Abstract
Objective:
To compare the capability of differentiation of small-cell lung cancer (SCLC) from non-SCLC (NSCLC) between diffusion-weighted imaging (DWI) and short tau inversion recovery (STIR) turbo spin-echo imaging.
Methods:
The institutional review board of Kobe University Hospital, Kobe, Japan, approved this study, and written informed consent was obtained from each patient. 49 patients with NSCLC (30 males and 19 females; mean age, 66.8 years) and 7 patients with SCLC (5 males and 2 females; mean age, 68.6 years) enrolled and underwent DWI and STIR. To quantitatively differentiate SCLC from NSCLC, apparent diffusion coefficient (ADC) values on DWI and contrast ratios (CRs) between cancer and muscle on STIR were evaluated. ADC values and CRs were then compared between the two cell types by Mann–Whitney's U-tests, and the diagnostic performances were compared by McNemar's test.
Results:
There were significant differences of mean ADC values (p < 0.001) and mean CRs (p = 0.003). With adopted threshold values, the specificity (85.7%) and accuracy (85.7%) of DWI were higher than those of STIR (specificity, 63.3%; p = 0.001 and accuracy, 66.1%; p = 0.001). In addition, the accuracy of combination of both indexes (94.6%; p = 0.04) could significantly improve as compared with DWI alone.
Conclusion:
DWI is more useful for the differentiation of SCLC from NSCLC than STIR, and their combination can significantly improve the accuracy in this setting.
Advances in knowledge:
Pulmonary MRI, including DWI and STIR, had a potential of the suggestion of the possibility as SCLC.
Lung cancer is the most common cause of cancer-related death among both males and females worldwide.1 Lung cancers are divided into non-small-cell cancer (NSCLC) and small-cell lung cancer (SCLC), and the differentiation between SCLC and NSCLC is important in clinical practice because their therapeutic strategies, clinical course and prognoses are different.2 In general, SCLC is usually determined with extensive hilar and mediastinal lymphadenopathy,3 and these cancers are mainly treated by chemotherapy or chemoradiotherapy.2,4
On the other hand, 5–10% of patients with SCLC were diagnosed as having solitary pulmonary nodules.5,6 In this situation, the assessments of distant metastases before treatment play an important role in deciding the treatment. At present, although there are some different reports for patients with NSCLC regarding the assessment of distant metastases before surgery,7–9 it is important to assess the distant metastases of these patients with SCLC because SCLC is known for its rapid doubling time, high growth fraction and early development of metastatic disease.10–12 If patients with SCLC are diagnosed at Stage I or possibly Stage II, clinicians consider their treatment as surgery and/or neoadjuvant chemotherapy.13–15 Therefore, the differentiation between SCLC and NSCLC and the suggestion of the possibility of SCLC may be important in routine clinical practice. However, the differentiation of SCLC from NSCLC is difficult on CT and positron emission tomography (PET) or PET/CT,5,6,16 and fiberoptic bronchoscopy and percutaneous biopsy are recommended, although their diagnostic sensitivities range from 67% to 100%.17–19
Recently, the image quality and diagnostic capability of chest MRI has improved because of the advancement of MR systems and sequences, and short tau inversion recovery (STIR) turbo spin-echo (SE) imaging and diffusion-weighted imaging (DWI) have been reported as useful in differentiating malignant nodules and lymph nodes from benign ones in several articles.20–25 Meanwhile, the utilities of chest MRI, including STIR and DWI, have been reported,26 and, in addition, meta-analysis report for pulmonary nodules by means of DWI have been published.27 However, to the best of our knowledge, there have been only reports of chest DWI regarding the differentiation between SCLC and NSCLC,22 but no major studies have reported a direct comparison of the use of DWI and STIR in chest MRI for the assessment of differentiation between SCLC and NSCLC. We hypothesized that both DWI and STIR were useful MR sequences for differentiation of SCLC from NSCLC and their combination might improve the differentiation capabilities. The aim of this study was to evaluate the diagnostic performances of DWI and STIR for differentiating between SCLC and NSCLC.
METHODS AND MATERIALS
Subjects
The institutional review board of Kobe University Hospital, Kobe, Japan, approved this study, and written informed consent was obtained from every subject before his or her enrolment in this study.
Between January 2006 and December 2011, 221 patients (139 males and 82 females; mean age, 65.3 years) who had been referred to our hospital, had been radiologically and pathologically diagnosed as having lung cancer and were considered to be candidates for surgical resection underwent chest MRI, including DWI and STIR. Of these 221 patients, 7 patients (5 males and 2 females; mean age, 68.6 years; age range, 62–73 years) were diagnosed with SCLC via pathological examinations of surgical specimens, and these patients were enrolled in this study. On the other hand, 60 patients with NSCLC were consecutively selected, and patients meeting the following criterion were excluded. The criterion was the air-containing lung adenocarcinoma (AD) based on CT appearance, because of the inability to measure the apparent diffusion coefficient (ADC) values of lesions located adjacent to air-containing organs because of susceptibility artefact in these cancers.28 Finally, according to this criterion 49 patients with NSCLC (30 males and 19 females; mean age, 66.8 years; age range, 42–82 years) were also enrolled in this study. All MR examinations were performed before the surgery treatment, and all MR images were not influenced by the therapeutic effect of radiation and/or chemotherapy. The details of these patients are shown in Table 1, and their pathological diagnoses were as follows: 30 ADs, 14 squamous cell carcinomas (SQs) and 5 large-cell neuroendocrine carcinomas (LCNECs). There was no significant difference of ages and diameters between patients with SCLC and patients with NSCLC by means of Mann–Whitney's U-test.
Table 1.
Details of the subjects in this study
| Characteristics | SCLC | Non-small-cell lung cancer | Large-cell neuroendocrine carcinoma | Squamous cell carcinoma | Adenocarcinoma |
|---|---|---|---|---|---|
| Male/female | 5/2 | 30/19 | 5/0 | 10/4 | 15/15 |
| Mean age (years) | 68.6 | 66.8 | 73.6 | 66.5 | 65.9 |
| Range (years) | 62–73 | 43–82 | 68–78 | 43–82 | 45–81 |
| p = 0.46a | p = 0.81b | p = 0.97b | p = 0.91b | ||
| Mean diameter (mm) | 35.0 | 34.2 | 38.0 | 33.4 | 33.9 |
| Range (mm) | 25–56 | 11–69 | 25–56 | 11–56 | 13–69 |
| p = 0.34a | p = 0.98a | p = 0.99a | p = 1.00a | ||
| Location | |||||
| Right upper lobe | 2 | 19 | 3 | 4 | 12 |
| Right middle lobe | N/A | 1 | N/A | N/A | 1 |
| Right lower lobe | 1 | 14 | 2 | 6 | 6 |
| Left upper lobe | 1 | 11 | N/A | 1 | 10 |
| Left lower lobe | 3 | 4 | N/A | 3 | 1 |
N/A, not applicable; SCLC, small-cell lung cancer.
aDifference with SCLC by using Mann–Whitney's U-test.
bDifference with SCLC by using the Tukey honest-significance test.
Chest MRI
Images of MR were obtained by a 1.5-T MRI machine (Gyroscan Intera; Philips Medical Systems, Best, Netherlands) and a four-channel sensitivity encoding body coil was used.
The sequentially re-ordered, half-Fourier, single-shot STIR SE echo-planar imaging sequence was performed for acquisition of DWI. The details of the sequence are as follows: free breathing acquisition; repetition time (TR), 5000 ms; echo time (TE), 70 ms; inversion time (TI), 180 ms; number of excitations (NEX), 5; echo train length, 41; slice thickness, 5 mm; slice gap, 1.5 mm; matrix size, 96 × 96; reconstruction matrix, 256 × 256; and b-values, 0 and 1000 s mm−2. Acquisition time was 5.0 min.
Yet, a centrically re-ordered, multishot, black blood STIR turbo SE sequence was performed for acquisition of STIR. The details of the sequence are as follows: breath holding acquisition; TR, 2–3 <R–R> ms; effective TE (TEeff), 8 ms; TI, 165 ms; NEX, 2; echo train length, 27; slice thickness, 5 mm; slice gap, 1.5 mm; matrix size, 256 × 256; reconstruction matrix size, 512 × 512; field of view, 320 mm; and reduction factor, 4. Mean acquisition time was 3.0 min (range, 2.5–3.5 min).
Pathological examinations and gold standard for pulmonary adenocarcinomas
Each resected lung specimen was fixed at end-inspiration volume. Owing to correlation of radiological findings with histopathological examinations, the specimens were cut into serial 1-mm-thick sections by referring to the axial radiological images. By pathologists with more than 15 years' experience, the specimens were stained with haematoxylin–eosin and diagnosed by the same pathologists.
Image analysis
All MR data were analysed with picture archiving and communication systems (ShadeQuest; Yokogawa, Tokyo, Japan).
For quantitative assessment of DWI, the ADC values were used. Meanwhile, the assessment of STIR was by using contrast ratios (CRs) for each nodule or mass and the muscle. The details of these methods are given in the following paragraphs.
Regions of interest (ROIs) were placed over each pulmonary lesion on DWI by one chest radiologist (HK) according to the consensus of two readers who had no knowledge of the pathological type of the lung cancer (HK and DT). A ROI was placed over the nodule or mass and encompassed the entire cross-sectional area of the nodule or mass, making it as large as possible and excluding necrotic lesions. The mean ADC value was then calculated using the following formula:
where Sh and Sl are the signal intensities in the ROI obtained with two different gradient factors (bh and bl). In this study, bh was 1000 s mm−2 and bl was 0 s mm−2.
STIR assessment was performed based on past literature.24,25 ROIs were placed over each pulmonary lesion and the rhomboid muscle by the same chest radiologist (HK) based on the consensus of two readers who lacked information on the pathological type of lung cancer (HK and DT). A ROI was placed over pulmonary lesions in a similar way as with DWI, whereas the ROI placed over the muscle was fixed at 120 mm2. The CR was then acquired following the formula:
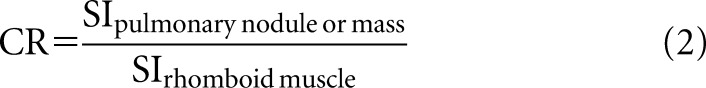 |
where SIpulmonary nodule or mass is the lung lesion signal intensity and SIrhomboid muscle is the muscle signal intensity.
Statistical analysis
Comparison of ADC values and CRs between SCLC and NSCLC
The ADC values between SCLCs and NSCLCs were compared by using Mann–Whitney's U-test. CRs of SCLCs and NSCLCs were also compared by same test. In addition, the ADC values and CRs of SCLCs were compared with those of the other pathological types by the Tukey honest-significance test.
Comparison of quantitatively differentiated capability
Receiver operating characteristic (ROC) analysis was used to evaluate the usefulness of ADC values and CRs as markers for distinguishing SCLCs from NSCLCs. Diagnostic capabilities, such as sensitivity, specificity, positive predictive value, negative predictive value and accuracy, were calculated for each level by varying the levels of indexes that signified a positive test (threshold value).29
To distinguish SCLCs from NSCLCs on DWI, sensitivity and specificity were defined as follows: sensitivity was the percentage of SCLCs with levels of indexes equal to or less than the given threshold level and specificity was the percentage of NSCLCs with levels of indexes greater than the threshold levels,29 because low ADC values are suggestive of hypercellularity.30 On the other hand, to distinguish SCLCs from NSCLCs on STIR, sensitivity and specificity were defined as follows: sensitivity was the percentage of SCLCs that had levels of indexes equal to or greater than the given threshold level and specificity was the percentage of NSCLCs that had levels of indexes less than the threshold levels,29 because many pathological lesions demonstrate increases in both T1 and T2.23,31,32 Therefore, the addition of these two types of contrasts to the STIR sequence produces a higher net tissue contrast.23,31,32 In the results, lung nodules or masses with high signal intensity on STIR are suggestive of malignant tumours, excluding necrosis and cystic change.
The feasible threshold values of ADC values and CRs for distinguishing SCLCs from NSCLCs were tested for their diagnostic capabilities and were compared using McNemar's test. In addition, diagnostic capabilities were obtained by a combination of both sequences for assessment of the utility of these sequences.
A p-value of <0.05 was considered to indicate a statistically significant difference for all statistical analyses. All statistical analyses were performed in Excel 2003 (Microsoft; Redmond, WA) and in statistical software based on Excel 2003 (StatMate III; ATMS Co. Ltd, Tokyo, Japan).
RESULTS
Comparison with ADC values and CRs between SCLC and NSCLC
The results of the comparison of the ADC values and CRs are shown in Table 2. The mean ADC values of SCLCs and NSCLCs were 0.79 × 10−3 mm2 s−1 (n = 7) and 1.33 × 10−3 mm2 s−1 (n = 49), respectively. The ADC values of SCLCs were significantly lower than those of NSCLCs (p < 0.001). On the other hand, the mean CRs of SCLC and NSCLC were 1.59 (n = 7) and 1.30 (n = 49), respectively. There were also significant differences between the CRs of SCLCs and NSCLCs (p = 0.003).
Table 2.
Comparison of apparent diffusion coefficient (ADC) values and contrast ratios (CRs)
| Parameters | SCLC | Non-small-cell lung cancer | Large-cell neuroendocrine carcinoma | Squamous cell carcinoma | Adenocarcinoma |
|---|---|---|---|---|---|
| Number | 7 | 49 | 5 | 14 | 30 |
| Mean diameter (mm) | 35.0 | 34.2 | 38.0 | 33.4 | 33.9 |
| Mean ADC values ± SD (×10−3) (mm2 s−1) | 0.79 ± 0.30 | 1.33a ± 0.38 | 1.46a ± 0.53 | 1.24 ± 0.40 | 1.35a ± 0.36 |
| Range (mm2 s−1) | 0.39–1.35 | 0.47–2.23 | 0.85–2.07 | 0.61–2.23 | 0.47–2.00 |
| Difference with SCLC | p < 0.001b | p = 0.020c | p = 0.060c | p = 0.005c | |
| Mean CR ± SD | 1.59 ± 0.21 | 1.30a ± 0.23 | 1.51 ± 0.11 | 1.30a ± 0.16 | 1.26a ± 0.25 |
| Range | 1.30–1.92 | 0.80–1.69 | 1.38–1.63 | 0.92–1.49 | 0.80–1.69 |
| Difference with SCLC | p = 0.003b | p = 0.920c | p = 0.030c | p = 0.004c |
ADC, apparent diffusion coefficient; CR, contrast ratio; SCLC, small-cell lung cancer; SD, standard deviation.
aSignificant difference with SCLC.
bDifference with SCLC by using Mann–Whitney's U-test.
cDifference with SCLC by using the Tukey honest-significance test.
Regarding the pathological type, the ADC values of SCLC were significantly different from those of LCNEC (p = 0.02) and AD (p = 0.005); however, there were no significant differences between those of SCLC and SQ (p = 0.06). On the other hand, CRs of SCLC were significantly different from those of SQ (p = 0.03) and AD (p = 0.004); yet, there were no significant differences between those of SCLC and LCNEC (p = 0.92).
Comparison of quantitatively differentiated capability
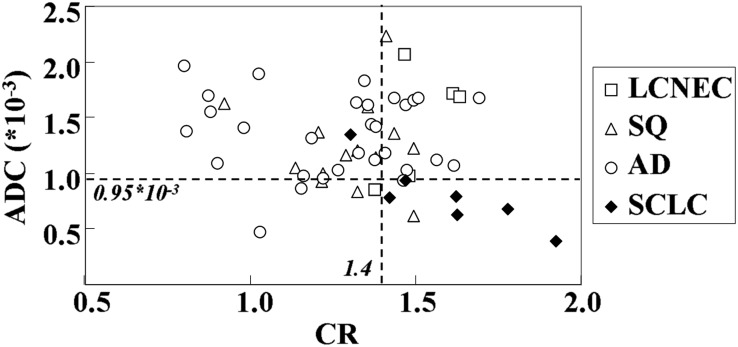
Two-dimensional scattergram of ADC values and CRs of SCLCs and NSCLCs, including LCNEC, SQ and AD, are shown in Figure 1. Based on the results of the ROC-based positive test that quantitatively distinguished SCLCs from NSCLCs, the feasible threshold values of qualitatively assessed DWI and STIR were determined to be 0.95 × 10−3 mm2 s−1 and 1.40, respectively. The threshold values for distinguishing SCLCs from NSCLCs were then adopted; the diagnostic capabilities are shown in Table 3. The specificity [85.7% (42/49)] and accuracy [85.7% (48/56)] of DWI were significantly higher than those of STIR [specificity, 63.3% (31/49) and accuracy, 66.1% (37/56)].
Figure 1.
Two-dimensional scattergram of apparent diffusion coefficient (ADC) values and contrast ratios (CRs) of small-cell lung cancers (SCLCs) and non-SCLCs. AD, adenocarcinoma; LCNEC, large-cell neuroendocrine carcinoma; SQ, squamous cell carcinoma.
Table 3.
Different capabilities of quantitatively distinguishing small-cell lung cancer from non-small-cell lung cancer
| Procedure | Feasible threshold value | Sensitivity (%) | Specificity (%) | Positive predicting value (%) | Negative predicting value (%) | Accuracy (%) |
|---|---|---|---|---|---|---|
| STIR | 1.40 | 85.7 (6/7) | 63.3 (31/49) | 25.0 (6/24) | 96.9 (31/32) | 66.1 (37/56) |
| DWI | 0.95 | 85.7 (6/7) | 85.7a (42/49) | 46.2 (6/13) | 97.7 (42/43) | 85.7a (48/56) |
| DWI + STIR | 0.95 (ADC × 10−3 mm2 s−1), 1.40 (contrast ratio) | 85.7 (6/7) | 95.9a (47/49) | 75.0 (6/8) | 97.9 (47/48) | 94.6a,b (53/56) |
ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; STIR, short tau inversion recovery.
aSignificant difference with STIR.
bSignificant difference with DWI.
When these threshold values were adopted, the accuracy of the combination of both sequences was significantly higher than that of only DWI [accuracy, 94.6% (53/56)].
Representative cases are shown in Figures 2–5.
Figure 2.
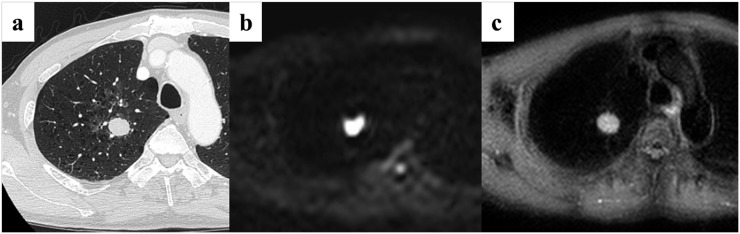
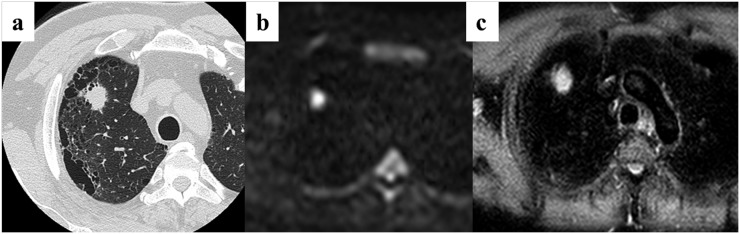
A 64-year-old male patient with small-cell lung cancer (SCLC) in the right upper lobe. (a) Thin-section CT shows a cancer with a diameter of 26 mm in the right upper lobe. (b) Diffusion-weighted imaging (DWI) shows remarkably high signal intensity. The apparent diffusion coefficient value for the cancer is 0.94 × 10−3 mm2 s−1. The quantitative assessment of DWI identified this case as SCLC. (c) Short tau inversion recovery (STIR) also shows a remarkable high intensity. The contrast ratio for the cancer is 1.47. Quantitative assessment of STIR identified this case as SCLC.
Figure 5.
A 59-year-old male patient with adenocarcinoma in the left upper lobe. (a) Thin-section CT shows a cancer with a diameter of 25 mm in the right upper lobe. (b) Diffusion-weighted imaging (DWI) shows high signal intensity. The apparent diffusion coefficient value for the cancer is 1.08 × 10−3 mm2 s−1. Quantitative assessment of DWI identified this case as non-small-cell lung cancer (NSCLC). (c) Short tau inversion recovery (STIR) shows a high intensity. The contrast ratio for the cancer is 1.28. Quantitative assessment of STIR identified this case as NSCLC.
Figure 3.
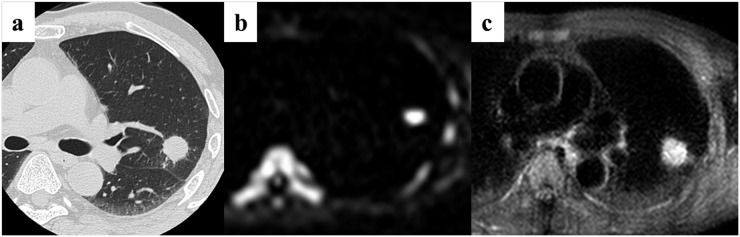
A 75-year-old male patient with large-cell neuroendocrine carcinoma in the right upper lobe. (a) Thin-section CT shows a cancer with a diameter of 38 mm in the right upper lobe. (b) Diffusion-weighted imaging (DWI) shows slightly high signal intensity. The apparent diffusion coefficient value for the cancer is 2.07 × 10−3 mm2 s−1. Quantitative assessment of DWI identified this case as non-small-cell lung cancer. (c) Short tau inversion recovery (STIR) also shows remarkable high intensity. The contrast ratio for the cancer is 1.46. This is a false-positive case on the quantitative assessment of STIR.
Figure 4.
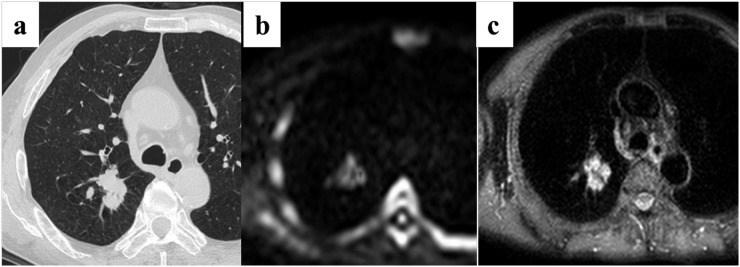
A 54-year-old male patient with squamous cell carcinoma in the right upper lobe. (a) Thin-section CT shows a cancer with a diameter of 26 mm in the right upper lobe. (b) Diffusion-weighted imaging (DWI) shows high signal intensity. The apparent diffusion coefficient value for the cancer is 1.07 × 10−3 mm2 s−1. Quantitative assessment of DWI identified this case as non-small-cell lung cancer. (c) Short tau inversion recovery (STIR) also shows remarkable high intensity. The contrast ratio for the cancer is 1.48. This is a false-positive case on the quantitative assessment of STIR.
DISCUSSION
In this study, we assessed the diagnostic capability of differentiating SCLC from NSCLC. The result was that the diagnostic capability of DWI was higher than that of STIR on the assessment of quantitative differentiation between SCLC and NSCLC. In addition, the combination of these two sequences increased diagnostic capability, and the specificity and accuracy were remarkably high. These facts indicate that chest MRI, particularly the DWI, could provide additional information in routine clinical practice.
Regarding the comparison of SCLC and NSCLC ADC values, SCLC ADC values were significantly lower than those of NSCLC. This may be explained by the fact that the tumour cellularity of SCLC seemed to be relatively high because tumour cellularity is an important factor influencing ADC values in viable tumour tissue.30 The ADC value is estimated to be lower in viable tumour tissue with densely packed, diffusion-hindering obstacles than that in tissue with less densely packed obstacles.30 In fact, Sugahara et al33 have reported that the ADC value of gliomas correlates significantly with tumour cellularity and that ADC values of high-grade gliomas are significantly lower than those of low-grade gliomas. Yet, Guo et al34 have revealed that tumour cellularity has a significant influence on ADC values obtained in both benign and malignant breast tumours. Considering these findings, our results were compatible with these studies, and ADC values might reflect the characteristics of lung tumours.
However, some reports have different results concerning ADC values of lung cancers. One study reported that the differentiation capabilities of pathological subtype classifications of pulmonary ADs using ADC values were low.25 Generally, avoiding susceptibility artefacts on DWI of lung cancers is difficult. Wang et al28 reported that air-containing areas within lung cancers could be considered to influence the measurement of ADC values because of inhomogeneities of the magnetic field; they were unable to measure the ADC values of lesions located adjacent to air-containing organs because of susceptibility artefacts. Considering these findings, as written in the report by Koyama et al,25 there is a limit to subtype classification, including AD in situ. However, the measurement of ADC values was actually higher, because the subjects did not have an air-containing nodule or mass in their CTs in this study.
Matoba et al20 reported that a significant negative linear correlation was found between tumour cellularity and the mean ADC value. However, the mean ADC value of SCLC tended to be higher than that of SQ and large-cell carcinoma in their study, and the mean ADC value was 2.09 × 10−3 ± 0.3 mm2 s−1. They suspected that the higher ADC values of SCLC were owing to the degree of necrosis and/or microstructural change.20 On the other hand, the ADC values of SCLC were low in other reports, and the ADC values were 1.06 × 10−3 ± 0.20 mm2 s−1.22 While the ADC value was 0.79 × 10−3 ± 0.30 mm2 s−1 in our study. Presuming the reason for the varying results is difficult; however, the setting of the b-value and the subject might have been factors. ADC values tended to be higher when low b-values were used because ADC values are greatly influenced by tissue perfusion and T2 time.20 In our study, because high b-values (1000 s mm−2) were used, the ADC values of SCLC might be lower than those found in the previous report.20,21 Regarding the subject problem, all subjects were enrolled based on diagnosis by surgical and pathological examinations in our study, and the selection of the subjects might have influenced the results. However, the presumption of the difference was not enough, and the patient population was relatively small. Therefore, further studies are needed to determine the factors influencing the ADC values of lung cancers.
STIR had a lower potential than that of DWI on the assessment of the difference between SCLC and NSCLC. Generally, many pathological lesions demonstrate an increase in both T1 and T2; the addition of these two types of contrasts to the STIR sequence produces a higher net tissue contrast.23,31,32 In addition, one study reported that the differentiation capabilities of pathological subtype classification of pulmonary ADs by STIR were higher than those of DWI.25 STIR seemed to be limited in its ability to reflect tumour cellularity of a solid nodule or mass in comparison with DWI on the assessment of differentiation between SCLC and NSCLC. However, STIR is also an important sequence in clinical practice, just as DWI is;23,24 in fact, the combination of DWI and STIR had a remarkably high diagnostic capability for the purposes of this study.
In this study, the combination of DWI and STIR increased diagnostic capability, and the diagnostic capabilities were remarkably high. These facts indicate that DWI and STIR provide additional information in routine clinical practice. For example, before biopsy and/or when adequate material has not been obtained for tissue diagnosis by means of fiberoptic bronchoscopy and percutaneous biopsy, radiologists can suggest the possibilities of SCLC. This might lead to better management for patients.
Our study has some limitations. First, the patient population was relatively small, and the patient selection was biased because the entry criteria were based on a surgical and pathological diagnosis of SCLC. Patients diagnosed with SCLC usually exhibited extensive hilar and mediastinal lymphadenopathy and distant metastases. Therefore, the SCLC patient population with solitary pulmonary nodules was relatively small. However, further studies involving a larger number of patients without potential selection bias should be performed. In addition, because this study was performed for a single centre cohort, multicentre studies will be needed in the near future.
Second, avoiding susceptibility artefacts on DWI of lung cancers is difficult, and the merit of ADC values is different according to the choice of b factors because they are influenced by tissue perfusion and T2 time.20 Although in this study two b factors were used as in previous reports,20,22,25 it may be desirable for accurate ADC measurement that one of the b-values is not 0 and that multiple b factors are used in the acquisition of DWI.35–37 In addition, although we chose the mean ADC value in this study, a minimal ADC value was chosen in some previous reports because the value within the entire tumour might not characterize the tumour because of its heterogeneity.38 Results might differ when these problems are solved.
Third, an analysis of the tumour cellularity was not performed in this study. Generally, ADC tumour cellularity is an important factor influencing the ADC values of viable tumour tissue.30 Although tumour cellularity of SCLC seemed to be relatively high, the result might not be strictly accurate. Meanwhile, some NSCLCs had high tumour cellularity according to the degree of tumour differentiation. In fact, the ADC values of NSCLC were broad in this study. Therefore, analysis should be performed in the future.
In conclusion, DWI and ADC values had high diagnostic capabilities on the quantitative assessment of differentiation between SCLC and NSCLC in comparison with STIR and CRs. Yet, the combination of ADC values and CRs increased diagnostic capability. DWI is a sensitive sequence for the differentiation of SCLC from NSCLC, and the combination of DWI and STIR served as a reliable diagnostic indicator for this purpose.
Acknowledgments
ACKNOWLEDGMENTS
Kazuyuki Kobayashi, MD, PhD, Yasuhiro Funada, MD, PhD, Yoshikazu Kotani, MD, PhD (Division of Respiratory Medicine, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe, Japan), Yasuhiro Sakai, MD, PhD (Division of Diagnostic Pathology, Kobe University Graduate School of Medicine, Kobe, Japan), Nobukazu Aoyama, BS and Hideaki Kawamitsu, BS (Division of Radiology, Kobe University Hospital, Kobe, Japan) are acknowledged for their contribution to this work.
FUNDING
This work was supported by Philips Healthcare.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Monnerat C, Turrisi AT 3rd, Perry MC, Leyvraz S. Small cell lung cancer: state of the art and future perspectives. Lung Cancer 2004; 45: 105–17. doi: 10.1016/j.lungcan.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 3.Fraser R, Müller N, Colman N, Paré P. Diagnosis of disease of the chest. 3rd edn. Philadelphia, PA: W.B. Saunders; 1999. pp. 1067–250. [Google Scholar]
- 4.Ihde D, Souhami B, Comis R, Gregor A, Hansen H, Johnson B, et al. Consensus report. Small cell lung cancer. Lung Cancer 1997; 17(Suppl. 1): S19–21. [DOI] [PubMed] [Google Scholar]
- 5.Quoix E, Fraser R, Wolkove N, Finkelstein H, Kreisman H. Small cell lung cancer presenting as a solitary pulmonary nodule. Cancer 1990; 66: 577–82. [DOI] [PubMed] [Google Scholar]
- 6.Yabuuchi H, Murayama S, Sakai S, Hashiguchi N, Murakami J, Muranaka T, et al. Resected peripheral small cell carcinoma of the lung: computed tomographic-histologic correlation. J Thorac Imaging 1999; 14: 105–8. [DOI] [PubMed] [Google Scholar]
- 7.Ichinose Y, Hara N, Ohta M, Motohiro A, Maeda T, Nobe T, et al. Preoperative examination to detect distant metastasis is not advocated for asymptomatic patients with stages 1 and 2 non-small cell lung cancer. Preoperative examination for lung cancer. Chest 1989; 96: 1104–9. [DOI] [PubMed] [Google Scholar]
- 8.Michel F, Solèr M, Imhof E, Perruchoud AP. Initial staging of non-small cell lung cancer: value of routine radioisotope bone scanning. Thorax 1991; 46: 469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatter J, Kohman LJ, Mosca RS, Graziano SL, Veit LJ, Coleman M. Preoperative evaluation of stage I and stage II non-small cell lung cancer. Ann Thorac Surg 1994; 58: 1738–41. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DH. Management of small cell lung cancer: current state of the art. Chest 1999; 116(Suppl. 6): 525S–30S. [DOI] [PubMed] [Google Scholar]
- 11.Elias AD. Small cell lung cancer: state-of-the-art therapy in 1996. Chest 1997; 112(Suppl. 4): 251S–8S. [DOI] [PubMed] [Google Scholar]
- 12.Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc 2008; 83: 355–67. doi: 10.4065/83.3.355 [DOI] [PubMed] [Google Scholar]
- 13.Waddell TK, Shepherd FA. Should aggressive surgery ever be part of the management of small cell lung cancer? Thorac Surg Clin 2004; 14: 271–81. doi: 10.1016/S1547-4127(04)00004-0 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura H, Kazuyuki S, Kawasaki N, Taguchi M, Kato H. History of limited resection for nonsmall cell lung cancer. Ann Thorac Cardiovasc Surg 2005; 11: 356–62. [PubMed] [Google Scholar]
- 15.Coolen L, Van den Eeckhout A, Deneffe G, Demedts M, Vansteenkiste J. Surgical treatment of small cell lung cancer. Eur J Cardiothorac Surg 1995; 9: 59–64. [DOI] [PubMed] [Google Scholar]
- 16.Bradley JD, Dehdashti F, Mintun MA, Govindan R, Trinkaus K, Siegel BA. Positron emission tomography in limited-stage small-cell lung cancer: a prospective study. J Clin Oncol 2004; 22: 3248–54. doi: 10.1200/JCO.2004.11.089 [DOI] [PubMed] [Google Scholar]
- 17.Johnston WW. Cytologic diagnosis of lung cancer. Principles and problems. Pathol Res Pract 1986; 181: 1–36. doi: 10.1016/S0344-0338(86)80184-4 [DOI] [PubMed] [Google Scholar]
- 18.Michel RP, Lushpihan A, Ahmed MN. Pathologic findings of transthoracic needle aspiration in the diagnosis of localized pulmonary lesions. Cancer 1983; 51: 1663–72. [DOI] [PubMed] [Google Scholar]
- 19.Delgado PI, Jorda M, Ganjei-Azar P. Small cell carcinoma versus other lung malignancies: diagnosis by fine-needle aspiration cytology. Cancer 2000; 90: 279–85. [PubMed] [Google Scholar]
- 20.Matoba M, Tonami H, Kondou T, Yokota H, Higashi K, Toga H, et al. Lung carcinoma: diffusion-weighted MR imaging—preliminary evaluation with apparent diffusion coefficient. Radiology 2007; 243: 570–7. [DOI] [PubMed] [Google Scholar]
- 21.Satoh S, Kitazume Y, Ohdama S, Kimula Y, Taura S, Endo Y. Can malignant and benign pulmonary nodules be differentiated with diffusion-weighted MRI? AJR Am J Roentgenol 2008; 191: 464–70. doi: 10.2214/AJR.07.3133 [DOI] [PubMed] [Google Scholar]
- 22.1Liu H, Liu Y, Yu T, Ye N. Usefulness of diffusion-weighted MR imaging in the evaluation of pulmonary lesions. Eur Radiol 2010; 20: 807–15. doi: 10.1007/s00330-009-1629-6 [DOI] [PubMed] [Google Scholar]
- 23.Ohno Y, Hatabu H, Takenaka D, Higashino T, Watanabe H, Ohbayashi C, et al. Metastases in mediastinal and hilar lymph nodes in patients with non-small cell lung cancer: quantitative and qualitative assessment with STIR turbo spin-echo MR imaging. Radiology 2004; 231: 872–9. doi: 10.1148/radiol.2313030103 [DOI] [PubMed] [Google Scholar]
- 24.Koyama H, Ohno Y, Kono A, Takenaka D, Maniwa Y, Nishimura Y, et al. Quantitative and qualitative assessment of non-contrast-enhanced pulmonary MR imaging for management of pulmonary nodules in 161 subjects. Eur Radiol 2008; 18: 2120–31. doi: 10.1007/s00330-008-1001-2 [DOI] [PubMed] [Google Scholar]
- 25.Koyama H, Ohno Y, Aoyama N, Onishi Y, Matsumoto K, Nogami M, et al. Comparison of STIR turbo SE imaging and diffusion-weighted imaging of the lung: capability for detection and subtype classification of pulmonary adenocarcinomas. Eur Radiol 2010; 20: 790–800. doi: 10.1007/s00330-009-1615-z [DOI] [PubMed] [Google Scholar]
- 26.Koyama H, Ohno Y, Seki S, Nishio M, Yoshikawa T, Matsumoto S, et al. Magnetic resonance imaging for lung cancer. J Thorac Imaging 2013; 28: 138–50. doi: 10.1097/RTI.0b013e31828d4234 [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Zhang J, Bao J, Zhang L, Hu X, Xia Y, et al. Meta-analysis of diffusion-weighted MRI in the differential diagnosis of lung lesions. J Magn Reson Imaging 2013; 37: 1351–8. doi: 10.1002/jmri.23939 [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, et al. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology 2001; 220: 621–30. doi: 10.1148/radiol.2202010063 [DOI] [PubMed] [Google Scholar]
- 29.Rosner BA. Fundamentals of biostatistics. 2nd edn. Boston, MA: Dexbury; 1986. pp. 584. [Google Scholar]
- 30.Herneth AM, Guccione S, Bednarski M. Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol 2003; 45: 208–13. [DOI] [PubMed] [Google Scholar]
- 31.Wiener JI, Chako AC, Merten CW, Gross S, Coffey EL, Stein HL. Breast and axillary tissue MR imaging: correlation of signal intensities and relaxation times with pathologic findings. Radiology 1986; 160: 299–305. doi: 10.1148/radiology.160.2.3726104 [DOI] [PubMed] [Google Scholar]
- 32.Fossel ET, Brodsky G, deLayre JL, Wilson RE. Nuclear magnetic resonance for the differentiation of benign and malignant breast tissues and axillary lymph nodes. Ann Surg 1983; 198: 541–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, et al. Usefulness of diffusion-weighted MRI with echoplanar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 1999; 9: 53–60. [DOI] [PubMed] [Google Scholar]
- 34.Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging 2002; 16: 172–8. doi: 10.1002/jmri.10140 [DOI] [PubMed] [Google Scholar]
- 35.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988; 168: 497–505. doi: 10.1148/radiology.168.2.3393671 [DOI] [PubMed] [Google Scholar]
- 36.Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J. Echo-planar imaging of intravoxel incoherent motion. Radiology 1991; 177: 407–14. doi: 10.1148/radiology.177.2.2217777 [DOI] [PubMed] [Google Scholar]
- 37.Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology 1999; 210: 617–23. doi: 10.1148/radiology.210.3.r99fe17617 [DOI] [PubMed] [Google Scholar]
- 38.Mori T, Nomori H, Ikeda K, Kawanaka K, Shiraishi S, Katahira K, et al. Diffusion-weighted magnetic resonance imaging for diagnosing malignant pulmonary nodules/masses: comparison with positron emission tomography. J Thorac Oncol 2008; 3: 358–64. [DOI] [PubMed] [Google Scholar]