Abstract
Objective:
To determine the clinical efficacy and toxicity of pemetrexed combined with low-dose cisplatin (CDDP) concurrent with late-course accelerated hyperfractionated (LCAF) intensity-modulated radiation therapy (IMRT) in patients with inoperable locally advanced oesophageal squamous cell carcinoma (ESCC).
Methods:
Patients with locally advanced ESCC (less than or equal to 75 years of age, clinical stages IIB–IVA and Karnofsky performance status ≥70) were enrolled into the study. A target group size of 22 was projected based on the estimation that 2-year overall survival (OS) would increase from 20% to 40%. Patients were treated with pemetrexed, low-dose CDDP and LCAF IMRT concurrently. The main objective of the study was for a 2-year OS, and the secondary objectives were progression-free survival (PFS), objective response, locoregional failure rate, and acute and late toxicities.
Results:
25 patients were recruited from October 2008 to July 2011. The median OS was 21 months, with 2- and 5-year OS rates of 44% and 44%, respectively. The median PFS was 18.2 months. The objective response rate was 96% (24/25), with 11 complete responses and 13 partial responses. The locoregional failure rate was 16%. Grades 4 and 5 acute toxicity rates were 8% and 4%, respectively, while no Grade 3 or greater late toxicity was observed.
Conclusion:
The findings of this Phase II study indicated that the therapeutic regimen appears to achieve an excellent response rate and favourable survival for locally advanced ESCC. However, the severe acute side effects should be considered cautiously in further studies.
Advances in knowledge:
To our knowledge, this is the first study that introduced pemetrexed and low-dose CDDP combined with LCAF IMRT to treat locally advanced ESCC. The 5-year OS rate was as high as 44%, which was more favourable than other studies.
Oesophageal carcinoma (EC) is one of the common malignant tumours all over the world, with China having a high incidence, for about 150,000 people die of it each year, accounting for nearly a quarter of all cancer deaths worldwide.1 According to a series of studies implemented by the Radiation Therapy Oncology Group (RTOG), such as the RTOG 85012 and RTOG 9405,3 concurrent chemoradiotherapy (CCRT) was established as a standard approach for locally advanced EC; however, the prognosis was still poor with a median overall survival (OS) of 14.1 and 18.1 months, respectively. In order to improve the prognosis, late-course accelerated hyperfractionated (LCAF) radiotherapy (RT) was scheduled on oesophageal squamous cell carcinoma (ESCC) by Shi et al4 in 1999. The results of LCAF RT were encouraging, with the 5-year survival rate varying from 26% to 33%.4–6 Furthermore, patients with ESCC who were treated with concurrent LCAF RT and chemotherapy had a 5-year survival rate of 40% and a median survival time of 30.8 months in the study by Zhao et al.7 Therefore, this study indicated better survival in patients who received concurrent LCAF RT and chemotherapy than in those receiving LCAF RT alone. However, Grades 3–4 and Grade 5 toxicity rates were 46% and 6%, respectively, which were more severe than that in LCAF RT alone and might be mostly owing to chemotherapy.
In order to reduce the adverse effects and improve the outcome, new chemotherapy regimens combined with LCAF RT should be investigated. Pemetrexed, a novel antitumour drug, acting as a multitargeted antifolate by inhibiting several key enzymes involved in nucleotide synthesis, has demonstrated broad antitumour activity in a wide variety of solid tumours.8 The interaction of pemetrexed and ionizing radiation has been investigated in vitro using different human tumour cell lines. It enhanced radiation-induced cell inactivation at moderately toxic exposures over several hours after drug removal.9 Myelosuppression and mucositis, the most significant toxicities induced by pemetrexed, have been significantly ameliorated by folic acid and vitamin B12 supplementation without compromising its antitumour effect. More importantly, vitamin supplementation has not demonstrated any adverse effects.8 Jatoi et al10 reported a clinical trial with concurrent pemetrexed, carboplatin and radiation followed by surgery to treat locally advanced EC and gastro-oesophageal-junction tumours. The pathological complete response (pCR) rate was 23%, higher than that in other studies.11,12
A Phase I clinical trial13 that combined pemetrexed and cisplatin (CDDP) with concurrent selective lymph node (SLN) LCAF RT was conducted in our institution (Shandong Cancer Hospital, Jinan, China). Although toxicities were common, the protocol was safe and well tolerated and achieved an encouraging outcome. To further determine the efficacy and side effects, the Phase II study was then performed in patients with locally advanced ESCC.
METHODS AND MATERIALS
Eligibility criteria
Patients with histologically confirmed ESCC without previous treatment, clinical stage ranging from IIB to IVA, indications for chemoradiotherapy, and who were inoperable or refused surgery were enrolled in this study. They all had bidimensionally measurable disease, lumen size >5 mm diameter, Karnofsky performance status ≥70 and a life expectancy of 3 months or longer. The criteria for laboratory examination were as follows: haemoglobin ≥10 g dl−1; absolute white blood cell count ≥4000 ml−1; platelet count ≥100,000 ml−1; total bilirubin level ≤1.5 mg dl−1; serum creatinine level ≤1.5 times the upper limit of normal; and aspartate/alanine aminotransferase levels ≤2.5 times the upper limit of normal.14
Exclusion criteria
Exclusion criteria were patients with distant metastases except for M1a, oesophageal perforation that reflected on radiographic imaging or oesophagoscope, and some other serious underlying medical conditions, such as significant cardiac disease, uncontrolled diabetes, serious infections, central nervous system disorders or psychological disability that cannot withstand the treatment protocol. Patients who participated in other clinical trials were also excluded.
Pre-treatment evaluation
Physical examination and a history inquiry were performed before treatment. Pre-treatment staging examination included barium oesophagogram, oesophagoscope, bone scan with single photon emission CT, CT scan of the brain, neck, chest and abdomen, and complete blood count with differential, serum chemistry tests, liver function tests, coagulation panel, urinalysis and electrocardiogram. Bronchoscopy was also performed if clinically necessary. Patients were staged according to the American Joint Committee on Cancer TNM Classification of Carcinoma of the Oesophagus and Oesophagogastric Junction (6th ed, 2002). All patients signed the informed consent form.
Chemotherapy
Chemotherapy began on Day 1, concurrent with the beginning of RT. Based on our Phase I study, patients were treated with 400 mg m−2 pemetrexed, which was administered intravenously over 10 min on Days 1 and 22, and 10 mg m−2 CDDP was given intravenously on Days 1–5 and 22–26. All patients received folic acid, vitamin B12 and steroid prophylaxis treatment.
Radiotherapy
RT was performed using a Varian® linear accelerator (Varian Medical Systems, Palo Alto, CA). All patients were immobilized in the supine position with the arms above the head in a vacuum-bag restriction system (Vac-Lock™; CIVCO Medical Solutions, Kalona, IA) and, then, consecutively underwent enhanced CT scanning under normal respiration with 5 mm slice thickness scans throughout the entire neck and thorax. All the CT images acquired were transferred to and registered in the treatment planning system. The targets and organs at risk were delineated according to the following criteria. The gross target volume (GTV) included primary tumour and metastatic lymph nodes. The SLN areas were defined as groups 104–107 and part of 108 in the upper thoracic oesophageal cancer; groups 104, 106–108, part of 110, 1 and 2 in the middle thoracic oesophageal cancer; and groups 104, part of 106 and 108, 1–3, 7 and 9 in the lower thoracic oesophageal cancer. The lymph node groups were named according to Japanese guidelines.15 For primary tumour, a 3 cm margin was added superiorly and inferiorly and 1 cm laterally to create a planning target volume (PTV), and for involved lymph nodes, a 1 cm margin was added around the entire lymph node. The fields of the first phase RT involved the above GTV, selective high-risk lymph node areas and PTV, which received 40 Gy in total, 2 Gy per fraction and five fractions a week. In the second phase, accelerated hyperfractionated radiation was employed with a total dose of 19.6 Gy, 1.4 Gy per fraction, twice a day with a minimum interval of 6 h, ten fractions a week. The selective high-risk lymph node areas were spared. The total dose of the 2-phase irradiation was 59.6 Gy per 34 fractions in 5.4 weeks. All plans aimed to achieve a minimum dose >95% and a maximum dose <107% of the prescribed dose, and that no 2 cc region (either within or more of the PTV) may receive >110% of the dose. The dose with inhomogeneity correction was prescribed to the 95% isodose line, which encompassed 95% PTVs. The dose–volume histogram constraints of the organs at risk were as follows: bilateral lung V20 ≤30%, spinal Dmax ≤50 Gy, heart V50 ≤33% and V45 ≤67%, hepatic V35 ≤50%, gastric Dmax ≤50 Gy and intestinal Dmax ≤50 Gy. No modifications in fraction size or total dose were permitted during the whole process.
Adverse effect assessment
During the intensity-modulated radiation therapy (IMRT) and chemotherapy period, assessment of the acute adverse effects was performed weekly using the National Cancer Institute Common Toxicity Criteria v. 3.0.16 3 months after the treatment, late RT effects were recorded according to the RTOG/EORTC late radiation morbidity scoring schema.17 Side effects were managed aggressively using standard supportive measurements; granulocyte colony stimulating factor and interleukin-11 were provided if medically necessary. Treatment interruption was allowed for patients with ≥Grade 3 toxicity lasting more than 7 days.
Follow-up
After treatment, patients were followed up every 2 months for the first year, every 4 months for the second year and every 6 months thereafter. Each visit included medical history, physical examination, complete blood count, chest X-ray, oesophageal barium radiography, and chest and abdomen CT. Biopsy of the primary tumour site was required when locoregional recurrence was suspected.
Study design and end points
A single centre open-label Phase II study was designed to further evaluate the effectiveness and safety of the recommended dose of pemetrexed and low-dose CDDP when given concurrently with SLN LCAF IMRT to the patients with locally advanced ESCC. This study was approved by the ethical committees of our hospital and registered with the Chinese Clinical Trial Registry (http://www.chictr.org/cn/); the registration number was ChiCTR-TRC-09000568.
6–8 weeks after completion of CCRT, the treatment response was evaluated using CT and barium oesophagogram. In accordance with the solid tumour's effect evaluation criterion of the World Health Organization, all responses were defined as follows: complete response (CR), complete disappearance of all tumours; partial response (PR), 50% decrease in the sum of products of the largest perpendicular diameters of all measurable lesions; stable disease (SD), failure to observe remission; and progressive disease, the appearance of new lesions or >25% increase in size of existing lesions.
The primary end point of this Phase II study was a 2-year OS. Secondary end points included progression-free survival (PFS), objective response, locoregional failure rate, and acute and late toxicities rates. OS was observed from the first day of treatment until death or last follow-up time, and PFS was observed from the first day of treatment until progress, death or last follow-up time.
Statistical analysis
Sample size was projected based on the estimate of the 2-year OS of 20% for CCRT. With our modified treatment protocol the 2-year OS was expected to increase to 40%. It was assumed that the one-sided Type I error was 10%, the statistical power was 0.80 and there was no dropout. Sample size was calculated by the Stata™ software v. 12.0 (StataCorp LP, College Station, TX), and 22 patients were expected to adequately detect the difference. The OS and PFS were estimated by the Kaplan–Meier model using SPSS® v. 17.0 (SPSS Inc., Chicago, IL).
RESULTS
Patient characteristics
25 patients (23 males and 2 females; with the following subsections: 3 cervical, 9 upper, 11 middle and 12 lower oesophageal squamous cell carcinoma) with Stages IIB–IVA were enrolled in this study from October 2008 to July 2011. The patients' characteristics are summarized in Table 1.
Table 1.
Clinical features of 25 patients with oesophageal squamous cell carcinoma
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 23 (92) |
| Female | 2 (8) |
| Age (years) | |
| Median | 62 |
| Range | 40–75 |
| Subsection | |
| Cervical | 3 (12) |
| Upper thoracic | 9 (36) |
| Middle thoracic | 11 (44) |
| Lower thoracic | 2 (8) |
| Stage | |
| IIB | 7 (28) |
| III | 11 (44) |
| IVA | 7 (28) |
| Karnofsky performance status | |
| Median | 90 |
| Range | 80–100 |
Treatment response
6–8 weeks after CCRT, the response was evaluated using both thoracic CT scan and barium oesophagogram. The CR, PR and SD were observed in 11 (44%) patients, 13 (52%) patients and 1 (4%) patient, respectively. So, the objective response rate was 96%.
Adverse effect
All patients were evaluated for toxicity. The acute toxicities for each patient are presented in Table 2. The major acute toxicities were myelosuppression, gastrointestinal reaction and oesophagitis. Grade 3 acute toxicity occurred in 13 (52%) patients, Grade 4 acute toxicity occurred in 2 (8%) patients and Grade 5 acute toxicity occurred in 1 (4%) patient. Two patients had a treatment interruption of less than 3 days owing to acute toxicities, and all the patients completed the treatment.
Table 2.
Acute toxicity
| Toxicity | Grade 0, n (%) | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) | Grade 5, n (%) |
|---|---|---|---|---|---|---|
| Skin reaction | 1 (4) | 24 (96) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Oesophagitis | 2 (8) | 17 (68) | 5 (20) | 1 (4) | 0 (0) | 0 (0) |
| Oesophageal stricture | 22 (88) | 3 (12) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fistula | 24 (96) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (4) |
| Nausea | 10 (40) | 9 (36) | 6 (24) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 17 (68) | 3 (12) | 4 (16) | 1 (4) | 0 (0) | 0 (0) |
| Pneumonia | 15 (60) | 9 (36) | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Leukopenia | 1 (4) | 3 (12) | 8 (32) | 12 (48) | 1 (4) | 0 (0) |
| Anaemia | 11 (44) | 10 (40) | 0 (0) | 4 (16) | 0 (0) | 0 (0) |
| Thrombocytopaenia | 8 (32) | 6 (24) | 6 (24) | 3 (12) | 2 (8) | 0 (0) |
| Maximum severity per patient | 0 (0) | 3 (12) | 6 (24) | 13 (52) | 2 (8) | 1 (4) |
Late radiation toxicities are listed in Table 3. The major late radiation toxicity was oesophageal injury. There were no observations of late toxicities >Grade 3.
Table 3.
Late radiotherapy toxicity
| Toxicity | Grade 0, n (%) | Grade 1, n (%) | Grade 2, n (%) |
|---|---|---|---|
| Skin | 22 (88) | 3 (12) | 0 (0) |
| Oesophagus | 20 (80) | 4 (16) | 1 (4) |
| Larynx | 24 (96) | 1 (4) | 0 (0) |
| Lung | 22 (88) | 2 (8) | 1 (4) |
| Maximum severity per patient | 17 (68) | 6 (24) | 2 (8) |
Survival and pattern of failure
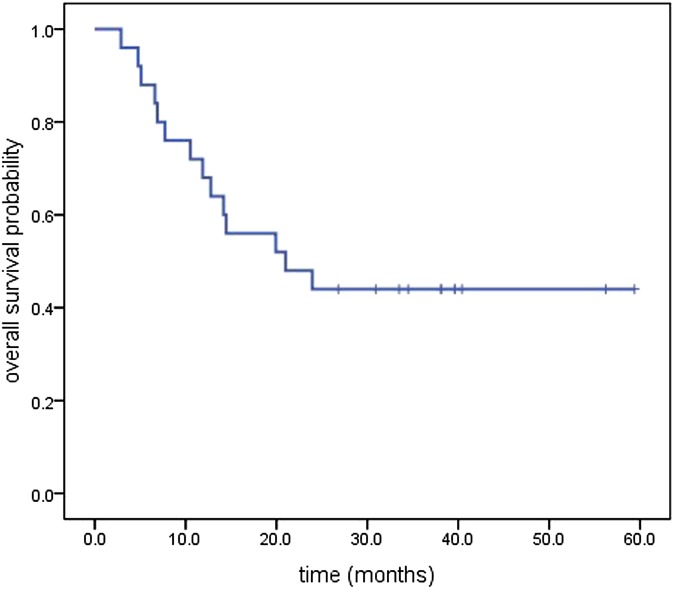
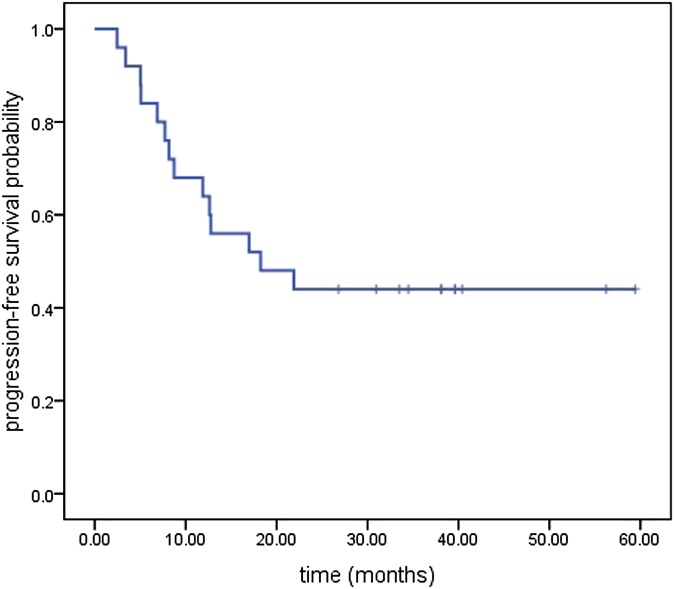
The median follow-up was 21 months, with a range of 2.9–59.4 months until the last follow-up date of September 2013. Median OS for all the patients was 21 months [95% confidence interval (CI), 5.56–36.439 months], and the median PFS was 18.23 months (95% CI, 3.33–33.13 months). The OS and PFS are illustrated in Figures 1 and 2, respectively. The Kaplan–Meier estimated l, 2 and 5 year OS rates and the PFS rates were 68%, 44%, 44% and 64%, 44%, 44%, respectively. The first failure patterns consisted of locoregional failure (16%) and distant metastasis (12%). Currently, a total of 11 patients are alive, 1 patient died of oesophageal fistula after therapy, 4 patients died of local disease recurrence, 3 patients died of distant metastasis, while the others died of non-tumour reasons (2 died of cardiac disease, 2 died of stroke and 2 died of unknown disease). The mean OS of the patients who died owing to non-tumour reasons was 8.42 months (ranging from 4.47 to 14.47 months). Of the living patients, there was no evidence of disease recurrence.
Figure 1.
Kaplan–Meier plot of overall survival.
Figure 2.
Kaplan–Meier plot of progression-free survival.
DISCUSSION
In this Phase II trial, we tested pemetrexed, low-dose CDDP and concomitant RT in patients with locally advanced ESCC. In the present study, patients achieved a 44% 2 year OS, which reached the value of expectation. The 5 year OS was as high as 44%, which was more favourable than other studies (Table 4).
Table 4.
Survival and toxicities of concurrent chemoradiotherapy for oesophageal cancer
| Studies | Year | Histology | Radiation dose (Gy) | Radiation style | Chemotherapy | Median OS months | 1 year OS rate (%) | 2 year OS rate (%) | 3 year OS rate (%) | 5 year OS rate (%) | Grade 3 toxicitya (%) | Grade 4 toxicitya (%) | Grade 5 toxicitya (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| al-Sarraf et al18 | 1997 | ADC, 10%; ESCC, 90% | 50.0 | CF RT | CDDP + 5-Fu | 14.1 | – | 36 | 30.0 | 27.0 | 46.00 (25.00) | 14 (4) | 2.0 (0.0) |
| ADC, 15%; ESCC, 85% | 64.0 | CF RT | – | 9.3 | – | 10 | 0.0 | 0.0 | 19.00 | 4 | 0.0 | ||
| ADC, 20%; ESCC, 80% | 50.0 | CF RT | CDDP + 5-Fu | 17.2 | – | – | 30.0 | – | 49.00 (15.00) | 13 (5) | 0.0 (0.0) | ||
| INT0123 (RTOG 9405); Minsky et al3 | 2002 | ESCC | 64.8 | CF RT | CDDP + 5-Fu | 13.0 | – | 31 | – | – | 46.00, 34.00 | 21, 11 | 9.0, 1.0 |
| 50.4 | CF RT | CDDP + 5-Fu | 18.1 | – | 40 | – | – | 43.00, 24.00 | 26, 13 | 2.0, 0.0 | |||
| Gao et al6 | 2002 | ESCC | 60.0 | LCAF RT | – | 25.4 | 73.2 | – | 34.2 | – | 2.40, 0.00 | 2.4, 0 | 0.0, 0.0 |
| CDDP | 32.6 | 80.7 | – | 40.0 | – | 10.00, 0.00 | 5, 0 | 0.0, 0.0 | |||||
| Ishikura et al19 | 2003 | ESCC | 60.0 | CF RT | CDDP + 5-Fu | 21.0 | – | – | 38.0 | 29.0 | 38.80, 9.35 | 5, 1.4 | 2.0, 1.4 |
| Zhao et al7 | 2005 | ESCC | 68.4 | LCAF RT | CDDP + 5-Fu | 30.8 | 67.0 | 58 | 44.0 | 40.0 | 40.00, 13.00 | 6, 2 | 6.0, 4.0 |
| – | 23.9 | 77.0 | 49 | 39.0 | 28.0 | 25.00, 23.00 | 0, 4 | 0.0, 4.0 | |||||
| Kang et al20 | 2007 | ADC, 1.6%; ESCC, 98.4% | 45.0–64.4 | CF RT | CDDP + 5-Fu | 14.0 | – | – | 16.0 | – | – | – | – |
| RTOG 8501; Cooper et al2 | 1999 | ADC, 21.5%; ESCC, 78.5% | 50.0 randomized | CF RT | CDDP + 5-Fu | – | 52.0 | 36 | 30.0 | 26.0 | –, 25.00 | 8, 4 | 2.0, 0.0 |
| 64.0 | CF RT | – | – | 34.0 | 10 | 0.0 | 0.0 | –, 19.00 | 2, 4 | 0.0, 0.0 | |||
| – | 50.0 non-randomized | CF RT | CDDP + 5-Fu | – | 62.0 | 35 | 26.0 | 14.0 | –, 23.00 | 4, 3 | 0.0, 0.0 | ||
| RTOG 0113; Ajani et al21 | 2008 | ADC, 65.28%; ESCC, 34.72% | 50.4 | CF RT | CDDP + 5-Fu + paclitaxel | 28.7 | 76.0 | 56 | – | – | 27 | 3.0 | |
| CF RT | Paclitaxel | 14.9 | 69.0 | 37 | – | – | 43.00 | 40 | 6.0 | ||||
| KROSG0101/JROSG021; Nishimura et al22 | 2009 | ADC, 2.17%; ESCC, 97.83% | 60.0 | Split for 1 week | CDDP + 5-Fu, short term | – | – | 46 | – | 35.0 | – | – | – |
| ESCC, 100% | 60.0 | Split for 1 week | CDDP + 5-Fu, protracted | – | – | 44 | – | 24.0 | – | – | – | ||
| Hurmuzlu et al23 | 2010 | ADC, 34.78%; ESCC, 65.22% | 66.0 | CF RT | CDDP + 5-Fu | 10.8 | – | 22 | 15.0 | 11.0 | 47.50 | 40 | 2.5 |
| Sheng et al24 | 2011 | ESCC | 64.0–69.0 | LCAF RT | Capecitabine | – | 85.7 | 55.6 | 30.2 | – | – | – |
5-Fu, 5-fluorouracil; ADC, adenocarcinoma; CDDP, cisplatin; CF RT, conventional fractionated radiotherapy; ESCC, oesophageal squamous cell carcinoma; LCAF RT, late course accelerated hyper-fractionated radiotherapy; OS, overall survival.
aToxicities were expressed as “acute toxicity rate, late toxicity rate” or “chemotherapy toxicity rate (radiotherapy toxicity rate)”.
Seiwert et al25 reported promising Phase I data with a combination of pemetrexed, carboplatin and radiation in patients with locally advanced oesophageal and lung cancers. The CR rate was 33%, and the incidence of oesophagitis as well as haematological, skin and pulmonary toxicities was lower than with other established chemoradiotherapy platforms. Jatoi et al10 also carried out a clinical trial with concurrent pemetrexed, carboplatin and radiation followed by surgery to treat locally advanced EC and gastro-oesophageal-junction tumours. The pCR rate was 23% and the median OS was 17.8 months. These data demonstrate that pemetrexed combined with platinum concurrent with RT achieved promising antineoplastic effects in patients with EC. However, the EC patients included in those studies were almost oesophageal adenocarcinomas. In the present study, we first applied pemetrexed in the treatment of ESCC and got a CR rate of 44% and a median OS of 21 months; the favourable results indicate that pemetrexed concurrent RT can be applied in the treatment of ESCC.
The studies RTOG 85012 and 94053 have established the standard role of CCRT in the treatment of locally advanced EC, where the median OS was 14.1–18.1 months. Compared with these outcomes, the results of our present study seemed much better. In addition, the chemoradiotherapy followed by surgery for oesophageal cancer (CROSS) group had conducted a study26 and found that neoadjuvant chemoradiotherapy followed by surgery to treat locally advanced EC resulted in a pCR rate of 29% and a 5-year OS rate of 57%. The finding indicated that patients with EC who underwent surgery could benefit from neoadjuvant chemoradiotherapy. Although the patients enrolled in our study were inoperable or refused surgery, the clinical stage was somewhat delayed, but the CR rate reached 44%, which was an encouraging outcome. The high response rate may be due to the LCAF RT with 59.6 Gy higher radiation dose than CF RT with 41.4–50.4 Gy in other studies.27 Shi et al4 compared the local control rate and the OS between CF RT and LCAF RT in patients with ESCC and demonstrated that the 5 year PFS in the LCAF RT group was improved significantly (42% vs 15%). The advantages of LCAF RT have been confirmed by many studies.28
It is undeniable that toxicities obtained in our study were relatively severe, but fortunately, most of the acute side effects were manageable. The incidence of Grade 3 oesophagitis (4%) in our study was not notably higher than that obtained in other studies.29 Although there were two patients who experienced treatment interruption, all patients completed the entire treatment process. There were no serious late toxicities, which may be attributed to the application of IMRT technology. Lin et al30 had confirmed that IMRT was better than three-dimensional conformal RT (3D-CRT) when combined with chemotherapy in patients with oesophageal cancer. Compared with patients treated with IMRT, patients treated with 3D-CRT had a significantly greater risk of dying (72.6% vs 52.9%; p < 0.0001) and locoregional recurrence (p = 0.0038).
Given the overall size of this study, the follow-up time and the occurrence rate of acute toxicities, the results have been reviewed cautiously. However, the high response rate and 5 year OS when administering pemetrexed, CDDP and LCAF RT were encouraging and support further Phase III trial testing in locally advanced ESCC. But the formulation of specific schemes still needs further consideration to reduce side effects. Moreover, using radioprotectors to protect normal tissue could be favourable to patients’ tolerability.
In conclusion, therapeutic regimens concurrent with SLN LCAF IMRT and pemetrexed with low-dose CDDP achieved excellent response rates and favourable survival rates for locally advanced ESCC. Further randomized studies could be carried out, but the severe and acute side effects should be reviewed cautiously, and additional measurements must also be taken to avoid and/or manage life-threatening acute toxicities.
REFERENCES
- 1.Zhou ZG, Gao XS, Qiao XY, Zhang P. Literature analysis of radiotherapy for esophageal cancer in China. Chin J Cancer 2010; 29: 873–81. [DOI] [PubMed] [Google Scholar]
- 2.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999; 281: 1623–7. [DOI] [PubMed] [Google Scholar]
- 3.Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) Phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002; 20: 1167–74. [DOI] [PubMed] [Google Scholar]
- 4.Shi XH, Yao W, Liu T. Late course accelerated fractionation in radiotherapy of esophageal carcinoma. Radiother Oncol 1999; 51: 21–6. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Shi XH, He SQ, Yao WQ, Guo XM, Wu GD, et al. Comparison between continuous accelerated hyperfractionated and late-course accelerated hyperfractionated radiotherapy for esophageal carcinoma. Int J Radiat Oncol Biol Phys 2002; 54: 131–6. [DOI] [PubMed] [Google Scholar]
- 6.Gao XS, Qiao XY, Yang XR, Asaumi J, Zhou ZG, Wang YD, et al. Late course accelerated hyperfractionation radiotherapy concomitant with cisplatin in patients with esophageal carcinoma. Oncol Rep 2002; 9: 767–72. [PubMed] [Google Scholar]
- 7.Zhao KL, Shi XH, Jiang GL, Yao WQ, Guo XM, Wu GD, et al. Late course accelerated hyperfractionated radiotherapy plus concurrent chemotherapy for squamous cell carcinoma of the esophagus: a Phase III randomized study. Int J Radiat Oncol Biol Phys 2005; 62: 1014–20. [DOI] [PubMed] [Google Scholar]
- 8.Adjei AA. Pemetrexed (ALIMTA), a novel multitargeted antineoplastic agent. Clin Cancer Res 2004; 10: 4276s–80s. doi: 10.1158/1078-0432.CCR-040010 [DOI] [PubMed] [Google Scholar]
- 9.Bischof M, Weber KJ, Blatter J, Wannenmacher M, Latz D. Interaction of pemetrexed disodium (ALIMTA, multitargeted antifolate) and irradiation in vitro. Int J Radiat Oncol Biol Phys 2002; 52: 1381–8. [DOI] [PubMed] [Google Scholar]
- 10.Jatoi A, Soori G, Foster NR, Hiatt BK, Knost JA, Fitch TR, et al. Phase II study of preoperative pemetrexed, carboplatin, and radiation followed by surgery for locally advanced esophageal cancer and gastroesophageal junction tumors. J Thorac Oncol 2010; 5: 1994–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajani JA, Correa AM, Walsh GL, Komaki R, Lee JH, Vaporciyan AA, et al. Trimodality therapy without a platinum compound for localized carcinoma of the esophagus and gastroesophageal junction. Cancer 2010; 116: 1656–63. doi: 10.1002/cncr.24935 [DOI] [PubMed] [Google Scholar]
- 12.Chiarion-Sileni V, Innocente R, Cavina R, Ruol A, Corti L, Pigozzo J, et al. Multi-center Phase II trial of chemo-radiotherapy with 5-fluorouracil, leucovorin and oxaliplatin in locally advanced esophageal cancer. Cancer Chemother Pharmacol 2009; 63: 1111–19. [DOI] [PubMed] [Google Scholar]
- 13.Li BS, Gong HY, Huang W, Yi Y, Zhang ZC, Li HS, et al. Phase I study of concurrent selective lymph node late course accelerated hyper-fractionated radiotherapy and pemetrexed, cisplatin for locally advanced esophageal squamous cell carcinoma. Dis Esophagus 2011; 24: 251–7. [DOI] [PubMed] [Google Scholar]
- 14.Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Urmacher C, Brennan MF. Preoperative staging of esophageal cancer: comparison of endoscopic US and dynamic CT. Radiology 1991; 181: 419–25. [DOI] [PubMed] [Google Scholar]
- 15.Esophageal Disease Research Society. Guidelines for clinical and pathologic studies on carcinoma of the esophagus, ninth edition: preface, general principles, part I. Esophagus 2004; 1: 61–88. [Google Scholar]
- 16.National Cancer Institute. Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE) [published 9 August 2006; accessed 13 January 2009]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf [Google Scholar]
- 17.Cox JD, Stetz J, Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6. [DOI] [PubMed] [Google Scholar]
- 18.al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle JS, Vaitkevicius VK, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol 1997; 15: 277–84. [DOI] [PubMed] [Google Scholar]
- 19.Ishikura S, Nihei K, Ohtsu A, Boku N, Hironaka S, Mera K, et al. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol 2003; 21: 2697–702. doi: 10.1200/JCO.2003.03.055 [DOI] [PubMed] [Google Scholar]
- 20.Kang SY, Han JH, Lee KJ, Choi JH, Park JI, Kim HI, et al. Low expression of Bax predicts poor prognosis in patients with locally advanced esophageal cancer treated with definitive chemoradiotherapy. Clin Cancer Res 2007; 13: 4146–53. [DOI] [PubMed] [Google Scholar]
- 21.Ajani JA, Winter K, Komaki R, Kelsen DP, Minsky BD, Liao Z, et al. Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol 2008; 26: 4551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimura Y, Mitsumori M, Hiraoka M, Koike R, Nakamatsu K, Kawamura M, et al. A randomized Phase II study of cisplatin/5-FU concurrent chemoradiotherapy for esophageal cancer: short-term infusion versus protracted infusion chemotherapy (KROSG0101/JROSG021). Radiother Oncol 2009; 92: 260–5. [DOI] [PubMed] [Google Scholar]
- 23.Hurmuzlu M, Monge OR, Smaaland R, Viste A. High-dose definitive concomitant chemoradiotherapy in non-metastatic locally advanced esophageal cancer: toxicity and outcome. Dis Esophagus 2010; 23: 244–52. [DOI] [PubMed] [Google Scholar]
- 24.Sheng W, Feng XZ, Han JQ. [Efficacy of late accelerated hyperfractionated conformal radiotherapy combined with capecitabine for esophageal carcinoma]. [In Chinese.] Zhonghua Zhong Liu Za Zhi 2011; 33: 702–6. [PubMed] [Google Scholar]
- 25.Seiwert TY, Connell PP, Mauer AM, Hoffman PC, George CM, Szeto L, et al. A Phase I study of pemetrexed, carboplatin, and concurrent radiotherapy in patients with locally advanced or metastatic non-small cell lung or esophageal cancer. Clin Cancer Res 2007; 13: 515–22. doi: 10.1158/1078-0432.CCR-06-1058 [DOI] [PubMed] [Google Scholar]
- 26.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–84. doi: 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 27.Ilson DH, Bains M, Kelsen DP, O'Reilly E, Karpeh M, Coit D, et al. Phase I trial of escalating-dose irinotecan given weekly with cisplatin and concurrent radiotherapy in locally advanced esophageal cancer. J Clin Oncol 2003; 21: 2926–32. [DOI] [PubMed] [Google Scholar]
- 28.Zhang YW, Chen L, Bai Y, Zheng X. Long-term outcomes of late course accelerated hyper-fractionated radiotherapy for localized esophageal carcinoma in Mainland China: a meta-analysis. Dis Esophagus 2011; 24: 495–501. [DOI] [PubMed] [Google Scholar]
- 29.Safran H, Suntharalingam M, Dipetrillo T, Ng T, Doyle LA, Krasna M, et al. Cetuximab with concurrent chemoradiation for esophagogastric cancer: assessment of toxicity. Int J Radiat Oncol Biol Phys 2002; 72: 958–9. [DOI] [PubMed] [Google Scholar]
- 30.Lin SH, Wang L, Myles B, Thall PF, Hofstetter WL, Swisher SG, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012; 84: 1078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]