Abstract
Tumour heterogeneity has, in recent times, come to play a vital role in how we understand and treat cancers; however, the clinical translation of this has lagged behind advances in research. Although significant advancements in oncological management have been made, personalized care remains an elusive goal. Inter- and intratumour heterogeneity, particularly in the clinical setting, has been difficult to quantify and therefore to treat. The histological quantification of heterogeneity of tumours can be a logistical and clinical challenge. The ability to examine not just the whole tumour but also all the molecular variations of metastatic disease in a patient is obviously difficult with current histological techniques. Advances in imaging techniques and novel applications, alongside our understanding of tumour heterogeneity, have opened up a plethora of non-invasive biomarker potential to examine tumours, their heterogeneity and the clinical translation. This review will focus on how various imaging methods that allow for quantification of metastatic tumour heterogeneity, along with the potential of developing imaging, integrated with other in vitro diagnostic approaches such as genomics and exosome analyses, have the potential role as a non-invasive biomarker for guiding the treatment algorithm.
Although continual improvements in diagnosis, surgical techniques and radiation oncology have together provided improved survival for many forms of human cancers, a majority of deaths from cancer are caused by the development and continuous growth of metastases that are resistant to conventional therapies. Similarly, although the use of systemic non-targeted and targeted adjuvant therapies has helped to prevent the spread of tumour cells from the primary site and is now a standard practice for many tumour types, the emergence of resistant disease continues to be a significant cause of patient mortality. These features provide an insight into the dynamic nature of the signalling network within the tumour cells,1 and human cancers are now being increasingly recognized as heterogeneous, characterized by distinct pathological, genomic, clinical and therapeutic features.
Nearly 150 years after the original theory of tumours originating from immature cells by Virchow,2 innovative technological approaches unequivocally demonstrate the cellular heterogeneity of tumours, composed of distinct subpopulations of cancer cells within (“intra”) and between (“inter”) tumours of individual patients. These subpopulations are characterized by specific genetic and morphological profiles, representing the clonal selection and evolution of that tumour.3,4 This heterogeneity provides a powerful internal mechanism through which tumour cells can ultimately escape environmental stresses, including oncological therapies, posing a considerable challenge for translational researchers.
There is considerable evidence that the tumour microenvironment actively contributes to tumour heterogeneity.5 Arguably the best example of this is the “pre-metastatic niche”, defined as the creation of an ideal thriving environment for the primary tumour to “seed” to. Through the secretion of cytokines, chemokines and growth factors, the primary tumour “primes” a distal site to become an ideal niche/target organ, favourable for future metastatic colonization.6 Although in some cases the target organ is already primed for metastatic spread and many organs may have “seeding” of cells, only a few will take “root”.7 Increasing understanding of tumour heterogeneity demands an effort from researchers to establish and understand pre-metastatic changes within distant organs and their major drivers.
This new paradigm of cancer heterogeneity has yet to be fully assimilated into everyday patient management. It has been well documented with certain cancers that imaging signals can show phenotypic tumour heterogeneity and have clinical implications; for example, in radio-iodine imaging of metastatic thyroid cancer, some metastatic lesions may not take up radio-iodine and therefore will be unaffected by radio-iodine therapy. However, for the majority of tumours, biopsies remain the standard of care for assessing tumour biology but cannot be expected to represent the entirety of a tumour in this tumour heterogenic era.4 Many physicians advocate the re-biopsy of metastatic disease at re-presentation for histological analysis and comparison with the primary, in an attempt to improve the choice of therapy upon relapse, having taken into account, for instance, intertumoral heterogeneity between the primary and metastatic disease.8 Repeated biopsy of tumour tissue is invasive, may be practically difficult, has resource implications and is clearly confounded by intratumoral heterogeneity. These shortcomings give huge potential to the recent advances in molecular imaging, which have the ability to visualize and quantify heterogeneity of tumour receptor expression, metabolism, apoptosis, blood flow or structure, non-invasively over time, i.e. at baseline and to assess response to treatment.
Owing to space constraints, we can only select a subset of imaging techniques for illustration purposes; a more comprehensive précis of the different image modalities has been reviewed elsewhere.8
VARIOUS IMAGING MODALITIES AND METHODS THAT CAN HELP TO MAP THE HETEROGENEITY IN TUMOUR METASTASIS
The development of metastasis is multifactorial and is dependent on the complex interaction between host factors and the tumour biology. This process is highly selective, and the metastatic lesion represents the end point of many sequential events that only a few cells can survive. Recent advances in next generation sequencing (NGS) have increased the understanding of (1) the clonal heterogeneity between primary and metastatic tumours and (2) the degree of genetic heterogeneity of metastatic tumours. For example, a study comparing sequences of primary tumours and metastases in lobular breast cancers revealed multiple mutations present only in metastases and several other mutations with increased frequency in metastatic sites.9 Similarly, a number of studies report on the discordance in oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (HER2) expression between different metastatic sites.10 As pointed out, histological analyses with repeated invasive biopsies have limitations. For instance, when different metastatic deposits are heterogeneous with respect to receptor expression and/or cellularity11 and are not all subjected to biopsy, then a clinical decision based on in vitro analysis of the biopsied material may be prone to undersampling error. However, recent advances in imaging techniques, image acquisition and image analysis have been used to measure quantitative imaging biomarkers that may be able to address the complexities of tumour heterogeneity better than a standard histological biopsy. Here, we critically appraise these strategies specifically in the context of heterogeneous metastatic disease.
18F-fludeoxyglucose–positron emission tomography/CT
Although CT is the imaging modality most widely used for tumour assessment, it provides very little in the way of distinct tumour activity information. The addition of positron emission tomography (PET) to CT can add such further information, and 18F-fludeoxyglucose (18F-FDG) is the most commonly used PET radiotracer, although there are many other radiotracers that examine different aspects of tumour biology. The ability of 18F-FDG-PET to detect cancer is based on elevated aerobic glycolysis in the malignant tissue in comparison with the normal tissue—also known as the Warburg12 effect. Although primarily reporting on tumour cell activity, 18F-FDG-PET has been shown to also inform on tumour heterogeneity. A retrospective study using 18F-FDG-PET/CT to monitor response among lesions in patients with bone-dominant metastatic breast cancer treated with systemic therapies reported that lesions showed heterogeneous metabolic response amongst responding and non-responding bony and non-bony lesions.13
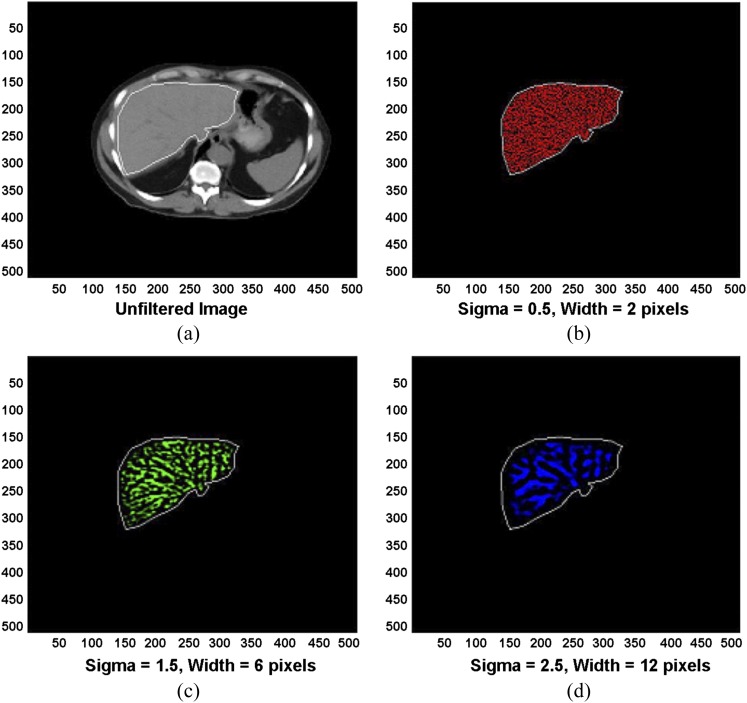
Novel utilization of 18F-FDG-PET/CT in recent years, such as texture analysis on CT imaging, has been shown to reflect tumour heterogeneity and associated prognosis. This has been examined in multiple tumour types, including lung,14–16 colorectal17–19 and oesophageal20 cancers. There are a number of ways to extract texture elements in images. One such CT textural analysis methodology utilizes a two-step filtration–histogram technique. The first stage uses a Laplacian of gaussian spatial band-pass filter to selectively extract and enhance features of different sizes corresponding to fine, medium and coarse texture scales, allowing detection of spatial differences within a tissue (arising from the different band of spatial frequencies employed). The Laplacian detects intensity changes (or edges) within an image, which have been first smoothened by the gaussian distribution, based on the spatial scale filter (SSF) value. A lower SSF value (e.g. 2 mm) extracts and enhances features of a “finer” texture scale, whereas an SSF value of 3, 4 or 5 mm extracts and enhances features of a “medium” texture scale and a higher SSF value (e.g. 6 mm) extracts and enhances features of a “coarser” texture scale, as shown in Figures 1 and 2. These novel texture analyses have also been applied to other imaging modalities, e.g. MRI,21,22 and will be discussed later (section on Simultaneous positron emission tomography/MR and textural analysis).
Figure 1.
(a) Conventional hepatic CT image. (b–d) Corresponding images selectively display (b) fine, (c) medium and (d) coarse texture obtained by using values for image filtration [spatial scale filter value (or sigma)] of 0.5 [width, 2 pixels (1.68 mm)], 1.5 [width, 6 pixels (5.04 mm)] and 2.5 [width, 12 pixels (10.08 mm)], respectively. Images should be viewed in the online format. Reproduced from Miles et al18 with permission from the Radiological Society of North America.
Figure 2.
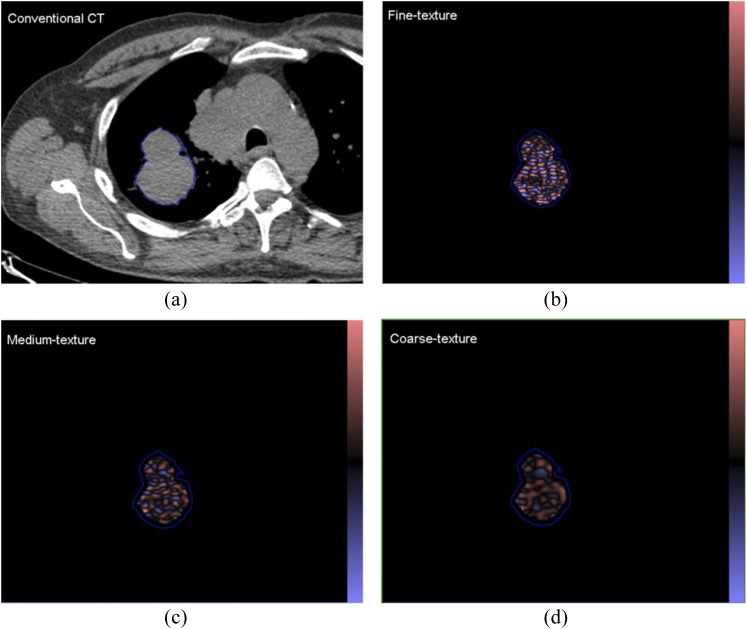
(a) A conventional CT (from a positron emission tomography/CT) image of a patient with a lung lesion and (b–d) corresponding images selectively displaying fine, medium and coarse texture obtained from TexRAD CT texture analysis (image heterogeneity) commercial research software (www.texrad.com, Radstock, UK). Images should be viewed in the online format.
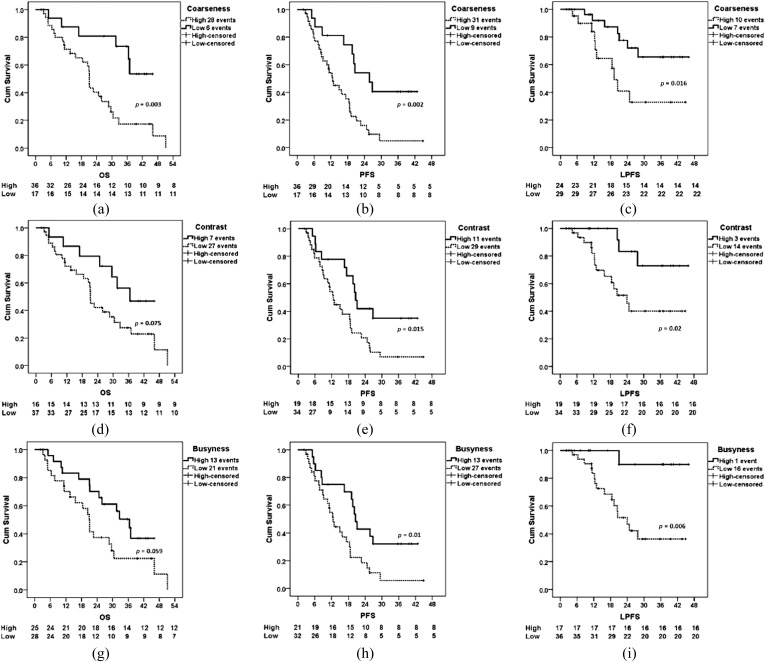
Generation of these texture parameters provides vital information on the image features themselves (reviewed in Miles et al23). Standard deviation (SD) increases approximately in proportion to the square root of the number of features highlighted and their mean intensity difference compared with background (i.e. dark and bright features are both positive). Skewness is related to the average brightness of the highlighted features (predominantly bright features give positive values, while predominantly dark objects give negative values), which tends to zero with increasing number of features highlighted and moves away from zero with intensity variation in highlighted features. Kurtosis is related inversely to the number of features highlighted (whether bright or dark) and increases by intensity variations in highlighted features. By quantifying these different image features (size, concentration and density variation of the features highlighted by the filter) within a lesion (representing the different aspects of tissue heterogeneity), computed image texture analysis algorithms have the potential to provide additional morphological information relating to tumour heterogeneity. The intratumoral variability assessed by this technique is at a scale where the measured heterogeneity is likely to reflect tumour vs stroma and/or tumour vs necrosis. These features could feasibly correlate with a metastatic phenotype, but more work is required in this area to understand the associations between tumour–stromal relationships and gene expression and/or metastatic potential (see section Molecular imaging of metastatic potential). Yet, the prognostic application of CT textural analysis has been validated in various tumours types, with coarser tumours pertaining to a poorer prognosis24 (Figure 3). In fact, overall survival (OS), progression-free survival (PFS) and local progression-free survival were all lower in individuals with high primary tumour coarseness.25 Analysis of tumour texture in pre- and post-chemotherapy treatment in colorectal patients, to examine response and prognosis, has revealed that tumours that respond to treatment have lower initial tumour coarseness.18 In addition to its correlation with survival, there is also limited pre-clinical literature which suggests that the application of these texture techniques can be used to analyse the surface heterogeneity of the primary tumour, and may yield non-invasive image parameters that may distinguish between metastatic and non-metastatic tumour phenotypes,26 with exciting translational potential which needs further investigation.
Figure 3.
Kaplan–Meier plots demonstrating differences in patients with high and low primary tumour 18F-fludeoxyglucose–positron emission tomography coarseness (a–c), contrast (d–f) and busyness (g–i). Differences in overall survival (OS) (a, d and g), progression-free survival (PFS) (b, e and h) and local progression-free survival (LPFS) (c, f and i) are demonstrated. Cum, cumulative. Reproduced from Cook et al25 with permission from SN Turiel & Associates, Inc. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.
PET texture analysis (PTA) can be conducted on the standardized uptake value (SUV) images used to measure the maximum SUV. The SUV images (individual pixel values) with initial units of uptake in Bq ml−1 can be converted (scaled) to SUV calibrated by patient weight and actual tracer activity (taking into consideration the initial tracer activity, amount of decay between the tracer measured time and scan time with respect to the half-life period of 18F-FDG) with final units of uptake in g ml−1. The tumour heterogeneity can be measured only on the SUV image without image filtration, using the histogram characteristics as described above in the section 18F-fludeoxyglucose–positron emission tomography/CT. Image filtration is not appropriate owing to the inherently poor resolution of PET (SUV) data. A recent study in non-small-cell lung cancer (NSCLC) using PET/CT image data sets has shown the ability of PTA to be a prognostic marker of survival.27 Other groups have shown that intra-tumour heterogeneity on PET via texture analysis predicts response to radiochemotherapy in oesophageal cancer (entropy, size, local and global heterogeneity and homogeneity, SUV),28 and lung cancer (coarseness, contrast, busyness, complexity).25 Given the poorer spatial resolution of PET compared with CT, the biophysical basis of metabolic textural features is not intuitive and requires further exploration.
Non-18F-fludeoxyglucose–positron emission tomography for imaging the metastatic potential of primary tumours and/or detecting tumour metastases
18F-fluoro-3′-deoxy-3′-l-fluorothymidine–positron emission tomography
18F-fluorothymidine (FLT) is a tracer used to examine cell proliferation. Pyrimidine analogue thymidine is incorporated in DNA, during the S phase of the cell cycle, where proliferating cells synthesize DNA. 18F-FLT is taken up by the cell and is phosphorylated by thymidine kinase 1. Thymidine kinase 1 activity is the highest during the cell division process in cells and takes place at a greater rate in malignant cells.29 Given the dependence of this radiotracer on thymidine kinase 1 activity, there can be issues when used in conjunction with certain cytotoxic drugs, which arrest cells in S phase,30 such as 5-fluorouracil. Various studies have been carried out on correlating imaging with histological findings and on immunostaining with Ki-67 to assess tumour proliferation rate. These studies have shown good correlation between the histological tumour proliferation rate and the 18F-FLT-PET image.31 Although 18F-FLT-PET is an excellent tool for measuring tumour proliferation, there are several theoretical limitations to its use in detecting micrometastatic disease in patients with cancer. While an increase in proliferation is important for the initiation and maintenance of primary tumours, growth inhibition could ultimately be crucial for survival of carcinoma cells in the circulation. Mechanistically, this apparent paradox is because of the dual function of cell cycle regulators, such as the well-known tumour suppressor gene p5332 and transcription factor YB-1,33 which also impact on the cell motility machinery. Additionally, metastatic cells in the target organ can enter into dormancy (i.e. a lag in tumour growth),34 thus the sensitivity of detecting tumour metastases is somewhat limited.35,36
11C-choline and 18F-fluorocholine–positron emission tomography
Some tumours have low glucose metabolism, and therefore standard FDG-PET imaging has difficulties in the assessment of disease and treatment response. In prostate cancers, choline-PET imaging has been especially useful for restaging. Choline- and fluorocholine-based tracers used in PET scanning utilize the principle that choline is an essential component of the phospholipid portion of the cell membrane. It is particularly of benefit in a selected group of individuals rather than as a staging method for all; namely, patients with minimal recurrent prostate-specific antigen (PSA) levels of ≥1 ng ml−1, those with short PSA doubling time (less than 3 months to a maximum of 6 months), and those with initial high recurrence risk tumour stage.37,38
Simultaneous positron emission tomography/MR
As discussed earlier in this review, PET image analysis traditionally focuses on the region of interest. The addition of MR to PET imaging can further add heterogeneity information regarding the tumour phenotype that is gathered from radionuclide-based studies.39 Dynamic contrast-enhanced (DCE) imaging differs from traditional MRI through the ability to acquire multiple images, before, during and after contrast injection (Figure 4). In the context of PET/MR, this imaging technique allows dynamic imaging of tumours to take place, with detailed imaging of tumour vascularity40 through the concomitant evaluation of αvβ3 expression and high glucose metabolism within tumours that can show perfusion heterogeneity.41 This form of imaging has also played a role in treatment assessment with vascular endothelial growth factor (VEGF) inhibitor use, which we discuss later on in further detail in this review (see section Molecular imaging of metastatic potential).
Figure 4.
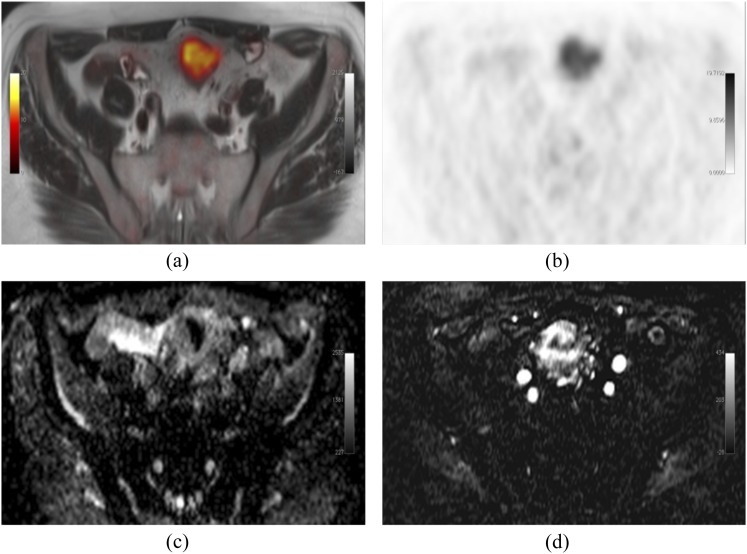
Produced from an imaging unit at the Institute of Nuclear Medicine, University College London, UK. Simultaneous 18F-fludeoxyglucose–positron emission tomography (PET)/MRI-acquired image of a patient with a sigmoid tumour. Fused axial T2 and PET (a), PET alone (b), MRI apparent diffusion coefficient map (c) and representative subtracted image from a dynamic contrast-enhanced MRI series (d); showing increased metabolism, cellularity and vascularity. Images should be viewed in the online format.
Ongoing developments in the combination of PET/MR with nanoparticle imaging have had further implications in the assessment of tumour heterogeneity.42 Given the above discussion on specific (FDG- and non-FDG-based) PET tracers that are potentially of use in mapping the heterogeneity of different tumour types, the combination of specific-tracer PET/MR holds particular interest in imaging molecular heterogeneity.
The combination of microstructural and vascular information afforded by MRI with specific metabolic PET tracers can now be achieved in a clinic with whole-body PET/MR scanners.43 Multiparametric imaging has well-recognized utility for microstructural and vascular tissue characterization and is rapidly establishing an expanding niche in the localization and management of prostate cancer.44,45 Yet, in general, it remains more difficult to assess metabolic activity with MRI than with PET; MR spectroscopy (MRS) is inconsistently used in clinics, as it requires significant expertise in acquisition and processing of the MR signal; whilst hyperpolarized (HP) MRI in addition requires significant investment in infrastructure. Studies validating the use of whole-body PET/MR compared with PET/CT have repeatedly shown increased sensitivity in early tumour detection, and using diffusion weighting on top of PET/MR can also detect treatment response at varying levels within metastases.46–50
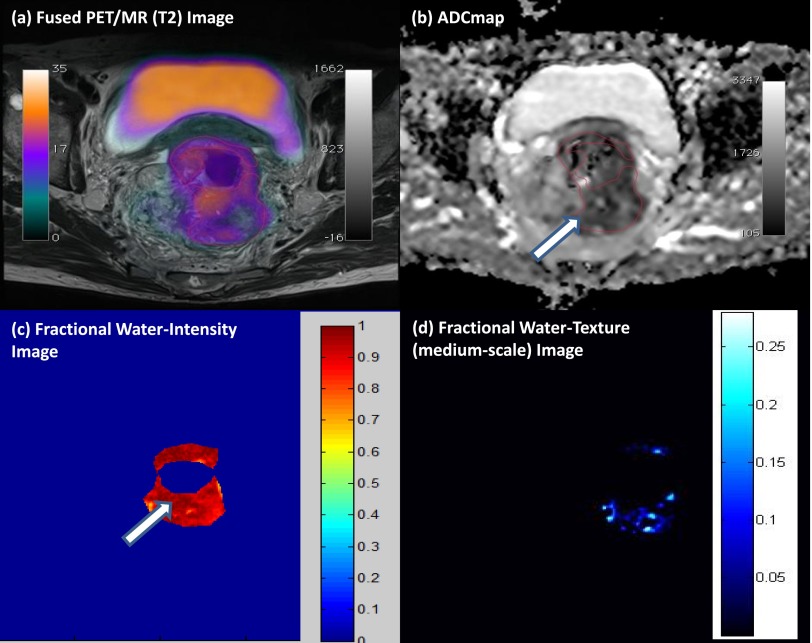
Multiparametric PET/MR performed by our group demonstrates the ability to assess glycolysis, cellularity and water content and intralesional heterogeneity (via texture analysis) within a single examination (Figure 5). In general, we found that tumours with more heterogeneous water distribution (i.e. higher SD and proportion of positive pixels) were more cellular (i.e. lower mean apparent diffusion coefficient) and glycolytic (i.e. higher SUVmean). Foci of high cellularity also correspond to areas of increased glycolysis. Textural filters applied to the fractional water images revealed features of around 3- to 4-mm bright objects, which may be associated with pockets of water content and tended to be higher within tumours having adverse biology (restricted diffusion and increased glucose uptake). Multiparametric PET/MRI data sets evaluating tissue microstructure, metabolism and heterogeneity are likely to contain prognostic information/relate to metastatic potential; both hypotheses require further work to validate.
Figure 5.
Multiparametric positron emission tomography (PET)/MRI of a rectal cancer. (a) High 18F-fludeoxyglucose uptake on fused PET/T2 MRI, with (b) a correspondingly patchy reduced apparent diffusion coefficient (ADC) in keeping with pockets of high cellularity within the tumour and (c) a fractional water image derived from source fat and water Dixon images of the same tumour confirms that areas of increased cellularity correlate with relatively increased water content (white arrows). (d) Application of a medium coarse textural filter highlights 3- to 4-mm bright objects on the fractional water image (medium texture map). Images should be viewed in the online format. (www.texrad.com, Radstock, UK.)
Furthermore, simultaneous PET/MRI offers the opportunity in the clinic to combine tissue characterization multiparametric MRI with specific molecular PET imaging, with the potential to assess dynamic biological relationships through multimodal imaging of, for example, tumour cellularity/cell turnover [diffusion-weighted imaging (DWI) or FLT-PET], hypoxia (blood oxygen level-dependent MRI or 18F-fluoromisonidazole PET ligand), vascularity (DCE/MRI or α-V-β-3 PET ligand) and glycolysis (18F-FDG-PET ligand or glucose chemical exchange saturation transfer MRI).51 Spatial heterogeneity of PET-MRI signals among metastases is often evident.11 Elucidating the mechanisms leading to heterogeneous multimodal metastatic phenotypes and the consequent therapeutic implications remains the remit of future research.
Imaging the link between metabolism and tumour signalling pathways that are associated with metastasis: hyperpolarized MRI
13C-MRS has been used in the investigation of metabolic processes in vivo for many years.52 Its limitations relate to the difficulty in the signal intensity of the proteins in question, mainly down to the physics of MRI and its use of the apparent diffusion coefficient of water. Hyperpolarization with the dynamic nuclear polarization technique can yield >10 000-fold signal increases in MR-active nuclei, allowing the detection of 13C-labelled substrates in vivo and also imaging of tissue distribution, in the absence of any background signal from non-polarized material. Pyruvate is a molecule involved in major metabolic and catabolic pathways in mammalian cells (Krebs cycle) and depending on anaerobic or aerobic metabolism can have various end products. 1-13C-pyruvate imaging can therefore detect lactate, alanine and carbon dioxide.53,54 The imaging data generated by this technique in a transgenic mouse model of prostate adenocarcinoma were shown to correlate with the histological grading of tumours and have been used to identify tumour necrosis and metastatic lymph nodes. The NCT01229618 clinical trial is examining the role of 1-13C-pyruvate imaging in patients with prostate cancer.55 HP 13C MR spectroscopic imaging, measuring the HP lactate-to-pyruvate ratios, can be used to monitor the heterogeneity in a major signalling pathway within cancers, namely the PI3K/AKT/mTOR pathway and its response to molecule-targeted therapeutics, such as Everolimus,56 and potentially inhibitors of other signalling pathways, e.g. hypoxia-inducible factor-1 and MYC, which are known to predispose tumour cells to metastasize under both normoxic and hypoxic conditions.57–59
Nanoparticle-based imaging
Nanoparticles are small, 1–100 nm, structures that in recent years have been explored in their capacity for imaging, drug delivery and monitoring of treatment outcome.60 Nanoparticles may be organic based (liposomes, polymeric nanoparticles, micelles, dendrimers and solid lipid nanoparticles), inorganic based (iron oxide nanoparticles, gold nanoparticles, semiconductor nanocrystals, ceramic nanoparticles and carbon nanotubes) or a hybrid of both. Nanoparticles have large surface to volume ratios contributing to their high loading capacity. As drug delivery systems, nanoparticles have been shown to improve drug solubility, prolong blood circulation half-life and control drug release.61 One of the major advantages with nanoparticle technology is that drug delivery and imaging probes can be combined into one delivery system.
Gold nanoparticles have high density and extinction coefficients and can be applied as contrast agents for CT, dark field imaging and photoacoustic imaging. The shape of gold nanoparticles can facilitate them to strongly absorb light in the near-infrared range, converting this energy into heat for photothermal therapy. Iron oxide-based nanoparticles are magnetic and therefore used as contrast agents to produce hypodense regions on T2/T2 weighted MR images.
Nanoparticles have also been used as a predictive tool in functional imaging. Superparamagnetic iron oxide nanoparticles (SPIONs), specifically reporting on tumour vasculature, have recently been used in predicting the likelihood of brain metastases in melanomas.62,63 Various imaging nanoparticles are currently undergoing human clinical trials; for example, 124I-labelled cRGDY silica nanoparticles in melanoma (NCT01266096), 99mTC-sulphur colloid nanoparticles in sentinel node mapping in breast cancers (NCT00438477) and ultrasmall (U)SPION in pre-operative pancreatic cancers (NCT00920023). All of the above are a mixture of imaging modalities, CT, MRI and single photon emission CT (SPECT), showing that nanoparticle imaging is not exclusive to one imaging modality. A specific application of these (U)SPIOs to characterize the heterogeneity of macrophage infiltration in the tumour microenvironment will be described in section Application of an integrated imaging–genomic approach to stratify cancer treatment—requirements for clinical translation.
Imaging tumour heterogeneity at a cellular level: intra-operative optical imaging
The basis of radio-guided surgery involves the deployment of a radiolabelled tumour tracer pre-operatively and the use of a detection probe intra-operatively. Intra-operative use of a gamma probe has been shown to reveal small (<10 mm) lesions within the abdomen that can be missed on traditional whole-body functional imaging. This technique has been shown to individualize surgical procedures intra-operatively, resulting in improved complete resection rates with subsequent effects on reducing recurrence rates.64–67 Moreover, to facilitate the visualization of cancer cells at a higher resolution, intra-operative tumour imaging has been successfully conducted with near-infrared dye-labelled molecule-targeted antibodies against various tumour cell targets, e.g. folate receptor, VEGF (bevacizumab) and HER2 (trastuzumab).68,69 The first-in-man ovarian cancer surgery was performed with an optical detection device that has a corresponding resolution varying between 150 and 30 μm.69 It allows for individual cellular clusters to be visualized and dissected. Further genomic investigations of these cellular clusters is likely to add further details to the degree of cell-to-cell tumour heterogeneity and its role in promoting resistance within an evolving tumour genome.
MOLECULAR IMAGING OF METASTATIC POTENTIAL
Early identification of patients at high risk of metastatic disease is arguably the most important task for improving cancer mortality. The pre-metastatic niche hypothesis comprises the creation of a supportive environment for circulating tumour cells to “seed” to.70 This dynamic process is thought to involve both chemokine secretion at the primary tumour site and subsequent activation of immune cells in the target tissue of metastasis. In response to tumour-secreted factors (TSFs), intra- and extramedullary haematopoiesis and consecutive immune cell differentiations are altered in order to promote the survival and outgrowth of disseminated tumour cells. Certain organs carry a greater susceptibility to specific tumours; for example, bone metastases are prevalent in breast and prostate cancers, whereas are rarer in others, such as ovarian. The understanding of tumour heterogeneity should allow us to not only assess the primary tumour at a molecular level but also examine distant organs for pre-metastatic changes.
Although targeted SPECT and PET probes mostly address surface markers or metabolic features of the primary tumour cells themselves, the same principles can be used for visualization of metastasis-associated changes of tissue composition or intercellular communication. Using a PET imaging probe for vascular cell adhesion molecule-1 (VLA-4), reportedly highly expressed in bone marrow-derived cells that have been implicated in establishing the pre-metastatic niche,71 Shokeen et al72 reported using imaging combined with immunohistochemistry, an enrichment of these haematopoietic progenitor cells (HPCs) at the sites of metastasis. Besides the HPCs, tumour-associated macrophage (TAM) accumulation in the tumour microenvironment has been linked to increased tumour invasiveness and therefore metastasis5 In primary tumours, visualization of TAM by MRI is established and frequently performed using macromolecular substances that are taken up by the target cells via phagocytosis, such as (U)SPIONs,73 as mentioned earlier in this review. Nevertheless, the limited sensitivity of MRI (compared with the extremely high sensitivity of PET microdosing), combined with the high background activity of phagocytic cells in typical target organs of metastasis, would, however, probably hinder the use of such techniques in imaging of pre-metastatic tissue priming.
Another aspect of the promoting effect of TAM on tumour metastasis is through enhanced angiogenesis, partly through an increase in VEGF secretion by macrophages.74–77 VEGF is an important signalling pathway in vasculogenesis and angiogenesis, and therefore plays a vital role in tumour growth, survival and metastases. In oncology, there have been multiple anti-VEGF therapies, of various forms, monoclonal antibodies (bevacizumab) and small tyrosine kinase inhibitors (pazopanib). The use of DCE/MR in vascular imaging has already been discussed in the assessment of angiogenesis. The lack of an appropriate biomarker for VEGF inhibitors has been a particular issue in the clinical setting. VEGF inhibitors are used widely in various tumour types, such as breast,78 colon,79 ovarian,80 renal cell81 and hepatocellular;82 however, treatment response can be very difficult to assess, especially in the maintenance setting. DCE/MRI allows non-invasive quantification of tumour microvasculature through dynamic imaging of enhancement and washout of injected contrast material. The vascular configuration in tumours promotes an initial faster and greater accumulation of contrast within the interstitial space and favours more rapid removal of contrast from the interstitium, as the concentration of contrast in the blood falls owing to renal excretion. These features can be fitted to pharmacokinetic models, and the derived variables have been directly related to VEGF modulation of vascular permeability.83,84
Imaging of mediators of inflammation, such as tumour necrosis factors or interleukin-1α, has been performed successfully in clinical and experimental imaging of inflammation.85,86 However, given the high background activity and relatively low specific accumulation of the respective tracers at the target site where there is a significant degree of inflammation, it is not hopeful that the subtle potential changes in pre-metastatic tissue could specifically be picked up using these or comparable approaches. It has recently been established that, in pre-metastatic lung tissue of tumour-bearing animals, the vessel permeability is locally altered in response to TSFs,87 resembling local inflammation. This permeability as well as the accompanied increase in extravascular cellularity (e.g. inflammatory cell content in the extravascular compartment) could in theory be visualized using established imaging approaches such as MRI.88,89 It would be of interest to see changes in tissue architecture and other MRI-based assessment of features, such as collagen content, consecutive mechanical characteristics, vessel architecture etc., that are revealed during the establishment of metastasis.
Moreover, further investigation of the cellular composition of the pre-metastatic niche and the main regulating factors is strongly required.90 It would potentially enable the use of specific MRI approaches for tissue characterization as well as an armament of specific probes for radionuclide and optical imaging of already established disease-associated target molecules and cells.91 In this context, exosomes are 40- to 100-nm-diameter membrane vesicles of endocytic origin that have been demonstrated to contain mRNA, miRNA and proteins, and are gaining increasing interest in terms of their translational research potential in cancer.92–96 They are released into the extracellular space from various cell types and body fluids and mediate intercellular transfer of RNAs and proteins. As such, exosome analysis is ideally suited for monitoring the evolving tumour longitudinally, in terms of its whole transcriptome, miRNome and proteome profiles.92,97 Exosomes have been shown to have an important role in intercellular communication, and they are involved in stimulation of the secretion of growth factors, cytokines. There is growing evidence that exosomes are generally involved in the manipulation of the pre-metastatic niche.96,98,99 Imaging the transfer of exosomes secreted by tumour cells into host cells in a cancer mouse model suggests that the tumour-derived exosomes contribute to the formation of a niche to promote tumour growth and metastasis.100 A number of current studies have shown that there is a correlation between exosomes and metastasis in different types of cancers. The detection and quantification of exosomes carrying tumour-relative antigens in melanoma patients may represent a potential tool for cancer screening and prediction of metastatic risk.101 Tumour-derived microvesicles from patients with head and neck cancer induce apoptosis of activated CD8+ T cells that correlated with disease activity and the presence of lymph node metastases.102 Furthermore, exosomes adapt to hypoxia in the local tumour microenvironment during cancer progression and thus reflect the hypoxic state of cancer cells. Under hypoxic conditions, a change of the protein cargo of exosomes secreted by tumour cells was observed that modulates the microenvironment and promotes angiogenesis and metastasis.95 In highly aggressive brain tumours, the analysis of exosomes from patient samples reveals the enrichment in exosomes of hypoxia-regulated mRNAs and proteins.103 In addition to the in vitro analysis of plasma/serum exosomes, the effect of the exosomes on pulmonary vascular permeability96 can be assessed by the aforementioned MR-based whole-body imaging techniques.
Clinically, exosomes are increasing in prominence for their diagnostic/predictive potential in cancers. For example, the tumour suppressor gene phosphatase and tensin homolog is only expressed in exosomes that circulate in the blood of patients with prostate cancer, but it is not detected in exosomes from normal subjects, and might be thus a potential biomarker for prostate cancer.104 In another study, potential diagnostic markers for human NSCLC were identified by proteomic analysis of purified microvesicles from pleural effusion in patients with NSCLC.102 Micro-RNA and protein profiling of brain metastasis cell-derived exosomes vs non-brain metastasis revealed changes in specific miRNA and proteins which may contribute to the discovery of new biomarkers for brain metastasis.105 Similarly, proteome profiling of exosomes from human primary and metastatic colorectal cancer reveal different expression of key metastatic factors.106 These examples demonstrate the increasing importance of exosomes in the identification of novel biomarkers in metastatic cancers, although imaging in patients is still a little way off clinical application.107
APPLICATION OF AN INTEGRATED IMAGING–GENOMIC APPROACH TO STRATIFY CANCER TREATMENT—REQUIREMENTS FOR CLINICAL TRANSLATION
Much energy has been expended recently in establishing the role of imaging biomarkers for evaluating treatment response in cancers. An ongoing collaborative effort by the American College of Radiology Imaging Network (ACRIN), Philadelphia, PA, Cancer and Leukaemia Group B, Chicago, IL, and the National Cancer Institute, Bethesda, MD, Specialized Programs of Research Excellence recently conducted the largest multicentre imaging trial (ACRIN 6657) as part of the I-SPY1 trial (Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging and Molecular Analysis). ACRIN 6657 utilizes MRI to measure treatment response in patients receiving neoadjuvant chemotherapy.108 Volumetric estimates of the tumour size, based on functional criteria applied to contrast-enhanced images, were seen to have greater sensitivity than linear tumour diameter measurements for predicting pathologically complete responses in patients completing neoadjuvant chemotherapy. The greatest difference in predictive ability occurred at the early time points, providing “proof of concept” that imaging parameters can serve as non-invasive predictive biomarkers of early treatment response. Its subsequent I-SPY2 clinical trial platform targeting the rapid focused clinical development of paired oncologic therapies and biomarkers now uses MR volumes to provide information about response to chemotherapy between regimens—information that cannot otherwise be obtained without surgical resection.109 Additionally, its sub-study, ACRIN 6698, combines both DCE and DWI MRI data to generate novel imaging biomarkers that may correlate with treatment response,110 and its results are eagerly awaited. Integration of these imaging biomarkers with genomic profiles of tumours are likely to prove essential for future clinical translation.
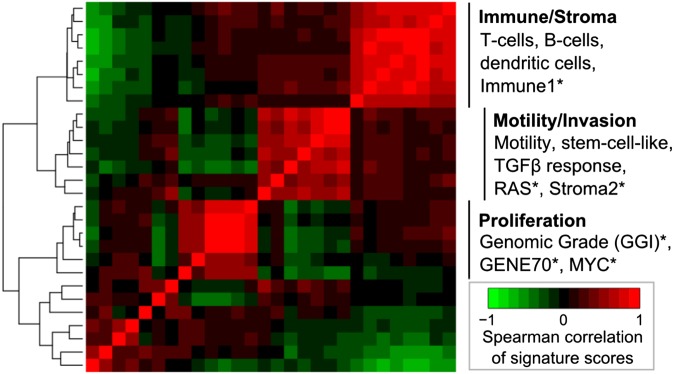
Transcriptomic analyses of primary solid tumours have revealed differential activation of gene expression signatures relating to cellular processes, including proliferation, cell migration and immune response (Figure 6) with the potential for prognostic and predictive stratification of tumours, which are phenotypically similar by current clinical methods.111,112,115 Meanwhile, putative associations between clinical imaging traits and gene expression profiles have been reported in solid tumours. Exploratory studies have reported correlations between selected image traits and the expression of individual genes or larger modules of co-expressed genes113,116 reviewed in Rutman and Kuo.117 Genomic copy number and other genomic aberrations exhibit variation between tumours from different patients118,119 and between subclones within a primary tumour4,120 Lesional and temporal variations in HER2 amplification and specific HER2 insertional mutations (such as HER2YVMA), for example, could have clinical implications for HER2-targeted treatment and monitoring in the metastatic setting.121,122 PET imaging using tracer-linked trastuzumab has been used to identify HER2-positive tumour and metastatic sites,123,124 indicating the potential for non-invasive monitoring of HER2-positive lesions. In the treatment–response setting, early metabolic response to trastuzumab (less than 48 h post treatment) was detected in a pre-clinical study using optical metabolic imaging but not FDG-PET.125 Many more genomic aberrations have been catalogued as part of large-cohort studies of primary solid tumours, revealing both recurrent mutations (e.g. p53, PIK3CA119,126) and recurrent dysregulation associated with a diversity of less frequent underlying genomic or transcriptional variation.121,127 Detection of intertumoral, interlesional and temporal variations may prove to be critical for describing and monitoring disease progression but would require methods for non-invasive detection. Non-invasive imaging, coupled to more advanced analyses, may in the future yield parameters that oncologists can monitor longitudinally, in conjunction with high-coverage NGS of plasma-derived DNA to monitor the evolution of tumour genomic profiles under treatment pressure.128 Some initial results have shown that there may be a correlation between some of these mutations (codons 12, 13 and 61 of KRAS, for instance) and various PET/CT-based parameters in colorectal cancers.129
Figure 6.
Intertumour heterogeneity of gene expression profiles associated with cellular processes and disease progression. Unsupervised hierarchical clustering showing pair-wise correlations of a panel of gene signature scores across primary breast tumour tissue samples (234 patients). Representative signatures are indicated for each cluster: T-cells, B-cells and dendritic cells, Immune1*, Motility, stem-cell-like, tumour growth factor β (TGFβ) response, RAS*, Stroma2*, GGI*, Gene70*, MYC* (*signature curated by Ignatiadis et al112). Recent studies report expression-based prognostic and predictive stratification of primary breast tumours, which are phenotypically similar according to current clinical methods.111,113,114 Images should be viewed in the online format.
TRANSLATION OF IMAGING TECHNOLOGIES IN ONCOLOGICAL TRIALS
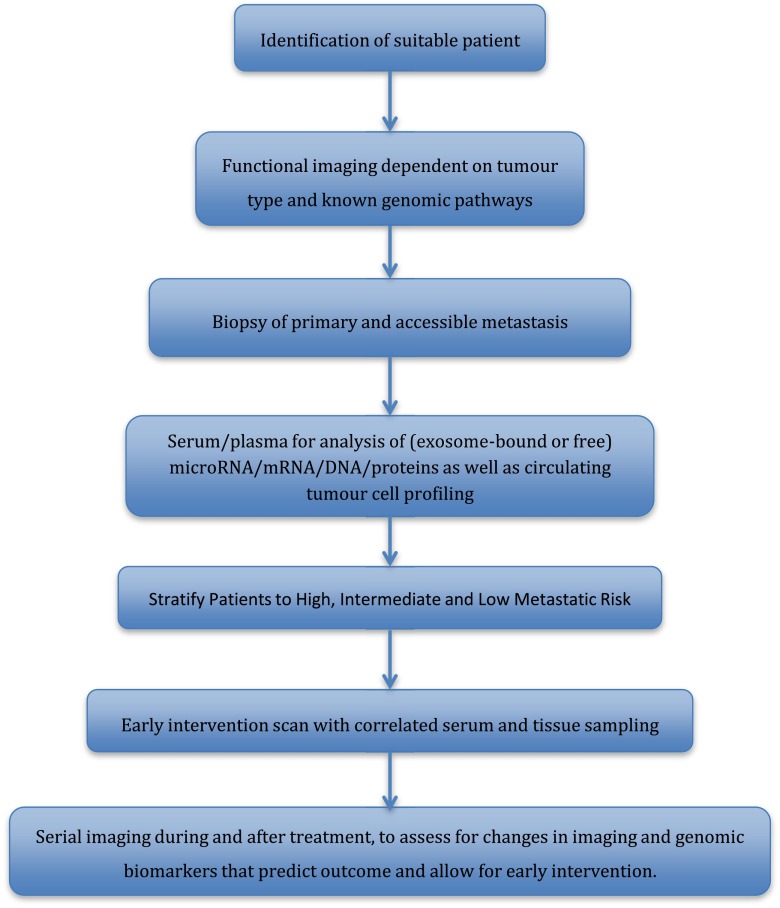
Given the lack of measurable biomarkers through patient sampling, the advances in molecular imaging provide an impetus for testing functional imaging as a cancer biomarker, in a way that is complementary to tissue- and blood-based biomarkers. Despite these rapid advances, the translation of such techniques into clinics continues to lag behind. Incorporation of functional imaging to evaluate tumour responses should play an important role in designing future trials (Figure 7). Strategically planned biomarker evaluations with access to functional imaging in early phase trials (Phase I/II) will allow for efficient Phase III clinical trial designs, increasing the chances of positive Phase III biomarker-driven trial results. Functional imaging can not only provide information on the treatment response but can also monitor mutational pathways and the various molecular pathway pressures on an individual tumour, allowing a more robust stratification of treatment pathways. A major setback for targeted therapy has been the duration of tumour response, as many patients go on to have progressive disease after a relatively short response period. At present, although we understand a small fraction of these tumour escape pathways, we are unable to respond in a clinical setting to early mutational changes. Functional imaging information can help identify and assess high-/at-risk patients non-invasively, allowing for implementation of appropriate management plans governed by their personal escape pathway and thereby improving patient outcome.
Figure 7.
Schematic of potential future trial design, incorporating functional imaging and tissue samples to further biomarker research.
At present robust large patient trials examining the methods we have discussed in this review are lacking. However, a few large trials are currently incorporating functional imaging within their remit. The NeoPHOEBE trial is a Phase II trial examining the application of FDG-PET as a biomarker of early response in the neoadjuvant setting in the treatment of HER2-positive breast cancer. Similarly, the FOCUS4 trial, currently recruiting, is a molecularly stratified randomized trial for patients with inoperable or metastatic colorectal cancer. It contains five arms [v-raf murine sarcoma viral oncogene homolog B (BRAF), PI3KCA, RAS, no mutation and non-classified] of randomization with prior histological analysis of patient pathology determining treatment. Despite the optimism behind these trials, the need for robust validation is crucial in order to offer patients lasting results.
The role of clinical trials should not be purely to review efficacy of treatment but also to aid the development of new research. To this end, the acquisition of patient samples at each step of the treatment paradigm plays a vital role in developing the translational application of research. As previously discussed, the role of exosomes in cancers is developing in prominence and understanding alongside the emerging role of circulating tumour cells and their reflection of the primary and metastatic tumour.
The integration of functional imaging, patient sampling and drug development together with wider research is likely to play a key role in fully understanding the nature of heterogeneity and ultimately how to control its effects to clinical advantage.
CONCLUSION
In this review, we have discussed the novel application of current imaging techniques in the assessment of heterogeneity especially in the context of examining metastasis and predicting metastatic potential. Although we have access to and are developing new tracers and new imaging techniques, there is a significant need for large patient trials and applications to fully determine their specific validity in the personalized patient treatment paradigm.
CONFLICTS OF INTEREST
B. Ganeshan and K.A. Miles are shareholders in TexRAD Ltd, a company developing and marketing the commercial (research) texture analysis software.
FUNDING
This work was supported by KCL Breakthrough Breast Cancer Research Unit/Sarah Green Fellowship funding (SI) and an endowment fund from Dimbleby Cancer Care to King's College London (TN). KL and ME were supported by the KCL-UCL Comprehensive Cancer Imaging Centre funding [CR-UK & EPSRC, in association with the MRC and DoH (England)]. HM was supported by an FP7-HEALTH-2010 European Union grant entitled “IMAGINT” (grant no. 259881). This work was undertaken at UCLH/UCL, which received a proportion of the funding from the UK's Department of Health's NIHR Biomedical Research Centre's funding scheme. Further funding was received from other NIHR sources.
REFERENCES
- 1.Fruhwirth GO, Fernandes LP, Weitsman G, Patel G, Kelleher M, Lawler K, et al. How Förster resonance energy transfer imaging improves the understanding of protein interaction networks in cancer biology. Chemphyschem 2011; 12: 442–61. [DOI] [PubMed] [Google Scholar]
- 2.Virchow R. Die cellular pathologie in ihrer begründung auf physiologische und pathologische gewebelehre. Berlin, Germany: August Hirschwald; 1858. [Google Scholar]
- 3.Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, et al. Tumour evolution inferred by single-cell sequencing. Nature 2011; 472: 90–4. doi: 10.1038/nature09807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366: 883–92. doi: 10.1056/NEJMoa1113205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19: 1423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer 2009; 9: 285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, et al. Systemic spread is an early step in breast cancer. Cancer Cell 2008; 13: 58–68. [DOI] [PubMed] [Google Scholar]
- 8.Patel GS, Kiuchi T, Lawler K, Ofo E, Fruhwirth GO, Kelleher M, et al. The challenges of integrating molecular imaging into the optimization of cancer therapy. Integr Biol (Camb) 2011; 3: 603–31. doi: 10.1039/c0ib00131g [DOI] [PubMed] [Google Scholar]
- 9.Shah SP, Morin RD, Khattra J, Prentice L, Pugh T, Burleigh A, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature 2009; 461: 809–13. doi: 10.1038/nature08489 [DOI] [PubMed] [Google Scholar]
- 10.Hoefnagel LD, van der Groep P, van de Vijver MJ, Boers JE, Wesseling P, Wesseling J, et al. Discordance in ERα, PR and HER2 receptor status across different distant breast cancer metastases within the same patient. Ann Oncol 2013; 24: 3017–23. [DOI] [PubMed] [Google Scholar]
- 11.Gaertner FC, Fürst S, Schwaiger M. PET/MR: a paradigm shift. Cancer Imaging 2013; 13: 36–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warburg O. On the origin of cancer cells. Science 1956; 123: 309–14. [DOI] [PubMed] [Google Scholar]
- 13.Huyge V, Garcia C, Alexiou J, Ameye L, Vanderlinden B, Lemort M, et al. Heterogeneity of metabolic response to systemic therapy in metastatic breast cancer patients. Clin Oncol (R Coll Radiol) 2010; 22: 818–27. doi: 10.1016/j.clon.2010.05.021 [DOI] [PubMed] [Google Scholar]
- 14.Ganeshan B, Goh V, Mandeville HC, Ng QS, Hoskin PJ, Miles KA. Non–small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology 2013; 266: 326–36. [DOI] [PubMed] [Google Scholar]
- 15.Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K. Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 2012; 22: 796–802. doi: 10.1007/s00330-011-2319-8 [DOI] [PubMed] [Google Scholar]
- 16.Ganeshan B, Abaleke S, Young RCD, Chatwin CR, Miles KA. Texture analysis of non-small cell lung cancer on unenhanced computed tomography: initial evidence for a relationship with tumour glucose metabolism and stage. Cancer Imaging 2010; 10: 137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganeshan B, Miles KA, Young RCD, Chatwin CR. Hepatic enhancement in colorectal cancer: texture analysis correlates with hepatic hemodynamics and patient survival. Acad Radiol 2007; 14: 1520–30. doi: 10.1016/j.acra.2007.06.028 [DOI] [PubMed] [Google Scholar]
- 18.Miles KA, Ganeshan B, Griffiths MR, Young RC, Chatwin CR. Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology 2009; 250: 444–52. doi: 10.1148/radiol.2502071879 [DOI] [PubMed] [Google Scholar]
- 19.Ganeshan B, Miles KA, Young RC, Chatwin CR. In search of biologic correlates for liver texture on portal-phase CT. Acad Radiol 2007; 14: 1058–68. doi: 10.1016/j.acra.2007.05.023 [DOI] [PubMed] [Google Scholar]
- 20.Cheng NM, Dean Fang YH, Chang JT, Huang CG, Tsan DL, Ng SH, et al. Textural features of pretreatment 18F-FDG PET/CT images: prognostic significance in patients with advanced T-stage oropharyngeal squamous cell carcinoma. J Nucl Med 2013; 54: 1703–9. [DOI] [PubMed] [Google Scholar]
- 21.Brown R, Zlatescu M, Sijben A, Roldan G, Easaw J, Forsyth P, et al. The use of magnetic resonance imaging to noninvasively detect genetic signatures in oligodendroglioma. Clin Cancer Res 2008; 14: 2357–62. doi: 10.1158/1078-0432.CCR-07-1964 [DOI] [PubMed] [Google Scholar]
- 22.Foroutan P, Kreahling JM, Morse DL, Grove O, Lloyd MC, Reed D, et al. Diffusion MRI and novel texture analysis in osteosarcoma xenotransplants predicts response to anti-checkpoint therapy. PLoS One 2013; 8: e82875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miles KA, Ganeshan B, Hayball MP. CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging 2013; 13: 400–6. doi: 10.1102/1470-7330.2013.9045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganeshan B, Skogen K, Pressney I, Coutroubis D, Miles K. Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol 2012; 67: 157–64. [DOI] [PubMed] [Google Scholar]
- 25.Cook GJ, Yip C, Siddique M, Goh V, Chicklore S, Roy A, et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med 2013; 54: 19–26. doi: 10.2967/jnumed.112.107375 [DOI] [PubMed] [Google Scholar]
- 26.Fan X, River JN, Zamora M, Tarlo K, Kellar K, Rinker-Schaeffer C, et al. Differentiation of nonmetastatic and metastatic rodent prostate tumors with high spectral and spatial resolution MRI. Magn Reson Med 2001; 45: 1046–55. [DOI] [PubMed] [Google Scholar]
- 27.Win T, Miles KA, Janes SM, Ganeshan B, Shastry M, Endozo R, et al. Tumor heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non-small cell lung cancer. Clin Cancer Res 2013; 19: 3591–9. doi: 10.1158/1078-0432.CCR-12-1307 [DOI] [PubMed] [Google Scholar]
- 28.Tixier F, Hatt M, Le Rest CC, Le Pogam A, Corcos L, Visvikis D. Reproducibility of tumor uptake heterogeneity characterization through textural feature analysis in 18F-FDG PET. J Nucl Med 2012; 53: 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boothman DA, Davis TW, Sahijdak WM. Enhanced expression of thymidine kinase in human cells following ionizing radiation. Int J Radiat Oncol Biol Phys 1994; 30: 391–8. [DOI] [PubMed] [Google Scholar]
- 30.Mirjolet JF, Barberi-Heyob M, Merlin JL, Marchal S, Etienne MC, Milano G, et al. Thymidylate synthase expression and activity: relation to S-phase parameters and 5-fluorouracil sensitivity. Br J Cancer 1998; 78: 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francis DL, Freeman A, Visvikis D, Costa DC, Luthra SK, Novelli M, et al. In vivo imaging of cellular proliferation in colorectal cancer using positron emission tomography. Gut 2003; 52: 1602–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roger L, Gadea G, Roux P. Control of cell migration: a tumour suppressor function for p53? Biol Cell 2006; 98: 141–52. [DOI] [PubMed] [Google Scholar]
- 33.Evdokimova V, Tognon C, Ng T, Sorensen PH. Reduced proliferation and enhanced migration: two sides of the same coin? Molecular mechanisms of metastatic progression by YB-1. Cell Cycle 2009; 8: 2901–6. [DOI] [PubMed] [Google Scholar]
- 34.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell 2013; 155: 750–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troost EGC, Vogel WV, Merkx MA, Slootweg PJ, Marres HA, Peeters WJ, et al. 18F-FLT PET does not discriminate between reactive and metastatic lymph nodes in primary head and neck cancer patients. J Nucl Med 2007; 48: 726–35. doi: 10.2967/jnumed.106.037473 [DOI] [PubMed] [Google Scholar]
- 36.Troost EG, Bussink J, Oyen WJ, Kaanders JH. 18F-FDG and 18F-FLT do not discriminate between reactive and metastatic lymph nodes in oral cancer. J Nucl Med 2009; 50: 490–1. doi: 10.2967/jnumed.108.055962 [DOI] [PubMed] [Google Scholar]
- 37.Umbehr MH, Müntener M, Hany T, Sulser T, Bachmann LM. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol 2013; 64: 106–17. doi: 10.1016/j.eururo.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 38.Yang Z, Sun Y, Zhang Y, Xue J, Wang M, Shi W, et al. Can fluorine-18 fluoroestradiol positron emission tomography–computed tomography demonstrate the heterogeneity of breast cancer in vivo? Clin Breast Cancer 2013; 13: 359–63. [DOI] [PubMed] [Google Scholar]
- 39.Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med 2008; 14: 459–65. [DOI] [PubMed] [Google Scholar]
- 40.Choyke PL, Dwyer AJ, Knopp MV. Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging 2003; 17: 509–20. [DOI] [PubMed] [Google Scholar]
- 41.Metz S, Ganter C, Lorenzen S, van Marwick S, Herrmann K, Lordick F, et al. Phenotyping of tumor biology in patients by multimodality multiparametric imaging: relationship of microcirculation, alphavbeta3 expression, and glucose metabolism. J Nucl Med 2010; 51: 1691–8. [DOI] [PubMed] [Google Scholar]
- 42.Glaus C, Rossin R, Welch MJ, Bao G. In vivo evaluation of (64)Cu-labeled magnetic nanoparticles as a dual-modality PET/MR imaging agent. Bioconjug Chem 2010; 21: 715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fraioli F, Punwani S. Clinical and research applications of simultaneous positron emission tomography and MRI. Br J Radiol 2014; 87: 20130464. doi: 10.1259/bjr.20130464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langer DL, van der Kwast TH, Evans AJ, Trachtenberg J, Wilson BC, Haider MA. Prostate cancer detection with multi-parametric MRI: logistic regression analysis of quantitative T2, diffusion-weighted imaging, and dynamic contrast-enhanced MRI. J Magn Reson Imaging 2009; 30: 327–34. [DOI] [PubMed] [Google Scholar]
- 45.Panebianco V, Barchetti F, Sciarra A, Musio D, Forte V, Gentile V, et al. Prostate cancer recurrence after radical prostatectomy: the role of 3-T diffusion imaging in multi-parametric magnetic resonance imaging. Eur Radiol 2013; 23: 1745–52. [DOI] [PubMed] [Google Scholar]
- 46.Reiner CS, Stolzmann P, Husmann L, Burger IA, Hüllner MW, Schaefer NG, et al. Protocol requirements and diagnostic value of PET/MR imaging for liver metastasis detection. Eur J Nucl Med Mol Imaging 2014; 41: 649–58. doi: 10.1007/s00259-013-2654-x [DOI] [PubMed] [Google Scholar]
- 47.Antoch G, Bockisch A. Combined PET/MRI: a new dimension in whole-body oncology imaging? Eur J Nucl Med Mol Imaging 2009; 36(Suppl. 1): S113–20. doi: 10.1007/s00259-008-0951-6 [DOI] [PubMed] [Google Scholar]
- 48.Wiesmüller M, Quick HH, Navalpakkam B, Lell MM, Uder M, Ritt P, et al. Comparison of lesion detection and quantitation of tracer uptake between PET from a simultaneously acquiring whole-body PET/MR hybrid scanner and PET from PET/CT. Eur J Nucl Med Mol Imaging 2013; 40: 12–21. doi: 10.1007/s00259-012-2249-y [DOI] [PubMed] [Google Scholar]
- 49.Chandarana H, Heacock L, Rakheja R, DeMello LR, Bonavita J, Block TK, et al. Pulmonary nodules in patients with primary malignancy: comparison of hybrid PET/MR and PET/CT imaging. Radiology 2013; 268: 874–81. doi: 10.1148/radiol.13130620 [DOI] [PubMed] [Google Scholar]
- 50.Ohno Y, Koyama H, Yoshikawa T, Matsumoto K, Aoyama N, Onishi Y, et al. Diffusion-weighted MRI versus 18F-FDG PET/CT: performance as predictors of tumor treatment response and patient survival in patients with non-small cell lung cancer receiving chemoradiotherapy. AJR Am J Roentgenol 2012; 198: 75–82. [DOI] [PubMed] [Google Scholar]
- 51.Walker-Samuel S, Ramasawmy R, Torrealdea F, Rega M, Rajkumar V, Johnson SP, et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat Med 2013; 19: 1067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golman K. Molecular imaging using hyperpolarized 13C. Br J Radiol 2003; 76(Suppl. 2): S118–27. [DOI] [PubMed] [Google Scholar]
- 53.Brindle KM, Bohndiek SE, Gallagher FA, Kettunen MI. Tumor imaging using hyperpolarized 13C magnetic resonance spectroscopy. Magn Reson Med 2011; 66: 505–19. [DOI] [PubMed] [Google Scholar]
- 54.Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, et al. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med 2007; 13: 1382–7. [DOI] [PubMed] [Google Scholar]
- 55.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci Transl Med 2013; 5: 198ra108. doi: 10.1126/scitranslmed.3006070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaumeil MM, Ozawa T, Park I, Scott K, James CD, Nelson SJ, et al. Hyperpolarized 13C MR spectroscopic imaging can be used to monitor Everolimus treatment in vivo in an orthotopic rodent model of glioblastoma. Neuroimage 2012; 59: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dafni H, Larson PE, Hu S, Yoshihara HA, Ward CS, Venkatesh HS, et al. Hyperpolarized 13C spectroscopic imaging informs on hypoxia-inducible factor-1 and myc activity downstream of platelet-derived growth factor receptor. Cancer Res 2010; 70: 7400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu S, Balakrishnan A, Bok RA, Anderton B, Larson PE, Nelson SJ, et al. 13C-pyruvate imaging reveals alterations in glycolysis that precede c-Myc-induced tumor formation and regression. Cell Metab 2011; 14: 131–42. doi: 10.1016/j.cmet.2011.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolfer A, Ramaswamy S. MYC and metastasis. Cancer Res 2011; 71: 2034–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berry CC, Curtis ASG. Functionalisation of magnetic nanoparticles for applications in biomedicine. J Phys D: Appl Phys 2003; 36: R198–206. [Google Scholar]
- 61.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano 2009; 3: 16–20. [DOI] [PubMed] [Google Scholar]
- 62.Sundstrøm T, Daphu I, Wendelbo I, Hodneland E, Lundervold A, Immervoll H, et al. Automated tracking of nanoparticle-labeled melanoma cells improves the predictive power of a brain metastasis model. Cancer Res 2013; 73: 2445–56. [DOI] [PubMed] [Google Scholar]
- 63.Weinstein JS, Varallyay CG, Dosa E, Gahramanov S, Hamilton B, Rooney WD, et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab 2010; 30: 15–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarikaya I, Povoski SP, Al-Saif OH, Kocak E, Bloomston M, Marsh S, et al. Combined use of preoperative 18F FDG-PET imaging and intraoperative gamma probe detection for accurate assessment of tumor recurrence in patients with colorectal cancer. World J Surg Oncol 2007; 5: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Epenetos AA, Kosmas C. Monoclonal antibodies for imaging and therapy. Br J Cancer 1989; 59: 152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nieroda CA, Mojzisik C, Sardi A, Ferrara PJ, Hinkle G, Thurston MO, et al. Radioimmunoguided surgery in primary colon cancer. Cancer Detect Prev 1990; 14: 651–6. [PubMed] [Google Scholar]
- 67.Nieroda CA, Mojzisik C, Hinkle G, Thurston MO, Martin EW. Radioimmunoguided surgery (RIGS) in recurrent colorectal cancer. Cancer Detect Prev 1991; 15: 225–9. [PubMed] [Google Scholar]
- 68.Terwisscha van Scheltinga AG, van Dam GM, Nagengast WB, Ntziachristos V, Hollema H, Herek JL, et al. Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J Nucl Med 2011; 52: 1778–85. [DOI] [PubMed] [Google Scholar]
- 69.Van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med 2011; 17: 1315–19. [DOI] [PubMed] [Google Scholar]
- 70.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res 2006; 66: 11089–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005; 438: 820–7. doi: 10.1038/nature04186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shokeen M, Zheleznyak A, Wilson JM, Jiang M, Liu R, Ferdani R, et al. Molecular imaging of very late antigen-4 (α4β1 integrin) in the premetastatic niche. J Nucl Med 2012; 53: 779–86. doi: 10.2967/jnumed.111.100073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daldrup-Link H, Coussens LM. MR imaging of tumor-associated macrophages. Oncoimmunology 2012; 1: 507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol 2009; 21: 154–65. [DOI] [PubMed] [Google Scholar]
- 75.Obeid E, Nanda R, Fu YX, Olopade OI. The role of tumor-associated macrophages in breast cancer progression (review). Int J Oncol 2013; 43: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol 2008; 84: 623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tartour E, Pere H, Maillere B, Terme M, Merillon N, Taieb J, et al. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev 2011; 30: 83–95. [DOI] [PubMed] [Google Scholar]
- 78.Miles DW, Chan A, Dirix LY, Cortés J, Pivot X, Tomczak P, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2010; 28: 3239–47. [DOI] [PubMed] [Google Scholar]
- 79.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008; 26: 2013–19. doi: 10.1200/JCO.2007.14.9930 [DOI] [PubMed] [Google Scholar]
- 80.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007; 25: 5165–71. [DOI] [PubMed] [Google Scholar]
- 81.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356: 115–24. doi: 10.1056/NEJMoa065044 [DOI] [PubMed] [Google Scholar]
- 82.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–90. [DOI] [PubMed] [Google Scholar]
- 83.Hirashima Y, Yamada Y, Tateishi U, Kato K, Miyake M, Horita Y, et al. Pharmacokinetic parameters from 3-Tesla DCE-MRI as surrogate biomarkers of antitumor effects of bevacizumab plus FOLFIRI in colorectal cancer with liver metastasis. Int J Cancer 2012; 130: 2359–65. [DOI] [PubMed] [Google Scholar]
- 84.Kelly RJ, Rajan A, Force J, Lopez-Chavez A, Keen C, Cao L, et al. Evaluation of KRAS mutations, angiogenic biomarkers, and DCE-MRI in patients with advanced non-small-cell lung cancer receiving sorafenib. Clin Cancer Res 2011; 17: 1190–9. doi: 10.1158/1078-0432.CCR-10-2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barrera P, van der Laken CJ, Boerman OC, Oyen WJ, van de Ven MT, van Lent PL, et al. Radiolabelled interleukin-1 receptor antagonist for detection of synovitis in patients with rheumatoid arthritis. Rheumatology 2000; 39: 870–4. [DOI] [PubMed] [Google Scholar]
- 86.Barrera P, Oyen WJ, Boerman OC, van Riel PL. Scintigraphic detection of tumour necrosis factor in patients with rheumatoid arthritis. Ann Rheum Dis 2003; 62: 825–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hiratsuka S, Ishibashi S, Tomita T, Watanabe A, Akashi-Takamura S, Murakami M, et al. Primary tumours modulate innate immune signalling to create pre-metastatic vascular hyperpermeability foci. Nat Commun 2013; 4: 1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kiessling F, Farhan N, Lichy MP, Vosseler S, Heilmann M, Krix M, et al. Dynamic contrast-enhanced magnetic resonance imaging rapidly indicates vessel regression in human squamous cell carcinomas grown in nude mice caused by VEGF receptor 2 blockade with DC101. Neoplasia 2004; 6: 213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ehling J, Lammers T, Kiessling F. Non-invasive imaging for studying anti-angiogenic therapy effects. Thromb Haemost 2013; 109: 375–90. doi: 10.1160/TH12-10-0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rafii S, Lyden D. S100 chemokines mediate bookmarking of premetastatic niches. Nat Cell Biol 2006; 8: 1321–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature 2008; 452: 580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soekmadji C, Russell PJ, Nelson CC. Exosomes in prostate cancer: putting together the pieces of a puzzle. Cancers (Basel) 2013; 5: 1522–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lorentzen E, Conti E. The exosome and the proteasome: nano-compartments for degradation. Cell 2006; 125: 651–4. doi: 10.1016/j.cell.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 94.Makino DL, Halbach F, Conti E. The RNA exosome and proteasome: common principles of degradation control. Nat Rev Mol Cell Biol 2013; 14: 654–60. [DOI] [PubMed] [Google Scholar]
- 95.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics 2010; 9: 1085–99. doi: 10.1074/mcp.M900381-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18: 883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiao D, Ohlendorf J, Chen Y, Taylor DD, Rai SN, Waigel S, et al. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One 2012; 7: e46874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rana S, Malinowska K, Zöller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia 2013; 15: 281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl) 2013; 91: 431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suetsugu A, Honma K, Saji S, Moriwaki H, Ochiya T, Hoffman RM. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev 2013; 65: 383–90. doi: 10.1016/j.addr.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 101.Logozzi M, De Milito A, Lugini L, Borghi M, Calabrò L, Spada M, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One 2009; 4: e5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bergmann C, Strauss L, Wieckowski E, Czystowska M, Albers A, Wang Y, et al. Tumor-derived microvesicles in sera of patients with head and neck cancer and their role in tumor progression. Head Neck 2009; 31: 371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A 2013; 110: 7312–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gabriel K, Ingram A, Austin R, Kapoor A, Tang D, Majeed F, et al. Regulation of the tumor suppressor PTEN through exosomes: a diagnostic potential for prostate cancer. PLoS One 2013; 8: e70047. doi: 10.1371/journal.pone.0070047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Camacho L, Guerrero P, Marchetti D. MicroRNA and protein profiling of brain metastasis competent cell-derived exosomes. PLoS One 2013; 8: e73790. doi: 10.1371/journal.pone.0073790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ji H, Greening DW, Barnes TW, Lim JW, Tauro BJ, Rai A, et al. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics 2013; 13: 1672–86. [DOI] [PubMed] [Google Scholar]
- 107.Matusiak N, van Waarde A, Bischoff R, Oltenfreiter R, van de Wiele C, Dierckx RA, et al. Probes for non-invasive matrix metalloproteinase-targeted imaging with PET and SPECT. Curr Pharm Des 2013; 19: 4647–72. [DOI] [PubMed] [Google Scholar]
- 108.Hylton NM, Blume JD, Bernreuter WK, Pisano ED, Rosen MA, Morris EA, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—results from ACRIN 6657/I-SPY TRIAL. Radiology 2012; 263: 663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther 2009; 86: 97–100. doi: 10.1038/clpt.2009.68 [DOI] [PubMed] [Google Scholar]
- 110.Hylton N, Partridge S, Rosen M, Kim E, L'Heureux D, Esseman L. OT2-03-06: ACRIN 6698 MR Imaging Biomarkers for Assessment of Breast Cancer Response to Neoadjuvant Chemotherapy: A Sub-Study of the I-SPY 2 TRIAL (Investigation of Serial Studies To Predict Your Therapeutic Response with Imaging And moLecular Analysis). Cancer Res 2012; 71(Suppl). [Google Scholar]
- 111.Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol 2007; 8: R157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ignatiadis M, Singhal SK, Desmedt C, Haibe-Kains B, Criscitiello C, Andre F, et al. Gene modules and response to neoadjuvant chemotherapy in breast cancer subtypes: a pooled analysis. J Clin Oncol 2012; 30: 1996–2004. doi: 10.1200/JCO.2011.39.5624 [DOI] [PubMed] [Google Scholar]
- 113.Lee JD, Yun M, Lee JM, Choi Y, Choi YH, Kim JS, et al. Analysis of gene expression profiles of hepatocellular carcinomas with regard to 18F-fluorodeoxyglucose uptake pattern on positron emission tomography. Eur J Nucl Med Mol Imaging 2004; 31: 1621–30. [DOI] [PubMed] [Google Scholar]
- 114.Ignatius MS, Chen E, Elpek NM, Fuller AZ, Tenente IM, Clagg R, et al. In vivo imaging of tumor-propagating cells, regional tumor heterogeneity, and dynamic cell movements in embryonal rhabdomyosarcoma. Cancer Cell 2012; 21: 680–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karn T, Pusztai L, Holtrich U, Iwamoto T, Shiang CY, Schmidt M, et al. Homogeneous datasets of triple negative breast cancers enable the identification of novel prognostic and predictive signatures. PLoS One 2011; 6: e28403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Segal E, Sirlin CB, Ooi C, Adler AS, Gollub J, Chen X, et al. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat Biotechnol 2007; 25: 675–80. doi: 10.1038/nbt1306 [DOI] [PubMed] [Google Scholar]
- 117.Rutman AM, Kuo MD. Radiogenomics: creating a link between molecular diagnostics and diagnostic imaging. Eur J Radiol 2009; 70: 232–41. [DOI] [PubMed] [Google Scholar]
- 118.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature 2007; 446: 153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, et al. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nik-Zainal S, Van Loo P, Wedge DC, Alexandrov LB, Greenman CD, Lau KW, et al. The life history of 21 breast cancers. Cell 2012; 149: 994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri S, Lau C, Zaidinski M, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012; 18: 4910–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Perera SA, Li D, Shimamura T, Raso MG, Ji H, Chen L, et al. HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proc Natl Acad Sci U S A 2009; 106: 474–9. doi: 10.1073/pnas.0808930106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 2010; 87: 586–92. doi: 10.1038/clpt.2010.12 [DOI] [PubMed] [Google Scholar]
- 124.Tamura K, Kurihara H, Yonemori K, Tsuda H, Suzuki J, Kono Y, et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med 2013; 54: 1869–75. doi: 10.2967/jnumed.112.118612 [DOI] [PubMed] [Google Scholar]
- 125.Walsh AJ, Cook RS, Manning HC, Hicks DJ, Lafontant A, Arteaga CL, et al. Optical metabolic imaging identifies glycolytic levels, subtypes, and early-treatment response in breast cancer. Cancer Res 2013; 73: 6164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011; 333: 1157–60. doi: 10.1126/science.1208130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hofree M, Shen JP, Carter H, Gross A, Ideker T. Network-based stratification of tumor mutations. Nat Methods 2013; 10: 1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diaz LA, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012; 486: 537–40. doi: 10.1038/nature11219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miles KA, Ganeshan B, Rodriguez-Justo M, Goh VJ, Ziauddin Z, Engledow A, et al. Multifunctional imaging signature for V-KI-RAS2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations in colorectal cancer. J Nucl Med 2014; 55: 386–91. doi: 10.2967/jnumed.113.120485 [DOI] [PubMed] [Google Scholar]