Abstract
Objective:
To investigate the association of pericardial fat volume (PFV) with coronary artery disease (CAD) in patients with intermediate pre-test probability of ischaemic heart disease assessed by coronary CT angiography.
Methods:
From a total of 115 consecutive Iraqi patients who underwent 64-multislice multidetector CT angiography examinations, only 74 patients (females, 38% and males, 68%) with a mean age of 54 ± 8 years were found to be eligible for statistical analysis. The patients were divided into two groups according to the median value of PFV (above and below 100 ml).
Results:
The median value of PFV in our study was 100 ml (range, 17–319 ml). A significant association was observed between high PFV and significant coronary artery stenosis (p = 0.005), between high PFV and significant left circumflex stenosis (p = 0.021) and between high PFV and the presence of coronary plaque (p = 0.005). Whereas there was no significant correlation between high PFV and coronary calcium score (p = 0.188), between high PFV and number of diseased coronary vessels (p > 0.3), and between high PFV and body weight and body mass index.
Conclusion:
Increased PFV is strongly associated with the presence and severity of CAD.
Advances in knowledge:
Our study highlights the role of pericardial fat as an emerging biomarker in cardiovascular risk assessment and supports its association with the magnitude of CAD.
Pericardial fat is an adipose tissue surrounding the heart, with anatomic proximity to the epicardial coronary arteries. Several studies1–5 show growing evidence that pericardial adipose tissue may be associated with increased left ventricular mass, metabolic syndrome, endothelial dysfunction, insulin resistance and the presence of coronary artery disease (CAD) via local secretion of numerous pro-inflammatory hormones and cytokines.
Coronary CT angiography is a non-invasive method that has been used for cardiovascular risk stratification based on the presence and severity of coronary calcium and atherosclerotic plaque with a reported sensitivity of 94–95% and specificity of 82%. Pericardial fat volume (PFV) can be assessed by direct measurement that depends on the detection of the thin pericardium or as a component of heart-level thoracic fat volume.6,7
Pericardial fat has been found to have a higher lipolysis and lipogenesis activity than that of other fat tissues with a strong correlation between PFV and triglyceride concentration in the myocardium.8
Furthermore, recent data have shown that PFV assessed by CT angiography is significantly associated with severe coronary stenosis, plaque burden even in asymptomatic individuals, high-risk plaque features and an increased risk of future cardiac events.9–11
The aim of this study was to investigate the association of PFV with CAD, cardiac risk factors and calcium scoring index in patients with an intermediate pre-test probability of ischaemic heart disease assessed by coronary CT angiography.
METHODS AND MATERIALS
This cross-sectional study was performed in the Cardiology centre in AL-Sader Teaching Hospital in AL-Najaf City, Iraq between January 2013 and June 2013. All patients gave their written informed consent for taking part in this study. The study was approved by the Ethical Committee of Medicine College of Kufa University, Najaf, Iraq.
A total of 115 consecutive Iraqi patients, with an intermediate pre-test probability of ischaemic heart disease, who underwent 64-multislice multidetector CT (MDCT) angiography examinations for the assessment of CAD were enrolled in this study.
Of these, only 74 patients were found to be eligible for statistical analysis and 41 patients were excluded owing to missing or lost data (31 patients), poor examination technique (6 patients) and difficulty in accurate PFV calculation or segmentation of fat (4 patients).
Exclusion criteria for MDCT examination included a history of cardiac surgery, iodine-based contrast allergy, renal failure (creatinine, >1.5 mg dl−1), atrial fibrillation or other unstable heart rhythm, inability to perform breath holding, haemodynamically unstable patients, presence of intracardiac devices (pacemaker), pregnant females and contraindications for the use of β-blocker.
Using standard physician-based questionnaires, a history of conventional cardiac risk factors for CAD was obtained from each patient at the time of coronary CT angiography examination, including a positive family history of premature atherosclerosis (occurring in males before the age of 55 years and before 65 years in females), current smoking history (>10 cigarettes per day in the past year), a history of hypertension or use of antihypertension medications, hyperlipidaemia (defined as levels of total cholesterol ≥200 mg dl−1 or triglyceride ≥150 mg dl−1) or use of lipid-lowering drugs, a history of diabetes mellitus or use of insulin or diabetic-lowering drugs, and obesity with a body mass index (BMI) of ≥30 kg m2.
For coronary CT analysis, plaques with a structure of >1 mm within and/or adjacent to vessel lumen were used, and plaque types were classified into calcified (plaque consisting of only calcium), non-calcified or soft plaque (plaque that was free of calcium), and multiple plaques (more than one plaque in the same patient).
Severity of coronary artery stenosis was visually graded as normal (normally appearing lumen), non-significant with a mean lumen diameter reduction of <50% or significant with a mean lumen diameter reduction of ≥50% in a single vessel by comparing the lumen diameter of the narrowest segment with that of a more proximal or distal normal segment in two orthogonal projections. This cutoff value of coronary stenosis had been used and investigated by several studies to define the severity of coronary stenosis in patients who had undergone multislice computed tomographic coronary angiography.2,12
CT scan protocol and pericardial fat volume quantification
CT coronary angiography was performed with a 64-slice scanner (Aquilion™ 64, v. 4.51 ER 010; Toshiba Medical Systems, Tochigi, Japan) with retrospective electrocardiography (ECG) gating. Before multislice CT angiography, a non-contrast CT was acquired to measure the calcium score according to the Agatston method using a sequence scan with a slice thickness of 3 mm. When the patients' heart rate was >65 beats per minute, a β-blocker (metoprolol, 20–60 mg orally) was administered before the scan. A bolus of 80 ml of contrast medium (Omnipaque™; GE Healthcare, Waukesha, MI; 350 mg ml−1 of iodine) was injected intravenously at a rate 5 ml s−1, followed by 30 ml of normal saline. The scan was obtained from the aortic arch to the level of the diaphragm during a single breath hold. When ECG-triggered scanning protocol was performed, the following parameters were used: collimation width, 32.5 × 32.5 cm; slice thickness, 0.5 mm; rotation time, 0.35 s; tube voltage, 120 kV; maximum effective tube current, 890 mA; and table feed, 0.3 mm/rotation. The duration of examination was about 10 s.
CT images were reconstructed using a smooth kernel (B25f) with a slice thickness of 0.5 mm (increment, 0.3 mm). CT data sets were transferred to a dedicated workstation (Vitrea® 2 workstation; Vital Image Inc., Plymouth, MN) for image analysis.
PFV was measured using a three-dimensional (3D) contrast-enhanced phase. The layer of the pericardium was manually traced, and a 3D image of the heart was constructed. Then, the PFV was quantified by calculating the total volume of the tissue whose CT density ranged from −250 to −20 HU within the pericardium by using a 3D workstation statistical analysis.
STATISTICAL ANALYSES
Data are presented as mean ± standard deviation or as numbers with percentages. The calcium score was determined by using the Agatston method. Categorical data are expressed as frequencies and were compared with Pearson's χ2 test. Continuous variables are presented as mean ± standard deviation and were compared using Student's t-test or analysis of variance. A probability (p) value of <0.05 was considered as statistically significant.
RESULTS
Overall, 74 patients (females, 38% and males, 62%; mean age, 54 ± 8 years) were enrolled in this study. Coronary CT angiography was performed for the evaluation of patients with an intermediate pre-test probability of ischaemic heart disease presenting with symptoms suggestive of CAD. PFV ranged from 17 to 319 ml, and the median value of PFV in our patients was 100 ml, which had been used as a cutoff value to separate patients into two groups for statistical analysis (high PFV ≥100 ml group and low PFV <100 ml group). The clinical characteristics of the patients enrolled in the study are shown in Table 1.
Table 1.
Patients' characteristics
| Parameters | Low PFV group, n (%) | High PFV group, n (%) | p-value |
|---|---|---|---|
| Male | 21 (58.3) | 25 (65.8) | 0.500 |
| Female | 15 (41.7) | 13 (34.2) | 0.500 |
| Age (years) | 53.39 ± 8.8 | 55.9 ± 9.1 | |
| Risk factors | |||
| Hypertension | 3 (60) | 2 (40) | 2.900 |
| Smoking | 3 (75) | 1 (25) | 0.200 |
| Diabetes | 2 (100) | 0 | 0.800 |
| Hyperlipidaemia | 2 (67) | 1 (33) | 0.800 |
| Family history | 4 (67) | 2 (33) | 0.500 |
| Body mass index ≥30 (kg m2) | 2 (50) | 2 (50) | >0.100 |
| More than one risk factor | 5 (33) | 10 (67) | 0.030 |
| Stenosis severity in all coronary arteries | |||
| Normal | 21 (68) | 10 (32) | 0.005 |
| Non-significant stenosis | 4 (50) | 4 (50) | 0.500 |
| Significant stenosis | 11 (31) | 24 (69) | 0.005 |
| Number of diseased coronary vessels | |||
| Normal coronaries | 21 (68) | 10 (32) | 0.043 |
| Single-vessel disease | 7 (39) | 11 (61) | 0.380 |
| Two-vessel disease | 4 (29) | 10 (71) | >0.500 |
| Three-vessel disease | 4 (36) | 7 (64) | >0.500 |
PFV, pericardial fat volume.
Dividing our patient collective into PFVs of < 100 ml or ≥ 100 ml (high vs low PFV group), we found a significant association (<0.05) with more than one traditional cardiac risk factor, whereas there was no significant correlation reported between PFV and individual cardiac risk factors (Table 1).
Patients with normal coronary arteries were noted to have a lower PFV value than that of patients with stenotic lesions (p = 0.005) (Table 1). Although significant stenoses were more common in the high PFV group, no significant correlation could be seen with the number of vessels (Table 1).
Regarding individual CAD, a significant association was reported between high PFV and significant left circumflex (LCX) artery stenosis (p = 0.021), but an association like this was not found with other coronary vessels (Table 2).
Table 2.
Relationship between pericardial fat volume (PFV) and individual coronary artery stenosis severity
| Coronary artery | Low PFV group, n (%) | High PFV group, n (%) | p-value |
|---|---|---|---|
| Left main stem artery stenosis | |||
| Normal | 28 (56) | 22 (44) | >0.100 |
| Non-significant stenosis | 3 (43) | 4 (57) | >0.100 |
| Significant stenosis | 5 (29) | 12 (71) | >0.100 |
| Left anterior descending artery stenosis | |||
| Normal | 26 (60.5) | 17 (39.5) | >0.050 |
| Non-significant stenosis | 2 (25) | 6 (75) | >0.050 |
| Significant stenosis | 8 (35) | 15 (65) | >0.050 |
| Left circumflex artery stenosis | |||
| Normal | 29 (54) | 25 (46) | >0.500 |
| Non-significant stenosis | 5 (71) | 2 (29) | >0.500 |
| Significant stenosis | 2 (15) | 11 (85) | 0.021 |
| Right coronary artery stenosis | |||
| Normal | 28 (56) | 22 (44) | >0.100 |
| Non-significant stenosis | 3 (43) | 4 (57) | >0.100 |
| Significant stenosis | 5 (29.5) | 12 (70.5) | >0.100 |
There was no statistically significant association between PFV and the coronary calcium score (Table 3).
Table 3.
Relationship of the calcium score grading with pericardial fat volume (PFV)
| Ca+2 score | PFV | p-value | |
|---|---|---|---|
| Low (<100), n (%) | High (≥100), n (%) | ||
| 0 | 19 (59.4) | 13 (40.6) | 0.065 |
| 1–9 | 4 (66.7) | 2 (33.3) | 0.357 |
| 10–99 | 7 (36.8) | 12 (63.2) | 0.232 |
| 100–400 | 6 (40.0) | 9 (60.0) | 0.453 |
| >400 | 0 (0.0) | 2 (100.0) | 0.163 |
| Total | 36 (48.6) | 38 (51.4) | |
Patients with high PFV had a statistically strong correlation with the presence of coronary plaque (p = 0.005), whereas there was no correlation reported between high PFV and the plaque type (Tables 4 and 5).
Table 4.
Relationship between pericardial fat volume (PFV) and the presence of plaque
| Presence of plaque | PFV | p-value | |
|---|---|---|---|
| Low (<100), n (%) | High (≥100), n (%) | ||
| Absent | 21 (67.7) | 10 (32.3) | 0.005 |
| Present | 15 (34.9) | 28 (65.1) | |
| Total | 36 (48.6) | 38 (51.4) | |
Table 5.
Relationship between pericardial fat volume (PFV) and plaque type
| Plaque type | PFV | p-value | |
|---|---|---|---|
| Low (<100), n (%) | High (≥100), n (%) | ||
| No plaque | 21 (67.7) | 10 (32.3) | 0.005 |
| Soft | 2 (25.0) | 6 (75.0) | 0.156 |
| Calcified | 10 (38.5) | 16 (61.5) | 0.197 |
| Multiple | 3 (33.3) | 6 (66.7) | 0.327 |
| Total | 36 (48.6) | 38 (51.4) | |
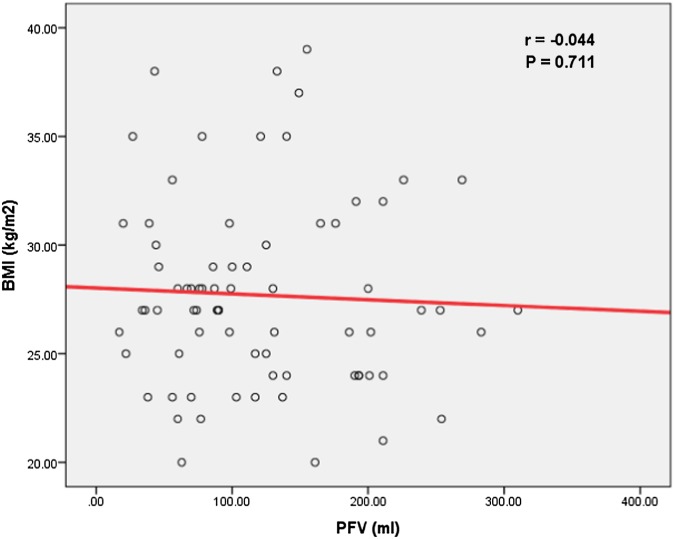
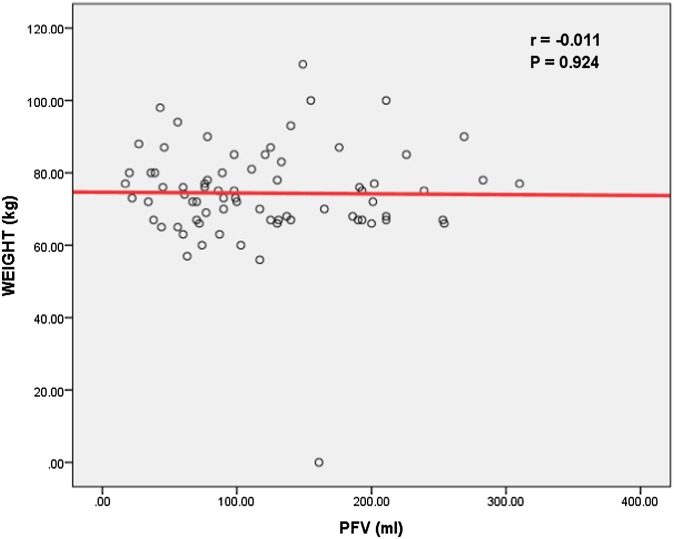
No significant correlation was found between PFV and weight and BMI (Figures 1 and 2).
Figure 1.
No significant correlation could be found between pericardial fat volume (PFV) and body mass index (BMI).
Figure 2.
No significant correlation could be found between pericardial fat volume (PFV) and body weight.
DISCUSSION
Nowadays, there are several non-invasive modalities that can be used to quantify and image pericardial fat such as echocardiography, cardiac MR and MDCT without a firm consensus on the gold standard modality for the quantification of pericardial fat.13
However, to evaluate the pericardial fat, cardiac CT provides a true volumetric quantification with high spatial resolution and 3D views of the heart without the use of contrast.14,15
Currently, there is no consensus regarding the upper limit of normality for PFV, and the reported values are highly variable among different patient populations and studies because of discrepancies and ambiguities in the definition and nomenclature of fat deposits around the heart.15
According to recent population-based studies,16,17 the mean volume of cardiac fat ranges from 68 ± 34 to 124 ± 50 ml However, several reported papers found a significant difference and association (p < 0.05) when a discriminative value around 100 ml for PFV had been used for predicting the relative risk for CAD.
Thanassoulis et al18 published community-based research regarding the prevalence and distribution of pericardial fat deposits among 3312 participants from the Framingham Heart Study Offspring and Third-Generation Cohorts who participated in the MDCT substudy and found that the median values for PFVs in males and females were 117.5 and 93.9 cm3, respectively.
Moreover, there is a broad individual variation in the amount and distribution of cardiac fat accumulation that may be attributable to the influence of different clinical and demographic factors. Reduction in body weight in patients undergoing bariatric surgery decreased the cardiac fat thickness, whereas pericardial fat seems to increase with increasing age. In addition, ethnicity may contribute to the variation of PFV and distribution especially in African–American individuals who have less central obesity than whites despite their greater resistance to insulin.19
However, there is no consensus in the literature on the impact of gender on the volume of pericardial fat based on the data from the Framingham study.20
In this study, increased PFV was associated with the presence of more than one traditional cardiac risk factor in the same patient, significant coronary artery stenosis and significant LCX coronary stenosis.
Several studies21 have linked pericardial fat deposits to the presence and severity of coronary artery atherosclerosis. The present findings are consistent with other studies13 that have demonstrated an association between cardiac fat deposits and CAD by angiography. Among 203 patients with known CAD who underwent echocardiography and cardiac catheterization, pericardial fat thickness was correlated with the severity of angiographic coronary disease.22
Additionally, the Multi-Ethnic Study of Atherosclerosis (MESA) was able to determine an association between PFV and coronary artery atherosclerosis after correcting for known risk factors in a community-based population with a prospective study design.23
A preferential association between epicardial fat volume in the left atrioventricular groove and atherosclerosis in the embedded LCX was determined, indicating a local atherogenic role of cardiac fat accumulated in the left atrioventricular groove.24
A growing body of evidence reveals that cardiac fat deposits may be associated with multiple metabolic risk factors, such as hypertension, hyperlipidaemia and diabetes, with an unfavourable cardiometabolic risk profile suggesting that a high pericardial fat accumulation might play a major role as a biomarker for cardiovascular risk assessment and in the pathogenesis of CAD.25
Another main finding of our study was the strong association between PFV and the presence of coronary plaque. However, no preference for a certain plaque form (soft, calcified or mixed plaque) could be shown.
It has been postulated that paracrine effect of pericardial fat may be a potent determinant of coronary plaque development and progression. Pericardial fat, owing to the anatomical proximity with coronary arteries and heart, has been found to contain higher levels of inflammatory markers and adipokines that can locally accelerate the atherosclerotic process by endothelial dysfunction, local proliferation of smooth muscle cells and increased plaque instability through apoptosis and neovascularization.26,27
Moreover, according to the MESA cohort study, PFV has been positively related to coronary atherosclerotic plaque burden even in asymptomatic individuals.9
Mahabadi et al28 demonstrated that pericoronary fat is independently associated with the presence of coronary plaque, irrespective of plaque type, and this association has been supported by recent studies showing that specific structural plaque characteristics identified on coronary CT angiography are associated with culprit coronary lesions and an increased risk of future cardiac events.29
Although pericardial fat has been shown to be correlated with coronary plaque and coronary stenosis, whether or not cardiac fat is related to the plaque type is a subject of ongoing investigation, and current uncertainty is the association and the clinical relevance of PFV with coronary plaque type, dimension, degree of remodelling and specific plaque characteristics in patients with stable ischaemic heart disease who are referred for CT angiography.30
Our study showed that there was no statistically significant association between PFV and the coronary calcium score, body weight and BMI.
Wassel et al31 studied the relationship of the PFV with the coronary calcium score in 600 patients using chest CT scans at two time points with an interval of about 4 years and found that PFV was not associated with the presence, severity and progression of coronary artery calcium.
A recent study by Kristoffersen et al32 reported that increased prevalence of silent CAD was strongly associated with PFV, whereas coronary calcifications seem less useful as screening tools for the detection of myocardial ischaemia in HIV patients with asymptomatic ischaemic heart disease.
A similar negative association between cardiac fat deposition and coronary calcification was demonstrated in patients with rheumatoid arthritis at an increased risk of CAD, which was assessed by cardiac CT.33
PFV increment has been observed to have a strong association with various inflammatory marker changes and early coronary endothelial dysfunction that may precede coronary calcification and the development of mature atherosclerotic changes. Whereas the coronary calcium score has been reported to have a weak relationship with these inflammatory markers and endothelial changes revealing that the presence of mere coronary calcification could represent a stable phase of atherosclerosis, and pericardial fat, however, may be more strongly associated with an active process.34,35
Consequently, PFV quantification may be used in addition to the coronary calcium score to identify patients with CAD even in the absence of coronary calcification.34
Furthermore, Janik et al35 showed that cardiac fat volume assessed by CT was an independent predictor of ischaemia and outperformed the coronary calcium score in a CAD-naive population at an intermediate pre-test probability of heart disease suggesting that the coronary calcium score might have a different significance in other population groups particularly for patients with lower Framingham score.13,36
The differences in anatomic description (pericardial vs epicardial fat) and measurement techniques (volume or thickness) may be attributed to a weak correlation of PFV with the coronary calcium score, and it is possible that a volume measurement of cardiac fat by CT imaging may be different than a linear measure in the correlation with coronary calcification and cardiovascular risk assessment.5
Our results support the idea that cardiac fat accumulation might represent a useful marker and a better predictor of CAD beyond the standard adiposity measures of BMI and body weight.
BMI or body weight might not accurately or adequately reflect body composition or the development of cardiovascular disease, and several studies found a positive correlation between cardiac fat deposits and waist circumference rather than BMI based on MESA cohort results.23
Currently, sagittal abdominal diameter, which has emerged as a surrogate marker of visceral obesity, accurately estimated cardiac fat deposits when compared with BMI and, thus, represents a clinically helpful non-invasive parameter that can identify patients with cardiac fat deposits.37
Pericardial fat has been reported to contain higher levels of inflammatory markers than does subcutaneous fat, and several studies have suggested that pericardial fat has a local pathogenic effect on surrounding coronary arteries, whereas subcutaneous fat exerts a systemic effect in enhancing the insulin resistance.21
No significant correlation existed between the number of coronary vessel involvement and PFV, and patients with normal coronary arteries were noted to have lower PFV values than patients with stenotic lesions in our study.
Evidence had been reported that coronary artery stenotic lesions were absent in the segments of coronary arteries deficient in fat deposits as compared with coronary artery segments surrounded by abundant fat.35
The crosslink correlation of the pericardial fat accumulation with the severity of coronary lesions evaluated by the number of coronary vessels involved by atherosclerosis was not recorded in previous studies.35
LIMITATIONS
There were several limitations in this study. First, our study was a single-centre investigation, and the population was non-randomly selected since it involved only patients with intermediate pre-test probability for ischaemic heart disease suggesting a possibility of selection bias. Second, the number of patients was relatively small especially when the study population is divided into subgroups. Third, we did not have invasive angiographic data from our patients to further describe and confirm CAD severity. Fourth, our algorithm for quantifying PFV was not completely automatic and still required user interaction.
Larger studies with follow-up are needed to further investigate the role of pericardial fat deposits as an imaging marker with prognostic significance and also report the effect of weight-modifying strategies on PFV.
CONCLUSION
High PFV assessed by CT angiography was shown to be significantly associated with CAD, especially LCX coronary stenosis, and the presence of coronary plaque. PFV might be an important imaging biomarker in assessing the risk and extent of CAD.
REFERENCES
- 1.Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S, et al. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis 2001; 157: 203–9. [DOI] [PubMed] [Google Scholar]
- 2.Djaberi R, Schuijf JD, van Werkhoven JM, Nucifora G, Jukema JW, Bax JJ. Relation of epicardial adipose tissue to coronary atherosclerosis. Am J Cardiol 2008; 102: 1602–7. doi: 10.1016/j.amjcard.2008.08.010 [DOI] [PubMed] [Google Scholar]
- 3.Gorter PM, de Vos AM, van der Graaf Y, Stella PR, Doevendans PA, Meijs MF, et al. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol 2008; 102: 380–5. doi: 10.1016/j.amjcard.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 4.Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005; 29: 251–5. doi: 10.1016/j.cyto.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 5.Nelson MR, Mookadam F, Thota V, Emani U, Al Harthi M, Lester SJ, et al. Epicardial fat: an additional measurement for subclinical atherosclerosis and cardiovascular risk stratification?. J Am Soc Echocardiogr 2011; 24: 339–45. [DOI] [PubMed] [Google Scholar]
- 6.Bluemke DA, Achenbach S, Budoff M, Gerber TC, Gersh B, Hillis LD, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the american heart association committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention, and the councils on clinical cardiology and cardiovascular disease in the young. Circulation 2008; 118: 586–606. [DOI] [PubMed] [Google Scholar]
- 7.Dey D, Wong ND, Tamarappoo B, Nakazato R, Gransar H, Cheng VY, et al. Computer-aided non-contrast CT-based quantification of pericardial and thoracic fat and their associations with coronary calcium and metabolic syndrome. Atherosclerosis 2010; 209: 136–41. doi: 10.1016/j.atherosclerosis.2009.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malavazos AE, Di Leo G, Secchi F, Lupo EN, Dogliotti G, Coman C, et al. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am J Cardiol 2010; 105: 1831–5. doi: 10.1016/j.amjcard.2010.01.368 [DOI] [PubMed] [Google Scholar]
- 9.Miao C, Chen S, Ding J, Liu K, Li D, Macedo R, et al. The association of pericardial fat with coronary artery plaque index at MR imaging: the Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2011; 261: 109–15. doi: 10.1148/radiol.11110346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Wang LJ, Peng YP, Zhang LJ, Jiang SS, Gong JB. Association of pericardial adipose tissue volume with presence and severity of coronary atherosclerosis. Clin Invest Med 2013; 36: E143–50. [DOI] [PubMed] [Google Scholar]
- 11.Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R, et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis 2012; 220: 223–30. doi: 10.1016/j.atherosclerosis.2011.09.041 [DOI] [PubMed] [Google Scholar]
- 12.Büyükterzi M, Türkvatan A, Büyükterzi Z. Frequency and extent of coronary atherosclerotic plaques in patients with a coronary artery calcium score of zero: assessment with CT angiography. Diagn Interv Radiol 2013; 19: 111–18. [DOI] [PubMed] [Google Scholar]
- 13.Davidovich D, Gastaldelli A, Sicari R. Imaging cardiac fat. Eur Heart J Cardiovasc Imaging 2013; 14: 625–30. doi: 10.1093/ehjci/jet045 [DOI] [PubMed] [Google Scholar]
- 14.Saremi F, Mekhail S, Sefidbakht S, Thonar B, Malik S, Sarlaty T. Quantification of epicardial adipose tissue: correlation of surface area and volume measurements. Acad Radiol 2011; 18: 977–83. [DOI] [PubMed] [Google Scholar]
- 15.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J 2007; 153: 907–17. doi: 10.1016/j.ahj.2007.03.019 [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Cheng X, Hong K, Huang C, Wan L. How to interpret epicardial adipose tissue as a cause of coronary artery disease: a meta-analysis. Coron Artery Dis 2012; 23: 227–33. doi: 10.1097/MCA.0b013e328351ab2c [DOI] [PubMed] [Google Scholar]
- 17.Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol 2009; 29: 781–6. doi: 10.1161/ATVBAHA.108.180653 [DOI] [PubMed] [Google Scholar]
- 18.Thanassoulis G, Massaro JM, Hoffmann U, Mahabadi AA, Vasan RS, O'Donnell CJ, et al. Prevalence, distribution, and risk factor correlates of high pericardial and intrathoracic fat depots in the Framingham heart study. Circ Cardiovasc Imaging 2010; 3: 559–66. doi: 10.1161/CIRCIMAGING.110.956706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertaso AG, Bertol D, Duncan BB, Foppa M. Epicardial fat: definition, measurements and systematic review of main outcomes. Arq Bras Cardiol 2013; 101: e18–28. doi: 10.5935/abc.20130138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakazato R, Rajani R, Cheng VY, Shmilovich H, Nakanishi R, Otaki Y, et al. Weight change modulates epicardial fat burden: a 4-year serial study with non-contrast computed tomography. Atherosclerosis 2012; 220: 139–44. doi: 10.1016/j.atherosclerosis.2011.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dey D, Nakazato R, Li D, Berman DS. Epicardial and thoracic fat—noninvasive measurement and clinical implications. Cardiovasc Diagn Ther 2012; 2: 85–93. doi: 10.3978/j.issn.2223-3652.2012.04.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK, et al. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J 2007; 71: 536–9. [DOI] [PubMed] [Google Scholar]
- 23.Soliman EZ, Ding J, Hsu FC, Carr JJ, Polak JF, Goff DCJr. Association between carotid intima-media thickness and pericardial fat in the Multi-Ethnic Study of Atherosclerosis (MESA). J Stroke Cerebrovasc Dis 2010; 19: 58–65. doi: 10.1016/j.jstrokecerebrovasdis.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang TD, Lee WJ, Shih FY, Huang CH, Chen WJ, Lee YT, et al. Association of epicardial adipose tissue with coronary atherosclerosis is region-specific and independent of conventional risk factors and intra-abdominal adiposity. Atherosclerosis 2010; 213: 279–87. doi: 10.1016/j.atherosclerosis.2010.07.055 [DOI] [PubMed] [Google Scholar]
- 25.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 2008; 117: 605–13. doi: 10.1161/CIRCULATIONAHA.107.743062 [DOI] [PubMed] [Google Scholar]
- 26.Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol.2010; 10: 191–6. doi: 10.1016/j.coph.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003; 108: 2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5 [DOI] [PubMed] [Google Scholar]
- 28.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J 2009; 30: 850–6. doi: 10.1093/eurheartj/ehn573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pflederer T, Marwan M, Schepis T, Ropers D, Seltmann M, Muschiol G, et al. Characterization of culprit lesions in acute coronary syndromes using coronary dual source CT angiography. Atherosclerosis 2010; 211: 437–44. [DOI] [PubMed] [Google Scholar]
- 30.Rajani R, Shmilovich H, Nakazato R, Nakanishi R, Otaki Y, Cheng VY, et al. Relationship of epicardial fat volume to coronary plaque, severe coronary stenosis, and high-risk coronary plaque features assessed by coronary CT angiography. J Cardiovasc Comput Tomogr 2013; 7: 125–32. doi: 10.1016/j.jcct.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wassel CL, Laughlin GA, Araneta MR, Kang E, Morgan CM, Barrett-Connor E, et al. Associations of pericardial and intrathoracic fat with coronary calcium presence and progression in a multiethnic study. Obesity (Silver Spring) 2013; 21: 1704–12. doi: 10.1002/oby.20111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristoffersen US, Lebech AM, Wiinberg N, Petersen CL, Hasbak P, Gutte H, et al. Silent ischemic heart disease and pericardial fat volume in HIV-infected patients: a case-control myocardial perfusion scintigraphy study. PLoS One 2013; 8: e72066. doi: 10.1371/journal.pone.0072066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ormseth MJ, Lipson A, Alexopoulos N, Hartlage GR, Oeser AM, Bian A, et al. Association of epicardial adipose tissue with cardiometabolic risk and metabolic syndrome in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013; 65: 1410–15. doi: 10.1002/acr.22027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamirani YS, Pandey S, Rivera JJ, Ndumele C, Budoff MJ, Blumenthal RS, et al. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis 2008; 201: 1–7. doi: 10.1016/j.atherosclerosis.2008.04.045 [DOI] [PubMed] [Google Scholar]
- 35.Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care 2011; 34(Suppl. 2): S371–9. doi: 10.2337/dc11-s250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janik M, Hartlage G, Alexopoulos N, Mirzoyev Z, McLean DS, Arepalli CD, et al. Epicardial adipose tissue volume and coronary artery calcium to predict myocardial ischemia on positron emission tomography-computed tomography studies. J Nucl Cardiol 2010; 17: 841–7. [DOI] [PubMed] [Google Scholar]
- 37.Vasques AC, Souza JR, Yamanaka A, de Oliveira Mda S, Novaes FS, Pareja JC, et al. Sagittal abdominal diameter as a marker for epicardial adipose tissue in premenopausal women. Metabolism 2013; 62: 1032–6. doi: 10.1016/j.metabol.2013.01.022 [DOI] [PubMed] [Google Scholar]