Abstract
Objective:
Occupational radiation doses from fluoroscopic procedures are some of the highest doses of exposure amongst medical staff using radiography. Protective equipment and dose monitoring are used to minimize and control the risk from these occupational doses. Other studies have considered the effectiveness of this protection, but this study further considers whether protection is adequate for the lower leg and foot and the extent to which these doses can be reduced.
Methods:
Scatter air kerma profiles at toe level were measured with an ionization chamber. Thermoluminescent dosemeters and lower extremity phantoms were used to estimate the dose variation with the height of patient couch. A 7-week period of in situ toe dose monitoring of four radiologists was also undertaken.
Results:
The use of protective curtains effectively reduced the exposure to most of the lower extremities. Toe doses were found to be high and increased with increase in couch height. In situ monitoring indicated annual toe doses of 110 mSv for two of the four radiologists monitored.
Conclusion:
Protective curtains should be used, but they might have limitations with respect to toe doses. Annual toe doses approaching the classification threshold of 150 mSv were measured for two radiologists. Caution should be exercised when there is a gap below curtains and, when possible, staff should step back from the couch. Lower legs and toes should be included in local radiation protection programmes.
Advances in knowledge:
Toe doses in interventional radiology may be higher than expected and may have to be included in radiation protection programmes.
The occupational radiation doses from interventional procedures with fluoroscopic image guidance are the highest dose exposures amongst medical staff using radiography.1 Fluoroscopic screening times per procedure vary owing to many factors, including procedure complexity, the experience of the interventionist and the equipment used. Interventional procedures generally necessitate more imaging than diagnostic procedures.2 Lengthy screening times may result in significant overall doses to the staff, most notably the interventionist, who remains in close proximity to the patient throughout a procedure and is therefore at risk from exposure to both primary and scattered radiation. The risk from primary radiation is minimized in staff avoiding the main beam; scattered radiation is therefore the main source of radiation exposure in staff. The monitoring of radiation exposure is a legal requirement in some jurisdictions,3 but the implementation of radiation safety procedures and the exact methods of monitoring vary between centres. Methods include personnel and dosimetry monitoring programmes, along with the use of personal protective equipment (PPE) in the form of aprons, eye shields, mobile and fixed shields and protective curtains. Some areas of the body, such as the hands, are difficult to protect; hand doses to staff are often monitored using finger dosemeters to ensure that dose limits are not exceeded. However, lower extremities are neither routinely protected nor monitored.
Protection at this level is almost solely reliant on a protective lead curtain hanging from the patient couch. In the UK, these curtains are typically manufacturer-standard supplied protection made from 0.5-mm lead-equivalent rubber with multiple- or single-piece blades. The dimensions of the blades are typical, but the attachment and drop may vary depending on the manufacturer. Protective lead curtains are, however, detachable and optional.
From anecdotal feedback, the operator's toes have been observed to protrude under the curtain, particularly if a high couch height is used, as required for certain procedures, and for taller interventionists. This suggests a limitation to the effectiveness of using curtains in reducing the radiation exposure to an operator's lower extremities.
The International Commission on Radiological Protection4 recommends annual hand and feet dose limits of 500 mSv against deterministic risks of erythema at 2 Gy and temporary epilation at 3 Gy; these limits have been adopted by the UK Ionising Radiations Regulations 1999 (IRR99).3 The IRR99 also requires employers to classify employees who are exposed to doses exceeding three-tenths of this limit, which for hands and feet is 150 mSv. The IRR99 instructs employers to take all necessary steps to restrict the extent to which its employees are exposed to ionizing radiation to as low as reasonably practicable (ALARP).
For fluoroscopy-guided interventional procedures, the recommended positioning for a vertical X-ray beam is to orientate the C-arm, such that the radiographic tube is under the couch and the detector is over the couch.5 This orientation is recommended to protect the hands and eyes of the medical staff because the scattered radiation reaching these areas would have been attenuated by the patient. Radiation is scattered from the patient in all directions. This implies that a component that is largely unattenuated by the patient, and therefore more intense, is scattered downwards back through the couch.6 In interventional fluoroscopy, there are two further types of radiation to consider in addition to the scattered radiation; these are primary radiation and leakage radiation. The primary radiation is not incident upon an operator's lower extremities in procedures where this set-up is used. A portion of the leakage radiation from the radiographic tube is incident on the lower extremities of the radiologists; however, under clinical conditions, the amount of leakage radiation will be considerably less than that of the scattered radiation.7 Scattered radiation is therefore the main source of concern regarding safety against radiation exposure in lower extremities.
Radiation shielding can provide some protection from the radiation backscattered underneath the couch to the lower extremities; this includes protective curtains attached to the side of a patient couch, portable radiographic screens and shin guards. Further radiation shielding available includes patient drapes and protective aprons, which provide protection from the radiation scattered on the exit side of the patient but do not protect the lower extremities [assuming a posteroanterior (PA) projection]. Portable shields are available, fixed to the patient couch, for protecting the lower extremities in regions where an interventionist's toes can protrude under the curtain; however, these are not widely in use. The use of shielding varies between interventional radiology departments, and only the protective curtains are in widespread use to shield against lower extremity doses. The use of protective equipment available depends on local factors, including staff diligence and awareness, the degree of involvement of the radiation protection adviser and the commitment of the radiation protection supervisor.8
Compared with research on radiation doses to upper extremities and eyes, research on lower extremity doses in interventional radiology is limited. Whitby and Martin9 carried out air kerma measurements and monitored the hand and foot doses to radiologists for a range of procedures. Thermoluminescent dosemeters (TLDs) were fixed 80 mm below the patella and on the upper aspect of each foot during monitoring, although it was not stated where on the upper aspect of the foot. The group found that, without protection, the lower limb dose was frequently greater than the hand dose, with a mean leg dose between 0.19 and 2.61 mSv per procedure without any protection and between 0.02 and 0.5 mSv per procedure with a protective curtain. The group also identified that, during biliary stent procedures, a greater couch height was required than during other clinical procedures, which resulted in a larger lower extremity dose, even if a protective curtain was used.
Shortt et al10 monitored nine interventional radiologists with TLDs positioned just above the ankle for 1 month before and 1 month after the installation of a protective curtain to the patient couch. Extrapolation of the results by monitoring for a month before installation of a lead curtain showed that a radiologist would have exceeded the annual dose threshold for both lower extremities for a non-classified worker of 150 mSv. The mean monthly left foot dose of the nine radiologists was 6.50 mSv before and 2.31 mSv after curtain installation. The group reported “alarmingly” high lower extremity doses, which were reduced by 64% after the introduction of a protective curtain. The group did not specifically address toe doses or the presence of a gap between the curtain and the floor.
A study focused on the radiation exposure to various anatomical locations by Hausler et al11 identified that exposure of the extremities needs more attention. Furthermore, they stated that the lower extremity dose should not restrict the annual number of procedures; however, this is based on a 500-mSv dose limit. They did not state whether they were suggesting that the 150-mSv non-classified extremity dose threshold could be exceeded. They concluded that the hanging of shielding blades from the couch “may give sufficient shielding”. Ubeda et al12 focused on paediatric interventional cardiology and measured scatter dose rates at the radiologist's eye and lower extremity levels without shielding. Scatter dose rates at the lower extremities were around double those at eye level, and a lower extremity dose of 0.1 mSv per paediatric cardiology single-plane procedure and 1 mSv per biplane procedure was estimated.
Tsapaki et al13 reported mean cardiologist foot doses of 0.036 mSv per coronary angiography (CA) procedure and 0.046 mSv per percutaneous transluminal coronary angioplasty (PTCA) procedure when a protective curtain was used. Without the use of a curtain, the group reported foot doses of 0.245 mSv for each CA and 0.479 mSv for each PTCA procedure. They did not state where on the foot the TLDs were positioned.
Schueler et al7 conducted a study to investigate how operator exposure in interventional radiology is affected by various common fluoroscopic imaging conditions. Stray radiation around a C-arm was measured in clinically representative conditions, and the isodose curves were plotted. The measurements were taken in a vertical plane at a number of distances above the floor level; notably, the lowest height measured was 30 cm. The study did not therefore address radiation dose levels at the floor level.
A study by Domienik et al14 estimated maximum annual doses of 55 mSv to the knees and 328 mSv to the ankles of physicians working across three hospitals. The group emphasized the need for the proper use of PPE. Efstathopoulos et al15 monitored the radiation doses to personnel working in interventional radiology and cardiology over 25 procedures. They included monitoring of both legs using TLDs positioned below a person's lead apron, and, in all procedures, a protective curtain was fixed to the patient table. The group's findings of interventionists' leg doses were 0.124 mSv (average) and 1.459 mSv (maximum) per procedure. Koukorava et al16 monitored 2 operators for 50 procedures and reported the maximum leg dose for a single procedure of 0.6 mSv, which was measured using TLDs positioned around 5 cm below the bottom of the operator's lead apron.
A pilot study in our institution was implemented prior to embarking on the main body of work described in this publication. Upper foot dose monitoring of 4 radiologists for a total of 12 interventional fluoroscopic procedures was implemented. Local clinical practice involves the use of a protective curtain hanging from the side of the patient couch on the side that the radiologist is working. Local clinical practice does not require further shielding of lower extremities. An aluminium oxide TLD of a minimum reportable dose of 0.01 mSv and a radiographic photon energy response range of 5 keV–6 MeV (Landauer Inc., Glenwood, IL) was positioned on the upper surface of each foot, around midway, distally between the toe and ankle of a radiologist. The ranges of right and left foot results of 0–0.12 and 0–0.26 mSv per procedure were similar to the results published by Whitby and Martin9 and Tsapaki et al13 and demonstrated a need for further investigation.
The body of literature indicates that the use of protective curtains hanging from the couch does effectively reduce the radiation dose to the lower leg, as summarized in Table 1. However, the published data also raise concerns that foot doses may be close to, or exceeding, the extremity radiation dose limit. This is further endorsed by early stage measurements in our institution where foot doses monitored using TLDs were as high as 0.26 mSv per procedure, which if sustained would reach the UK classification level in 577 exposures.
Table 1.
Published effective lower-leg exposures (mSv) for comparison where per procedure dose is reported
| Study | Position of dosemeters | Mean procedure dose (mSv) | Maximum procedure dose (mSv) |
|---|---|---|---|
| Efstathopoulos et al15 | Lower leg | 0.143 | 1.959 |
| Koukorava et al16 | Lower leg | 0.100 | 0.600 |
| Tsapaki et al13 (PTCA) | Foot | 0.046 | 0.100 (mean + 1 standard deviation) |
| Whitby and Martin9 | Lower leg | 0.020 | 0.500 |
| Ubeda et al12 | Ankle | 0.100 (single plane) | 1.000 (bi-plane) |
| Domienik et al14 | Ankle | 0.100 | 1.459 |
PTCA, percutaneous transluminal coronary angioplasty.
Only results where protective curtains were used are included.
The aim of this study was to monitor radiation doses to the lower extremities, particularly the toes, as these are the most distal part of the foot and are prone to protruding under the protective curtain. The results will provide information to determine whether classification levels of radiation were being exceeded at our centre and whether we and the other centres should be doing more to achieve the IRR99 requirement of keeping staff doses ALARP.3
METHODS AND MATERIALS
Scatter air kerma rate profiles
Scatter air kerma rate profiles around a Philips Allura fluoroscopic C-arm, flat plate, radiographic system (Royal Philips Electronics, Amsterdam, Netherlands) were measured using a calibrated Keithley Triad™ dosimetry system with a 150-cc parallel plate ionization chamber (Fluke Electronics Corp., Everett, WA).
Fluoroscopic factors were chosen to replicate those used clinically for fluoroscopy in our institution for abdominal imaging. An anthropomorphic “Alderson RANDO®” phantom (Radiology Support Devices Inc., Long Beach, CA) was used to simulate a patient. Automatic exposure control was used for fluoroscopic screening and resulted in a tube voltage of 75 kV and tube current of 5 mA pulsed at 7.5 frames per second (low dose), with system-selected 0.9-mm Cu and 1-mm Al filtration. A low dose rate, PA projection setting was adopted, as this is the main clinical projection5 and reflected the worst-case scenario with respect to lower body exposure.
The field size at the detector was 48 cm (diagonally) with no collimation within the field. The source-to-image distance (SID) was 92 cm. Only the table-mounted lead screen was used, which is constructed from a 75 × 63 cm detachable single-piece curtain containing 0.5 mm lead.
A grid of 53 squares of side length 13 cm was used for positioning the chamber as illustrated in the Results section. Squares E4–E7 were aligned with the side of the radiographic tube housing. The distance between the pedestal and the nearside of square H7 was 4.5 cm. The far sides of squares E7, F7, G7 and H7 were in line with the centre of the pedestal. The average dose rate, in micro-Gray per minute, in each square of the grid was measured using the following experimental set-ups.
Set-up 1
The ion chamber was mounted 6 cm above floor level and orientated parallel to the floor to represent the upper surface of the foot. No curtain was used at baseline.
Set-up 2
As mentioned in set-up 1, but using the protective curtain. The height of the couch was set to a “typical” height that left a 13.5-cm gap between the floor and the curtain.
Phantom measurements of lower extremity doses with varying couch height
A pair of knee-high waterproof boots were filled with water to act as a tissue-equivalent lower-leg phantom. A combination of two types of TLDs was used to assess the radiation dose to the anterior aspect of the phantom (Mirion Technologies Inc., San Ramon, CA, and Landauer Inc., Glenwood, IL). Both sets of TLDs had a minimum reportable dose of 0.01 mSv and a radiographic photon energy response range of 5 keV–6 MeV. The TLDs were fixed to the upper surface of the foot and to the anterior surface of the shin region. At the toes, the height above the floor level of the TLD was 6 cm and the most superior TLD position on the shin was 32 cm. The left boot was positioned in squares F3 and F4 as shown in the Results section and the right boot in the centre of squares G3, G4, H3 and H4. The toe-ends of the boots were 3 cm from the vertical plane of the curtain (i.e. not protruding under). The boot circumference at the level of the top dosemeter was 44 cm, and at the ankle the circumference was 36 cm.
The same fluoroscopic factors were used as for the scatter measurements. Screening was for 15 min at SIDs of 92, 100 and 108 cm corresponding to floor–curtain gaps of 10.5, 18.5 and 26.5 cm, respectively. The SID range was considered achievable in clinical use, although the actual SID range is operator and procedure dependent. An additional baseline was taken without the lead curtain.
In situ toe dose monitoring
Three lithium fluoride TLDs (Regional Radiation Physics and Protection Service, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK) with a minimum reportable dose specified by the dosimetry service of 0.2 mSv were secured in both of the personally identified theatre clogs of four interventional radiologists for 7 weeks. The radiologists were briefed on the necessity of using the clogs containing the TLDs during the monitoring period. The use of the clogs was also noted alongside the procedure entry in the system log books. The TLDs were attached to the inner upper surface of each of the clogs, at the distal end of the clogs, using a heavy-duty tape. The locations of the TLDs are shown in Figure 1.
Figure 1.
A theatre clog showing the type and location of the thermoluminescent dosemeters. For clarity these are shown on the exterior of the clog but were inside for the in situ monitoring.
The clogs were worn for a range of procedures during the data collection period (Table 2).
Table 2.
The number of procedures undertaken in the monitoring period for each interventionist
| Procedure | Radiologist 1 | Radiologist 2 | Radiologist 3 | Radiologist 4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Embolization | 1 | 2 | 1 | 2 | |||||
| EVAR | 0 | 2 | 2 | 0 | |||||
| FEVER | 0 | 1 | 0 | 1 | |||||
| Fistuloplasty | 0 | 1 | 1 | 0 | |||||
| IVC filter | 0 | 0 | 1 | 1 | |||||
| Other | 1 | 5 | 0 | 2 | |||||
| PTA | 3 | 1 | 3 | 5 | |||||
| RIG | 0 | 0 | 0 | 2 | |||||
| Sclerotherapy | 0 | 5 | 0 | 1 | |||||
| Stent | 2 | 3 | 6 | 4 | |||||
| TEVAR | 1 | 0 | 0 | 1 | |||||
| TIPPS | 1 | 1 | 0 | 0 | |||||
| Venogram | 0 | 1 | 0 | 1 |
EVAR, endovascular aneurysm repair; FEVAR, fenestrated endovascular aneurysm repair; IVC, inferior vena cava; PTA, percutaneous transluminal angioplasty; RIG, radiologically inserted gastrostomy; TEVAR, thoracic endovascular aneurysm repair; TIPPS, transjugular intrahepatic portosystemic shunt.
All rooms used for the in situ measurements used the same size of table-attached curtains, with the same level of protection, except in one where the system manufacturer attached the curtain slightly lower than the others.
The type of PPE used was not controlled or monitored during the data collection period, but there is a requirement in the Local Rules for appropriate PPE to be worn. For all the examinations in this study, this was a 0.35-mm lead-equivalent apron in either a tabard or a “kilt and top” style dependent on operator preference. Neither apron type would have affected the dose received by the toes.
Thyroid shields were also available. The results were scaled up to represent an annual dose based on each radiologist's workload. The annual workload for each radiologist was estimated based on their individual workload for the preceding calendar year, as recorded in the system log books.
RESULTS
Scatter air kerma rate profiles
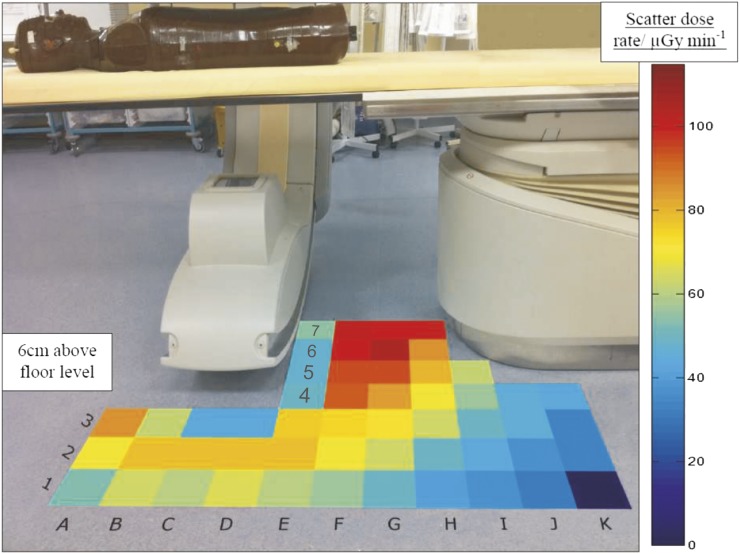
The scatter air kerma rate profile measured at approximate toe height without the use of the protective curtain is shown in Figure 2. This shows that, in the absence of the curtain, dose rates approaching 100 μGy min−1 at a 6-cm height (toe height) were measured where the interventionist stands.
Figure 2.
Scatter air kerma rate (μGy min−1) profile measured using an ionization chamber mounted 6 cm above floor level and orientated parallel to the floor to represent the upper surface of the foot. A protective curtain was not used for these measurements.
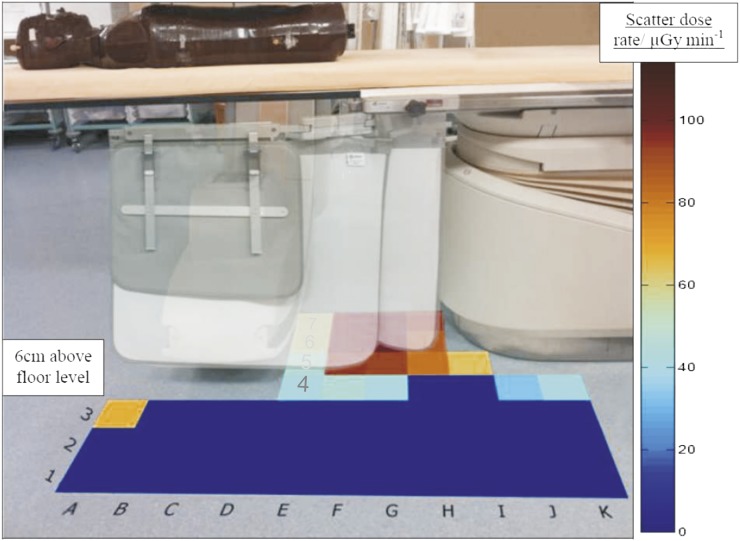
Figure 3 shows that the use of a protective curtain reduces these doses by approximately 50% in the toe region. Region A3 has a measured dose rate of approximately 70 μGy min−1, but all other areas in front of the protective screen received a dose rate of <15 μGy min−1.
Figure 3.
Scatter air kerma rate (μGy min−1) profile measured with the ionization chamber mounted 6 cm above floor level and orientated parallel to the floor to represent the upper surface of the foot measured using a protective curtain. The floor to curtain gap is 10.5 cm. Note that the protective curtain is transparent for illustrative purposes.
Phantom measurements of lower extremity doses with varying couch height
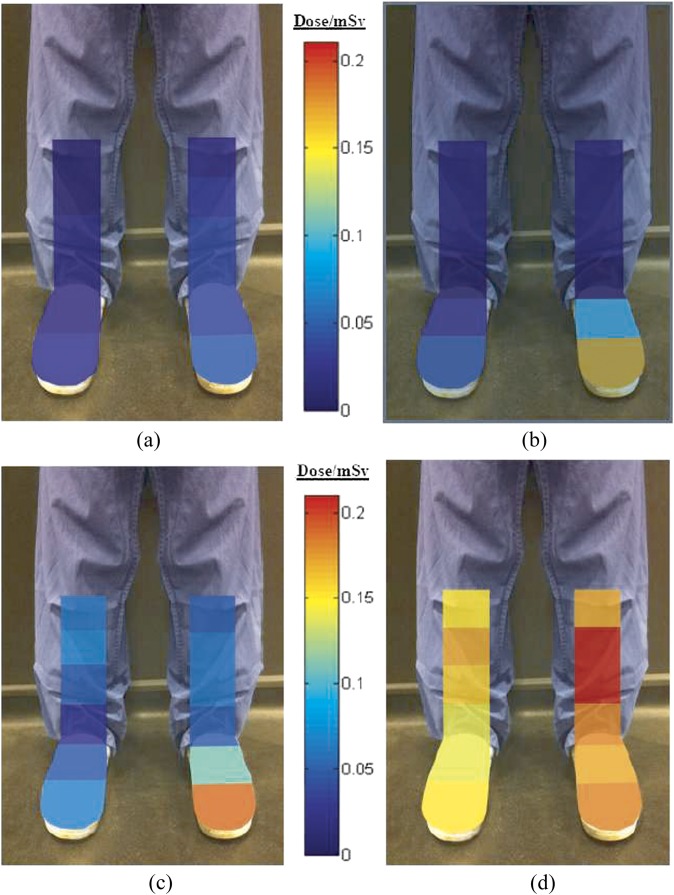
The equivalent radiation doses measured using TLDs attached to the lower-leg water phantom, irradiated using varying couch heights, are shown superimposed onto their anatomical position in Figure 4.
Figure 4.
Illustrative representation of the effective dose distribution (mSv) to the lower extremities after 15 min fluoroscopic screening with floor to curtain gaps of (a) 10.5 cm, (b) 18.5 cm, (c) 26.5 cm and (d) no protective curtain. The left foot was nearest the X-ray tube.
The results show that dose to the lower extremities increases as the table height increases. The operator's left toe region also consistently received the highest dose whilst the protective curtain was in use. Without the protective curtain, the highest dose received was to the left shin area.
In situ toe dose monitoring
The in situ radiation doses measured over the 7-week dosimetric monitoring period are shown in Table 3; this shows that two of the four radiologists have a projected annual toe exposure of 110 mSv, which is approaching the classification level in the UK of 150 mSv. Radiologist 4 has an estimated annual toe exposure of <10% of this but had been observed to consistently step back during exposures.
Table 3.
Effective radiation toe dose (mSv) results for four radiologists monitored during a sample of procedures performed within a 7-week period in our institution
| Radiologist | Left foot (mSv) |
Right foot (mSv) |
No. of procedures monitored | No. of annual procedures | Estimated annual toe dose (mSv) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fifth toe | Third toe | Hallux | Hallux | Third toe | Fifth toe | ||||
| 1 | 3.0 | 3.2 | 3.6 | 4.9 | 4.1 | 3.7 | 9 | 199 | 110 |
| 2 | 9.3 | 9.9 | 9.4 | 6.0 | 5.5 | 4.8 | 22 | 157 | 70 |
| 3 | 3.8 | 4.0 | 4.4 | 7.2 | 7.6 | 8.9 | 14 | 167 | 110 |
| 4 | 0.5 | 0.6 | 0.5 | 0.4 | 0.3 | 0.3 | 20 | 137 | <10 |
The thermoluminescent dosemeter (TLD) positions are approximated to the nearest toe. The estimated annual dose is based on the highest TLD result and the number of procedures performed by the corresponding radiologist over the preceding calendar year.
DISCUSSION
The aims of this study were to evaluate the lower extremity radiation doses to interventionists working in interventional fluoroscopy and to address how and to what extent these radiation doses could be reduced. Owing to the nature of interventional radiology, there is no standard procedure type, and the variations between procedure parameters are wide. This produces difficulties when attempting to simulate a typical clinical environment for experimentation and necessitates in situ dosimetry in addition to experimental data collection.
There are no similar studies in the literature with which to compare the scatter air kerma rate profiles illustrated in Figures 2 and 3. Whitby and Martin9 produced dose rate contour plots in the volume around the C-arm, whereas we focused on the floor area providing greater detail over a smaller volume. They reported dose rates near the floor level of 10–50 μGy min−1. Their floor level data appear to have largely been obtained in the midline of the radiographic tube, which, as shown in Figure 2, provides shielding and reduces dose rates within its immediate vicinity. This may explain the relatively lower dose rates at floor level that they reported.
As the data in Figures 2 and 3 represent air kerma measurements that have not been converted to equivalent dose measurements, they cannot be used to estimate personnel annual doses. The data provided an indication of the relative doses in different areas of the room with, and without, the curtain. The scatter air kerma rates in this study were measured with an X-ray beam uncollimated within the field size. In reality, the interventionist is encouraged to collimate the beam appropriately. Further work could involve investigating the effect of variation of field size with scatter dose to the lower extremities. Other sources that could increase or decrease the interventionist's lower extremity dose include patient size, use of acquisitions, procedure complexity and the interventionist's experience. Furthermore, some clinical procedures require angulation of the radiographic tube with respect to the patient, which can result in the use of the protective curtain being infeasible.
Mobile shields and other floor-mounted protection were not considered in this study, because they were not available in our institution. The use of such shields will depend on institution and operator preference, and the utility of them could influence the toe dose either way. This is an area that could be explored in future work.
The results from Table 1 indicate that there is a clear benefit for the interventionist in stepping back from the X-ray source both with and without the use of the protective curtain. This is further supported by considering the dose rate of 100 μGy min−1 on the patient side of the curtain reducing to <15 μGy min−1 on the interventionist side of the curtain. If the toes are protruding under the curtain, stepping back a few centimetres to ensure the toes are on the protected side would therefore provide an 85% reduction in toe dose. The results for Radiologist 4, who had been observed to consistently step back during procedures, support this, as this radiologist had the lowest extremity dose results at <10% of that of the highest two radiologists' results. This can be accounted for by the observations that Radiologist 4 was much more aware of the scattered radiation risk and, whenever possible, stepped back from the couch.
The anomaly in square A3 in Figure 3 exhibits a higher scatter dose. This can be accounted for by the lack of protection from the self-shielding effect of the radiographic tube or, where applicable, the protective curtain. This area corresponds to a region in which other staff may stand. The radiation dose to staff groups other than the interventionist should be further investigated and was not addressed by the in situ dosimetric monitoring in this study.
The dosimetric consequences of a varied couch height were investigated showing that there is a greater risk of lower extremity radiation dose when using higher couch positions. The left toe closest to the tube region always had the highest radiation doses when a protective curtain was used. When no protective curtain was used, the highest radiation doses were measured at the left shin region.
Of concern is that the in situ toe dose monitoring results show that two of the four radiologists studied indicated estimated annual lower extremity doses of 110 mSv, despite the use of a protective curtain on the side of the treatment couch. This exceeds our local investigation level of 100 mSv and is approaching the classification level of 150 mSv. Monitoring of a larger sample size could identify interventionists who unknowingly exceed the classification threshold.
No other studies reporting long-term toe radiation monitoring have been published; however, a number of groups have reported lower extremity doses, as discussed earlier in this article. If these results are extrapolated using the relative toe, ankle and leg doses as found in Figure 4 (there is an approximate factor of 2 increase to toe dose), then it is possible that many interventionists could be receiving lower extremity radiation doses in excess of 150 mSv annually.
This study demonstrates that current radiation protection for the toe region is not always adequate and does not always result in doses ALARP. It is suggested that a theatre clog constructed with a shielding material in the toe region, combined with the correct use of the protective curtain, should protect the interventionist from the majority of lower extremity radiation doses. Matching the curtain length to the operator height and procedure type may be a practical solution, particularly if the curtain was height adjustable. But care should be taken to prevent “gathering” of the curtain at the floor, as this may produce an obstruction to the operator or gaps in the protection due to folds.
CONCLUSIONS
This study has demonstrated the need for radiation protection and dosimetric monitoring considerations to be made regarding the lower extremities, particularly the toes, of staff members working in interventional radiology. Even with the use of current radiation protection devices, such as a protective curtain, the annual radiation doses were determined to be almost three-quarters of the dose level necessitating classification of staff. This exposure is attributed to the presence of a gap between the protective curtain and the floor, the size of which is dependent on the height of the treatment couch. Consequently, for procedures requiring a higher couch height, interventionists may receive an increased lower extremity radiation dose. Stepping back from the couch appears to be an effective method of reducing toe dose; if this is not practicable, the use of a protective clog with a protective toe insert could be considered without changing current operator practice. If an institution's local risk assessment identifies that similar dose levels may be reached, it is recommended that routine dosimetric monitoring of the lower leg/toes is required in order to verify that the applicable national dose limits are not being breached. Other methods such as mobile screens may be appropriate but were not investigated in this study.
FUNDING
This work is supported by the Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Acknowledgments
ACKNOWLEDGMENTS
The authors thank all staff who contributed within the trust. Resource contributions were kindly donated by: Landauer Europe (Oxford, UK); Mirion Technologies (IST) Ltd (Farnborough, UK); and Toffeln Global LLP (Bristol, UK). The authors would like to thank these companies for their support.
REFERENCES
- 1.Vano E. Radiation exposure to cardiologists: how it could be reduced. Heart 2003; 89: 1123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanhavere F, Carinou E, Donadille L, Ginjaume M, Jankowski J, Rimpler A, et al. An overview on extremity dosimetry in medical applications. Radiat Prot Dosimetry 2008; 129: 350–5. doi: 10.1093/rpd/ncn149 [DOI] [PubMed] [Google Scholar]
- 3.HMSO. The ionising radiations regulations 1999. Norwich, UK: The Stationery office; 1999. Available from: www.legislation.gov.uk/uksi/1999/3232/contents/made
- 4.International Commission on Radiological Protection. 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication no. 60. Ann ICRP 1991; 21: 1–3. [PubMed] [Google Scholar]
- 5.Miller DL. Interventional fluoroscopy: reducing radiation risks for patients and staff. Society for Interventional Radiology. J Vasc Interv Radiol 2009; 20 (Suppl. 7): S274. doi: 10.1016/j.jvir.2009.04.057 [DOI] [PubMed] [Google Scholar]
- 6.Schueler BA. Operator shielding: how and why. Tech Vasc Interv Radiol 2010; 13: 167–71. doi: 10.1053/j.tvir.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 7.Schueler BA, Vrieze TJ, Bjarnason H, Stanson AW. An investigation of operator exposure in interventional radiology. Radiographics 2006; 26: 1533–41. doi: 10.1148/rg.265055127 [DOI] [PubMed] [Google Scholar]
- 8.Martin CJ, Whitby M. Application of ALARP to extremity doses for hospital workers. J Radiol Prot 2003; 23: 405–21. [DOI] [PubMed] [Google Scholar]
- 9.Whitby M, Martin CJ. Radiation doses to the legs of radiologists performing interventional procedures: are they a cause for concern? Br J Radiol 2003; 76: 321–7. [DOI] [PubMed] [Google Scholar]
- 10.Shortt CP, Al-Hashimi H, Malone L, Lee MJ. Staff radiation doses to the lower extremities in interventional radiology. Cardiovasc Intervent Radiol 2007; 30: 1206–9. doi: 10.1007/s00270-007-9071-0 [DOI] [PubMed] [Google Scholar]
- 11.Hausler U, Czarwinski R, Brix G. Radiation exposure of medical staff from interventional x-ray procedures: a multicentre study. Eur Radiol 2009; 19: 2000–8. [DOI] [PubMed] [Google Scholar]
- 12.Ubeda C, Vano E, Gonzalez L, Miranda P, Valenzuela E, Leyton F, et al. Scatter and staff dose levels in paediatric interventional cardiology: a multicentre study. Radiat Prot Dosimetry 2010; 140: 67–74. doi: 10.1093/rpd/ncq039 [DOI] [PubMed] [Google Scholar]
- 13.Tsapaki V, Kottou S, Vano E, Komppa T, Padovani R, Dowling A, et al. Occupational dose constraints in interventional cardiology procedures: the DIMOND approach. Phys Med Biol 2004; 49: 997–1005. [DOI] [PubMed] [Google Scholar]
- 14.Domienik J, Brodecki M, Rusicka D. A study of the dose distribution in the region of the eye lens and extremities for staff working in interventional cardiology. Radiat Meas 2012; 47: 130–8. [Google Scholar]
- 15.Efstathopoulos EP, Pantos I, Andreou M, Gkatzis A, Carinou E, Koukorava C, et al. Occupational radiation doses to the extremities and the eyes in interventional radiology and cardiology procedures. Br J Radiol 2011; 84: 70–7. doi: 10.1259/bjr/83222759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koukorava C, Carinou E, Simantirakis G, Vrachliotis TG, Archontakis E, Tierris C, et al. Doses to operators during interventional radiology procedures: focus on eye lens and extremity dosimetry. Radiat Prot Dosimetry 2011; 144: 482–6. doi: 10.1093/rpd/ncq328 [DOI] [PubMed] [Google Scholar]