Abstract
Objective:
Anatomical changes during radiotherapy (RT) might introduce discrepancies between planned and delivered doses. This study evaluates the need for adaptive treatment in lung cancer RT.
Methods:
15 patients with non-small-cell lung cancer, undergoing radical RT with or without concurrent chemotherapy, consecutively underwent planning CT scans at baseline and after 44–46 Gy. Target volumes were delineated on both scans. Phase I delivered 44–46 Gy to the initial planning target volume (PTV). Two Phase II plans for 16–20 Gy were developed on initial and mid-treatment scans, the treatment being delivered with the mid-treatment plan. The second CT structure set was fused with the initial scan data set using dose wash. Volumetric and dosimetric changes in target volumes and critical structures were assessed.
Results:
There was significant reduction in primary gross tumour volume (34.00%; p = 0.02) and PTV (34.70%; p < 0.01) in the second scan. In Plan 2, delivering the same dose to the initial PTV would have resulted in a significantly higher dose to the lung PTV (V20, 52.18%; V5, 21.76%; mean, 23.93%), contralateral lung (mean, 29.43%), heart (V10, 81.47%; V5, 56.62%; mean, 35.21%) and spinal cord (maximum dose, 37.53%).
Conclusion:
Treatment replanning can account for anatomical changes during RT and thereby enable better normal tissue sparing, while allowing radical target doses with the possibility of maximizing local control.
Advances in knowledge:
This study supports the sparse dosimetric data regarding the quantitative tumour volume reduction, re-emphasizing the need for adaptive replanning for minimizing normal tissue toxicity without compromising local control, and adds to the existing body of literature.
Lung cancer is the commonest cause of cancer mortality worldwide.1 Annually, 1.4 million new cases are diagnosed, accounting for 12% of all cancer cases.2 Chemoradiotherapy remains the standard of care for patients presenting with an advanced stage and for those whose tumours are inoperable owing to medical reasons, although this results in a dismal prognosis, and many patients succumb to locoregional failure or distant metastases.3 Several dose escalation trials4,5 have reported better local control with increased freedom from relapse and survival. On the other hand, RTOG 06176 has shown a higher locoregional failure rate and shorter overall survival (OS), possibly owing to deaths related to the effects from high-dose three-dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiation therapy (IMRT) on normal lungs and perhaps the heart. A meta-analysis by Auperin et al7 has confirmed that improved local control rates influence OS. With increments of every 1 Gy above the conventional prescription dose the 3- to 5-year survival rates improve by 1% with a decrease in hazard from death by 3%.5 However, dose escalation is limited by the tolerance of normal tissues, such as the lung, heart, oesophagus and spinal cord.6,8 One important dose-limiting toxicity in lung cancer is radiation pneumonitis. In the modern era of radiotherapy (RT) technology, its occurrence and severity correlates well with V20 and mean lung dose.9,10
Modern RT techniques, such as 3D-CRT, IMRT, stereotactic body RT (SBRT) and volumetric-modulated arc therapy (VMAT), can potentially improve target coverage with a much steeper dose gradient and minimize irradiated normal tissue volumes.11–13 Precision of radiation therapy is challenged by substantial geometrical uncertainties in accuracy of imaging, treatment planning, treatment delivery and tumour shrinkage during treatment. Tumour volume shrinkage during treatment for lung cancer is well known.14,15 Woodford et al16 reported that replanning can better normal tissue sparing and improve the therapeutic ratio further if the gross tumour volume (GTV) decreases by ≥30%.
In the current study, we aimed to quantify the degree of tumour volume change during the course of RT and its impact on normal tissue sparing. In addition, we attempted to estimate the clinical benefit in patients treated with adaptive RT.
METHODS AND MATERIALs
15 patients with histologically proven non-small-cell lung cancer (NSCLC) who were consecutively treated at our institute, between October 2011 and December 2012, were prospectively included in the study.
All patients underwent RT planning (RTP) based on a contrast-enhanced CT (CECT) scan prior to treatment and a repeat scan after 44–46 Gy. CT images were transferred to the FOCAL® SIM v. 4.64 (Elekta, Kungstensgatan, Sweden) contouring platform via digital imaging and communications in medicine. Diagnostic/staging positron emission tomography (PET)-CECT was fused with RTP scans when available. The GTV was delineated on the CT images, with guidance from regions of high fludeoxyglucose (18F-FDG) uptake in PET images. Central necrosis within the tumour was not excluded from GTV. Lymph nodes were defined to be involved if their short axis was >10 mm on CT or if they had high 18F-FDG uptake on PET images. GTV P was the gross primary disease, and GTV N was the gross nodal disease as seen on imaging. The clinical target volume (CTV) for both gross primary tumour and gross nodes was generated by adding a 6–8 mm margin to the respective GTV in all dimensions. Internal target volume (ITV) was generated for primary CTV by adding a 7–10 mm radial margin and 10–15 mm craniocaudal margin to the CTV (departmental protocol, as per motion observed according to tumour location and experience). The planning target volume (PTV) was generated with a 5 mm radial and 10 mm superior and inferior expansion of ITV as per the institutional protocol for setup uncertainties. No elective irradiation of the lymphatic regions was conducted. On mid-treatment scans, similar steps were performed. The targets as well as normal tissue contours (ipsilateral lung) were modified on the second scan to adapt to tumour volume change. For GTV N, the nodes considered significant on the baseline scan were identified and contoured, irrespective of the size criteria. Normal structures (lung PTV, ipsilateral lung, heart, oesophagus and spinal cord) were contoured.
Treatment planning was performed in Monaco® v. 3.0 (Elekta, Crawley, UK). The IMRT plan consisted of five to seven coplanar beams of 6 MV photons, and beam angles were selected such that they would transmit through a minimal amount of normal tissue before reaching the tumour, at the same time avoiding collisions with the table and patient. Monte Carlo algorithm-based IMRT optimization was then performed, and the final dose distribution was calculated. All initial and mid-treatment plans aimed to deliver the 95% isodose volume (at a minimum) as closely as possible to the PTV, while respecting the dose constraints to the organs at risk. Specifically, for the combined plan, the tissue volume outside the PTV constrained to receive 20 Gy was <35% of the lung volume and <20% of heart volume, the maximal spinal cord dose was limited to 45 Gy.
The dose constraints for each phase were given separately to obtain the abovementioned combined doses and are given below:
• Phase I: ipsilateral lung (outside PTV) V20 <20% and V15 <30%, contralateral lung V5 <20%, both lung mean dose <9 Gy, heart V15 <20%, spinal cord maximum dose <35 Gy and oesophagus mean dose <25 Gy.
• Phase II: ipsilateral lung (outside PTV) V10 <20% and V5 <30%, contralateral lung V5 <10%, both lung mean dose <4 Gy, heart V5 <20%, spinal cord maximum dose <10 Gy and oesophagus mean dose <10 Gy.
Combined plan doses were also evaluated, and the Phase II plan was accepted if they fulfilled the following criteria: lung outside PTV V20 <35%, heart V20 <20% and maximal spinal cord dose <45 Gy at any point.
In Phase I, fractionated IMRT plans were generated on Monaco v. 3.0 for 44–46 Gy to the initial PTV. Phase II plans were developed on the initial scan PTV (Plan 1) and new mid-treatment scan PTV (Plan 2) for 16–20 Gy. Patients were treated with the new plan (Plan 2). The goal of treatment planning in this study was to develop a plan where at least 95% isodose entirely covered the PTV and minimized dose to the surrounding normal tissue. The mid-treatment RTP scan was co-registered with the initial scan using the Monaco treatment planning system with multiple match points (lung apices, aortic arch, heart, spine, sternum, carina, diaphragm and tumour) to ensure the best possible match. FOCAL SIM uses the “Auto Fusion” algorithm, which optimally registers corresponding anatomical details in two three-dimensional study sets of the same patient's anatomy, using a volume of interest function limited to the region of the tumour. The registration criterion is mutual information, i.e. a measure of the statistical similarity of the overlapping data. The “best registration” is the transformation resulting from an optimization process that gives the maximum value of the mutual information. Use of a mask function allows the optimization algorithms to consider only relevant patient data, and it maximizes the chances of achieving a “true” transformation. Co-registration was always performed by one radiation oncologist and the quality of alignment assessed by three experienced readers. The reduction in doses to ipsilateral lung PTV, ipsilateral lung, contralateral lung and heart [V5, V20 and mean dose (Dmean)], oesophagus (Dmean) and spinal cord (Dmax and dose exceeding 2% of DVH points, surrogate for maximum dose) was assessed. X-ray volume imaging (XVI) for setup verification was done in all patients for the first five fractions. In 4 patients the setup error was less, hence in the rest of the treatment weekly imaging was performed, whereas in 11 patients XVI was done during the entire course of treatment. Response assessment was performed on whole body PET-CECT done at 10–12 weeks following completion of RT using response evaluation criteria in solid tumours criteria v. 1.1.17 Toxicity was recorded using common toxicity criteria v. 4.0.18
Data collection and statistical analysis
Target volumes (including GTV and PTV), prior to and after 44–46 Gy, were measured on FOCAL SIM. SPSS® v. 12.0 (SPSS Inc., Chicago, IL) software for Windows (Microsoft, Redmond, CA) was used for statistical analysis. Paired t-test was used to compare the volume reduction. Wilcoxon signed-rank test was used to compare parameters of the dose–volume histogram (DVH) between the two Phase 2 plans (Plan 1 and Plan 2). The statistical difference was considered significant at p < 0.05.
RESULTS
Demographic data
Mean age of the patient cohort (13 males and 2 females) was 59.5 years (range, 31–83 years). Out of 15 patients, 1 patient had Stage IIB, 5 patients had IIIA and 9 patients had IIIB disease. Ten patients had node-positive disease and nine had right-sided primary tumours. 13 patients received concurrent chemotherapy (weekly cisplatin 40 mg m−2 or carboplatin area under curve (AUC); in 2 patients, intravenous with appropriate hydration and supportive medication). Patients and tumour characteristics are summarized in Table 1.
Table 1.
Demographic profile (n = 15 patients with non-small-cell lung cancer)
| Characteristic | n |
|---|---|
| Age (years) [mean (range)] | 59.5 (31–83) |
| Gender | |
| Male | 13 |
| Female | 2 |
| Nodal involvement | |
| Positive | 10 |
| Negative | 5 |
| Laterality | |
| Right | 9 |
| Left | 6 |
| The American Joint Committee on Cancer stage | |
| IIB | 1 |
| IIIA | 5 |
| IIIB | 9 |
| Concurrent chemotherapy | |
| Yes | 13 |
| No | 2 |
Volumetric changes
Shrinkage in target volume was observed for all patients in the mid-treatment scan. The median GTV P, GTV N and PTV on the initial scans were 313, 33 and 773 cm3, respectively. On mid-treatment scans, median GTV P reduced to 113 cm3 (p = 0.02; median reduction, 34.0%), median GTV N reduced to 8 cm3 (p = 0.14; median reduction, 49.0%) and median PTV reduced to 565 cm3 (p ≤ 0.01; median reduction, 34.7%). Our patient cohort of 15 had 5 node-negative cases. Perhaps for this small number of patients, GTV N volume change was not statistically significant despite >49% median reduction (Table 2).
Table 2.
Tumour volume
| Delineated structure | Pre-treatment median (range) | Mid-treatment median (range) | Median reduction (%) (range) | p-value |
|---|---|---|---|---|
| GTV primary (cm3) | 313 (45.0–829.0) | 113 (30.0–691.0) | 34.00 (13.8–73.0) | 0.02 |
| GTV nodal (cm3) | 33 (9.4–141.0) | 8 (4.5–20.0) | 49.00 (6.0–53.0) | 0.14 |
| PTV (cm3) | 773 (169.0–1826.0) | 565 (130.0–1272.0) | 34.70 (32.0–76.0) | 0.00 |
GTV, gross tumour volume; PTV, planning target volume.
Dosimetric changes
Organ at risk
The two Phase II plans were compared for the same total dose 16–20 Gy in terms of the normal tissue DVH (ipsilateral lung PTV, contralateral lung, heart, oesophagus and spinal cord) (Figures 1 and 2). Table 3 compares the DVH parameters for Plan 1 and Plan 2. In comparison with Plan 1, in Plan 2, for ipsilateral lung PTV, mean V20 reduced to 114.05 cm3 from 238.51 cm3 (p = 0.01), mean V5 reduced to 399.49 from 510.65 cm3 (p = 0.05), and mean of mean lung dose reduced to 596.65 from 784.39 cGy (p < 0.001).
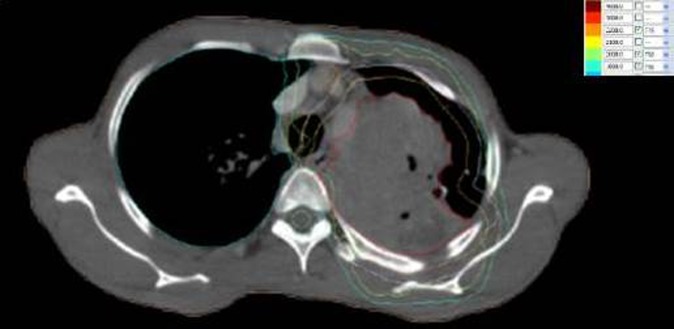
Figure 1.
Pre-treatment dose distribution.
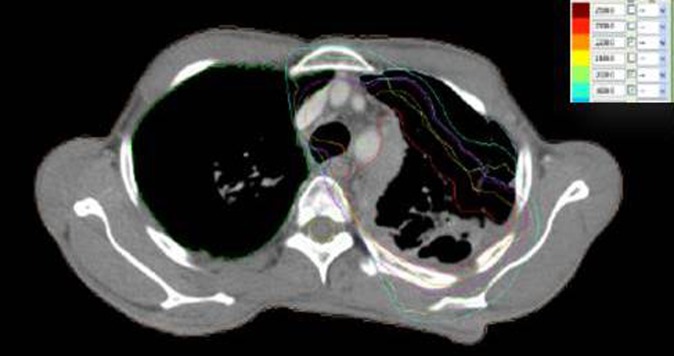
Figure 2.
Mid-treatment dose distribution.
Table 3.
Dose variation
| Dose parameters | Pre-treatment mean ± SD (range) | Mid-treatment mean ± SD (range) | Mean reduction (%) | p-value |
|---|---|---|---|---|
| Ipsilateral lung without PTV | ||||
| V20 (cm3) | 238.51 ± 149.25 (98–471) | 114.05 ± 116.63 (60–317) | 52.18 | 0.01 |
| V5 (cm3) | 510.65 ± 145.30 (285–912) | 399.49 ± 105.13 (233–612) | 21.76 | 0.05 |
| Mean (cGy) | 784.39 ± 302.05 (392–1182) | 596.65 ± 297.33 (345–943) | 23.93 | 0.00 |
| Contralateral lung | ||||
| V20 (cm3) | 21.80 ± 12.58 (0–30) | 1.24 ± 3.00 (0–9) | 42.85 | 0.15 |
| V5 (cm3) | 351.66 ± 222.65 (112–803) | 297.22 ± 200.67 (52.6–646) | 15.48 | 0.31 |
| Mean (cGy) | 402.49 ± 167.06 (192–697) | 284.00 ± 121.79 (78–546) | 29.43 | 0.01 |
| Heart | ||||
| V20 (cm3) | 16.86 ± 29.16 (0–85) | 3.12 ± 0.23 (0–4) | 81.47 | 0.13 |
| V10 (cm3) | 97.57 ± 65.56 (0–184) | 42.32 ± 32.90 (0–97) | 56.62 | 0.00 |
| V5 (cm3) | 169.98 ± 97.94 (0–276) | 115.88 ± 56.46 (0–234) | 31.82 | 0.04 |
| Mean (cGy) | 531.53 ± 447.03 (31–1477) | 344.38 ± 241.26 (12–736) | 35.21 | 0.02 |
| Oesophagus | ||||
| Mean (cGy) | 1020.00 ± 351.00 (616–1245) | 709.00 ± 370.00 (464–1139) | 19.03 | 0.17 |
| Spine | ||||
| D2 (cGy) | 1130.47 ± 160.03 (221–1701) | 706.18 ± 151.78 (152–1311) | 37.53 | 0.00 |
| Dmax (cGy) | 1215.02 ± 184.84 (336–1989) | 771.09 ± 178.69 (259–1817) | 36.53 | 0.00 |
Dmax, maximum dose; PTV, planning target volume; SD, standard deviation.
On comparing dose–volume parameter for heart, mean V10 decreased to 42.32 from 97.57 cm3 (p = 0.00), mean V5 to 115.9 from 170 cm3 (p = 0.04) and mean of mean heart dose decreased to 344.38 from 531.53 cGy (p = 0.02). For the spinal cord, mean of maximum dose (Dmax) changed to 771.09 from 1215.02 cGy (p = 0.00). Change in mean dose for the oesophagus was not significant.
Initial gross tumour volume coverage
Volume of initial GTV receiving 50 Gy in the combined plan (V50) was approximately 100% regardless of whether Plan 1 or Plan 2 was assessed for combined dose. However, V60 for this GTV in the combined plan was only 90.8% when a combination of Phase I and Plan 2 plans were evaluated.
Clinical benefits
3 out of 15 patients had complete response, and 12 had partial response at the locoregional site at 3 months. Three patients failed at distant sites (one each developed brain metastases, bone metastases and contralateral lung nodule).
All patients tolerated the treatment well. Ten patients developed Grade I skin toxicity. Ten patients developed Grade I pneumonitis and four patients developed asymptomatic Grade II pneumonitis. Nine patients developed Grade I, and five patients developed Grade II oesophagitis.
At a median follow-up of 11 months (range, 3–20 months), two patients died of progressive disease and two patients were lost to follow-up.
DISCUSSION
Management of locally advanced lung cancer remains a therapeutic challenge for the radiation oncologist. The treatment of lung cancer has undergone significant improvements over the past decade with better accuracy of imaging, treatment planning and treatment delivery. Modern RT techniques such as IMRT, VMAT and SBRT have enabled delivery of high doses to the target, while sparing the normal tissues. Tumour shrinkage has been studied using various imaging modalities, such as electronic portal imaging, repetitive CT scanning or PET-CT scanning. The daily regression rates reported in the literature vary between 0.6% and 2.4%. Fox et al19 showed a median reduction of 24.7% after 30 Gy and 44.3% after 50 Gy. Similarly, tumour regression by 1.2% per day was reported by Guckenberger et al.15 Ding et al20 also reported a median reduction by 35% (range, 3–95%) at 40 Gy. In our study, median reduction of GTV was 34.0% (range, 13.8–73.0%) over a 4–5 week period.
We know from tumour control probability (TCP) and normal tissue complication probability curves that there is a narrow therapeutic window where maximum tumour control can be achieved without increasing normal tissue complications.
With doses of ≥60 Gy, modern RT techniques have reported local control rates of <50%.5,21,22 The absence of locoregional control is a major concern, as it serves not only as a source of metastatic dissemination but also increases the risk of death due to local effects. In the study by Arriagada et al,23 the main cause of failure was local progression or relapse and correlated with poorer survival. High RT doses can potentially improve locoregional control. A study by the University of Michigan,24 Ann Arbor, MI, on 114 patients with medically inoperable node-negative NSCLC showed that there was a significant interaction between the radiation dose and GTV (p < 0.001). With a biologic equivalent dose (BED) of ≤79.2 Gy, the median OS was 18.2 and 23.9 months for patients with GTV >51.8 and ≤51.8 cm3, respectively (p = 0.015). For BED ≥79.2 and ≤79.2 Gy, the median OS was 30.4 and 18.2 months, respectively, in patients with GTV >51.8 cm3 (p < 0.001).
However, it is challenging to deliver a high dose owing to the accompanying increased risk of normal tissue complications. Recent advances in imaging (PET-CECT), RTP (IMRT, VMAT and adaptive RT) and delivery (IGRT) can aid us in dose escalation. Guckenberger et al15 have concluded that shrinking of GTV did not compromise dose coverage of volumes harbouring suspected microscopic disease. It can potentially increase the TCP by >40% compared with RTP without adaptive RT.
Also, in the current study, replanning during treatment achieved significant reduction in dose to normal tissue. Spoelstra et al25 did not observe any significant change in lung V20 and V5, and mean lung doses or maximum spinal cord dose in their study when replanning was done after 30 Gy. By contrast, we have replanned after a dose of 44–46 Gy, when a greater tumour reduction could possibly explain the decrease in lung and spinal cord doses. The treatment techniques used for our patients (IMRT or VMAT) would also have contributed to lower normal tissue doses. Guckenberger et al15 were able to achieve a mean lung dose reduction of 8% with two-fold adaptation in a 3D-CRT plan, whereas we achieved 23% with a single mid-treatment adaptation with an IMRT-based plan. A similar study by Ding et al20 also reported significant reduction in doses to the lung (V20, 31.60–29.40 cm3, p ≤ 0.00; mean, 17.20–15.90 Gy, p = 0.04), heart (V45, 15.50–15.00 cm3; p = 0.012) and the spinal cord (Dmax, 43.30–40.20 Gy; p ≤ 0.00). Replanning during treatment was deemed beneficial to protect organs at risk.
In our study, the incidence of Grade 2 pneumonitis was 26% and Grade 2 oesophagitis was 33%. None of our patients developed Grade 3 or 4 toxicity. Kong et al26 have reported a 15.6% incidence of ≥Grade 2 pneumonitis and 16.5% and 2.7% Grades 2 and 3 oesophagitis, respectively.
The main limitation of our study was the small patient number, which prevented us from carrying out an analysis based on tumour laterality, location with respect to lung lobes and proximity of tumour to critical structures. Thus, the results obtained from this cohort might not be directly applicable to the general population. We obtained a single CT data set for each patient, the scans in expiratory and inspiratory phases or four-dimensional CT that would have enabled individual ITV generation were not taken. The fusion and matching algorithm used is based on rigid co-registration, hence deformation of volumes in Phase II were not accounted for. Some institutions use deformable co-registration algorithms, which may be more reflective of actual volumetric changes; however, our institution does not have deformable registration at present.
CONCLUSIONS
Adaptive mid-treatment replanning should be considered in lung cancer RT for more accurate dose delivery, especially when appreciable reduction is noted on daily or weekly imaging. Significant changes in DVH can occur for normal structures as well as target volumes owing to anatomical changes during the course of treatment. Mid-treatment replanning has the potential to allow delivery of optimum target doses, while enabling better normal tissue sparing, hence creating the possibility of improved locoregional control and consequent survival benefits.
REFERENCES
- 1.Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy for non-small cell lung cancer. Cochrane Database Syst Rev 2000: CD002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide, in IARC Cancer Base. Lyon, France: IARC Press; 2004. [Google Scholar]
- 3.Kim TY, Yang SH, Lee SH, Park YS, Im YH, Kang WK, et al. A phase III randomized trial of combined chemoradiotherapy versus radiotherapy alone in locally advanced non-small-cell lung cancer. Am J Clin Oncol 2002; 25: 238–43. [DOI] [PubMed] [Google Scholar]
- 4.Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005; 63: 324–33. doi: 10.1016/j.ijrobp.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 5.Belderbos JS, Heemsbergen WD, De Jaeger K, Baas P, Lebesque JV. Final results of a phase I/II dose escalation trial in non-small-cell lung cancer using three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 2006; 66: 126–34. doi: 10.1016/j.ijrobp.2006.04.034 [DOI] [PubMed] [Google Scholar]
- 6.Bradley J, Paulus R, Komaki R, Masters GA, Forster K, Schild SE, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer: results on radiation dose in RTOG 0617. In: 49th Annual Meeting of the American Society of Clinical Oncology; 31 May–4 June 2013, Chicago, IL.
- 7.Auperin A, Rolland E, Curran W, Le Péchoux C, Furuse K, Fournel P, et al. Concomitant radio-chemotherapy (RT-CT) versus sequential RT-CT in locally advanced nonsmall cell lung cancer (NSCLC): a meta-analysis using individual patient data (IPD) from randomised clinical trials (RCTs): A1-05. J Thorac Oncol 2007; 2: S310. [Google Scholar]
- 8.Bradley J, Graham MV, Winter K, Purdy JA, Komaki R, Roa WH, et al. Toxicity and outcome results of RTOG 9311: a phase I–II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 2005; 61: 318–28. doi: 10.1016/j.ijrobp.2004.06.260 [DOI] [PubMed] [Google Scholar]
- 9.Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999; 45: 323–29. [DOI] [PubMed] [Google Scholar]
- 10.Martel MK, Ten Haken RK, Hazuka MB, Kessler ML, Strawderman M, Turrisi AT, et al. Dose-volume histogram and 3-D treatment planning evaluation of patients with pneumonitis. Int J Radiat Oncol Biol Phys 1994; 28: 575–81. [DOI] [PubMed] [Google Scholar]
- 11.Grills IS, Yan D, Martinez AA, Vicini FA, Wong JW, Kestin LL, et al. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys 2003; 57: 875–90. [DOI] [PubMed] [Google Scholar]
- 12.Chapet O, Kong FM, Lee JS, Hayman JA, Ten Haken RK. Normal \ modeling for acute esophagitis in patients treated with conformal radiation therapy for non-small cell lung cancer. Radiother Oncol 2005; 77: 176–81. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz M, Alber M, Lebesque JV, Mijnheer BJ, Damen EM. Dose heterogeneity in the target volume and intensity-modulated radiotherapy to escalate the dose in the treatment of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2005; 62: 561–70. doi: 10.1016/j.ijrobp.2005.02.011 [DOI] [PubMed] [Google Scholar]
- 14.Britton KR, Starkschall G, Tucker SL, Pan T, Nelson C, Chang JY, et al. Assessment of gross tumor volume regression and motion changes during radiotherapy for non-small-cell lung cancer as measured by four-dimensional computed tomography. Int J Radiat Oncol Biol Phys 2007; 68: 1036–46. [DOI] [PubMed] [Google Scholar]
- 15.Guckenberger M, Wilbert J, Richter A, Baier K, Flentje M. Potential of adaptive radiotherapy to escalate the radiation dose in combined radiochemotherapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 79: 901–8. doi: 10.1016/j.ijrobp.2010.04.050 [DOI] [PubMed] [Google Scholar]
- 16.Woodford C, Yartsev S, Dar AR, Bauman G, Van Dyk J. Adaptive radiotherapy planning on decreasing gross tumor volumes as seen on megavoltage computed tomography images. Int J Radiat Oncol Biol Phys 2007; 69: 1316–22. doi: 10.1016/j.ijrobp.2007.07.2369 [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 18.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published: 28 May 2009. Available from http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 19.Fox J, Ford E, Redmond K, Zhou J, Wong J, Song DY. Quantification of tumor volume changes during radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009; 74: 341–48. doi: 10.1016/j.ijrobp.2008.07.063 [DOI] [PubMed] [Google Scholar]
- 20.Ding XP, Zhang J, Li BS, Li HS, Wang ZT, Yi Y, et al. Feasibility of shrinking field radiation therapy through 18F-FDG PET/CT after 40 Gy for stage III non-small cell lung cancers. Asian Pac J Cancer Prev 2012; 13: 319–23. [DOI] [PubMed] [Google Scholar]
- 21.Sause W, Kolesar P, Taylor S, Johnson D, Livingston R, Komaki R, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000; 117: 358–64. [DOI] [PubMed] [Google Scholar]
- 22.Dillman RO, Herndon J, Seagren SL, Eaton WL Jr, Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst 1996; 88: 1210–5. [DOI] [PubMed] [Google Scholar]
- 23.Arriagada R, Le Chevalier T, Quoix E, Ruffie P, de Cremoux H, Douillard JY, et al. ASTRO (American Society for Therapeutic Radiology and Oncology) plenary: effect of chemotherapy on locally advanced non small cell lung carcinoma: a randomized study of 353 patients. GETCB (Groupe d'Etude et Traitement des Cancers Bronchiques), FNCLCC (Féderation Nationale des Centres de Lutte contre le Cancer) and the CEBI trialists. Int J Radiat Oncol Biol Phys 1991; 20: 1183–90. [DOI] [PubMed] [Google Scholar]
- 24.Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005; 63: 324–33. [DOI] [PubMed] [Google Scholar]
- 25.Spoelstra FOB, Pantarotto JR, Sornsen de Koste JR, Slotman BJ, Senan S. Role of adaptive radiotherapy during concomitant chemoradiotherapy for lung cancer: analysis of data from a prospective clinical trial. Int J Radiat Oncol Biol Phys 2009; 75: 1092–97. [DOI] [PubMed] [Google Scholar]
- 26.Kong FM, Hayman JA, Griffith KA, KalemKerian GP, Arenberg D, Lyons S, et al. Final toxicity results of a radiation-dose escalation study in patients with nonsmall-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys 2006; 65: 1075–86. doi: 10.1016/j.ijrobp.2006.01.051 [DOI] [PubMed] [Google Scholar]