Abstract
Objective:
Radiotherapy treatments of post-mastectomy chest walls are complex, requiring treatment close to skin, necessitating bolus use. Commonly used 5- and 10-mm-thick boluses develop full skin dose, needing removal for the latter half of treatment and requiring two treatment plans to be generated. Can a thinner bolus be used for all treatment fractions, requiring only one plan?
Methods:
Investigation of doses received using (A) a half-time 10-mm-thick Vaseline® bolus (current situation); (B) a brass mesh (Whiting & Davis, Attleboro Falls, MA) and (C) 3- and 5-mm Superflab™ (Mick Radio-Nuclear Instruments, Mount Vernon, NY) for 6 and 15 MV. Dosimetric measurements in Barts WT1 solid water and an anthropomorphic phantom, using ionization chambers and thermoluminescent dosemeters, were used to study the effect of different bolus regimes on the photon depth–dose curves (DDCs) and skin doses.
Results:
Measured skin doses for the current 10-mm-thick Vaseline bolus, brass mesh and 3-mm bolus were compared (5 mm bolus has been rejected). The brass mesh has the least effect on the DDC, with changes <0.7% for depths greater than dmax. Brass mesh conforms superiorly to skin surfaces. Measurements on an anthropomorphic phantom demonstrate an increased skin dose compared with our current treatment protocol.
Conclusion:
Brass mesh has the smallest effect on the DDC, whilst sufficiently increasing surface dose. It can be removed at any fraction, based on a clinical decision, without the need for generating a new plan. Treating with one plan significantly reduces planning times.
Advances in knowledge:
Quantification of skin doses required and achieved from wax-on/wax-off treatment compared with alternative available breast boluses.
Chest walls can be difficult to treat with radiotherapy because of irregular surface contours, large curvature and near-surface target volumes necessitating bolus. Bolus can either be used for the whole treatment or for part of the treatment course. Vaseline® and Superflab™ (Mick Radio-Nuclear Instruments, Mount Vernon, NY1) bolus materials are commonly used, but both affect the radiation distribution sufficiently that if one is using bolus for only a part of the treatment course, then two treatment plans are required, one for the bolus and one for the non-bolus fractions. A recent article published by Healy et al2 from the Radiation Oncology Department at the University of California (UCD), Sacramento, CA, presents clinical findings of using brass mesh for post-mastectomy chest wall irradiations (PMRT). The article discusses the skin dose effects of using brass mesh during PMRT at UCD from January 2008 to June 2011. The report states that only one treatment plan is required when using the brass mesh throughout the course of treatment since brass mesh does not substantially change the number of monitor units (MUs) associated with the plan. The mesh was only removed when brisk erythema or moist desquamation was achieved.
In the UK, the National Institute for Health and Clinical Excellence provides guidelines3 on the diagnosis and treatment of early and locally advanced breast cancer; however, no reference is given to the use of bolus material to aid treatment. In hospital centres, this is predominantly at the discretion of the clinician responsible for the treatment. The American Society of Clinical Oncology has also published guidelines for PMRT, but similarly no advice is given on the use of bolus.4 Various other articles reference the lack of guidance given to the application of bolus.5–7 A worldwide survey carried out in 2004 by the Department of Radiation Oncology, Sunnybrook Health Sciences Centre, Canada,8 reviewed the use of bolus in PMRT for oncologists practising in the USA, Canada, Europe and Australasia. The results showed a wide variation in the use of application of bolus in radiotherapy treatment. Respondents from the Americas were significantly more likely to always use a bolus (82%) than the Europeans (31%), as were the Australasians (65%). Europeans were significantly more likely to use a bolus for specific indications. The results also showed wide variation in the schedule of application [every day (33%) and alternate days (46%)] and thickness used [<1 cm (35%) and 1 cm (48%)]. For centres using bolus on every treatment fraction, only one treatment plan is required, with bolus simulated in the treatment planning system (TPS). However, for centres that use bolus on alternate days, two plans must be generated, increasing the workload. This is the case of our current protocol at St Bartholomew's Hospital, London, UK.
Currently, we use a 1-cm Vaseline bolus to provide adequate build-up of dose at the skin surface for breast treatments. A prescription dose of 50 Gy in 25 fractions is given for post-reconstructed breasts or a prescription dose of 40.05 Gy is given for non-reconstructed breasts, and bolus is applied for the first half of the course. We aim to establish a bolus method that will reproduce the current clinical doses, but without the requirement for two treatment plans.
The dosimetric qualities of using brass mesh for chest wall radiotherapy and its effect on surface skin dose have been previously published by Fessenden et al9 in 1978.
This article sets out to expand the work of UCD by adding a real world head-to-head comparison of available breast boluses. Quantification of skin doses achieved using a traditional wax-on/wax-off protocol is compared with doses achieved using alternative bolus materials. This work sets out to establish if using one sheet of brass mesh full time throughout treatment is equivalent to using a Vaseline bolus during the first half of treatment, thus reducing the workload from producing two plans to producing only one. An investigation into the skin doses received using (1) a half-time 10-mm-thick Vaseline bolus (current clinical practice); (2) a 3-mm Superflab synthetic oil–gel bolus; (3) a 5-mm Superflab synthetic oil–gel bolus; and (4) a brass mesh bolus for both 6- and 15-MV treatment beams was carried out. Dosimetric measurements in solid water and an anthropomorphic phantom using an ionization chamber and thermoluminescent dosemeters (TLDs) were used to study the effect of the different bolus regimes on the photon depth–dose curves (DDCs). Results from the build-up region of the curve were used to assess skin surface doses, whilst results from depths greater than dmax were used to assess whether a single plan solution to chest wall radiotherapy with this bolus regime was feasible.
METHODS AND MATERIALS
Bolus materials and current clinical data
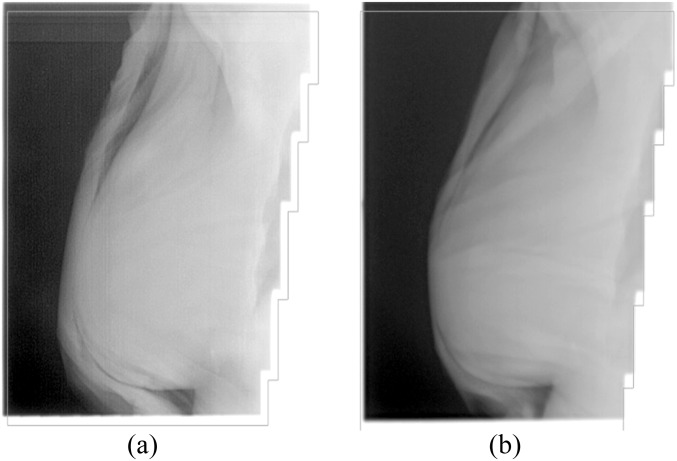
Vaseline bolus is very pliable and can be easily compressed, which makes it difficult to maintain a uniform thickness across the skin surface. Figure 1 shows two megavoltage images taken during two consecutive chest wall treatment fractions for one patient. Figure 1a demonstrates the air gaps introduced between the skin surface and bolus on application of the material. Figure 1b shows the difficulty in achieving a uniform thickness across the bolus surface when Vaseline bolus is used. The bolus is very thin at the most anterior part of the breast, leading to less build-up and therefore less surface dose at the skin.
Figure 1.
(a,b) Megavoltage image taken during patient treatment demonstrating interfraction positioning of bolus.
An alternative to Vaseline is Superflab bolus material. It is made of a synthetic oil–gel which is much less pliable than Vaseline and therefore maintains a good uniformity of thickness. Its disadvantage is that it cannot conform to the patient's skin surface since folds appear where the material tries to conform to a three-dimensional convex surface. Air gaps between the bolus material and skin surface are also common with this material, leading to reduced build-up of the radiation to the chest wall.
Bolus materials that are thick enough to significantly affect the DDC require two radiotherapy plans to be produced for each patient (one with bolus and without) to achieve the appropriate clinical dose distribution.
The most noticeable advantage of using brass mesh is its superior conformity to the skin surface in three dimensions, especially in the presence of scars and other surface irregularities. There are fewer air gaps and therefore the dose uniformity is superior. Figure 2 shows a close-up of the brass mesh and its ability to conform well to the skin.
Figure 2.
Brass mesh conforming to skin.
To assess the clinical skin doses currently being achieved, TLD measurements were carried out on eight patients. Four patients underwent chest wall irradiation post-reconstruction and four were treated having had no reconstruction. Treatment plans for each patient used a combination of 6- and 15-MV beams. The majority of bolus treatments for all patients were carried out using 15-MV beams. Five pairs of TLDs were positioned across the midline accordingly: (1) 1 cm from the medial field entry border; (2) medial central axis entry point; (3) anterior; (4) lateral central axis entry point; and (5) 1 cm from the lateral field entry border. Measurements were taken for each patient for one bolus treatment fraction and one “no bolus” treatment fraction.
Simple incident beam
Thermoluminescent dosemeter calibration for in vivo dosimetry measurements
At St Bartholomew's Hospital, LiF:Mg:Ti TLD-100 chips (3.2 × 3.2 × 0.89 mm) were used for in vivo dosimetry measurements. The TLDs were characterized for absolute dosimetry measurements by irradiating a subset of TLDs for use as calibration dosemeters. These dosemeters were irradiated under standard calibration conditions (6 MV, 10 × 10 cm field, source to surface distance = 100 cm, 100 MU) at 5-cm depth and a simultaneous Farmer® (model 2571; NE Technology, Reading, UK) chamber reading was recorded to determine the sensitivity of each chip to a known dose, when read out using a HARSHAW 5500 TLD reader (Thermo Scientific™, Erlangen, Germany). The average charge of the reference dosemeters was used to determine a reader calibration factor (in nC/Gy), converting charge to dose. The remaining “field” chips used for the measurements were then exposed under reference conditions at 5-cm depth, and their sensitivities normalized to the mean of the calibration chips, giving an element correction coefficient for each chip. The dose to each chip was determined using Equation (1), where qi is the integrated charge read-out from the exposed dosemeter i.
 |
This process was repeated to establish chip sensitivity values when exposed to a 15-MV beam. Field chips must agree to within 3% of the measured dose, otherwise they are removed from clinical use. Typically, the dose agreement was better than the maximum tolerance, with most dosemeters agreeing to within 2% of the expected dose.
Depth–dose curve measurements
DDC measurements using bolus types of Vaseline, Superflab 3 mm, Superflab 5 mm and brass mesh were carried out using a solid water phantom block and various thicknesses of solid water to simulate tissue-equivalent depths ranging from 1 to 10 mm in the build-up region. TLD pairs were positioned in plastic sachets and were centred on the beam central axis at each depth and the different bolus materials applied. The reference set-up conditions were used to irradiate the TLDs using both 6- and 15-MV beams with an exposure of 100 MU. At depths greater than dmax, Farmer ionization chamber measurements were carried out from 2- to 18-cm depths in 2-cm increments. An output measurement for each beam quality was performed and applied to all measurements. The TLDs were estimated to be equivalent to 1 mm of tissue, based on the thickness of the TLD (0.89 mm) and the packet containing them. All depths were corrected for 1 mm when plotting the DDC in the build-up region. The reference DDCs were measured with an electron diode in a water tank during linac commissioning.
Dose verification using ATOM® phantom
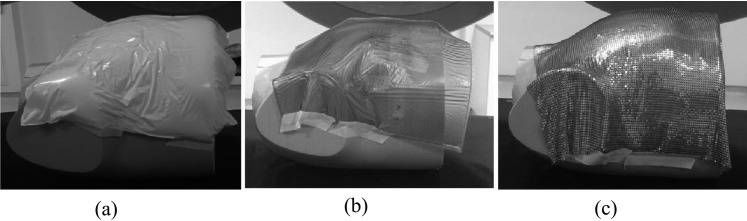
An ATOM anthropomorphic dosimetry phantom (Computerized Imaging Reference Systems, Norfolk, VA; model 70210) was used for verification of radiotherapy treatment plans to an adult female. Figure 4 shows an image of the ATOM phantom. It consists of tissue-equivalent epoxy resins to model all tissues and organs within the body. Tattoo marks were positioned laterally on the phantom as well as the anterior, close to the medial border of the field edge. This allowed a consistent reproducible set-up of the phantom. The phantom was scanned on a GE 64-slice CT scanner (GE Medical Systems, Hatfield, UK) at 120 kV with 150 mA and the data imported into Eclipse™ (Varian Medical Systems, Palo Alto, CA) TPS v. A10. Two external beam chest treatments plans were created and calculated [with analytic anisotropic algorithm (AAA) with v. 10.0.28] for each photon energy: one with 1 cm of Vaseline bolus and one without bolus, ensuring that the 95% isodose was covering the clinical treatment volume.
Figure 4.
(a) Vaseline® bolus moulded to the chest wall surface; (b) 3-mm Superflab™ (Mick Radio-Nuclear Instruments) bolus; (c) brass bolus.
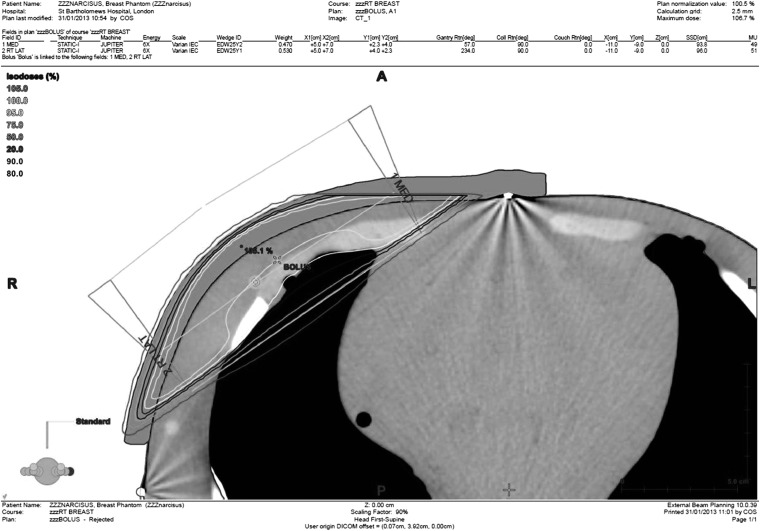
Figure 3 shows the created 6-MV Vaseline bolus plan. The optimal plan, based on the phantom geometry, uses only 6-MV beams. The same plan was recalculated by changing the beams to 15 MV to give a 15-MV plan. However, it must be noted that the 15-MV plan was not optimized for coverage. The prescription dose was chosen to be 40.05 Gy in 15 fractions (2.67 Gy per fraction) based on the current protocol at St Bartholomew's Hospital for non-reconstructed breasts. The source to skin distance to the medial tattoo was noted in addition to the X and Z shifts to the isocentre.
Figure 3.
6-MV Vaseline® bolus plan. L, left; R, right. Reproduced with permission from Varian Medical Systems.
TLDs were used to assess the dose at the skin surface for each bolus material. The chest phantom was transferred to the linac couch and the lateral and medial tattoos aligned to the lasers. Shifts to the isocentre were then made according to the treatment plan set-up. Cling-film packets, containing two TLDs side-by side, were attached to the chest wall of the phantom at nine positions within the treatment field. Four packets were positioned along the midline, three packets were positioned superior of the midline and two further packets were positioned inferior to the midline. These positions were chosen to be representative of the dose to the chest wall. The bolus materials were then placed over the chest wall successively and smoothed to aid the dose homogeneity at the surface. In Figure 4, the three bolus materials are shown applied to the chest wall; the Vaseline and Superflab materials do not conform optimally to curves on the surface, developing folds. This causes air gaps between the bolus and skin surface in addition to non-uniformity of thickness across the chest wall. By contrast, the brass bolus conforms exceptionally well to the curves and no folds are present, therefore minimizing air gaps. The bolus plan was delivered when the Vaseline bolus was positioned on the phantom chest wall. The “no bolus” plan was delivered without any bolus applied to the skin surface and when the 3-mm Superflab and brass boluses were present on the chest wall.
RESULTS
Current clinical practice
For the eight patients on treatment with the existing Vaseline bolus technique, the mean of the five TLD pair average doses was determined and the results are summarised in Table 1.
Table 1.
Summary of thermoluminescent dosemeter surface dose measurements carried out on eight patients
| Patient | No bolus (%) | Vaseline® bolus (%) | Combined skin dose (%) |
|---|---|---|---|
| 1 | 70.6 | 97.9 | 85.2 |
| 2 | 69.4 | 95.5 | 83.3 |
| 3 | 78.3 | 89.9 | 84.3 |
| 4 | 76.2 | 92.8 | 84.8 |
| 5 | 73.4 | 94.1 | 84.4 |
| 6 | 69.8 | 102.4 | 87.2 |
| 7 | 73.5 | 89.7 | 82.1 |
| 8 | 75.5 | 102.4 | 89.8 |
The results show the obvious trend that skin doses increase with bolus. Surface dose measurements at the most anterior position of the chest wall gave higher doses, as expected. The average combined skin doses were 85.1% for all patients.
Simple incident beam
Results: build-up region
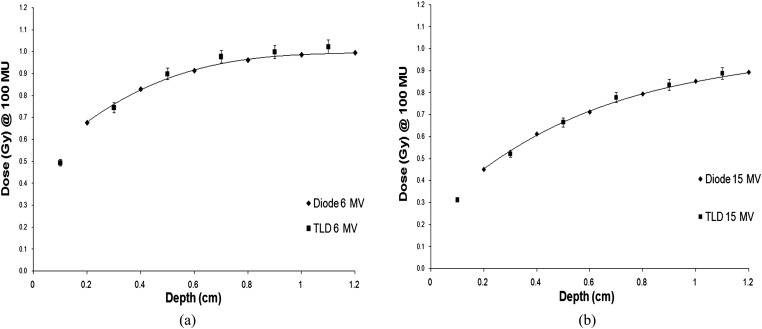
Figure 5 summarises the build-up region results when no bolus is applied and compares the TLD doses with diode measurements carried out in a water tank at commissioning (10 × 10 cm field). For both energies, the TLD measurements are in good agreement, lying within 3% of the diode results. These curves give confidence of the TLD calibration and the effectiveness of using TLDs to measure doses in steep gradients.
Figure 5.
(a) 6-MV build-up curve; (b) 15-MV build-up curve. TLD, thermoluminescent dosemeter.
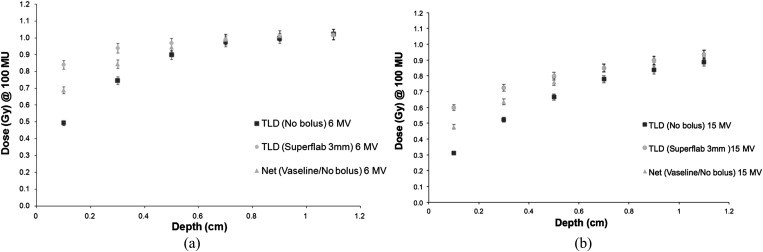
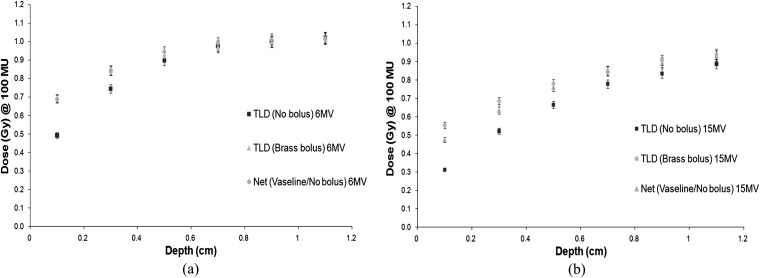
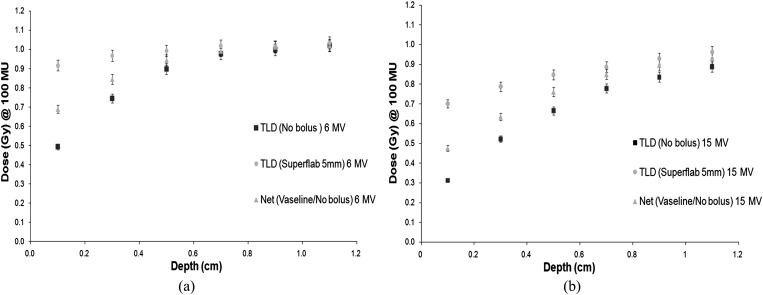
Next, measurements were carried out in the build-up region from 0.1 to 1.1 cm using each bolus type. The net dose was determined for our current protocol, which uses Vaseline bolus for half of the time and no bolus for half of the time to treat chest walls. A comparison was made of the doses at each depth for the Superflab and brass boluses when compared with the net Vaseline/no bolus doses. Figures 6–8 show the results.
Figure 6.
(a) 6-MV build-up curve Superflab™ (Mick Radio-Nuclear Instruments) 3-mm bolus. (b) 15-MV build-up curve Superflab 3-mm bolus. TLD, thermoluminescent dosemeter.
Figure 8.
(a) 6-MV build-up curve brass bolus. (b) 15-MV build-up curve brass bolus. TLD, thermoluminescent dosemeter.
Figure 7.
(a) 6-MV build-up curve Superflab™ (Mick Radio-Nuclear Instruments) 5-mm bolus. (b) 15-MV build-up curve Superflab 5-mm bolus. TLD, thermoluminescent dosemeter.
The results for Superflab 3 mm show that the doses at the skin surface and subsequent depths up to 0.6 cm are higher than the net dose given using the alternate Vaseline/no bolus treatment. The greatest dose differences are seen at the skin surface, with the dose difference reduced from 0.7 cm depth onwards. The Superflab 5-mm bolus achieves a greater dose at the surface than the 3-mm bolus skin doses. Compared with the Vaseline/no bolus treatment regime, there is a maximum dose difference of 23% for 6 MV and 28% for 15 MV for the 3-mm Superflab. A maximum dose difference of 35% (6 MV) and 51% (15 MV) for Superflab 5 mm was found.
Comparing the brass bolus build-up curve with the net Vaseline/no bolus treatment regimen, the results are more similar. The 6-MV results are in good agreement with the dose achieved using our current protocol. The 15-MV results show that more dose is achieved (+17%) at the skin surface using brass bolus. These results give confidence that the behaviour of the brass bolus is similar to that of our current Vaseline/no bolus treatment protocol.
Results: depth–dose curve after dmax
For each bolus material, Farmer ionization chamber measurements were used to determine the dose at depths greater than dmax. With the aim of carrying out treatment planning without accounting for bolus, the results must demonstrate that the dose after dmax is unaffected by the bolus material. A maximum allowed dose difference tolerance of 2% was set. This value was considered not to significantly alter the number of MUs calculated in a plan, for depths greater than dmax. TLD and ionization chamber measurements taken in solid water blocks were in good agreement with commissioned diode data. This served as a check that our measurements were consistent with the gold standard machine data. Table 2 summarises the maximum dose difference between the doses at depth achieved with the bolus compared with those achieved with the gold standard “no bolus data” set.
Table 2.
Maximum percentage dose difference between bolus depth–dose curves (DDCs) and gold standard “no bolus” DDCs
| Bolus | 6 MV (%) | 15 MV (%) |
|---|---|---|
| 3-mm Superflab™ | <2.0 | <1.9 |
| 5-mm Superflab | <2.8 | <2.7 |
| 10-mm Vaseline® | <2.8 | <2.7 |
| 1-mm brass | <0.7 | <0.7 |
Superflab™ was obtained from Mick Radio-Nuclear Instruments.
In summary, brass bolus gives the best results for matching of the DDC to the “no bolus” data for doses at depths greater than dmax. It is therefore the best candidate for a single plan solution and would not have to be modelled in the planning system. The 5-mm Superflab bolus modifies the doses at depth by up to 2.8%, therefore the bolus would need to be accounted for in the TPS. This bolus has therefore been excluded from any further investigations. The 3-mm Superflab bolus could be applied to the patient during the CT planning scan stage, and only one plan solution delivered; however, the skin doses at the surface would be significantly more than our current Vaseline/no bolus treatment regime.
ATOM phantom: clinical simulation
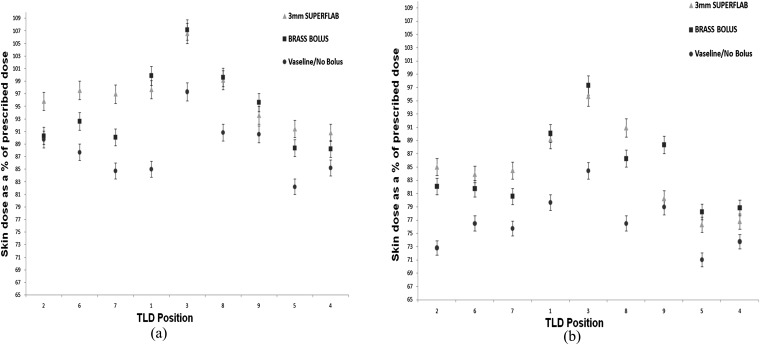
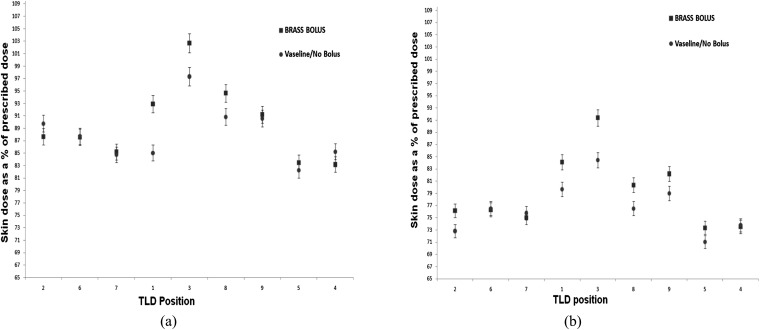
Figure 9 shows the skin dose achieved on the ATOM phantom using the 6- and 15-MV plans, respectively, for all bolus types. The TLD positions on the x-axis go from medial positions in the field (TLD positions 2, 5 and 7), through to the anterior (positions 1, 3 and 8) and lateral (positions 9, 5 and 4) positions. The net combined skin dose for the current protocol of using Vaseline bolus for the first eight fractions, and then the remaining seven fractions without bolus, is given by the circular data points. The brass bolus, (square data points) and 3-mm Superflab (triangular data points) skin doses assume that the bolus has been used throughout all treatment fractions.
Figure 9.
Skin doses measured in the treatment field for a 6-MV plan (a) and a 15-MV plan (b). TLD, thermoluminescent dosemeter.
For both treatment energies, the brass and Superflab boluses give higher doses at the skin surface than our current treatment regime with Vaseline/no bolus. Position 3 represents the most anterior dose measurement on the phantom. As expected, the doses here are the highest for all bolus types. The brass and Superflab bolus types have the greatest increase in dose at this position compared with the Vaseline/no bolus treatment dose. Positions 2, 6 and 7 represent the medial field doses, where the Superflab bolus achieves a greater skin surface dose than the brass bolus. Higher doses are expected here owing to the obliquity of the beam traversing the bolus materials. Anteriorly and laterally, the brass and Superflab TLD doses are comparable to one another, given the 3% error on the TLD measurements.
An average of all nine TLD measurements was determined and the results compared with those predicted by the TPS. All surface doses are stated as a percentage of the prescribed dose. TLD structures were created in Varian's Eclipse TPS at the measured positions and an average dose calculated by the planning system over all points within the structure. Table 3 gives the results for 6- and 15-MV plans.
Table 3.
Average skin doses (as a percentage of the prescribed dose) for 6- and 15-MV plans
| Bolus type | Average skin dose (% of prescribed dose) | ||
|---|---|---|---|
| Thermoluminescent dosemeter (%) | Treatment planning system (%) | Combined dose (%) | |
| 6-MV data | |||
| No bolus | 68.0 | 76.5 | 85.4 |
| Vaseline® | 100.7 | 103.0 | |
| 3-mm Superflab™ | 97.7 | 94.9 | 97.7 |
| Brass | 91.6 | N/A | 91.6 |
| 15-MV data | |||
| No bolus | 55.0 | 69.2 | 75.9 |
| Vaseline | 94.2 | 97.8 | |
| 3-mm Superflab | 86.5 | 85.3 | 86.5 |
| Brass | 83.3 | N/A | 83.3 |
N/A, not applicable.
Superflab™ was obtained from Mick Radio-Nuclear Instruments.
The results for the 6-MV plan show that the TPS and TLD dose values agree well, with the TPS doses marginally different. The “combined dose” column uses the measured TLD results to determine the total skin dose over all treatment fractions. Overall, the average skin dose is the highest when using 3-mm Superflab throughout all treatment fractions. The skin doses with the brass bolus on for all fractions are higher than our current method, but less than those measured with 3-mm Superflab. Comparing the 15-MV “no bolus” TLD readings with those determined by the TPS, the TPS results are higher. The AAA used by the TPS overestimates the dose at the surface when compared with the TLD measurements. A study of dose calculations in the build-up region by Oinam and Singh11 also found that the AAA overestimated the dose at depths <2 mm from the surface, when compared with TLD measurements. It was suggested that this overestimation of dose could be due to the electron contamination source used in the AAA optimization model. The combined dose results show that the 3-mm Superflab gives the highest dose to the skin, whilst the brass bolus gives a lesser dose than the Superflab, but more than our current protocol.
To compare our previous clinical patient skin dose measurements with those measured on the ATOM phantom, the combined phantom skin dose was determined. Since the clinical patients were treated with a combination of 6- and 15-MV beams (15-MV bolus plan and 6-MV no bolus plan), the combined percentage skin dose for the phantom was calculated as in Equation (2):
 |
The measured phantom skin dose is in close agreement with the average clinical patient skin dose result of 85.1%. These results give confidence in our treatment method and serve as a check between our clinical and ATOM phantom data.
Having discussed the options with the lead oncologist for chest wall treatments, the brass bolus was the favoured method for treating chest walls. Additional advantages include (1) significant reduction in workload for the whole multidisciplinary team; (2) allows for earlier start dates of patient's treatment; and (3) allows for an increased workload within the department.
Dose scaling
Since the brass bolus achieves skin doses greater than those achieved with the current Vaseline/no bolus protocol, an investigation was made into the number of fractions that the brass bolus would need to be kept on for, to give the same dose as the current protocol. It was found that similar skin doses were achieved when the brass bolus was kept on for 80% of all treatment fractions. Figure 10 shows the scaled dose results for both treatment energies.
Figure 10.
Skin doses achieved when brass bolus is used for 80% of treatment fractions and the remainder without bolus. (a) 6-MV, (b) 15-MV results. TLD, thermoluminescent dosemeter.
In the most anterior position (TLD positions 1, 3 and 8 in Figure 10), the dose achieved with the brass bolus is still greater than that achieved by the current Vaseline/no bolus treatment regime. However, it has been shown that it is very difficult to ensure that the Vaseline bolus is homogeneous across the chest wall (Figure 1). Experience has shown that, in many cases, the bolus is thinner at the anterior edge of the chest wall. Therefore, the Vaseline/no bolus skin doses are underestimated at these positions. The large dose difference between the brass bolus skin dose and the current protocol skin dose at position 3 is therefore likely to be overestimated and realistically is less than is shown.
CONCLUSION AND DISCUSSION
The aim of this work was to investigate the use of different bolus materials to optimize our current treatment protocol at St Bartholomew's Hospital. As there is no evidence for which skin dose is optimum, the skin surface dose levels from the current treatment regime of a 10-mm-thick Vaseline bolus for half of the time were used. Four bolus materials have been characterized by measuring DDCs and carrying out measurements on a representative phantom. Of the four bolus materials investigated, half-time 10-mm Vaseline and 5-mm Superflab attenuate the radiation beam sufficiently to require two treatment plans. The 3-mm Superflab bolus could be used without replanning but results in too high a skin dose for our criteria. Only the brass bolus meets all our criteria.
Therefore, our results show that a single plan solution for treating chest walls with brass bolus is feasible. The key points for use of brass bolus are:
• superior conformity to the skin surface;
• much fewer air gaps, giving a more uniform dose distribution;
• brass bolus does not significantly modify the DDC at depths greater than dmax (<1.0%);
• only one treatment plan required;
• no need to model brass in the TPS;
• use the bolus only on first 80% of treatment fractions.
For other centres using an alternate day bolus fractionation scheme, this work shows that it is possible to achieve similar skin doses using brass bolus and just one treatment plan. The number of fractions used with the brass bolus material can be modified to achieve the desired clinical result. The results of this work have prompted a clinical pilot study being carried out to trial the use of brass bolus on chest wall treatments. At the end of the pilot study, all results will be reviewed and a decision made on whether a change in protocol would benefit the department's workload and the patient's standard of care.
ACKNOWLEDGMENTS
The authors would like to thank Scott Dube for his expertise on the brass bolus.
REFERENCES
- 1.Mick Radio-Nuclear Instruments. Superflab bolus material. Available from: http://www.micknuclear.com/page_ external_beam/external_beam_accessories3.asp
- 2.Healy E, Anderson S, Cui J, Backett L, Chen AM, Perks J, et al. Skin dose effects of postmastectomy chest wall radiation therapy using brass mesh as an alternative to tissue equivalent bolus. Pract Radiat Oncol 2013; 3: 45–53. [DOI] [PubMed] [Google Scholar]
- 3.National Collaborating Centre for Cancer. Early and locally advanced breast cancer diagnosis and treatment. NICE clinical guideline 80. London, UK: National Institute for Health and Clinical Excellence; 2009. [PubMed] [Google Scholar]
- 4.Taylor M, Haffty B, Shank B, Halberg F, Martinez A, McCormick B, et al. Postmastectomy radiotherapy. American College of Radiology. ACR appropriateness criteria. Radiology 2000; 215(Suppl.) 1153–70. [PubMed] [Google Scholar]
- 5.Blitzblau RC, Horton JK. Treatment planning technique in patients receiving postmastectomy radiation therapy. Pract Radiat Oncol 2013; 3: 241–8. [DOI] [PubMed] [Google Scholar]
- 6.Tieu M, Browne L, Graham P, Chin Y. The effect of adjuvant postmastectomy radiotherapy bolus technique on local recurrence. Int J Radiat Oncol Biol Phys 2011; 81: e165–71. doi: 10.1016/j.ijrobp.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 7.Fundagul A, Yasemin O, Rima D, Sule BC, Emine I, Mehmet E. Evaluation of skin dose associated with different frequencies of bolus applications in post-mastectomy three-dimensional conformal radiotherapy. J Exp Clin Cancer Res 2009; 28: 41. doi: 10.1186/1756-9966-28-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vu T, Pignol JP, Rakovitch E, Spayne J, Paszat L. Variability in radiation oncologists’ opinion on the indication of a bolus in post-mastectomy radiotherapy: an international survey. Clin Oncol (R Coll Radiol) 2007; 19: 115–19. [DOI] [PubMed] [Google Scholar]
- 9.Fessenden P, Palos B, Karzmark J. Dosimetry for tangential chest wall irradiation. Radiology 1978; 128: 485–9. doi: 10.1148/128.2.485 [DOI] [PubMed] [Google Scholar]
- 10.Computerized Imaging Reference Systems, Inc. ATOM anthropomophic phantom. Available from: http://www.cirsinc.com/products/all/33/atom-dosimetry-verification-phantoms/
- 11.Oinam A, Singh L. Verification of IMRT dose calculations using AAA and PBC algorithms in dose buildup regions. J Appl Clin Med Phys 2010; 11: 3351. [DOI] [PMC free article] [PubMed] [Google Scholar]