6–10% fibrin present. After thirty days of implantation, one of seven aneurysms evaluated was composed of more than 50% fibrin, three of seven between 11–25% fibrin, and the remaining three had 6–10% residual fibrin (Figure 4 (A.30, B.30, C.30)). After ninety days, there was one aneurysm composed of between 11–25% fibrin, with the remaining five being composed of less than 5% fibrin (Figure 4 (A.90, B.90, C.90)).
Figure 4.
PTAH Staining (1X and 4X magnification): A.30) 1X PTAH staining of a bisected aneurysm after thirty days of implantation, B.30) 4X PTAH staining of the central core of the bisected aneurysm after thirty days of implantation, C.30) 4X PTAH staining of foam and parent artery interface after thirty days of implantation, A.90) 1X PTAH staining of a bisected aneurysm after ninety days of implantation, B.90) 4X PTAH staining of the central core of the bisected aneurysm after ninety minutes of implantation, C.90) 4X PTAH staining of foam and parent artery interface after ninety days of implantation.
3.3.2.3 Connective tissue within the aneurysm
The first four rows of Table 2 are a summary of the infiltration of dense cellular connective tissue within the aneurysms; it is expressed in the form of a percentage of infiltration of the aneurysms at each time point throughout the volume of the dome. There was no cellular infiltration at zero days. At thirty days, there was greater than 75% cellular infiltration throughout the aneurysm volume (Figure 5 (A.30, B.30 and C.30)). At ninety days, there was greater than 85% cellular infiltration in all aneurysms (Figure 5 (A.90, B.90 and C.90)).
Table 2. Two metrics of aneurysm composition: 1. Percentage of complete infiltration with dense, cellular connective tissue and 2. The amount of inflammatory cells present determined from histology and location within the aneurysm dome (anastomosis interface; inner core; apex).
1. For dense cellular connective tissue, ideal condition was defined as complete infiltration with dense, cellular connective tissue (score = 0). The following conditions are the intermediate gradations between ideal and less than ideal listed in descending order: >95% of the inner core that has been infiltrated with dense cellular connective tissue (score = 1), 86–95% of the inner core that has been infiltrated with dense cellular connective tissue (score = 2), 75–85% of the inner core that has been infiltrated with dense cellular connective tissue (score = 3). The less than ideal case was defined as <75% of the inner core had been infiltrated with dense cellular connective tissue (score = 4). 2. For inflammatory cells, ideal condition was defined as a lack of inflammation (score = 0). The following conditions are the intermediate gradations between ideal and less than ideal listed in descending order: <5% of the region is infiltrated by inflammatory cells (1 macrophage/lymphocyte per 0.025mm2) (score = 1), between 5–10% of the region is infiltrated by inflammatory cells (2–3 macrophages/lymphocytes or 1 neutrophil per 0.025mm2) (score = 2), between 10–25% of the region is infiltrated by inflammatory cells (4–5 macrophages/lymphocytes or 2 neutrophils per 0.025mm2) (score = 3). The less than ideal case was defined as >25% of the region is infiltrated by inflammatory cells (>6 macrophages/lymphocytes or >3 neutrophils per 0.025mm2) (score = 4).
| 30 days, n = 7 | 90 days, n = 8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Dense cellular connective tissue | ||||||||||
| Anastomosis | 0% | 0% | 43% | 57% | 0% | 0% | 0% | 12.5% | 87.5% | 0% |
| Inner core | 14% | 14% | 43% | 29% | 0% | 0% | 0% | 25% | 75% | 0% |
| Apex | 57% | 0% | 14% | 29% | 0% | 0% | 0% | 12.5% | 87.5% | 0% |
| 2. Inflammatory cells | ||||||||||
| Anastomosis | 0% | 29% | 71% | 0% | 0% | 0% | 0% | 50% | 50% | 0% |
| Inner core | 0% | 0% | 86% | 14% | 0% | 0% | 0% | 0% | 100% | 0% |
| Apex | 0% | 29% | 57% | 14% | 0% | 0% | 0% | 50% | 50% | 0% |
Figure 5.
Trichrome Staining (1X and 4X magnification): A.30) 1X Trichrome staining of a bisected aneurysm after thirty days of implantation, B.30) 4X Trichrome staining of the central core of the bisected aneurysm after thirty days of implantation, C.30) 4X Trichrome staining of foam and parent artery interface after thirty days of implantation, A.90) 1X Trichrome staining of a bisected aneurysm after thirty days of implantation, B.90) 4X Trichrome staining of the central core of the bisected aneurysm after 90 days of implantation, C.90) 4X Trichrome staining of foam and parent artery interface after ninety days of implantation.
3.3.2.4 Inflammation within and around the aneurysm
The second four rows of Table 2 are a summary of the inflammatory response elicited by the foam within the dome of the aneurysms as measured by the average number of inflammatory cells present per area evaluated at 250X or 0.025mm2. Inflammation induced by the presence of foam was evaluated at three locations throughout the volume which included the anastomosis, central core and the apex of the aneurysm. For all areas evaluated at zero days, there was no significant inflammation due to the short in vivo exposure time. At thirty days, most aneurysms exhibited a mild inflammation score for all three areas, which consisted of between 5–10% inflammatory cell infiltrates, which included approximately two to three macrophages or lymphocytes or one neutrophil per area evaluated. At ninety days, most aneurysms exhibited a minimal inflammation score for all three areas, which consisted of between less than 5% inflammatory cell infiltrates, which included approximately one or less macrophage or lymphocytes or one neutrophil per area evaluated.
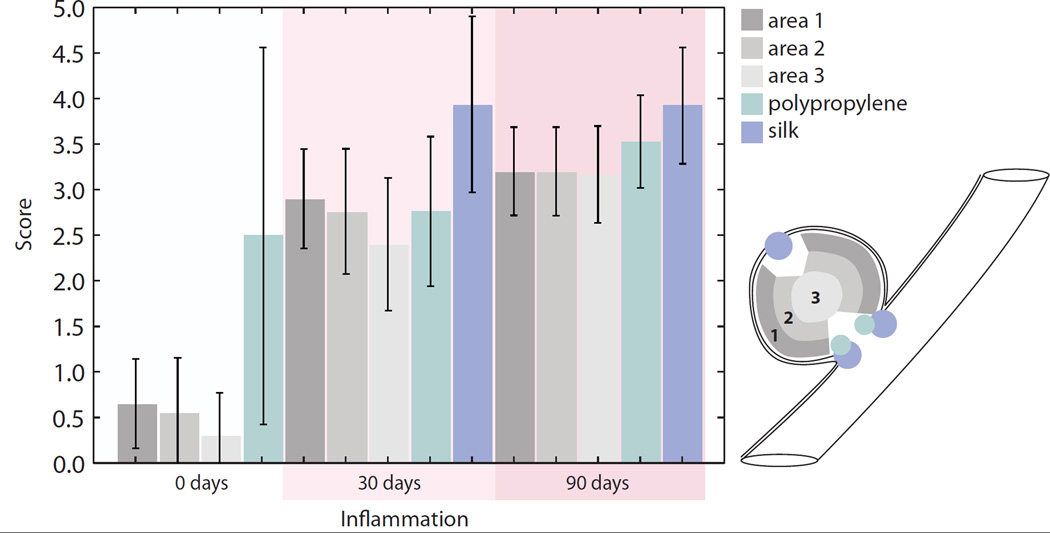
Figure 6 is a summary of not only the inflammatory response elicited by the foam, but also the inflammation at the suture sites at the periphery of the aneurysms. For the foam, at zero days the average score for all areas evaluated was approximately 0.5, which corresponded to zero to two leukocytes per area evaluated. At thirty days the foam had an average score of 2.5, which corresponded to three to eight leukocytes per area evaluated. At ninety days the foam had an average score of 3, which corresponded to five to eight leukocytes per area evaluated. For all time points, and all areas observed, the foam elicited an inflammation response which was slightly higher on the perimeter than within the core.
Figure 6.
Average pathology scoring of inflammation (250X magnification, or 0.025 mm2, error bars indicate standard deviation): indicated by the amount of leukocytes present throughout the aneurysm dome at zero, thirty and ninety days in Areas 1–3 and a comparison of the amount of inflammation elicited by suture materials used to create the aneurysm model. Area 1 represents the perimeter of the aneurysm excluding the areas proximal to sutures. Area 2 represents the area between the periphery and the core. Area 3 represents the core, or middle of the aneurysm dome. A score of 0 indicated a lack of leukocytes present in the area evaluated. A score of 1 indicated minimal or one to two leukocytes per area evaluated. A score of 2 indicated three to four leukocytes per area evaluated. A score of 3 indicated five to eight leukocytes per area evaluated. A score of 4 indicated eight to eleven leukocytes per area evaluated. A score of 5 indicated more than twelve leukocytes per area evaluated. Silk was not visible in the zero day time point.
Suture materials were also evaluated for their inflammation response. At zero days, polypropylene suture material was the only suture material evaluated do to a lack of silk incorporated into the tissue during histology, and there were three to eight leukocytes per area evaluated. At thirty days, polypropylene suture material induced inflammation reflected by presence of four to eight leukocytes per area evaluated. Silk suture at thirty days elicited eight to eleven leukocytes per area evaluated. At ninety days, there were five to eleven leukocytes per area evaluated for polypropylene sutures and eight to eleven leukocytes per area evaluated for silk. Additionally, focal mineralization was observed in relation to the polypropylene sutures after thirty days and around the silk sutures after ninety days.
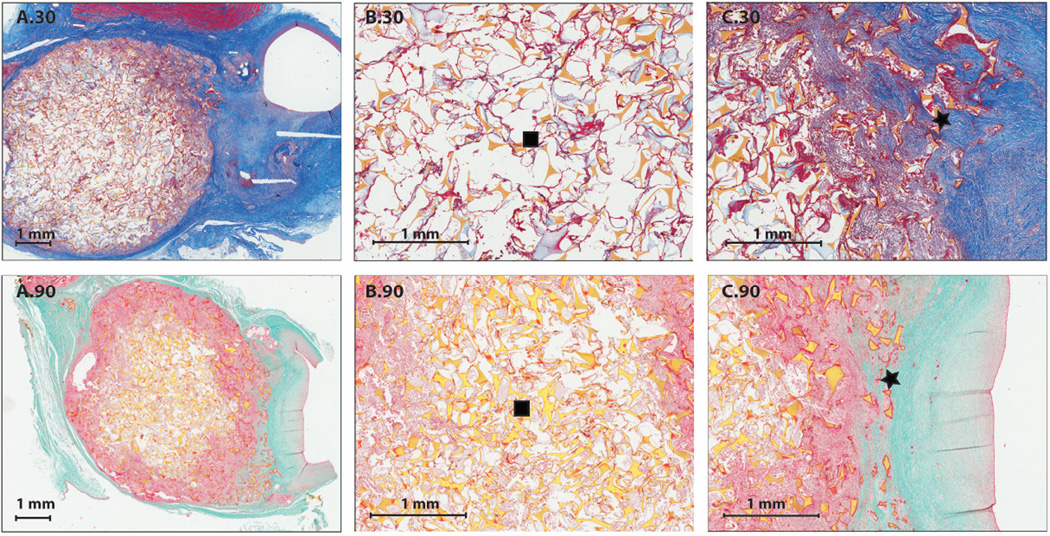
Visual comparison of polymer and suture material inflammatory response can be seen in Figure 7 (A.30, B.30, A.90 and B.90) for thirty and ninety day time points. Inflammation around the suture material was evident (Figure 7 (C.30, D.30, C.90 and D.90)), reflected by an abundance of multinucleated giant cells when compared to the foam (Figure 7 (B.30 and B.90)).
Figure 7.
Suture interaction in vivo (2X and 8X magnification): A.30) 2X H&E staining of a bisected aneurysm exhibiting the foam and suture interaction after thirty days of implantation, B.30) 8X H&E staining of a middle section of a bisected aneurysm after thirty days of implantation showing the foam-body interaction, C.30) 8X H&E staining of suture interaction after thirty days of implantation. Diamond indicates polypropylene suture and doughnut indicates focal mineralization adjacent to the polypropylene suture material, D.30) 8X H&E staining of suture interaction after thirty days of implantation. Diamond indicates silk suture A.90) 2X H&E staining of a bisected aneurysm after ninety days of implantation, B.90) 8X H&E staining of a middle section of a bisected aneurysm after ninety days of implantation showing the foam-body interaction, C.90) 8X H&E staining of suture interaction after ninety days of implantation. Diamond indicates polypropylene suture and doughnut indicates focal mineralization adjacent to the polypropylene suture material, D.90)8X H&E staining of suture interaction after ninety days of implantation. Diamond indicates silk suture.
3.3.2.5 Healing throughout the aneurysm dome based on neovascularization
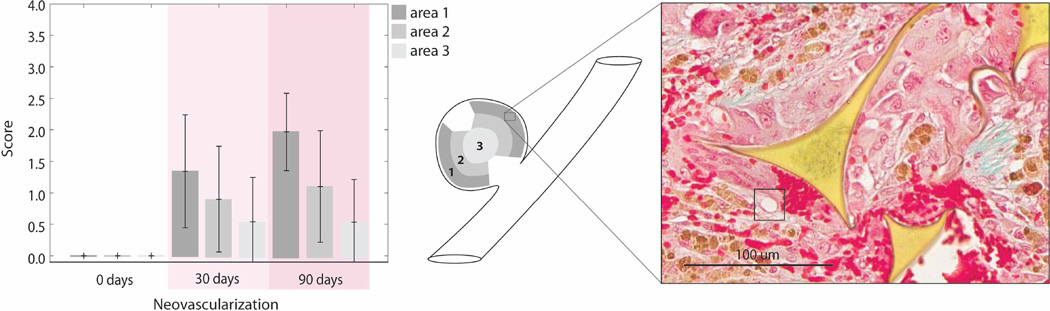
Figure 8 summarizes the amount neovascularization throughout the aneurysm domes by quantifying the amount of neovascularization at three locations from the periphery to the core. It was shown that at zero days there was no neovascularization, at thirty days there was approximately one neovascular bud per area evaluated (Figure 8), and at ninety days there was approximately two neovascular buds per area evaluated.
Figure 8.
Average pathology scoring of neovascularization throughout the aneurysm dome at zero, thirty and ninety days in Areas1-3. (250X magnification, or 0.025 mm2, error bars indicate standard deviation): Area 1 represents the perimeter of the aneurysm excluding the areas proximal to sutures. Area 2 represents the area between the periphery and the core. Area 3 represents the core, or middle of the aneurysm dome. A score of zero indicated no neovascular buds present per area evaluated. A score of one indicated minimal or one neovascular bud per area evaluated. A score of two indicated two to three neovascular buds per area evaluated. A score of three indicated four to five neovascular buds per area evaluated. A score of four indicated more than five neovascular buds per area evaluated. Black square indicates neovascular bud.
3.3.3 Neointima proliferation at the base of the aneurysms
The fourth row of Table 1 summarizes the amount of neointima proliferation at the neck of the aneurysm as determined via LV-SEM and histology. At zero days, there is no narrowing of the parent vessel lumen due to a lack of neointima formation (figure 3 (C.0)). There was approximately 5% narrowing of the parent vessel at thirty days, and for two aneurysms, there was between 5 and 20% narrowing (figure 3 (C.30)). At ninety days there was less than 5% narrowing of the parent vessel for all aneurysms (figure 3 (C.90)).
4.0 Discussion
The goals of this research study were twofold: 1. to assess whether polyurethane based SMP foam is biocompatible in vivo when implanted into a porcine vein pouch aneurysm model and 2. to demonstrate the feasibility of these materials as aneurysm filling devices. These devices were evaluated at three time points, zero, thirty, and ninety days. LV-SEM imaging and histology were performed for all aneurysms. Histology verified that the SMP foams are biocompatible and effective at providing a biological scaffold which seems to enhance the healing response as exhibited by presence of a predominantly connective tissue substrate within the foam at ninety days. Healing was also evidenced by neointima formation across the aneurysm neck, permanently excluding the aneurysm from the parent vessel, without compromise of the parent vessel lumen.
We have also shown using an internal control that the SMP foam has a reduced inflammatory response when compared to conventional suture materials (monofilament polypropylene and braided silk). Karaca, et al. showed that silk and polypropylene suture promote granulomatous inflammatory response with varying severity37. Inflammation noted around these suture materials consisted of a “purulent core surrounded by inflammatory cells and an outer fibrous encapsulation”37. These results are similar to those of Chu, et al. 38, who noted that in less than one month silk and polypropylene elicit a marked and moderate reaction, respectively. When implanted for up to twenty-four months, silk and polypropylene, such as were used during creation of the aneurysms in the model used for this study, elicit moderate and slight reactions respectively, similar to the findings at ninety days in this study38. The fibrous encapsulation of the foreign material is the hallmark of the end of inflammation induced by a material38. This encapsulation isolates the device/material from the surrounding tissues38.
When an implanted material induces a minimal or mild inflammatory response the connective tissue capsule is smaller and may even be nonexistent38. When directly comparing the SMP foam to the braided silk and to some extent the polypropylene sutures, the perimeter of biological tissues surrounding our SMP foam shows a thinner capsule and showed less inflammation than the suture used in this procedure as an internal control (Figure 7). The SMP foam induces less chronic-active inflammatory response when compared to silk. Whether or not an implant causes a sustained inflammatory response is dependent upon the host response to the implanted material38. For implanted devices, the physical and chemical properties of the device should ideally cause a host inflammatory response that is minimal to mild and of short duration, an indicator of biocompatibility. A chronic or chronic-active inflammatory reaction can lead to impairment of the function of the tissue in which the implant is located38. The mild inflammatory response seen with the SMP foam in this study leads to an earlier transition toward healing, evident by the laying down of collagen and elastin throughout the aneurysm and a reduced population of multinucleated giant cells. Transition to healing was also present in the areas where sutures were used, but as compared to the SMP foam, there was a greater cellular inflammatory response.
Granulation tissue (early stage of healing) was present throughout the volume of the SMP foam-filled aneurysm dome. In general, granulation tissue was comprised of collagen, neovascular buds, a small number of macrophages and/or multinucleated giant cells, and, to a lesser extent, eosinophils and neutrophils38, which was seen with these implants. In the later stages of healing, type III collagen is predominant38. This fibrous connective tissue substrate with minimal inflammatory cells is the final transition to the host/biomaterial stable state38.
Neovascular bud infiltration throughout the SMP foam was also evaluated. The presence of neovascularization indicates an active healing process, or the intermediate step prior to full encapsulation. In the current study, by ninety days there was an increased number of neovascular buds. Similar results were reported after six months in a study of polyglycolic acid/lactic acid copolymer coated platinum aneurysm coils (Matrix coils, Boston Scientific Neurovascular, Fremont, California [now Stryker Neurovascular]) 12. These results suggested that polymers could promote the healing process to a greater degree than bare metal coils when implanted in aneurysms12. Another study conducted by Kipshidze, et al., compared a cylindrical reticulated polycarbonate polyurethane based, non-SMP foam (Biomerix, vascular occlusion device, New York, New York), to stainless steel metal coils39. They showed that intravascular implantation of the foams resulted in faster and safer aneurysm occlusion39. Both of these previous studies support the use of polymers to promote healing, achieve occlusion, and stabilize aneurysms in vivo. A Head-to-Head comparison of bare platinum coils to these polyurethane SMP foams with in an aneurysm model would be required in order to make conclusive statements about biocompatibility, which would include aneurysm occlusion, healing and stabilization.
Compaction of coils within a treated aneurysm is a well-documented phenomenon, particularly in the setting of larger aneurysms and lower packing density, and this compaction results in recanalization of the aneurysm, with varying degrees of filling and potential risk of rupture. There was no visual compaction of foam throughout our study, and within ninety days, neointima formed over the neck of the aneurysm, excluding the aneurysm from the circulation. There was also no gross or microscopic evidence of active thrombogenesis at the endothelialized neck, which implies that the foam is stable and not affected by blood flow. Previously, it has been proposed that aneurysm recurrence14 is facilitated by compaction of the coils4 within the aneurysm sac due to the constant impingement by arterial blood flow and/or the lack of complete filling during treatment8. Compaction of metal coils occurs due to the fact that there has not been neointima formation over the coils at the aneurysm neck or tissue ingrowth from the dome and periphery of the aneurysm; this lack of stabilization and healing is most likely due to the lack of biological activity of the platinum coils.
In conclusion, we achieved both goals introduced in the beginning of the discussion. First, we demonstrated that polyurethane based SMP foam is a biocompatible aneurysm filling device when implanted into a porcine vein pouch aneurysm model. Second, these initial pathological results demonstrate the feasibility of these materials as aneurysm filling devices for clinical application. This was concluded based on their biocompatibility demonstrated at ninety day pathological results and complete endothelial cap at the base of the aneurysm. Additionally, the ability of these materials to be compressed to dimensions that allow them to be delivered via catheter also demonstrated feasibility as being developed into a clinical aneurysm filling device. While speculative, we believe that aneurysm filling by polyurethane based SMP foams have the potential to be a superior endovascular aneurysm treatment when compared to bare metal coils based on the positive pathological results demonstrated by this study.
Supplementary Material
Table S1: Summary of residual fibrin remaining in the aneurysm dome determined by histology. Scores were defined as: 0 is an absence of fibrin, 1 is <5% of the field of view contained fibrin, 2 is between 5–10% of the of the field of view contained fibrin, 3 is between 10–25% of the of the field of view contained fibrin, 4 is between 25–50% of the field of view contained fibrin, and 5 is >50% of the field of view contained fibrin.
Acknowledgements
This work was supported by the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering Grant R01EB000462 and partially performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. The authors would like to than Jordan Conway for his assistance in the preparation of the final manuscript.
References
- 1.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):3–9. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 2.Bederson JB, Connolly ES, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE, Harbaugh RE, Patel AB, Rosenwasser RH. Guidelines for the management of aneurysmal subarachnoid hemorrhage. Stroke. 2009;40(3):994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 3.Horowitz M, Samson D, Purdy P. Does electrothrombosis occur immediately after embolization of an aneurysm with Guglielmi detachable coils? American Journal of Neruroradiology. 1997;18(3):510–513. [PMC free article] [PubMed] [Google Scholar]
- 4.Cognard C, Weill A, Spelle L, Piotin M, Castaings L, Rey A, Moret J. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999;212(2):348–356. doi: 10.1148/radiology.212.2.r99jl47348. [DOI] [PubMed] [Google Scholar]
- 5.Johnston SC, Zhao S, Dudley RA, Berman MF, Gress DR. Treatment of unruptured cerebral aneurysms in California. Stroke. 2001;32(3):597–605. doi: 10.1161/01.str.32.3.597. [DOI] [PubMed] [Google Scholar]
- 6.Brinjikji WRAA, Lanzino G, Kallmes DF, Cloft HJ. Effect of age on outcomes of treatment of unruptured cerebral aneurysms: a study of the national inpatient sample 2001-2008. Stroke. 2011;42(5):1320–1324. doi: 10.1161/STROKEAHA.110.607986. [DOI] [PubMed] [Google Scholar]
- 7.Brinjikji WRAA, Lanzino G, Kallmes DF, Cloft HJ. Patient outcomes are better for unruptured cerebral aneurysms treated at centers that preferentially treat with endovascular coiling: a study of the national inpatient sample 2001-2007. American Journal of Neruroradiology. 2011;32(6):1065–1070. doi: 10.3174/ajnr.A2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortega JMD, Wilson T, Tsai W, Savas O, Saloner D. Vascular Dynamics of a Shape Memory Polymer Foam Aneurysm Treatment Technique. Annals of Biomedical Engineering. 2007;35(11):1870–1884. doi: 10.1007/s10439-007-9358-y. [DOI] [PubMed] [Google Scholar]
- 9.Kallmes DF, Fujiwara NH, Yuen D, Dai D, Li ST. A collagen-based coil for embolization of saccular aneurysms in a New Zealand white rabbit model. American Journal of Neuroradiology. 2003;24(4):591–596. [PMC free article] [PubMed] [Google Scholar]
- 10.Doerfler A, Wanke I, Egelhof T, Dietrich U, Asgari S, Stolke D, Forsting M. Aneurysmal rupture during embolization with Guglielmi detachable coils: causes, management, and outcome. American Journal of Neuroradiology. 2001;22(10):1825–1832. [PMC free article] [PubMed] [Google Scholar]
- 11.Murayama Y, Viñuela F, Tateshima S, Song JK, Gonzalez NR, Wallace MP. Bioabsorbable polymeric material coils for embolization of intracranial aneurysms: a preliminary experimental study. Journal of Neurosurgery. 2001;94(3):454–463. doi: 10.3171/jns.2001.94.3.0454. [DOI] [PubMed] [Google Scholar]
- 12.Szikora I, Seifert P, Hanzely Z, Kulcsar Z, Berentei Z, Marosfoi M, Czirjak S, Vajda J, Nyary I. Histopathologic evaluation of aneurysms treated with Guglielmi detachable coils or matrix detachable microcoils. American Journal of Neuroradiology. 2006;27(2):283–288. [PMC free article] [PubMed] [Google Scholar]
- 13.Horowitz M, Purdy P, Kopitnik T, Dutton K, Samson D. Aneurysm retreatment after Guglielmi detachable coil and nondetachable coil embolization: report of nine cases and review of the literature. Neurosurgery. 1999;44(4):712–719. doi: 10.1097/00006123-199904000-00013. discussion 719-20. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa M, Murayama Y, Duckwiler GR, Gobin YP, Guglielmi G, Viñuela F. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. Journal of Neurosurgery. 2000;93(4):561–568. doi: 10.3171/jns.2000.93.4.0561. [DOI] [PubMed] [Google Scholar]
- 15.Gunnarsson T, Klurfan P, terBrugge KG, Willinsky RA. Treatment of intracranial aneurysms with hydrogel coated expandable coils. Canadian Journal of Neurological Science. 2007;34(1):38–46. doi: 10.1017/s0317167100018710. [DOI] [PubMed] [Google Scholar]
- 16.Findlay JMD. T. E. Endovascular management of cerebral aneurysms: work in progress. Canadian Journal of Neurological Science. 2007;34(1):1–2. doi: 10.1017/s0317167100005709. [DOI] [PubMed] [Google Scholar]
- 17.Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, Martin N, ViÃuela F. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. Journal of Neurosurgery. 2003;98(5):959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- 18.Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, Lamoureux J, Chagnon M, Roy D. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34(6):1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 19.van der Schaaf I, Algra A, Wermer MJ, Molyneux A, Clarke M, van Gijn J, Rinkel G. Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid hemorrhage. Stroke. 2006;37(2):572. [Google Scholar]
- 20.Macdonald RL. Evidence-based treatment of subarachnoid hemorrhage: current status and future possibilities. Clinical Neurosurgery. 2006;53:257–266. [PubMed] [Google Scholar]
- 21.Wang CXX. Treatment of an unraveled intracerebral coil. Catheterization and Cardiovascular Interventions. 2010;76(5):746–750. doi: 10.1002/ccd.22643. [DOI] [PubMed] [Google Scholar]
- 22.Dalyai RTRC, Ghobrial G, Gonzalez LF, Tjoumakaris SI, Dumont AS, Rosenwasser RH, Jabbour P. Redefining Onyx HD 500 in the flow diversion era. International Journal of Vascular Medicine. 2012:9. doi: 10.1155/2012/435490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molyneux AJ, Cekirge S, Saatci I, Gal G. Cerebral Aneurysm Multicenter European Onyx (CAMEO) Trial: Results of a prospective observational study in 20 European centers. American Journal of Neuroradiology. 2004;25(1):39–51. [PMC free article] [PubMed] [Google Scholar]
- 24.Metcalfe A, Desfaits A-C, Salazkin I, Yahia L, Sokolowski WM, Raymond J. Cold hibernated elastic memory foams for endovascular interventions. Biomaterials. 2003;24(3):491–497. doi: 10.1016/s0142-9612(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 25.Hampikian JM, Heaton BC, Tong FC, Zhang Z, Wong CP. Mechanical and radiographic properties of a shape memory polymer composite for intracranial aneurysm coils. Materials Science and Engineering: C. 2006;26(8):1373–1379. [Google Scholar]
- 26.Rodriguez JN, Yu YJ, Miller MW, Wilson TS, Hartman J, Clubb FJ, Gentry B, Maitland DJ. Opacification of shape memory polymer foam designed for treatment of intracranial aneurysms. Annals of Biomedical Engineering. 2012;40(4):883–897. doi: 10.1007/s10439-011-0468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortega J, Maitland D, Wilson T, Tsai W, Savas O, Saloner D. Vascular dynamics of a shape memory polymer foam aneurysm treatment technique. Annals of Biomedical Engineering. 2007;35(11):1870–1884. doi: 10.1007/s10439-007-9358-y. [DOI] [PubMed] [Google Scholar]
- 28.Pierot LCC, Ricolfi F, Anxionnat R. Mid-term anatomic results after endovascular treatment of ruptured intracranial aneurysms with Guglielmi detachable coils and Matrix coils: analysis of the CLARITY series. American Journal of Neuroradiology. 2012;33(3):469–473. doi: 10.3174/ajnr.A2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coley S, Sneade M, Clarke A, Mehta Z, Kallmes D, Cekirge S, Saatci I, Roy D, Molyneux A. Cerecyte coil trial: Procedural safety and clinical outcomes in patients with ruptured and unruptured intracranial aneurysms. American Journal of Neuroradiology. 2012;33(3):474–480. doi: 10.3174/ajnr.A2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhee JY, Trocciola SM, Dayal R, Lin S, Chaer R, Kumar N, Mousa A, Bernheim J, Christos P, Prince M, et al. Treatment of type II endoleaks with a novel polyurethane thrombogenic foam: Induction of endoleak thrombosis and elimination of intra-aneurysmal pressure in the canine model. Journal of Vascular Surgery. 2005;42(2):321–328. doi: 10.1016/j.jvs.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 31.Guglielmi G, Ji C, Massoud TF, Kurata A, Lownie SP, F. Viñuela F, Robert J. Experimental saccular aneurysms. II. A new model in swine. Neuroradiology. 1994;36(7):547–550. doi: 10.1007/BF00593518. [DOI] [PubMed] [Google Scholar]
- 32.Osterman FAJ, Bell WR, Montali RJ, Novak GR, White RIJ. Natural history of autologous blood clot embolization in swine. Investigative Radiology. 1976;11(4):267–276. doi: 10.1097/00004424-197607000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Singhal P, Rodriguez JN, Small W, Eagleston S, Van de Water J, Maitland DJ, Wilson TS. Ultra low density and highly crosslinked biocompatible shape memory polyurethane foams. Journal of Polymer Science, Part B: Polymer Physics. 2012;50(10):724–737. doi: 10.1002/polb.23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang W, Volk BL, Akberali F, Singhal P, Criscione JC, Maitland DJ. Estimation of aneurysm wall stresses created by treatment with a shape memory polymer foam device. Biomechanics and Modeling in Mechanobiology. 2011:1–15. doi: 10.1007/s10237-011-0345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown GG, Carnes WH. Primer of Histopathologic Technique. New York: Appleton-Century-Crofts; 1969. p. 224. [Google Scholar]
- 36.Putt FA. Manual of histopathological staining methods. New York: John Wiley & Sons; 1972. p. 335. [Google Scholar]
- 37.Karaca E, Hockenberger AS, Yildiz H. Investigating changes in mechanical properties and tissue reaction of silk, polyester, polyamide, and polypropylene sutures in vivo. Textile Research Journal. 2005;75(4):297–303. [Google Scholar]
- 38.Chu CC, von Fraunhofer JA, Greisler HP. Wound closure biomaterials and devices. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- 39.Kipshidze N, Sadzaglishvili K, Panarella M, Elias A, Rivera MHS, Virmani R, Leon MB. Evaluation of a novel endoluminal vascular occlusion device in a porcine model: early and late follow-up. Journal of Endovascular Therapy. 2005;12(4):486–494. doi: 10.1583/05-1543.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Summary of residual fibrin remaining in the aneurysm dome determined by histology. Scores were defined as: 0 is an absence of fibrin, 1 is <5% of the field of view contained fibrin, 2 is between 5–10% of the of the field of view contained fibrin, 3 is between 10–25% of the of the field of view contained fibrin, 4 is between 25–50% of the field of view contained fibrin, and 5 is >50% of the field of view contained fibrin.