Abstract
Objective:
To ascertain the progress being made towards the implementation of stereotactic ablative body radiotherapy (SABR) treatment in the UK, to obtain details of current practice in centres with an active treatment programme and to assess the projected future provision.
Methods:
In August 2012, an online questionnaire was sent to all 65 UK radiotherapy institutions. The included questions covered the current number of patients being treated and the intended number of patients for each clinical site; immobilization and motion management methods; CT scanning protocols; target and organ-at-risk delineation; treatment planning; image-guidance and treatment protocols; and quality assurance methods.
Results:
48/65 (74%) institutions responded by the end of November 2012, with 15 indicating an active SABR programme. A further four centres indicated that a SABR protocol had been established but was not yet in clinical use. 14 of the 29 remaining responses stated an intention to develop a SABR programme in the next 2 years.
Conclusion:
The survey responses confirm that SABR provision in the UK is increasing and that this should be expected to continue in the next 2 years. A projection of the future uptake would suggest that by the end of 2014, UK SABR provision will be broadly in line with international practice.
Stereotactic ablative body radiotherapy (SABR) uses hypofractionated dose schedules (three to eight fractions) and high-precision treatment delivery to improve local control of disease. The radiobiological rationale for hypofractionation, that delivery of a few large fractions over a short overall treatment time will achieve a greater therapeutic ratio than delivery of standard treatment regimes of 20 or more fractions, is indicated in numerous studies for a range of clinical indications.1,2 This evidence is most robust in patients with non-small-cell lung cancer (NSCLC), where a recent systematic review found 2-year survival and local control rates after SABR of 70% and 91%, respectively.3 Despite the lack of randomized trial evidence, SABR is now a recognized standard of care for inoperable patients with early-stage peripheral lung tumours,4 while a recent systematic review of the use of SABR for treatment of central lung tumours found local control rates >85% for biologically equivalent tumour doses >100 Gy, with low rates of treatment-related toxicity.5 These promising outcomes have led to suggestions that SABR could be an acceptable alternative to surgery in operable patients with NSCLC, potentially enabling a balance to be struck between the risk of morbidity and mortality of lobectomy, with the increased risk of locoregional recurrence from SABR.6–8
However, there are significant risks associated with introducing SABR in a clinic owing to the high-dose fractions, the non-standard method of dose prescription and the complex nature of both planning and delivery.9,10 Post-SABR radiological changes might differ with the delivery technique used.11 In particular, volumetric modulated arc therapy (VMAT) compared with intensity-modulated radiotherapy (IMRT) offers improvement in delivery efficiency owing to a reduction in delivery time, as well as a reduction in monitor unit (MU) usage, which results in a subsequent reduction of integral radiation dose to the rest of the body.12 Although initial reports on SABR (then termed stereotactic body radiotherapy) for thoracic tumours originated from the Karolinska Institute, Stockholm, Sweden, in the mid-1990s,13 the first treatments in the UK started as recently as 2009.
The UK SABR Consortium was established in early 2008 with the aim of achieving a consensus on how best to develop, implement and research SABR in the UK. An initial version of the UK SABR Consortium guidelines,14 which were intended to standardize UK implementation and ensure safe delivery of SABR to early-stage lesions in the peripheral lung, were written to be consistent with, or drawn from, the RTOG 023615 and ROSEL trial16 protocols. Updating this guidance is part of the ongoing work of the consortium, and the most recent version is available online (http://actionradiotherapy.org/). Subsequently, the 2011 National Radiotherapy Implementation Group (NRIG) report17 acknowledged that SABR had become the standard of care for the management of early-stage medically inoperable peripheral NSCLC. However, for other treatment sites, additional research data are required and, therefore, SABR should be delivered only within a clinical trial setting.
The NRIG report proposed that, amongst other functions, the UK SABR Consortium should develop an interdepartmental audit programme to support widespread clinical implementation. To this end, in April 2012, in collaboration with the NCRI Radiotherapy Trials Quality Assurance (RTTQA) group and the National Physical Laboratory (NPL), the Consortium appointed an experienced multidisciplinary QA group to establish and address the QA needs of the Consortium membership. Subgroup membership consisted of five supporting centres, representing a range of treatment platforms.
After frequent requests from within the Consortium for assistance in developing the SABR protocols (at least 29 requests for support, ranging from e-mail advice to performing a full-external audit), the QA subgroup decided to circulate a detailed questionnaire to the Consortium membership to address several issues:
• Quantify the number of UK centres actively treating with SABR and the number intending to develop a SABR service in the next 2 years, so that the need for a formal mentoring programme could be assessed.
• Identify current and future clinical sites being treated with SABR.
• Identify the equipment used in different institutions so that mentors and mentees could be efficiently matched.
• Quantify the resource implications of a SABR service.
• Determine if the Consortium guidelines are being adhered to, guide best practice and alert outlying centres to possible improvements in workflow and quality.
This would build on a previous questionnaire circulated by the Consortium in June 2010, the results of which were reported by Baker et al18 in 2011 and identified seven treating centres in the UK, with a further six centres intending to treat using this technique in the near future.
METHODS AND MATERIALS
An online questionnaire was sent to the Heads of Radiotherapy Physics at all 65 UK radiotherapy centres in August 2012. The survey took approximately 30 min to complete and aimed to ascertain the progress being made in the implementation of SABR treatment and to obtain details of current practices in centres with active treatment programmes. Non-responders were followed up by e-mail in October 2012, which increased the response rate from 31% to 74% (48/65 UK institutions). Centres that reported having established a SABR treatment pathway but that the pathway was not yet active were not included in the data analysis, except in relation to four-dimensional CT (4D-CT) scanning, where significant experience had been gained in all such cases.
The questionnaire covered several areas, often in considerable detail: current and intended number of patients being treated for each clinical site; immobilization and motion management methods; CT scanning protocols; target and organ-at-risk (OAR) delineation; treatment planning; image-guidance and treatment protocols; and QA methods.
The following analysis of the questionnaire and the reporting of results are influenced by the fact that all UK centres delivering SABR are providing a service for peripheral lung, whereas only a minority are doing so for other extracranial sites. Where the data suggest a systematically different practice for non-lung indications, this has been highlighted. Even amongst “treating” centres, the response rate to some questions was partial, possibly because of the wording of the questionnaire. Results are therefore quoted as a fraction of the centres responding to each question. Where appropriate, the results are compared with those of published surveys from elsewhere in the world to put UK practice into an international context.
RESULTS
Current status and future expectations of UK stereotactic ablative body radiotherapy provision
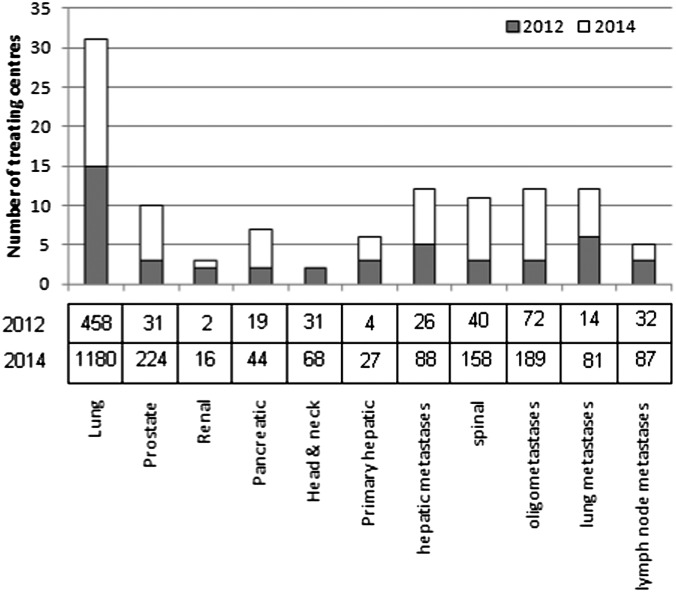
Response data were collated at the end of November 2012, at which point, 48/65 (74%) centres had responded. 15 of the 48 responding centres (23% of the UK centres) were treating patients with SABR. On average, these centres were each treating 31 patients for lung SABR per annum (range, 4–180 patients). The 8/15 centres currently treating <25 per annum, which is the minimum level of provision recommended by the NRIG,17 could perhaps be explained by limited initial provision as confidence and experience are acquired. Four UK centres stated that they had developed a treatment pathway but that the SABR programme was not yet active. 14 of the remaining 29 centres that responded to the questionnaire plan to introduce SABR by the end of 2014, taking the total number of treating centres to 33. Previous national surveys from Japan and the USA suggest that despite a comparatively rapid uptake, current UK SABR provision (23%) still lags behind that of other countries: approximately 56% of Japanese institutions in 200919 and 64% of US oncologists in 201120 had adopted SABR.
The responses shown in Figure 1 suggest that this rapid early uptake is expected to continue, with a projected doubling of the number of UK centres providing SABR by the end of 2014 and >1000 patients being treated annually using standardized protocols based on the Consortium guidelines14 (only 4/33 centres stated an intention to treat less than the 25 patient annual workload recommended by NRIG once their SABR programmes are active). These data might represent an optimistic projection of future uptake but would suggest that UK SABR provision will be broadly in line with international practice within 2 years. However, even this projection is still at the lower end of the NRIG guidance for commissioners, which estimated a demand of 1000–3000 patients for inoperable peripheral lung cases.17
Figure 1.
A graph showing the results from the current survey, indicating current levels and 2-year projections of the number of UK centres with an active stereotactic ablative body radiotherapy programme. The current and predicted annual numbers of treated patients is tabulated below the graph. Adapted with permission from the British Institute of Radiology.
Managing tumour motion and CT simulation
Immobilization and management of tumour motion is essential to accurately deliver a high dose to the target.
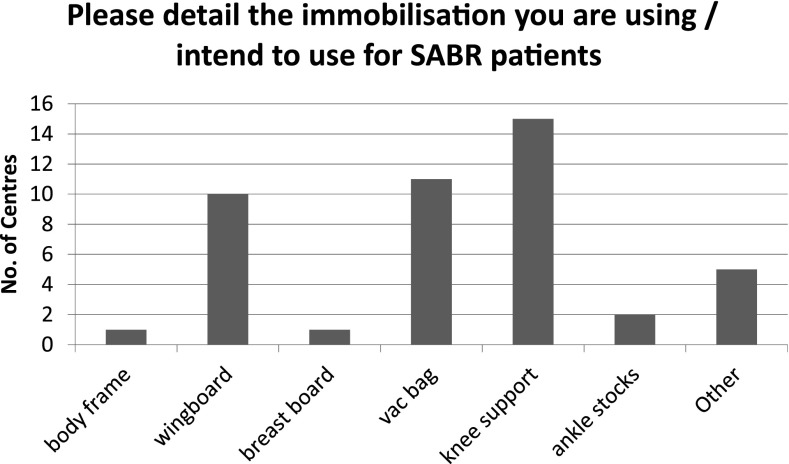
A range of immobilization devices are used throughout the UK, as indicated in Figure 2 [with “other” including thermoplastic shells, BodyFix® (Medical Intelligence, Medizintechnik GmbH, Schwabmunchen, Germany) frames and mattresses]. 12/15 centres indicated the use of a knee support for patients undergoing lung treatments, while it is used universally for other SABR treatments. Vacbag® (CIVCO Medical Solutions, Orange City, IA) and wingboard are used for lung treatments by 10/15 and 9/15 centres, respectively. Several centres used a combination of devices with the most popular combination being the use of a wingboard with vacuum immobilization. This differs markedly from reported practice in Japan,19 where the body frame was used by 68% of centres, compared with just 1/15 centres in the UK. However, a US survey21 showed that although 24% of respondents used the stereotactic bodyframe, 52% used an alpha-cradle/vacuum-lock system alone. This may be owing to the fact that 62% of centres in Japan (2009)19 were using two-dimensional portal film for treatment verification compared with 74% of US centres (2013) using three-dimensional cone beam CT (CBCT), and therefore the Japanese centres were more reliant on rigid immobilization.
Figure 2.
Immobilization devices currently used in the UK (15 treating centres). SABR, Stereotactic ablative body radiotherapy.
Of the 19 centres that stated they had an established treatment process for SABR patients (15 of which were already treating patients with SABR), 15 centres use 4D-CT, while 1 centre intended to do so in the future but currently controlled respiratory motion using abdominal compression. Three other centres use CyberKnife™ Synchrony® (Accuray Inc., Sunnyvale, CA) tracking during treatment instead. This is broadly in line with the recommendation of the Consortium guidelines,14 which states that all centres should move towards the use of 4D-CT for treatment of peripheral lung, although it might not always be possible, for example, in patients with slow, shallow or irregular breathing cycles, or even necessary, for example, in patients with very superior tumours that have been observed not to move under fluoroscopy. However, 4D-CT is not only limited to use with lung patients but also tends to be used for other SABR indications where respiratory motion might have an impact, specifically hepatic and oligometastatic lesions. The widespread UK use of 4D-CT reflects the recent uptake of this scanning technique, which can inherently account for tumour motion by the delineation of an internal target volume (ITV). This perhaps explains why gating, breath holding and abdominal compression are rarely used in UK SABR practice, in sharp contrast to the Japanese survey from 2009,19 where the use of 4D-CT is not mentioned. However, the US survey of SABR21 practice suggests that 4D-CT (used in 75% of centres) is often used in combination with abdominal compression (51%) or gating (31%).
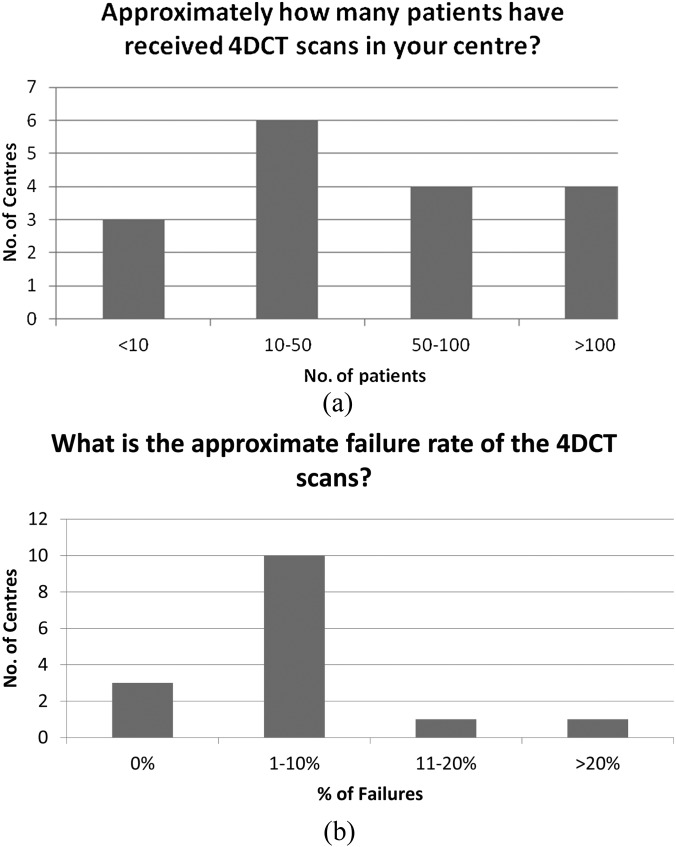
UK centres have a range of experience with 4D-CT (Figure 3a). The failure rate tends to be <10% (Figure 3b), and questionnaire responses indicate that this is primarily due to issues of patient compliance or breathing irregularity. 4D-CT scans are largely used to image the entire scan volume (14/17) rather than just the section containing the tumour, and contrast is used either as standard or in selected lung cases in 14/17 centres. 11 centres provided dose–length product (DLP) values to indicate the typical 4D-CT scan dose, with the values found to vary by almost an order of magnitude (range, 400–3840 mGy cm). Further investigation suggested a possible dependence on manufacturer (Table 1) perhaps due to differences in their implementation of 4D-CT, whereas the experience of the centre in terms of the number of 4D-CT scans performed, as well as the choice of scan length, was not seen to correlate with DLP.
Figure 3.
(a) Current provision of four-dimensional CT (4DCT) amongst stereotactic ablative body radiotherapy–active centres in the UK and (b) approximate failure rate of 4DCT scans in the UK.
Table 1.
Four-dimensional CT scan dose–length product (DLP) values grouped by scanner manufacturers
| Manufacturers | n | DLP mean (mGy cm) | DLP range (mGy cm) |
|---|---|---|---|
| Philips Healthcare (Best, Netherlands) | 4 | 648 | 400–800 |
| GE Healthcare (Buckinghamshire, UK) | 5 | 2756 | 1440–3840 |
| Siemens Healthcare (Erlangen, Germany) | 2 | 1550 | 1500–1600 |
Delineation and treatment planning
12/15 centres delineate an ITV to contain respiratory-induced tumour motion within the treated region, either directly from the maximum intensity projection or from the union of gross tumour volumes on all or a selection of individual phases (one of these centres uses this ITV strategy in combination with others). The three centres not using an ITV-based approach to target delineation are instead using the CyberKnife Synchrony motion management system.
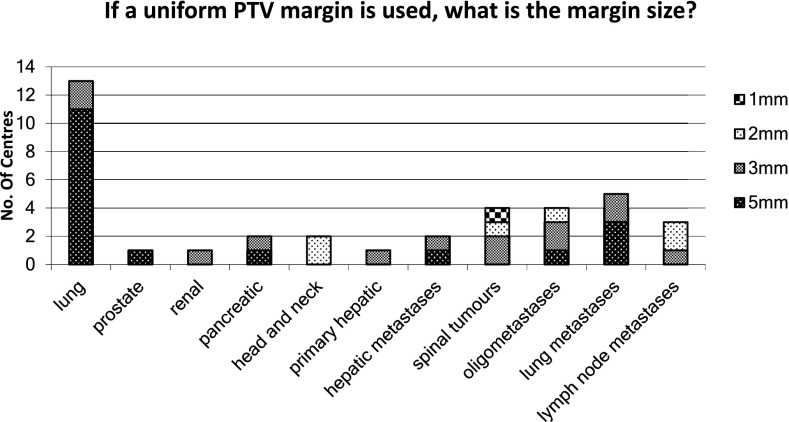
It is most common to have uniform planning target volume (PTV) expansion margins, except in the prostate where a smaller posterior margin is often used. Minimum margins for different clinical sites are not mandated by the Consortium guidelines,14 as the optimal values will depend on the immobilization, scanning protocol and delivery method used in each centre. However, centres are encouraged to establish appropriate margins and audit their ongoing suitability as per “on target”22 recommendations. Reported PTV margins vary from 1 to 5 mm, with 5 mm being more common in treatment sites where there is uncertainty due to breathing motion, e.g. lung and liver lesions (Figure 4). This might also explain the wide range of margins used for oligomets, which might depend more on the location of the met than on the hospital at which they are treated.
Figure 4.
The planning target volume (PTV) margin size across a number of treatment sites.
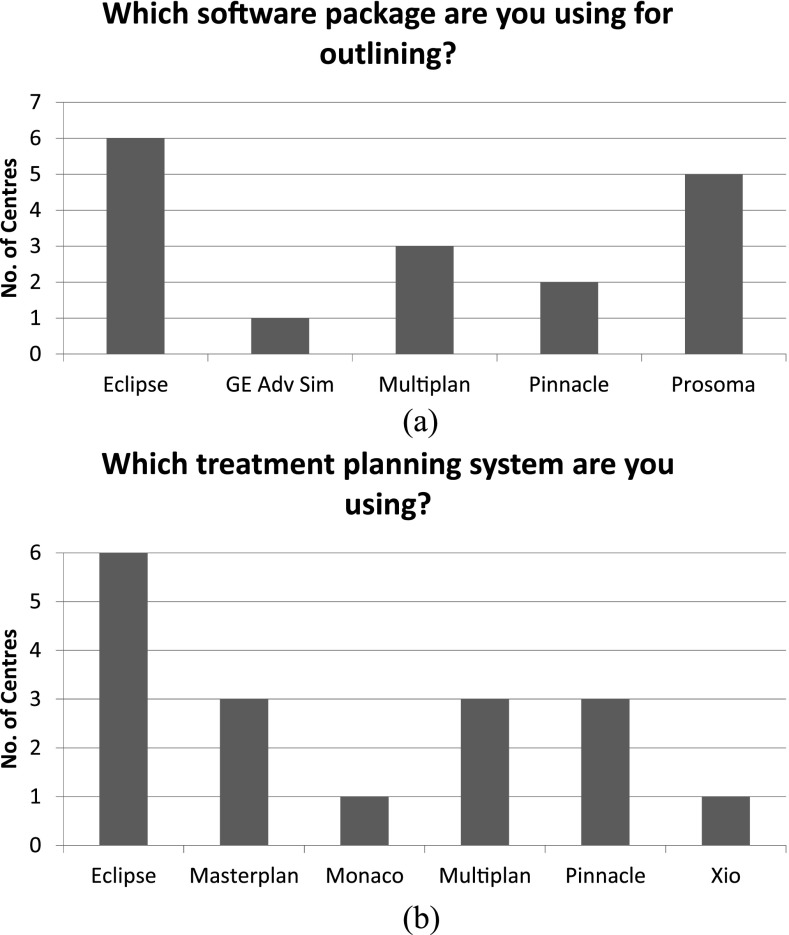
Figure 5a,b, respectively, illustrate the diversity of equipment used throughout the UK to delineate structures and plan lung SABR treatments. 5/15 centres use some form of auto-segmentation software to aid delineation (with 2 centres using this for non-lung SABR plans), whereas only 4/15 rely entirely on the clinician for lung SABR delineation.
Figure 5.
Illustrating the diversity of equipment used throughout the UK to (a) delineate structures and (b) plan lung stereotactic ablative body radiotherapy treatments (responses from 15 treating centres). Some centres used multiple systems. Eclipse™ (Varian Medical Systems, Palo Alto, CA), GE Adv Sim (GE Health Care, Buckinghamshire, UK), Monaco® (Elekta AB, Stockholm, Sweden), Multiplan® (Accuray Incorporated, Sunnyvale, CA), Pinnacle® (Philips Healthcare, Best, Netherlands), ProSoma (Oncology Systems Limited, Shropshire, UK), Oncentra® Masterplan (Nucletron™, Netherlands), Xio (Elekta AB).
7/14 centres use VMAT delivery, whereas 10/14 use a form of inverse-planned solution in some or all cases. Only 5/14 centres use exclusively fixed-field techniques and 6 centres use non-coplanar beams for at least some of their patients. The Japanese survey of 200919 reported IMRT/VMAT solutions in 21% of centres, but it is perhaps to be expected that this proportion might have increased significantly in the intervening period and that the same trend towards increased delivery complexity as is seen in the UK might also be occurring. In the more recent US survey,21 although 48% are still using static non-coplanar beams, >40% are using IMRT/VMAT solutions. For lung SABR, VMAT has been shown to offer improved conformity, significantly reduced treatment times and comparable or reduced OAR doses.23–25 It is therefore anticipated that the use of this delivery technique will continue to increase.
For SABR indications other than lung, the tendency is for non-coplanar beam arrangements to be used, which perhaps reflects the high proportion of CyberKnife-equipped centres treating these patients.
The 3/15 centres treating with CyberKnife, which provides both Type A and Monte Carlo algorithms, had elected at the time of the questionnaire to plan using a Type A algorithm, which does not account for variations in electron transport with the density of the medium. All other centres use a more robust Type B algorithm as recommended by the Consortium guidelines.14 This reduction in the use of Type B algorithms compared with the reported 94% of centres in Japan probably reflects the increased use of CyberKnife in the UK.
Verification and treatment
Of the treating centres, 60% (9/15) are performing treatment verification with patients on the linac prior to Fraction 1 (i.e. checking patient compliance, practicalities of plan delivery and immobilization etc.). This step, often referred to as “Day 0”, is recommended in the Consortium guidelines,14 so the reduced implementation perhaps reflects centres with more experience, having greater confidence and feeling able to remove this time-consuming process (mean duration, 34 min; range, 20–50 min).
SABR lung treatments in the UK tend to be delivered on conventional linear accelerators with CBCT imaging facilities, with equal numbers, 5/12 responders, using Elekta® Synergy (Elekta AB, Stockholm, Sweden) or Varian® OBI/TE (Varian Medical Systems, Palo Alto, CA). A minority of centres (2/12) reported the use of CyberKnife delivery, which probably reflects the current lower install base of this technology in the UK.
Treatment of other extracranial sites with SABR is low with standard linacs, with prostate, pancreatic, renal and head and neck SABR being performed exclusively in CyberKnife centres. The use of ExacTrac® imaging on the Novalis® (Brainlab LG, Fedkirchen, Germany; Varian Medical Systems, Palo Alto, CA) is reported for treating spinal tumours and there is some use of standard linacs for treating both primary and metastatic liver disease.
Image guidance is mainly with a soft tissue registration for lung SABR (11/14 centres) with some centres also using a preliminary automatic match to a region of interest around the PTV. In other areas of the body the use of implanted fiducial markers is more common (all centres treating prostate, renal or pancreatic cancer do so using fiducial implants), while bony anatomy is used for indications where it is likely to provide a good surrogate for the tumour position (e.g. head and neck and spinal tumours). Image guidance for liver SABR (both primary and metastatic) tends to use implanted maker techniques, sometimes in combination with soft tissue registration.
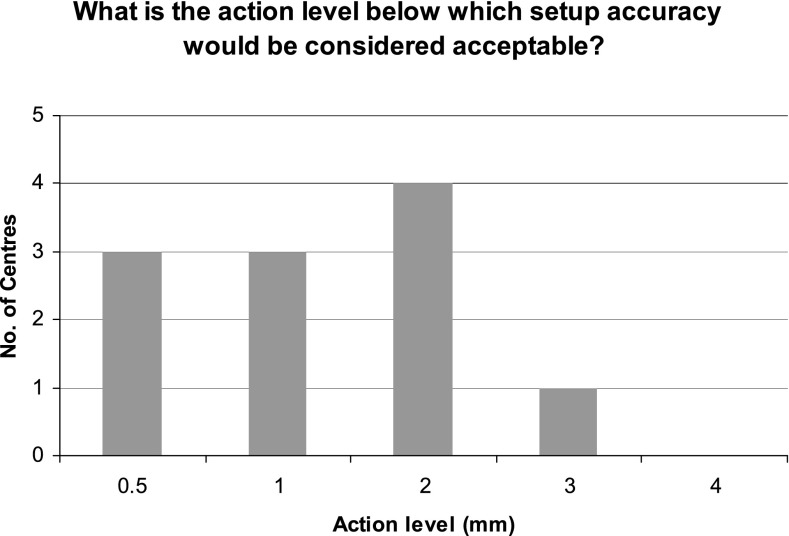
The guidelines state that centres should assess the accuracy of immobilization device/s used for positioning patients for SABR and that systematic setup errors should be within 3–5 mm. The exact values will depend on the accuracy of equipment/couch repositioning together with interobserver variability of image registration. For example, CyberKnife reports an accuracy of 0.5 mm, whilst TrueBeam™ (Varian Medical Systems) tolerance for couch repositioning is 0.5 mm, and Varian On-Board Imager®/Elekta couch tolerances are both 1.0 mm. The reported imaging tolerance levels for lung SABR, as shown in Figure 6, tended to be higher (mean, 1.41 mm) than those for other sites (mean, 0.83 mm). This is likely to be in part as a result of the tumour motion associated with lung treatments and might also represent the variation in imaging techniques between the mainly linac-based lung SABR and the higher proportion of CyberKnife deliveries for other clinical indications. All centres reported that the tolerances chosen were achievable.
Figure 6.
Action level below which setup accuracy would be considered acceptable for patients for lung treatments. As the number of centres treating non-lung stereotactic ablative body radiotherapy is low, these values have not been displayed.
Imaging during and/or following treatment is suggested in the Consortium guidelines14 to verify that patient positioning is maintained. For conventional linac-based lung SABR, this recommendation is largely being followed with 3/11 centres performing post-correction imaging, 6/11 performing mid-treatment imaging and 9/11 imaging after the treatment has finished. The three centres treating with CyberKnife use tumour tracking, which involves regular verification of tumour position. For other clinical sites, the post-correction and post-treatment imaging is rare but mid-treatment imaging is almost universal, perhaps reflecting the different imaging modalities available on the CyberKnife and Novalis, which are predominately used to treat these lesions and can acquire low-dose planar tube potential images between treatment fields.
Quality assurance
Across all treatments sites, >50% of treating centres are still performing patient-specific QA for all patients. This response is in line with replies from a National SABR Dosimetry Questionnaire in early 2012 (G Distefano, Royal Surrey County Hospital, Guildford, UK, Personal Communication), where >50% of centres were using two or more methods for patient-specific QA. Eight centres are using a homogeneous phantom and four a semi-anthropomorphic phantom. 8/9 centres said that additional routine linac QA is performed by a member of the physics staff.
Timings for entire process of lung SABR
To help new centres allow for the extra time resources required for the implementation of a SABR technique, the questionnaire asked each centre to state the time required for each major step in the SABR workflow. Whilst the outlining/planning and QA takes longer than it does for conventional treatments, the total treatment time can be significantly shorter owing to the reduced number of fractions.
Only 3/19 centres use a simulator session as preparation for 4D-CT lung scans, the duration of which can be anything from <20 to 60 min, with a trend being observed towards the use of a simulator session leading to shorter subsequent scanner sessions. The length of 4D-CT scan sessions themselves, which can be very variable (Table 2), appears to be unaffected by the choice of immobilization and only weakly dependent on the centre's experience.
Table 2.
A summary of the most frequent time at each stage of the stereotactic ablative body radiotherapy process in the UK compared with that in Japan
| Stages | UK mode (min) | Japan mode (min) |
|---|---|---|
| 4D-CT | 30–40 | |
| Target and OAR delineation | 30–60 | |
| Treatment planning | >91 | 61–120 |
| Patient-specific plan QA | 30–45 | 50–60 |
| Daily treatment delivery | 21–30 | 30 |
4D-CT, four-dimensional CT; OAR, organ at risk; QA, quality assurance.
The time required for treatment planning was not seen to clearly decrease with the SABR-related experience of the centre or the use of a particular treatment planning system. Treatment planning times for other extracranial sites were broadly similar to those shown in Table 2.
For a treatment to become widely available in busy hospitals as part of the National Health Service, it must be deliverable in a reasonable time. Treatment times for delivery of SABR vary considerably across the country (Table 2), perhaps representing the wide range of treatment techniques and imaging protocols used. Lung SABR is on average quicker than for other sites and mean times compare well to results from a previous Japanese Survey in 200919 (Table 2).
DISCUSSION
The survey benefited from a good response rate (74%) and can be assumed to accurately reflect both current practice and the aspirations of future provision of SABR in the UK. The data are considered robust for treatment of peripheral lung owing to the higher number of treating centres, but perhaps provide only an indication of the future direction of UK provision for other extracranial sites. It would be very useful to repeat this survey in 2015, to assess how realistic the intentions expressed in the current survey have been and whether further investment would be required to increase provision of this clinically advantageous technique. As outlined by a recent review article written by UK SABR Consortium members that describes the context in which this treatment technique is developing in the UK,10 the data on SABR provision that have been acquired from the questionnaire have already been used to inform successful negotiations regarding the need to fund a mentorship scheme for centres implementing a SABR programme and to assign appropriate mentors based on the equipment used at each centre. Furthermore, the information gathered has been used, in collaboration with the NPL and RTTQA, to guide the successful development and implementation of a formal dosimetry audit, which has been carried out in six centres using a range of planning and delivery platforms. A follow-up audit using a semi-anthropomorphic thorax phantom together with alanine and radiochromic film is currently underway across 20 treating centres.
The survey also provides reassurance that SABR treatments in the UK are largely performed according to the guidelines established by the UK SABR Consortium, albeit with some clinical indications being treated where no formal guidance is offered.
The clinical commissioning policy for SABR, published by the NHS Commissioning Board in April 2013,26 reports that there is sufficient evidence to routinely commission SABR for the subset of patients with early NSCLC meeting explicit inclusion and exclusion criteria, indicating that treatment of this indication will be routinely funded. This is likely to progress the development of SABR treatment for early NSCLC in the UK.
ACKNOWLEDGMENT
We thank the UK SABR Consortium members for their input to this article.
REFERENCES
- 1.Høyer M, Swaminath A, Bydder S, Lock M, Méndez Romero A, Kavanagh B, et al. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys 2012; 82: 1047–57. doi: 10.1016/j.ijrobp.2011.07.020 [DOI] [PubMed] [Google Scholar]
- 2.Buyyounouski MK, Price RA Jr, Harris EE, Miller R, Tomé W, Schefter T, et al. Stereotactic body radiotherapy for primary management of early-stage, low- to intermediate-risk prostate cancer: report of the American Society for Therapeutic Radiology and Oncology Emerging Technology Committee. Int J Radiat Oncol Biol Phys 2010; 76: 1297–304. [DOI] [PubMed] [Google Scholar]
- 3.Soldà F, Lodge M, Ashley S, Whitington A, Goldstraw P, Brada M. Stereotactic radiotherapy (SABR) for the treatment of primary non-small cell lung cancer; systematic review and comparison with a surgical cohort. Radiother Oncol 2013; 109: 1–7. doi: 10.1016/j.radonc.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 4.Louie AV, Senthi S, Palma DA. Surgery versus SABR for NSCLC. Lancet Oncol 2013; 14: e491. doi: 10.1016/S1470-2045(13)70446-7 [DOI] [PubMed] [Google Scholar]
- 5.Senthi S, Haasbeek CJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 2013; 106: 276–82. doi: 10.1016/j.radonc.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 6.Shirvani SM, Chang JY, Roth JA. Can stereotactic ablative radiotherapy in early stage lung cancers produce comparable success as surgery? Thorac Surg Clin 2013; 23: 369–81. doi: 10.1016/j.thorsurg.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 7.Donington J, Ferguson M, Mazzone P, Handy J Jr, Schuchert M, Fernando H, et al.; Thoracic Oncology Network of American College of Chest Physicians; Workforce on Evidence-Based Surgery of Society of Thoracic Surgeons. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest 2012; 142: 1620–35. [DOI] [PubMed] [Google Scholar]
- 8.Senan S, Paul MA, Lagerwaard FJ. Treatment of early-stage lung cancer detected by screening: surgery or stereotactic ablative radiotherapy? Lancet Oncol 2013; 14: e270–4. doi: 10.1016/S1470-2045(12)70592-2 [DOI] [PubMed] [Google Scholar]
- 9.Martin A, Gaya A. Stereotactic body radiotherapy: a review. Clin Oncol (R Coll Radiol) 2010; 22: 157–72. doi: 10.1016/j.clon.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 10.Jain P, Baker A, Distefano G, Scott AJD, Webster GJ, Hatton MQ. Stereotactic ablative radiotherapy in the UK: current status and developments. Br J Radiol 2013; 86: 20130331. doi: 10.1259/bjr.20130331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senthi S, Dahele M, van de Ven P, Slotman B, Senan S. Late radiological changes after stereotactic ablative radiotherapy for early stage lung cancer: a comparison of fixed-beam versus arc delivery techniques. Radiat Oncol 2013; 109: 77–81. doi: 10.1016/j.radonc.2013.08.034 [DOI] [PubMed] [Google Scholar]
- 12.Teoh M, Clark C, Wood K, Whitaker S, Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol 2011; 84: 967–96. doi: 10.1259/bjr/22373346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blomgren H, Lax I, Näslund I, Svanström R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995; 34: 861–70. [DOI] [PubMed] [Google Scholar]
- 14.Stereotactic Ablative Body Radiotherapy (SABR): A Resource, SABR UK Consortium. Available from: http://actionradiotherapy.org/wp-content/uploads/2014/03/UK-SABR-Consortium-Guidelines.pdf
- 15.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Toxicity analysis of RTOG 0236 using stereotactic body radiation therapy to treat medically inoperable early stage lung cancer patients. Int J Radiat Biol 2007; 69: S86. [Google Scholar]
- 16.Hurkmans CW, Cuijpers JP, Lagerwaard FJ, Widder J, van der Heide UA, Schuring D, et al. Recommendations for implementing stereotactic radiotherapy in peripheral stage IA non-small cell lung cancer: report from the Quality Assurance Working Party of the randomised phase III ROSEL study. Radiat Oncol 2009; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Radiotherapy Implementation Group Report. SBRT Guidelines for Commissioners. London, UK: NRIG: 2010. [Google Scholar]
- 18.Baker A, Appleton L, Scott A, Jain P. Current and future impact of stereotactic body radiotherapy on UK oncology services. Imaging Oncol 2011; 14–22. [Google Scholar]
- 19.Yasushi N, Masahiro H, Takashi M, Yuichiro N, Yukinori M, Yoshiki N, et al. Survey of stereotactic body radiation therapy in Japan by the Japan 3-d conformal external beam radiotherapy group. Int J Radiat Oncol Biol Phys 2009; 75: 343–7. doi: 10.1016/j.ijrobp.2009.02.087 [DOI] [PubMed] [Google Scholar]
- 20.Pan H, Simpson D, Mell L, Mundt A, Lawson J. A Survey of stereotactic radiation therapy in the United States. Cancer 2011; 117: 4566–72. doi: 10.1002/cncr.26067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daly ME, Perks JR, Chen AM. Patterns-of-care for thoracic stereotactic body radiotherapy among practicing radiation oncologists in the United States. J Thoracic Oncol 2013; 8: 202–7. doi: 10.1097/JTO.0b013e318279155f [DOI] [PubMed] [Google Scholar]
- 22.Merrow C, Wang I, Podgorsak M. A dosimetric evaluation of VMAT for the treatment of non-small cell lung cancer. J Appl Clin Med Phys 2012; 14: 228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verbakel W, Senan S, Cuijpers J, Slotman B, Lagerwaard F. Rapid delivery of stereotactic radiotherapy for peripheral lung tumours using volumetric intensity-modulated arcs. Radiat Oncol 2009; 93: 122–4. doi: 10.1016/j.radonc.2009.05.020 [DOI] [PubMed] [Google Scholar]
- 24.Ong CL, Verbakel W, Cuipers J, Slotman B, Lagerwaard F, Senan S, Stereotactic radiotherapy for peripheral lung tumours: a comparison of volumetric arc therapy with 3 other delivery techniques. Radiat Oncol 2010; 97: 437–42. doi: 10.1016/j.radonc.2010.09.027 [DOI] [PubMed] [Google Scholar]
- 25.The Royal College of Radiologists, Institute of physics and Engineering in Medicine, Society and College of Radiographers. On target: ensuring geometric accuracy in radiotherapy. London, UK: The Royal College of Radiologists; 2008. Available from: http://www.rcr.ac.uk/docs/oncology/pdf/BFCO(08)5_On_target.pdf [Google Scholar]
- 26.NHS Commissioning Board. Clinical Commissioning Policy: stereotactic Ablative Body Radiotherapy for Non-Small-Cell Lung Cancer (Adult). NHSCB/B01/P/a. 2013.