Abstract
Objective
We sought to identify the major risk factors associated with mortality in Roux-en-Y gastric bypass (RYGB) surgery.
Background
Bariatric surgery has become an established treatment for extreme obesity. Bariatric surgery mortality has steadily declined with current rates of less than 0.5%. However, significant variation in the mortality rates has been reported for specific patient cohorts and among bariatric centers.
Methods
Clinical outcome data from 185,315 bariatric surgery patients from the Bariatric Outcome Longitudinal Database were reviewed. Of these, 157,559 patients had either documented 30 or more day follow-up data, including mortality. Multiple demographic, socioeconomic, and clinical factors were analyzed by univariate analysis for their association with 30-day mortality after gastric bypass. Variables found to be significant were entered into a multiple logistic regression model to identify factors independently associated with 30-day mortality. On the basis of these results, a RYGB mortality risk score was developed.
Results
The overall 30-day mortality rate for the entire bariatric surgery cohort was 0.1%. Of the 81,751 RYGB patients, the mortality rate was 0.15%. Factors significantly associated with 30-day gastric bypass mortality included increasing body mass index (BMI) (P < 0.0001), increasing age (P < 0.005), male gender (P < 0.001), pulmonary hypertension (P < 0.0001), congestive heart failure (P = 0.0008), and liver disease (P = 0.038). When the RYGB risk score was applied, a significant trend (P < 0.0001) between increasing risk score and mortality rate is found.
Conclusions
Increasing BMI, increasing age, male gender, pulmonary hypertension, congestive heart failure, and liver disease are risk factors for 30-day mortality after RYGB. The RYGB risk score can be used to determine patients at greater risk for mortality after RYGB surgery.
Keywords: bariatric surgery, mortality, risk factors
Bariatric surgery is now widely accepted as an effective treatment for the adverse health and quality-of-life issues caused by extreme obesity. The steady improvement in bariatric surgery outcomes since these procedures became accepted therapy in 1991 has been well documented. In 1991, accepted mortality rates for open bariatric surgery were 0.5% to 1.5%.1 Recent reports from established bariatric treatment centers and a variety of clinical and administrative registry reviews confirm mortality rates of less than 0.5% for bariatric surgery.2–5 The improved safety of these procedures is likely due to the contribution of a variety of factors such as the use of advanced laparoscopic techniques, the formalization of minimally invasive surgery training, the credentialing process for bariatric surgeons, and the better integration of medical and surgical care in comprehensive treatment programs.
However, despite the favorable improvement in overall surgical mortality rates, significant variations in outcomes exist among individual reporting centers and less favorable outcomes have been documented in patient with higher body mass index (BMI),6 older age,7 Medicare insurance coverage,8 and other comorbid conditions.9 These reports underscore the need to identify risk factors associated with surgical mortality. Development of a risk stratification system would allow for comparison of outcomes among centers and could serve as a tool for measuring quality by comparing observed to expected outcomes for comparable patient subsets.
Previously, the Obesity Surgery Mortality Risk Score emerged as a useful tool for simple identification of patients who may be at increased risk for postoperative mortality.10 DeMaria et al11 developed and validated the risk score in 2007, from a multivariate analysis of a single institution experience with 2075 gastric bypass patients and 31 fatalities with a 30-day mortality rate of 1.5%. The purpose of this study was to take advantage of a robust, current clinical bariatric surgery registry derived from the experience of high-volume credentialed bariatric centers of excellence to investigate patient factors, which impact surgical risk in bariatric surgery. The goal was to define overall 30-day mortality rate in current bariatric surgery practice, to perform multivariate analysis of risk factors for mortality in Roux-en-Y gastric bypass (RYGB) surgery, and to create a simple clinical scoring system to predict 30-day mortality.
METHODS
Study Population
This study is based on clinical outcome data from the Bariatric Outcomes Longitudinal Database (BOLD),12 a large clinical registry devoted entirely to the metabolic and bariatric surgery specialty. This registry contained data entered prospectively by University and Community bariatric centers throughout the country that participated in the American Society for Metabolic and Bariatric Surgery Bariatric Surgery Center of Excellence (BSCOE) program. Clinical data were derived from the medical record including preoperative assessment data, perioperative process, and care variables, as well as later outcomes.
Primary BOLD data were generally collected in medical charts (paper or electronic) by a health care provider. Each surgical practice designated individuals, many of whom may be involved in the care of patients, who were responsible for ensuring high-quality data entry into BOLD. This assignment of data entry responsibility varied across bariatric programs. Training was available to assist with data entry and promote consistent reporting of outcomes. A portion of the data was verified by on-site inspection of patient charts before BSCOE designation and on designation renewal every 3 years. During site inspection, all operations reported in BOLD during the 12-month designated inspection period were verified with the hospital surgical record; complications and readmissions reported in BOLD were verified by chart review; and 10% of total cases were selected at random for chart review. Any unreported reoperations, readmissions, deaths, transfers, or revisions found during chart review triggered a 100% chart review. Inconsistencies noted during site inspection were reported to the Bariatric Surgery Review Committee, a group of practicing surgeons responsible for reviewing all applications to the BSCOE program.
Study Design
Clinical information was reviewed from 185,315 bariatric surgery patients who had a surgery performed between June 2007 and February 2011, all of whom were older than 18 years, had a body mass index (BMI) greater than 35 kg/m2, and the bariatric procedure was a primary (and not revision) procedure. To eliminate any loss of follow-up, this sample was limited to the 157,559, or 85% with either documented death within 30 days of discharge from the hospital or a documented follow-up visit after discharge that was 30 or more days after surgery. For this sample, the following data were reviewed and analyzed:
Surgical procedure and approach
30-day mortality
Patient demographics (age, race, BMI)
Socioeconomic factors (type of insurance coverage and employment status)
Comorbid conditions (hypertension, congestive heart failure, ischemic heart disease, lower extremity edema, thromboembolism, abnormal glucose metabolism, lipid abnormalities, gout, obstructive sleep apnea syndrome, gastroesophageal reflux [GERD], liver disease, pseudotumor cerebri, abdominal hernia, functional status)
Mental health status (substance abuse, psychological impairment, depression, mental health diagnosis, alcohol use, tobacco use)
Comorbid and mental health conditions were assessed at both preoperative and postoperative visits to the surgical practice using a modified version of a scoring system developed to evaluate obesity-related conditions in bariatric surgery patients.13 The system assigns a numerical value (0–5) to indicate the severity of the disease as diagnosed by a health care provider and allows tracking of changes in the disease state over time. For the current study, the scale was dichotomized to define the presence (Yes) or absence (No) of the condition as indicated in Table 1. In the dichotomization, care was taken to review the classification for each comorbid condition and to include the comorbid condition as present only when there was as much documentation as possible.
TABLE 1.
Definitions of Comorbidities, Mental health Status, and Socioeconomic Factors
| Abdominal hernia | N = No hernia |
|---|---|
| Abdominal hernia | N = No hernia |
| Y = Asymptomatic hernia with no prior operation, Symptomatic hernia with or without incarceration, Successful repair, Recurrent hernia or size > 15 cm, Chronic evisceration through large hernia with associated complication or multiple failed hernia repair | |
| Alcohol use | N = None, Rare |
| Y = Occasional, Frequent | |
| Angina assessment | N = No chest pain symptoms/angina |
| Y = Anginal chest pain only with extreme exertion (eg, running, swimming, etc), Anginal chest pain occurs with moderate activity or exertion, Anginal chest pain with minimal exertion (eg, walking across a room) or “at rest,” Unstable angina, Previous myocardial infarction by history or by current workup (eg, wall motion abnormality, etc) | |
| Mental health diagnosis | N = None |
| Y = Bipolar disorder, Anxiety/panic disorder, Personality disorder, Psychosis | |
| Congestive heart failure | N = No history or symptoms of congestive heart failure |
| Y = Class I: Symptoms with more than ordinary activity, Class II: Symptoms with ordinary activity, Class III: Symptoms with minimal activity, Class IV: Symptoms at rest | |
| Depression | N = No symptoms of depression, Mild and episodic not requiring treatment |
| Y = Moderate accompanied by some impairment that may require treatment, Moderate with significant impairment and treatment indicated, Severe and definitely requiring intensive treatment, Severe requiring hospitalization | |
| DVT/PE | N = No history of DVT/PE |
| Y = History of DVT resolved with anticoagulation, Recurrent DVT long-term anticoagulation medications Previous PE, Recurrent PE, decreased function, hospitalization, Vena Cava filter | |
| Functional status | N = No impairment of functional status |
| Y = Able to walk 200 ft with assistance device (cane or crutch), Cannot walk 200 ft with assistance device (cane or crutch), Requires wheelchair, Bedridden | |
| GERD | N = No history of GERD, Intermittent or variable symptoms with no medication, Intermittent medication |
| Y = H2 blockers or low-dose PPI, High-dose PPI, Meet criteria for antireflux surgery or prior surgery for GERD | |
| Diabetes | N = No symptoms or evidence of diabetes, Elevated fasting glucose |
| Y = Diabetes controlled with oral medication, Diabetes controlled with insulin, Diabetes controlled with insulin and oral medication, Diabetes with severe complications (retinopathy, neuropathy, renal failure, blindness) | |
| GOUT/Hyperuricemia | N = No symptoms of gout/hyperuricemia, Hyperuricemia with no symptoms, Hyperuricemia with medications |
| Y = Arthropathy, Destructive joints, Disability, unable to walk | |
| Hypertension | N = No history of hypertension, Borderline with no medication, Diagnosis of hypertension with no medication |
| Y = Treatment with a single medication, Treatment with multiple medications, Poorly controlled by medications, organ damage or dysfunction | |
| Ischemic heart disease | N = No history of ischemic heart disease, Abnormal ECG with no active ischemia |
| Y = History of MI or anti-ischemia medication percutaneous coronary intervention, CABG, Active ischemia | |
| Hyperlipidemia | N = Not present, Present but no treatment required, Controlled with lifestyle change including step 1 or step 2 diet |
| Y = Controlled with a single medication, Controlled with multiple medication, Not controlled | |
| Liver disease | N = No history of liver disease, Hepatomegaly modest with normal LFT and fatty change category 1 |
| Y = Modest or greater hepatomegaly with liver function test alteration and fatty change; or moderate to marked hepatomegaly with fatty change; or mild inflammation and mild fibrosis; or definite nonalcoholic steatohepatitis with cirrhosis and abnormal liver function; or Hepatic Failure with transplant indicated or done. | |
| Lower extremity edema | N = No symptoms of lower extremity edema, Intermittent lower extremity edema but not requiring treatment |
| Y = Symptoms requiring treatment (diuretics, elevation, or hose), Stasis ulcers, Disability, decreased function, hospitalization | |
| Obesity hypoventilation syndrome | N = No symptoms of obesity hypoventilation |
| Y = Hypoxemia/hypercarbia on room air, Severe hypoxemia or hypercarbia, Pulmonary hypertension, Right heart failure, Right heart failure-left ventricular dysfunction | |
| Obstructive sleep apnea syndrome | N = No symptoms or evidence of obstructive sleep apnea syndrome, Sleep apnea symptoms (negative sleep study or not done) |
| Y = Sleep apnea diagnosis by sleep study (no oral appliance), Sleep apnea requiring oral appliance such as CPAP, Sleep apnea with significant hypoxia or oxygen dependent, Sleep apnea with complications (pulmonary hypertension, etc) | |
| Peripheral vascular disease | N = No symptoms or peripheral vascular disease |
| Y = Asymptomatic with bruit, Claudication, anti-ischemic medication, Transient ischemic attack or rest pain, Procedure for peripheral vascular disease, Stroke with loss of tissue secondary to ischemia | |
| Pseudotumor cerebri | N = No symptoms of pseudotumor cerebri, Headaches with dizziness, nausea, and/or pain behind the eyes with no visual symptoms, Headaches with visual symptoms and/or controlled with diuretic |
| Y = Patient has had MRI to confirm pseudotumor cerebri, is well controlled with oral diuretics, Patient is well controlled with stronger medications, Patient requires narcotics or had had (or needs) surgical intervention | |
| Psychosocial impairment | N = No impairment, Mild impairment in psychosocial functioning but able to perform all primary task |
| Y = Moderate impairment in psychosocial functioning but able to perform most primary task, Moderate impairment in psychosocial functioning and unable to perform some primary task, Severe impairment in psychosocial functioning and unable to perform most primary task, Severe impairment in psychosocial functioning and unable to function | |
| Pulmonary hypertension | N = No symptoms or indication of pulmonary hypertension, Symptoms associated with PH (tiredness, shortness of breath, dizziness, fainting) |
| Y = Confirmed PH diagnosis, Well controlled on anticoagulants and/or calcium channel blockers, Stronger medications and/or oxygen, Patient needs or has had lung transplant | |
| Substance abuse | N = None |
| Y = Rare, Occasional, Frequent | |
| Tobacco use | N = None, Rare |
| Y = Occasional, Frequent |
Y = criteria for the presence of the comorbid condition; N = absence criteria
Thirty-day mortality in RYGB patients was evaluated in the context of patient demographics, socioeconomic factors, comorbid conditions, and mental health conditions. These univariate relationships were assessed using χ2 tests. Variables that were significant in univariate analysis were considered for inclusion in a multiple logistic regression model, which was used to identify the set of variables that independently predicted 30-day mortality. Variables were selected for inclusion in the model using manual, forward, stepwise approach after considering whether they had previously been associated with 30-day mortality, the strength of association in univariate analysis, their correlation with variables already in the model, and the frequency of occurrence. All tests were 2 –sided, and P < 0.05 was considered significant. SAS version 9.2 (SAS Institute, Cary, NC) was used for statistical analyses.
RYGB Risk Score
A RYGB risk score was developed using the results of the multiple logistic regression model. The scoring system accounted for the relative contribution of each variable to the final model using the size of the odds ratios (eg, odds ratios between 2.00 and 3.99 were given a score of 1 point, odds ratios between 4.00 and 5.99 were given a score of 2 points, odds ratios greater than 6.00 were given a score of 3 points). The score was then calculated for each of the RYGB patients in the study by totaling their points across the items in the final logistic regression model. The distribution of the study patients across risk scores was evaluated, and the 30-day mortality rate at each risk score level was assessed using a Cochran-Armitage trend test. The RYGB risk score was also compared with the Obesity Surgery Mortality Risk Score, with the number of total patients and mortality rate in each risk score group for each risk score was calculated. To evaluate the RYGB risk score and Obesity Surgery Mortality Risk Score in regard to differentiating among risk groups, measures of discrimination and reclassification were calculated. C-statistics were used to evaluate discrimination, which assesses the ability of each mortality score to distinguish between those who died and those who survived RYGB at 30 days.14 The net reclassification index was used to examine whether using the RYGB mortality risk score appropriately reclassified cases (ie, those who died) into the highest numerical risk groups and reclassified noncases (ie, survivors) into the lowest risk groups as compared with the Obesity Surgery Mortality Risk Score.15,16
RESULTS
The focus of the analysis was 30-day postoperative mortality. There were 158 mortalities in the entire cohort of 157,559 patients resulting in an overall mortality rate of 0.1%. Table 2 shows the 30-day mortalities expressed by surgery type. The most common procedure performed was RYGB (N = 81,751), and the majority of the overall mortalities (78%) occurred in this group of patients. The approach to RYGB was laparoscopic in 93% of cases and open in 6%, with a 1% conversion rate. Mortality rate was not influenced significantly by the surgical approach (χ2 P = 0.48).
TABLE 2.
Bariatric Surgical Procedures and 30-Day Mortality Rate
| Surgery Type | No. Surgeries | No. Deaths Within 30 Days (%) |
|---|---|---|
| Gastric bypass (Roux-en-Y) | 81,751 | 123 (.15) |
| Gastric banding, adjustable | 63,669 | 13 (.02) |
| Sleeve gastrectomy | 7323 | 10 (.13) |
| BPD with duodenal switch | 1660 | 6 (.36) |
| Gastric bypass banded | 1407 | 1 (.07) |
| Other | 1749 | 5 |
| TOTAL | 157,559 | 158 (.1) |
The relationship between patient demographics and BMI with 30-day mortality in RYGB was determined by univariate analysis (Table 3). Statistically significant predictors of 30-day mortality included male gender (P < 0.0001), increasing age (P < 0.0001), and baseline BMI (P < 0.0001). In addition, the analysis revealed that differences in insurance coverage and employment status are associated with statistically significant differences in RYGB mortality (P < 0.0013, and P < 0.0001 respectively).
TABLE 3.
Unadjusted Relationships Between Demographics, BMI, and 30-Day Death Rate for RYGB
| Gastric Bypass (Roux-en-Y)
|
|||
|---|---|---|---|
| N | % Deaths Within 30 Days | P | |
| Total | 81,751 | 0.15% | |
| Gender | |||
| Male | 17,641 | 0.30% | <.0001 |
| Female | 64,110 | 0.11% | |
| Age, y | |||
| 18–29 | 7147 | 0.07% | <.0001 |
| 30–39 | 19,443 | 0.07% | |
| 40–49 | 23,552 | 0.11% | |
| 50–59 | 21,322 | 0.20% | |
| 60–69 | 9554 | 0.35% | |
| 70–75 | 733 | 0.55% | |
| Race | |||
| Caucasians | 62,682 | 0.14% | 0.642 |
| African American | 8527 | 0.19% | |
| Hispanic | 6400 | 0.19% | |
| Other | 4142 | 0.14% | |
| Baseline BMI, kg/m2 | |||
| 35–39 | 10,446 | 0.08% | <.0001 |
| 40–49 | 44,472 | 0.12% | |
| 50–59 | 20,104 | 0.18% | |
| 60–69 | 5191 | 0.37% | |
| ≥70 | 1538 | 0.52% | |
| Insurance | |||
| Private | 57,345 | 0.12% | 0.0013 |
| Government* | 12,578 | 0.28% | |
| Self-pay | 1420 | 0.21% | |
| Other† | 3125 | 0.19% | |
| Missing | 7283 | 0.12% | |
| Employment | |||
| Employed | 49,183 | 0.10% | <.0001 |
| Unemployed | 6146 | 0.21% | |
| Student | 1376 | 0.07% | |
| Homemaker | 3529 | 0.06% | |
| Disabled | 6966 | 0.30% | |
| Retired | 5641 | 0.39% | |
| Unknown | 8910 | 0.18% | |
P values resulted from χ2 test.
Includes Medicare, Medicaid, and Tricare.
More than 1 method was used.
The presence of specific comorbid conditions and impaired functional status also showed a statistically significant relationship with 30-day mortality in the univariate analysis (Table 4). The associated comorbidities included hypertension (P < 0.0001), congestive heart failure (P < 0.0001), ischemic heart disease (P = 0.0001), peripheral vascular disease (P = 0.0044), diabetes (P < 0.0001), hyperlipidemia (P = 0.0003), obesity hypoventilation syndrome (P = 0.0003), pulmonary hypertension (P < 0.0001), liver disease (P = 0.017), psychosocial impairment (P = 0.047), abdominal hernia (P = 0.0099), and impaired functional status (P < 0.0001).
TABLE 4.
Unadjusted Relationships Between Preoperative Comorbidities and Other Factors With 30-Day Death for 81,751 RYGB Surgeries
| N (%) With Each Comorbidity
|
% Death Within 30-Days | P | |||
|---|---|---|---|---|---|
| n | % | ||||
| Hypertension | N | 39,224 | 48.0% | 0.09% | <0.0001 |
| Y | 42,527 | 52.0% | 0.21% | ||
| Congestive heart failure | N | 79,822 | 97.6% | 0.13% | <0.0001 |
| Y | 1929 | 2.4% | 0.83% | ||
| Ischemic heart disease | N | 79,286 | 97.0% | 0.14% | 0.0001 |
| Y | 2465 | 3.0% | 0.45% | ||
| Angina assessment | N | 79,341 | 97.1% | 0.15% | 0.464 |
| Y | 2410 | 2.9% | 0.21% | ||
| Peripheral vascular disease | N | 80,743 | 98.8% | 0.15% | 0.0044 |
| Y | 1008 | 1.2% | 0.50% | ||
| Lower extremity edema | N | 71,105 | 87.0% | 0.14% | 0.286 |
| Y | 10,646 | 13.0% | 0.19% | ||
| DVT/PE | N | 79,278 | 97.0% | 0.15% | 0.230 |
| Y | 2473 | 3.0% | 0.24% | ||
| Diabetes | N | 55,974 | 68.5% | 0.11% | <0.0001 |
| Y | 25,777 | 31.5% | 0.24% | ||
| Hyperlipidemia | N | 59,204 | 72.4% | 0.12% | 0.0003 |
| Y | 22,547 | 27.6% | 0.23% | ||
| GOUT/Hyperuricemia | N | 80,879 | 98.9% | 0.15% | 0.018 |
| Y | 872 | 1.1% | 0.46% | ||
| Obstructive sleep apnea syndrome | N | 51,969 | 63.6% | 0.13% | 0.056 |
| Y | 29,782 | 36.4% | 0.18% | ||
| Obesity hypoventilation syndrome | N | 80,124 | 98.0% | 0.14% | 0.0003 |
| Y | 1627 | 2.0% | 0.49% | ||
| Pulmonary hypertension | N | 81,312 | 99.5% | 0.14% | <0.0001 |
| Y | 439 | 0.5% | 1.59% | ||
| GERD | N | 61,528 | 75.3% | 0.15% | 0.742 |
| Y | 20,223 | 24.7% | 0.16% | ||
| Liver disease | N | 79,792 | 97.6% | 0.15% | 0.017 |
| Y | 1959 | 2.4% | 0.36% | ||
| Psychosocial impairment | N | 78,594 | 96.1% | 0.15% | 0.047 |
| Y | 3157 | 3.9% | 0.29% | ||
| Depression | N | 61,022 | 74.6% | 0.14% | 0.429 |
| Y | 20,729 | 25.4% | 0.17% | ||
| Mental Health diagnosis | N | 72,505 | 88.7% | 0.16% | 0.265 |
| Y | 9246 | 11.3% | 0.11% | ||
| Alcohol use | N | 70,901 | 86.7% | 0.15% | 0.725 |
| Y | 10,850 | 13.3% | 0.14% | ||
| Substance abuse | N | 81,399 | 99.6% | 0.15% | 0.466 |
| Y | 352 | 0.4% | 0.00% | ||
| Tobacco use | N | 77,704 | 95.0% | 0.15% | 0.705 |
| Y | 4047 | 5.0% | 0.17% | ||
| Pseudotumor cerebri | N | 81,563 | 99.8% | 0.15% | 0.177 |
| Y | 188 | 0.2% | 0.53% | ||
| Abdominal hernia | N | 77,384 | 94.7% | 0.14% | 0.0099 |
| Y | 4367 | 5.3% | 0.30% | ||
| Functional status | N | 78,788 | 96.4% | 0.14% | <0.0001 |
| Y | 2963 | 3.6% | 0.44% | ||
Variables shown to be significantly associated with increased 30-day mortality by univariate analysis were included stepwise in a multivariate model. Results of the multivariate analysis showed that increasing BMI, increasing age, and male gender were strong predictors of mortality (Table 5), with BMI and age correlated with increased mortality risk in a continuous relationship. In addition, after adjusting for age, BMI, and gender, comorbid conditions associated with increased mortality risk in this model included pulmonary hypertension (P = 0.0001), congestive heart failure (P = 0.0008), and liver disease (P = 0.038). Patient factors such as insurance and employment status, functional status, and the remaining comorbid diseases found to be statistically significant in the univariate analysis lost statistical significance in the multivariate model.
TABLE 5.
Multivariate Logistic Regression Results for 30-Day Death in 81,751 RYGB Patients
| Risk Factor | Odds Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| BMI, kg/m2 | |||
| 35–39 | Reference | ||
| 40–49 | 1.65 | 0.78, 3.45 | 0.187 |
| 50–59 | 2.48 | 1.15, 5.37 | 0.021 |
| 60–69 | 5.31 | 2.30, 12.27 | <0.0001 |
| 70+ | 7.53 | 2.76, 20.51 | <0.0001 |
| Age | |||
| 18–29 | Reference | ||
| 30–39 | 0.96 | 0.34, 2.69 | 0.934 |
| 40–49 | 1.51 | 0.58, 3.96 | 0.401 |
| 50–59 | 2.84 | 1.12, 7.22 | 0.028 |
| 60–69 | 4.74 | 1.82, 12.30 | 0.0014 |
| ≥70 | 7.20 | 1.90, 27.37 | 0.0037 |
| Gender | |||
| Female | Reference | ||
| Male | 2.14 | 1.49, 3.08 | <0.0001 |
| Pulmonary hypertension | 4.94 | 2.20, 11.08 | 0.0001 |
| Congestive heart failure | 2.63 | 1.50, 4.63 | 0.0008 |
| Liver disease | 2.26 | 1.08, 4.87 | 0.038 |
We then developed a RYGB risk score based upon the size of the odds ratios found for each of the variables according to the following algorithm:
1 point for each decade older than 40 years (eg, 50 = 1, 60 = 2, 70 = 3).
1 point for each 10 BMI units greater than 40 (eg, 50 = 1, 60 = 2, 70 = 3).
1 point for male gender.
1 point for CHF.
1 point for liver disease
2 points for pulmonary hypertension.
The RYGB risk score developed in this study and the Obesity Surgery Mortality Risk Score were then calculated for each of the 81,751 RYGB patients and compared with actual mortalities in each score category. A significant trend between mortality rate and increasing risk score (Cochran-Armitage trend test P < 0.0001) was found for both the risk score derived in this study and the Obesity Surgery Mortality Risk Score (Fig. 1). The RYGB risk score also demonstrated better discrimination (C-statistic 0.761 vs 0.722, P = 0.016) than the Obesity Surgery Mortality Risk Score. The RYGB risk score also reclassified an additional 3.3% of cases into the highest risk group and an additional 13.7% of the noncases into the lowest risk group. This resulted in an overall net reclassification index of 17.0% (95% confidence interval = 11.5%–22.5%, P < 0.0001).
FIGURE 1.
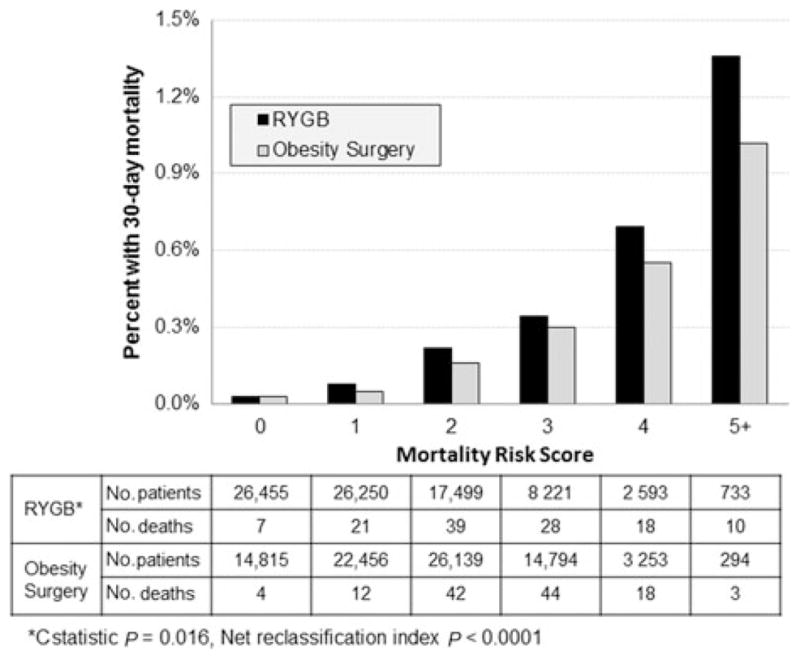
Comparison of Current Gastric Bypass Risk Score with the Obesity Mortality Risk Score 10
DISCUSSION
This study utilized a robust bariatric clinical registry comprising detailed information about obesity-related comorbid conditions and perioperative clinical data obtained from participants in the American Society for Metabolic and Bariatric Surgery BSCOE program. This is the largest sample size yet analyzed to determine bariatric surgical outcomes. The overall 30-day mortality rate of 0.1% we found is consistent with recent data from the Michigan Bariatric Surgery Collaborative with a 0.09% mortality rate also from a large clinical registry (N = 25,469),2 the 0.12% rate from the Nationwide Inpatient Sample, an administrative database based on a group of 304,515 patients,5 and the National Surgical Quality Improvement Program report of 11,023 patients with mortality of 0.2%.4 These outcomes reflect the continuing trend toward declining mortality rates after high-risk surgical procedures.17
Among the bariatric procedures we reviewed, the procedure with the lowest 30-day mortality rate was adjustable gastric banding (0.02%), followed by sleeve gastrectomy (0.13%), RYGB (0.15%), and biliopancreatic diversion with duodenal switch (BPD-DS) (0.36%). The increased mortality rate associated with BPD-DS found in this study is similar to the findings in the Michigan Bariatric Surgery Collaborative data (0.3%),2 and lower than the National Surgical Quality Improvement Program data set (1.7%),5 although the numbers of patients undergoing BPD-DS are relatively small in each cohort. In this study, RYGB was the only procedure with a large enough sample size to provide sufficient power to analyze surgical mortality.
We found that higher BMIs and age were strong predictors of risk for 30-day mortality in RYGB patients, confirming the work of others.6,7,10 However, this is the first risk analysis to demonstrate a continuous “dose-response” relationship between increasing BMI and increasing age with mortality risk. Our findings support the findings of the Obesity Mortality Risk Score analysis, which identified BMI greater than 50 as the strongest predictor of mortality with an odds ratio of 3.6.10 We found that the highest BMI cohort (>70) was the strongest risk predictor with an odds ratio of 7.53.
The contribution of the obesity disease burden to surgical mortality risk is much more difficult to study. Factors contributing to this difficulty include how individual comorbid conditions are defined; the severity and duration of individual comorbid conditions; and the established relationship between increasing age, increasing BMI, and male gender with more serious comorbid conditions.18 Comorbid conditions previously linked to surgical risk include chronic venous stasis disease,9 pulmonary embolus,6 and hypertension.6 The Obesity Mortality Risk Score defined the risk of postoperative pulmonary embolus (combining venous stasis disease, obesity hypoventilation syndrome, right heart failure, pulmonary hypertension, previous thromboembolism, and inferior vena cava filter) as a mortality predictor.10 The Longitudinal Assessment of Bariatric Surgery study also identified a history of thromboembolism, a diagnosis of obstructive sleep apnea, and impaired functional status as risk factors for a 30-day adverse composite endpoint including mortality.3
Our findings indicate that, after controlling for BMI, age and gender, pulmonary hypertension was a strong independent risk factor for mortality (odds ratio = 4.94), whereas congestive heart failure and liver disease were somewhat weaker predictors of mortality. In our multivariate analysis, we assessed the collective components for thromboembolism risk as defined by DeMaria10 and were unable to confirm thromboembolism as a risk for mortality. Although thromboembolism risk may well be a contributor, its impact on 30-day mortality appears to be reduced in this cohort. There are several plausible explanations for this finding. The percent with thromboembolism risk observed by DeMaria was 7.2% as compared with 3.6% in our cohort. This might reflect changes in patient selection driven by thromboembolism risk factors and more aggressive use of thromboembolism prophylaxis in contemporary bariatric practice as a result of DeMaria’s findings. In addition, the learning curve for minimally invasive bariatric procedures is now completed in credentialed bariatric centers and procedures are shorter with possibly less thromboembolism risk, and open procedures are now less than 10%. Finally, because of the work of DeMaria and others, patients with thromboembolism risk are now identified as high risk, which may result in better preoperative preparation and postoperative surveillance.
We also identified pulmonary hypertension as a strong predictor with male gender, congestive heart failure, and liver disease as additional, less powerful but statistically significant risk predictors. Although pulmonary hypertension and heart failure have been associated with increased risk in obese patients for other surgical procedures,19 little has been documented regarding the implications of pulmonary hypertension in bariatric surgery outcomes. The prevalence of pulmonary hypertension in this cohort was 0.5%, and only patients with a confirmed diagnosis were included in the analysis. Pulmonary hypertension has multiple causes, including idiopathic, drugs, left heart disease, lung disease (including sleep disordered breathing), hypoxia, and chronic thromboembolism.20 Furthermore, the diagnosis, which is usually based on intravascular ultrasound findings, can often be subject to technical limitations and operator experience.
Congestive heart failure is a proven surgical risk factor in the general population undergoing noncardiac surgery. The prevalence of congestive heart failure in this study cohort was 2.4%, which is comparable to the prevalence of this comorbid condition in other series of bariatric patients. However, in this cohort, the diagnosis is based on degree of symptoms, likely because the clinical diagnosis of congestive heart failure in patients with extreme obesity is difficult because of the extensive subcutaneous fat, which hides distended neck veins, limits estimation of hepatojugular reflux, and renders adventitious heart sounds inaudible. In addition, many patients with extreme obesity may have limiting exertional symptoms solely on the basis of obesity and deconditioning. Long-standing extreme obesity is a known risk factor for congestive heart failure, and varying degrees of cardiac remodeling are often detected in morbidly obese patients being considered for bariatric surgery.21 On the basis of these data, future efforts to better identify, define, and stratify pulmonary hypertension and congestive heart failure and their relationship to bariatric surgery seem warranted.
Although cirrhosis is a known risk factor for postoperative mortality in bariatric surgery,22,23 this is the first study that documents liver disease of lesser degrees than cirrhosis as a risk factor for 30-day mortality after RYGB. Nonalcoholic fatty liver disease is a very common finding in a population with extreme obesity. In a large bariatric surgery cohort, liver steatosis based on biopsy was present in 67%, fibrosis in 25%, and cirrhosis in 2%.24 The data we analyzed had a much lower overall prevalence of liver disease (<3%) that was assessed preoperatively without the use of systematic intraoperative liver biopsy. The discrepancy likely reflects liver disease diagnosed only on the basis of relatively insensitive nonhistological methods such as serum alanine and aspartate aminotransferase levels as well as liver ultrasound findings. The relationship between nonalchoholic fatty liver disease and RYGB mortality may be related to proinflammatory and prothrombotic states.25
The Obesity Surgery Mortality Risk Score developed by DeMaria was based on data from an earlier era of bariatric surgery, at the beginning of the transition from open to minimally invasive approaches and was based upon data from a single institution and a limited cohort of 2075 patients with 31 mortalities.10 In contrast, this study takes advantage of a much larger sample size and a similarly larger number of mortalities. Despite the differences in sample size and time period, the 2 risk analyses identified increasing age and BMI as well as male gender as mortality risk factors. Although surgical mortality rates are influenced by many factors including surgeon experience and expertise, procedure volume, structure and process of care, and patient-specific risk factors,26 our use of a much larger clinical data registry derived from established high-volume bariatric surgery centers may account for differences with previous studies. Importantly, we found that the RYGB mortality risk score was able to classify a significantly greater number of patients into the highest and lowest mortality risk categories. More than twice as many patients were classified in the highest risk category of the RYGB risk score, which had a correspondingly higher mortality rate. Almost twice as many patients were classified in the lowest risk category by the RYGB score, which in absolute terms translates into a large number of patients. Any improvement in our ability to more accurately identify high- and low-risk patients has obvious implications regarding patient selection and preparation for surgery as well as cost-effective allocation of resources.
The strengths of this study include the robust sample size, the large number of mortalities, and the favorable ratio of predictors to events, which strengthens the multivariate analysis. The major limitations of this study relate to method of data collection in this large cohort, and to the specific definitions, diagnosis, and stratification of comorbid conditions including pulmonary hypertension, congestive heart failure, and liver disease. It is hoped that the findings in this study will be confirmed and augmented by future studies utilizing similar statistical methodology, but with standardized criteria for the definition and severity assessment of the obesity-related comorbid diseases.
A recent report has raised questions regarding the long-term benefits of bariatric surgery in high-risk patients.27 In the current era when patient access to bariatric surgery is now a major national issue, the ability to better characterize surgical risk in candidates for bariatric surgery will improve patient selection for surgery, enhance the implementation of risk-reducing strategies, and favorably influence allocation of resources.
Acknowledgments
The authors thank Mss Christina Manney and Jamie Seiler for their assistance with data collection and management. Drs Benotti, Still, and Gerhard and Mr Wood had full access to all of the data in the study and they take responsibility for the integrity of the data and the accuracy of the data analysis. The contributions of all the authors were as follows: Study concept and design: Benotti, Still, Wood, Gerhard, Petrick, and Winegar; Acquisition of data: Wood, Benotti, and Winegar; Statistical analysis and interpretation: Benotti, Still, Wood, Gerhard; Drafting of the manuscript: Benotti, Wood, Gerhard, Still; Critical revision of the manuscript for important intellectual content: Benotti, Wood, Winegar, Petrick, Still, Argyropoulos, Gerhard; Obtaining funding: Still, Gerhard; Administrative, technical or material support: Wood; and Study supervision: Benotti, Gerhard, Still.
Footnotes
Disclosure: Dr Still receives grant and consulting support from Ethicon-Endosurgery. Dr Petrick has educational grants from Covidien and Ethicon-Endosurgery. This work was supported by funds from Geisinger Clinic, the Weis Center for Research, the Geisinger Obesity Research Institute, and grants DK072488 (GSG and CDS) and DK088231 (GSG) from the National Institute of Health. The authors declare no conflicts of interest.
References
- 1.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956–961. [PubMed] [Google Scholar]
- 2.Finks JF, Kole KL, Yenumula PR, et al. Predicting risk for serious complications with bariatric surgery: results from the Michigan Bariatric Surgery Collaborative. Ann Surg. 2011;254:633–640. doi: 10.1097/SLA.0b013e318230058c. [DOI] [PubMed] [Google Scholar]
- 3.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta PK, Franck C, Miller WJ, et al. Development and validation of a bariatric surgery morbidity risk calculator using the prospective, multicenter NSQIP dataset. J Am Coll Surg. 2011;212:301–309. doi: 10.1016/j.jamcollsurg.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen NT, Masoomi H, Laugenour K, et al. Predictive factors of mortality in bariatric surgery: data from the Nationwide Inpatient Sample. Surgery. 2011;150:347–351. doi: 10.1016/j.surg.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez AZ, Jr, Demaria EJ, Tichansky DS, et al. Multivariate analysis of risk factors for death following gastric bypass for treatment of morbid obesity. Ann Surg. 2004;239:698–702. doi: 10.1097/01.sla.0000124295.41578.ab. discussion 702–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston EH, Huerta S, Arthur D, et al. Male gender is a predictor of morbidity and age a predictor of mortality for patients undergoing gastric bypass surgery. Ann Surg. 2002;236:576–582. doi: 10.1097/00000658-200211000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flum DR, Salem L, Elrod JA, et al. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA. 2005;294:1903–1908. doi: 10.1001/jama.294.15.1903. [DOI] [PubMed] [Google Scholar]
- 9.Sugerman HJ, Sugerman EL, Wolfe L, et al. Risks and benefits of gastric bypass in morbidly obese patients with severe venous stasis disease. Ann Surg. 2001;234:41–46. doi: 10.1097/00000658-200107000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMaria EJ, Portenier D, Wolfe L. Obesity surgery mortality risk score: proposal for a clinically useful score to predict mortality risk in patients undergoing gastric bypass. Surg Obes Relat Dis. 2007;3:134–140. doi: 10.1016/j.soard.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 11.DeMaria EJ, Murr M, Byrne TK, et al. Validation of the obesity surgery mortality risk score in a multicenter study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity. Ann Surg. 2007;246:578–582. doi: 10.1097/SLA.0b013e318157206e. discussion 583–584. [DOI] [PubMed] [Google Scholar]
- 12.DeMaria EJ, Pate V, Warthen M, et al. Baseline data from American Society for Metabolic and Bariatric Surgery-designated Bariatric Surgery Centers of Excellence using the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis. 2010;6:347–355. doi: 10.1016/j.soard.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Ali MR, Maguire MB, Wolfe BM. Assessment of obesity-related comorbidities: a novel scheme for evaluating bariatric surgical patients. J Am Coll Surg. 2006;202:70–77. doi: 10.1016/j.jamcollsurg.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 16.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305:1553–1559. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 17.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benotti P, Wood GC, Still C, et al. Obesity disease burden and surgical risk. Surg Obes Relat Dis. 2006;2:600–606. doi: 10.1016/j.soard.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation. 2009;120:86–95. doi: 10.1161/CIRCULATIONAHA.109.192575. [DOI] [PubMed] [Google Scholar]
- 20.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Ashrafian H, le Roux CW, Darzi A, et al. Effects of bariatric surgery on cardiovascular function. Circulation. 2008;118:2091–2102. doi: 10.1161/CIRCULATIONAHA.107.721027. [DOI] [PubMed] [Google Scholar]
- 22.Brolin RE, Bradley LJ, Taliwal RV. Unsuspected cirrhosis discovered during elective obesity operations. Arch Surg. 1998;133:84–88. doi: 10.1001/archsurg.133.1.84. [DOI] [PubMed] [Google Scholar]
- 23.Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:897–901. doi: 10.1016/j.cgh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Gerhard GS, Chokshi R, Still CD, et al. The influence of iron status and genetic polymorphisms in the HFE gene on the risk for postoperative complications after bariatric surgery: a prospective cohort study in 1,064 patients. Patient Saf Surg. 2011;5:1. doi: 10.1186/1754-9493-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 26.Birkmeyer JD, Dimick JB. Understanding and reducing variation in surgical mortality. Annu Rev Med. 2009;60:405–415. doi: 10.1146/annurev.med.60.062107.101214. [DOI] [PubMed] [Google Scholar]
- 27.Maciejewski ML, Livingston EH, Smith VA, et al. Survival among high-risk patients after bariatric surgery. JAMA. 2011;305:2419–2426. doi: 10.1001/jama.2011.817. [DOI] [PubMed] [Google Scholar]


